On the Potential of a Poly(vinylidenefluoride-co-hexafluoropropylene) Polymer Inclusion Membrane Containing Aliquat® 336 and Dibutyl Phthalate for V(V) Extraction from Sulfate Solutions
Abstract
:1. Introduction
2. Methods
2.1. Reagents
2.2. Instrumentation
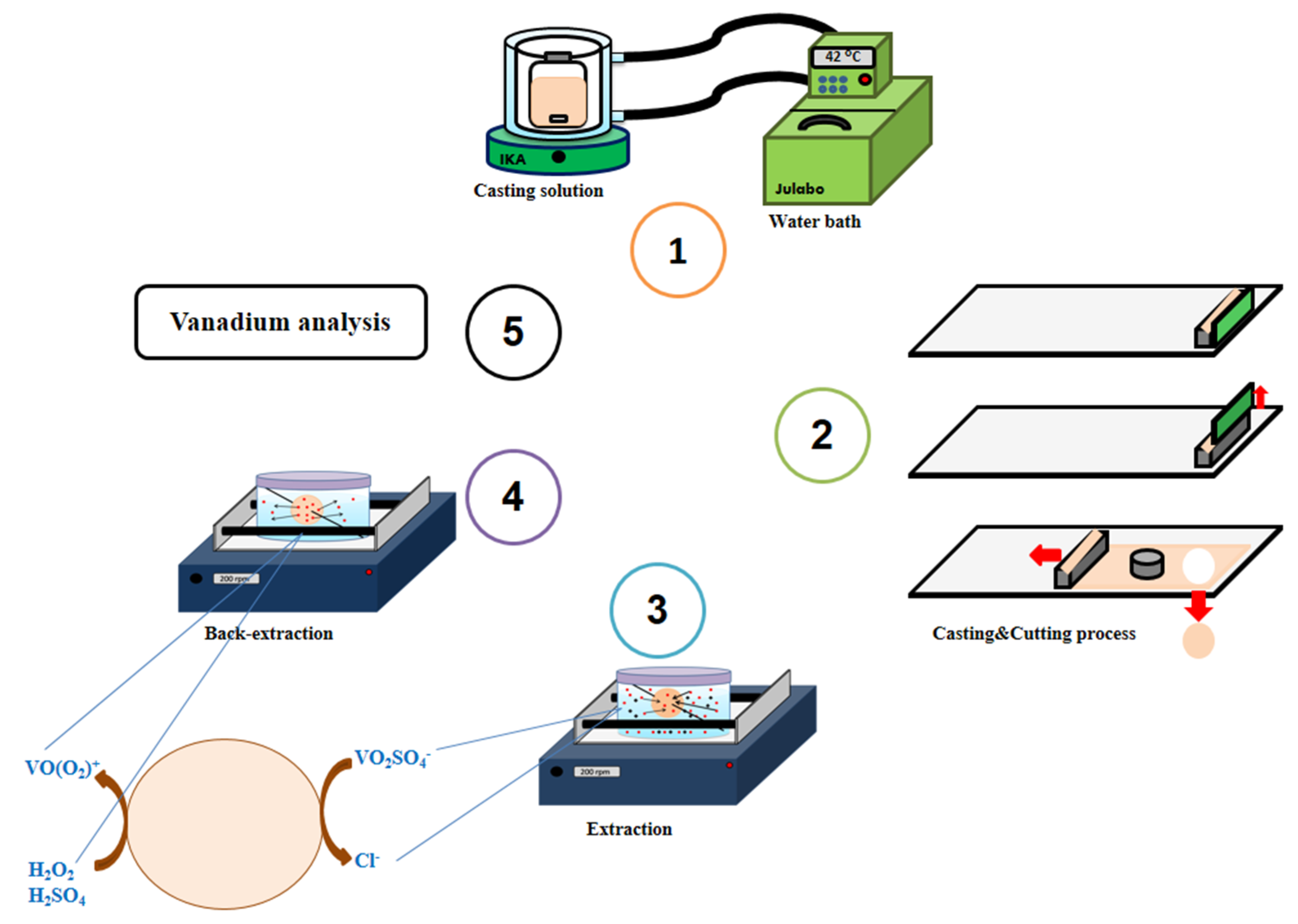
2.3. Membrane Preparation
2.4. Extraction and Back-Extraction Experiments
2.5. Eliminating the Co-Extraction Mo(VI) with V(V)
3. Results and Discussion
3.1. Preliminary Extraction Experiments
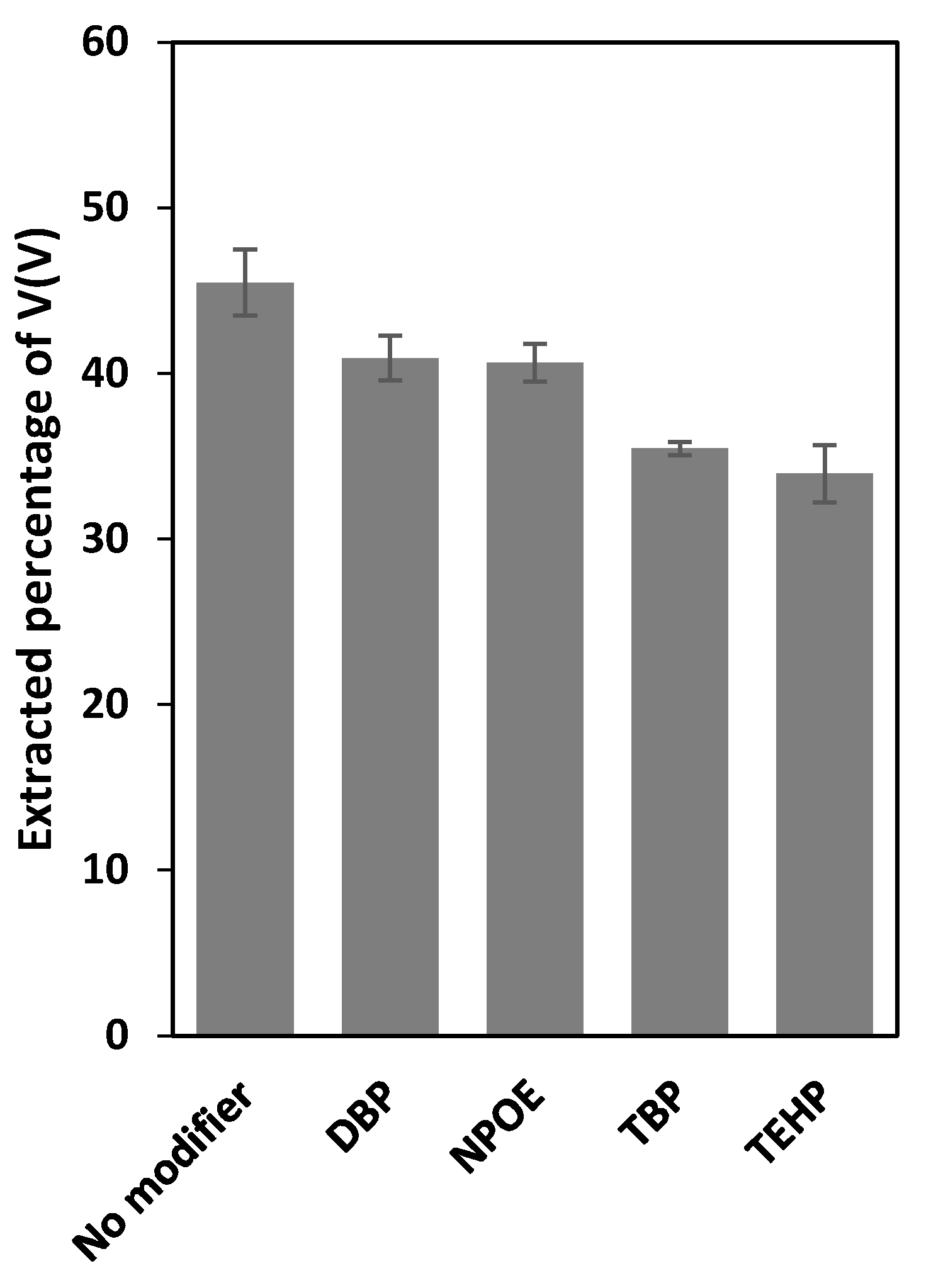
3.2. Selection of a Plasticizer/Modifier
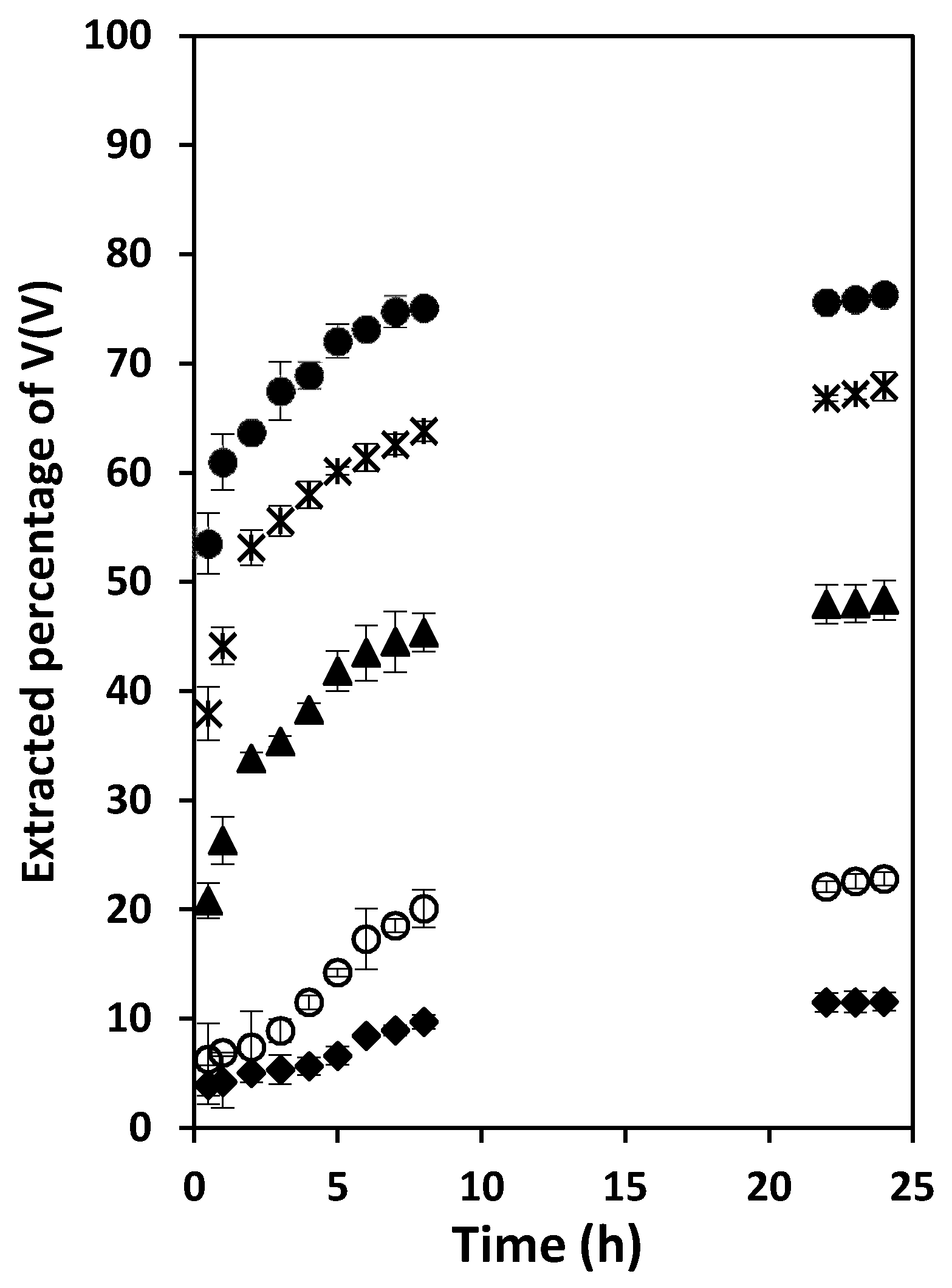
3.3. Optimization of the PIMs Composition
3.4. Effect of the Aqueous Solution pH on the Extraction Rate of V(V)
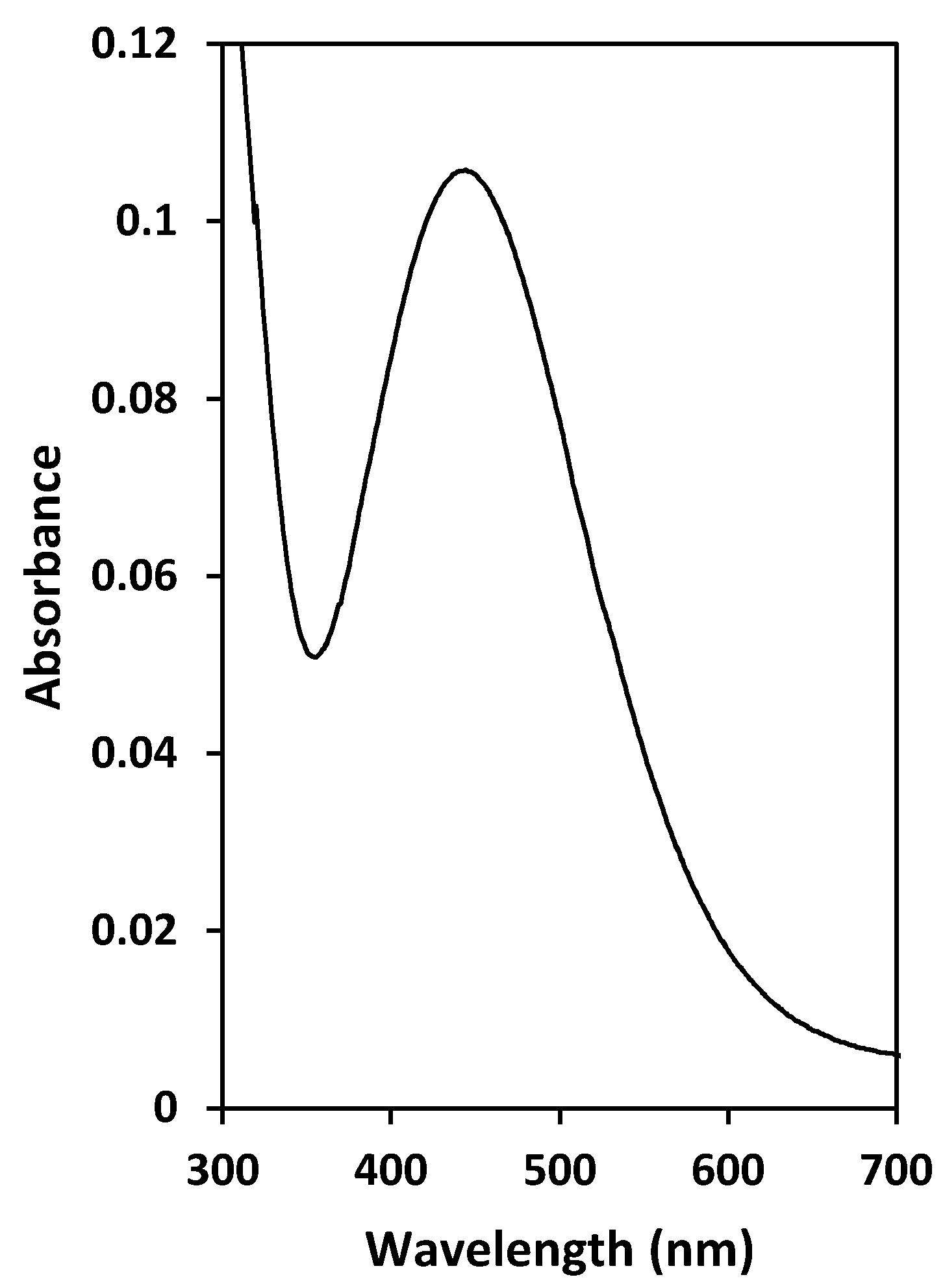
3.5. Effect of the Sulfate ion Concentration and Characterization of the Extracted Species
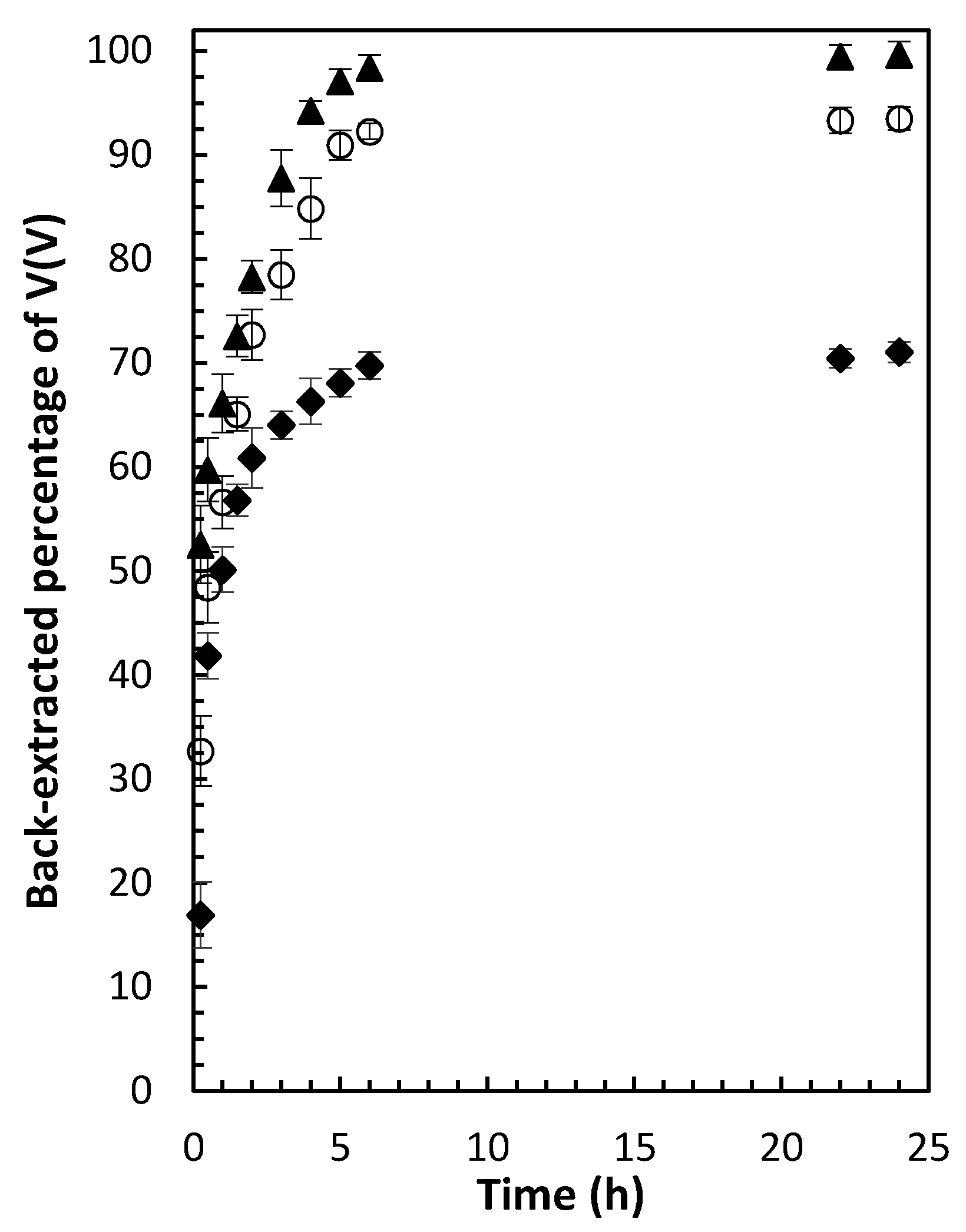
3.6. Back-Extraction Studies
3.7. PIM Selectivity
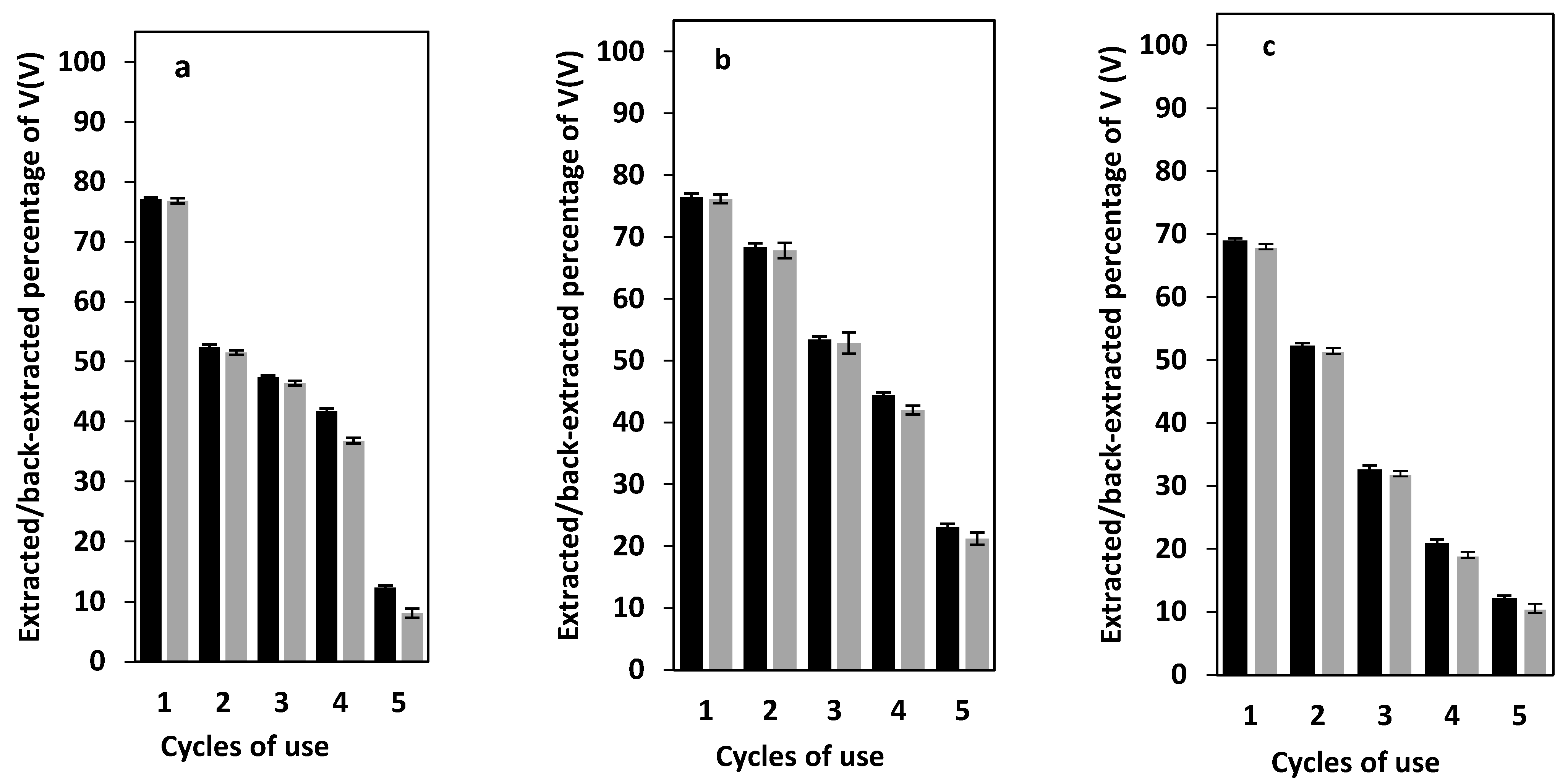
3.8. PIM Reusability and Stability
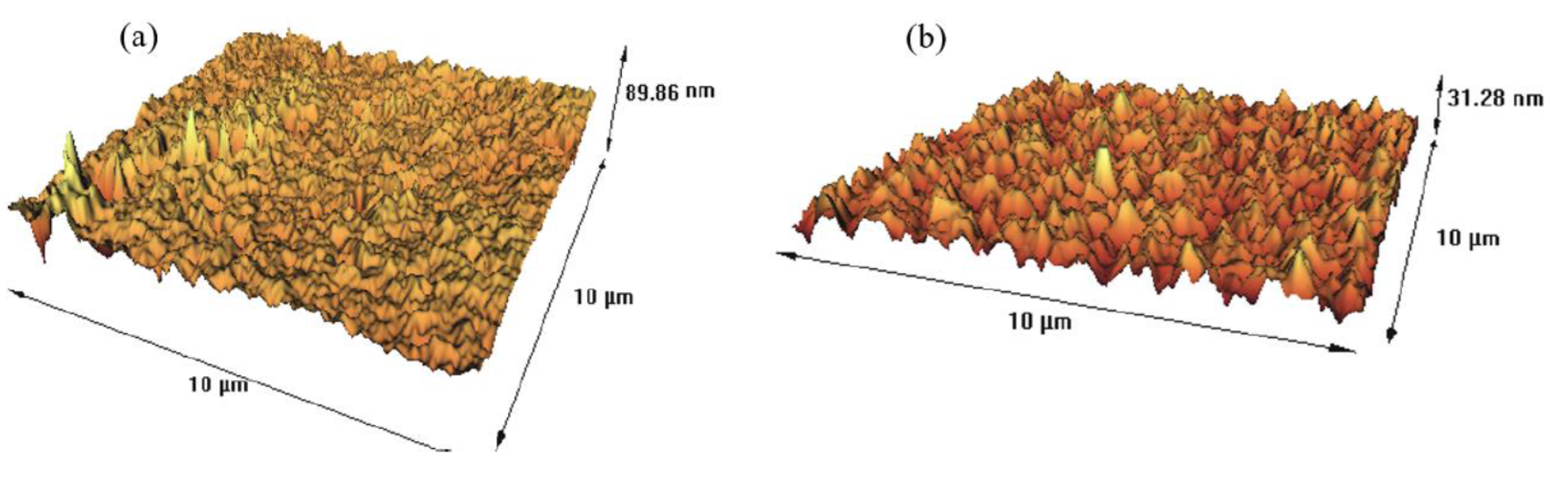
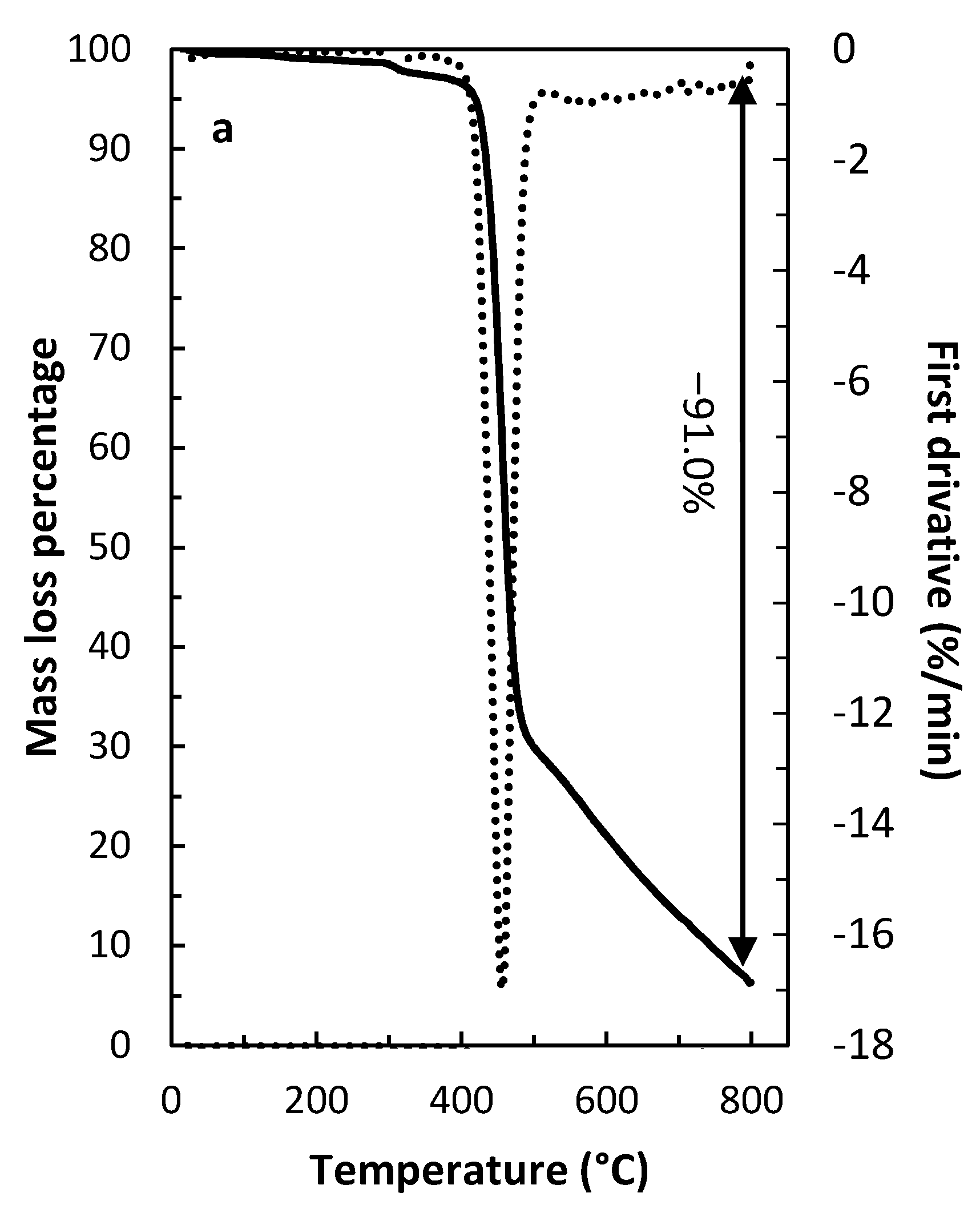
3.9. PIM’s Characterization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Moskalyk, R.R.; Alfantazi, A.M. Processing of vanadium: A review. Miner. Eng. 2003, 16, 793–805. [Google Scholar] [CrossRef]
- Zeng, L.; Cheng, C.Y. Recovery of molybdenum and vanadium from synthetic sulphuric acid leach solutions of spent hydrodesulphurisation catalysts using solvent extraction. Hydrometallurgy 2010, 101, 141–147. [Google Scholar] [CrossRef]
- Zhou, X.; Wei, C.; Li, M.; Qiu, S.; Li, X.J.H. Thermodynamics of vanadium–sulfur–water systems at 298 K. Hydrometallurgy 2011, 106, 104–112. [Google Scholar] [CrossRef]
- Rehder, D. The potentiality of vanadium in medicinal applications. Future Med. Chem. 2012, 4, 1823–1837. [Google Scholar] [CrossRef]
- Akcil, A.; Vegliò, F.; Ferella, F.; Okudan, M.D.; Tuncuk, A. A review of metal recovery from spent petroleum catalysts and ash. Waste Manag. 2015, 45, 420–433. [Google Scholar] [CrossRef]
- Crans, D.; Amin, S.; Keramidas, A. Chemistry of Relevance to Vanadium in the Environment; Nriagu, J., Ed.; John Wiley and Sons: New York, NY, USA, 1998. [Google Scholar]
- Lazaridis, N.K.; Jekel, M.; Zouboulis, A.I. Removal of Cr(VI), Mo(VI), and V(V) ions from single metal aqueous solutions by sorption or nanofiltration. Sep. Sci. Technol. 2003, 38, 2201–2219. [Google Scholar] [CrossRef]
- Cseh, L.; Ingerman, L.; Keith, S.; Taylor, J. Toxicological Profile for Vanadium; The U.S. Department of Health and Human Services: Atlanta, GA, USA, 2012.
- Zeng, L.; Cheng, C.Y. A literature review of the recovery of molybdenum and vanadium from spent hydrodesulphurisation catalysts: Part I: Metallurgical processes. Hydrometallurgy 2009, 98, 1–9. [Google Scholar] [CrossRef]
- Sirviö, J.A.; Hasa, T.; Leiviskä, T.; Liimatainen, H.; Hormi, O. Bisphosphonate nanocellulose in the removal of vanadium(V) from water. Cellulose 2016, 23, 689–697. [Google Scholar] [CrossRef]
- Naeem, A.; Westerhoff, P.; Mustafa, S. Vanadium removal by metal (hydr)oxide adsorbents. Water Res. 2007, 41, 1596–1602. [Google Scholar] [CrossRef]
- Padilla-Rodríguez, A.; Hernández-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L.; Perales-Pérez, O.; Román-Velázquez, F.R. Synthesis of protonated chitosan flakes for the removal of vanadium(III, IV and V) oxyanions from aqueous solutions. Microchem. J. 2015, 118, 1–11. [Google Scholar] [CrossRef]
- Barik, S.P.; Park, K.H.; Nam, C.W. Process development for recovery of vanadium and nickel from an industrial solid waste by a leaching–solvent extraction technique. J. Environ. Manag. 2014, 146, 22–28. [Google Scholar] [CrossRef]
- Ning, P.; Lin, X.; Wang, X.; Cao, H. High-efficient extraction of vanadium and its application in the utilization of the chromium-bearing vanadium slag. Chem. Eng. J. 2016, 301, 132–138. [Google Scholar] [CrossRef]
- Kumar, V.; Sahu, S.K.; Pandey, B.D. Prospects for solvent extraction processes in the Indian context for the recovery of base metals. A review. Hydrometallurgy 2010, 103, 45–53. [Google Scholar] [CrossRef]
- St John, A.M.; Cattrall, R.W.; Kolev, S.D. Extraction of uranium(VI) from sulfate solutions using a polymer inclusion membrane containing di-(2-ethylhexyl) phosphoric acid. J. Membr. Sci. 2010, 364, 354–361. [Google Scholar] [CrossRef]
- Kolev, S.D. Liquid Membranes. In Encyclopedia of Analytical Science; Worsfold, P., Townshend, A., Poole, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Kislik, V.S. Liquid Membrans. In Principles and Applications in Chemical Separations and Water Treatment; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Nagul, E.A.; Croft, C.F.; Cattrall, R.W.; Kolev, S.D. Nanostructural characterisation of polymer inclusion membranes using X-ray scattering. J. Membr. Sci. 2019, 588, 117208. [Google Scholar] [CrossRef]
- Bahrami, S.; Yaftian, M.R.; Najvak, P.; Dolatyari, L.; Shayani-Jam, H.; Kolev, S.D. PVDF-HFP based polymer inclusion membranes containing Cyphos® IL 101 and Aliquat® 336 for the removal of Cr(VI) from sulfate solutions. Sep. Purif. Technol. 2020, 250, 117–251. [Google Scholar] [CrossRef]
- Sellami, F.; Kebiche-Senhadji, O.; Marais, S.; Lanel, C.; Fatyeyeva, K. Novel Poly (Vinylidene Fluoride)/Montmorillonite Polymer Inclusion Membrane: Application to Cr (VI) Extraction from Polluted Water. Membranes 2021, 11, 682. [Google Scholar] [CrossRef]
- Shirzad, M.; Karimi, M.; Abolghasemi, H. Polymer inclusion membranes with dinonylnaphthalene sulfonic acid as ion carrier for Co(II) transport from model solutions. Desalination Water Treat. 2019, 144, 185–200. [Google Scholar] [CrossRef]
- Shirzad, M.; Karimi, M. Statistical analysis and optimal design of polymer inclusion membrane for water treatment by Co(II) removal. Desalination Water Treat. 2020, 182, 194–207. [Google Scholar] [CrossRef]
- Almeida, M.I.G.; Cattrall, R.W.; Kolev, S.D. Polymer inclusion membranes (PIMs) in chemical analysis-A review. Anal. Chim. Acta 2017, 987, 1–14. [Google Scholar] [CrossRef]
- Almeida, M.I.G.; Cattrall, R.W.; Kolev, S.D. Recent trends in extraction and transport of metal ions using polymer inclusion membranes (PIMs). J. Membr. Sci. 2012, 415, 9–23. [Google Scholar] [CrossRef]
- Mishra, R.K.; Rout, P.C.; Sarangi, K.; Nathsarma, K.C. Solvent extraction of Fe(III) from the chloride leach liquor of low grade iron ore tailings using Aliquat 336. Hydrometallurgy 2011, 108, 93–99. [Google Scholar] [CrossRef]
- Mondal, S.K.; Saha, P. Separation of hexavalent chromium from industrial effluent through liquid membrane using environmentally benign solvent: A study of experimental optimization through response surface methodology. Chem. Eng. Res. Des. 2018, 132, 564–583. [Google Scholar] [CrossRef]
- Chaouchi, S.; Hamdaoui, O. Acetaminophen extraction by emulsion liquid membrane using Aliquat 336 as extractant. Sep. Purif. Technol. 2014, 129, 32–40. [Google Scholar] [CrossRef]
- Fontàs, C.; Anticó, E.; Salvadó, V.; Valiente, M.; Hidalgo, M. Chemical pumping of rhodium by a supported liquid membrane containing Aliquat 336 as carrier. Anal. Chim. Acta 1997, 346, 199–206. [Google Scholar] [CrossRef]
- Bal, Y.; Bal, K.; Cote, G.J. Kinetics of the alkaline stripping of vanadium(V) previously extracted by Aliquat® 336. Miner. Eng. 2002, 15, 377–379. [Google Scholar] [CrossRef]
- El-Nadi, Y.; Awwad, N.; Nayl, A. A comparative study of vanadium extraction by Aliquat-336 from acidic and alkaline media with application to spent catalyst. Int. J. Mineral. Processing 2009, 92, 115–120. [Google Scholar] [CrossRef]
- Yaftian, M.R.; Almeida, M.I.G.S.; Cattrall, R.W.; Kolev, S.D. Selective extraction of vanadium(V) from sulfate solutions into a polymer inclusion membrane composed of poly(vinylidenefluoride-co-hexafluoropropylene) and Cyphos® IL 101. J. Membr. Sci. 2018, 545, 57–65. [Google Scholar] [CrossRef]
- Bonggotgetsakul, Y.Y.N.; Cattrall, R.W.; Kolev, S.D. Recovery of gold from aqua regia digested electronic scrap using a poly(vinylidene fluoride-co-hexafluoropropene) (PVDF-HFP) based polymer inclusion membrane (PIM) containing Cyphos® IL 104. J. Membr. Sci. 2016, 514, 274–281. [Google Scholar] [CrossRef]
- Sellami, F.; Kebiche-Senhadji, O.; Marais, S.; Couvrat, N.; Fatyeyeva, K. Polymer inclusion membranes based on CTA/PBAT blend containing Aliquat 336 as extractant for removal of Cr(VI): Efficiency, stability and selectivity. React. Funct. Polym. 2019, 139, 120–132. [Google Scholar] [CrossRef]
- Lozano, L.J.; Godínez, C.; Alguacil, F.J. Facilitated transport of vanadium(V) by supported liquid membranes. Hydrometallurgy 2005, 80, 196–202. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.; Bao, S.; Shen, C. Separation and recovery of vanadium from a sulfuric-acid leaching solution of stone coal by solvent extraction using trialkylamine. Sep. Purif. Technol. 2016, 164, 49–55. [Google Scholar] [CrossRef]
- Butler, A.; Clague, M.J.; Meister, G.E. Vanadium Peroxide Complexes. Chem. Rev. 1994, 94, 625–638. [Google Scholar] [CrossRef]
- Fritz, J.S.; Topping, J.J. Chromatographic separation of vanadium, tungsten and molybdenum with a liquid anion-exchanger. Talanta 1971, 18, 865–872. [Google Scholar] [CrossRef]
- Baczyńska, M.; Regel-Rosocka, M.; Coll, M.T.; Fortuny, A.; Sastre, A.M.; Wiśniewski, M. Transport of Zn(II), Fe(II), Fe(III) across polymer inclusion membranes (PIM) and flat sheet supported liquid membranes (SLM) containing phosphonium ionic liquids as metal ion carriers. Sep. Sci. Technol. 2016, 51, 2639–2648. [Google Scholar] [CrossRef]
- Yaftian, M.R.; Almeida, M.I.G.; Cattrall, R.W.; Kolev, S.D. Flow injection spectrophotometric determination of V(V) involving on-line separation using a poly (vinylidene fluoride-co-hexafluoropropylene)-based polymer inclusion membrane. Talanta 2018, 181, 385–391. [Google Scholar] [CrossRef]
- Kaya, A.; Onac, C.; Alpoğuz, H.K.; Agarwal, S.; Gupta, V.K.; Atar, N.; Yilmaz, A. Reduced graphene oxide based a novel polymer inclusion membrane: Transport studies of Cr(VI). J. Mol. Liq. 2016, 219, 1124–1130. [Google Scholar] [CrossRef]
- Chau, T.T.; Bruckard, W.J.; Koh, P.T.L.; Nguyen, A.V. A review of factors that affect contact angle and implications for flotation practice. Adv. Colloid Interface Sci. 2009, 150, 106–115. [Google Scholar] [CrossRef]
- Kaya, A.; Onac, C.; Alpoguz, H.K.; Yilmaz, A.; Atar, N. Removal of Cr(VI) through calixarene based polymer inclusion membrane from chrome plating bath water. Chem. Eng. J. 2016, 283, 141–149. [Google Scholar] [CrossRef]
- Kebiche-Senhadji, O.; Mansouri, L.; Tingry, S.; Seta, P.; Benamor, M. Facilitated Cd(II) transport across CTA polymer inclusion membrane using anion (Aliquat 336) and cation (D2EHPA) metal carriers. J. Membr. Sci. 2008, 310, 438–445. [Google Scholar] [CrossRef]







| PIM | PVDF-HFP (wt%) | Aliquat® 336 (wt%) | DBP (wt%) | Extracted V(V) ± SD (%) |
|---|---|---|---|---|
| 1 | 50 | 35 | 15 | 61.41 ± 0.82 |
| 2 | 55 | 35 | 10 | 61.48 ± 1.06 |
| 3 | 55 | 40 | 5 | 62.19 ± 0.49 |
| 4 | 50 | 40 | 10 | 63.80 ± 0.42 |
| Solution | Metallic Species | Extraction (%) | Back-Extraction (%) |
|---|---|---|---|
| 1 | V(V) | 76.3 ± 0.6 | 76.1 ± 0.6 |
| 2 | V(V) Mo(VI) | 52.2 ± 2.1 98.6 ± 2.6 | 51.7 ± 1.5 20.0 ± 2.0 |
| 3 | V(V) Al(III) | 72.3 ± 0.7 2.6 ± 0.9 | 71.4 ± 0.4 ND |
| 4 | V(V) Co(II) | 73.9 ± 1.1 0.6 ± 2.0 | 73.6 ± 1.3 ND |
| 5 | V(V) Cu(II) | 74.4 ± 1.9 ND | 74.0 ± 0.7 ND |
| 6 | V(V) Fe(III) | 75.2 ± 2.1 ND | 75.0 ± 1.8 ND |
| 7 | V(V) Mn(II) | 76.1 ± 1.6 ND | 76.0 ± 1.8 ND |
| 8 | V(V) Ni(II) | 73.2 ± 2.2 ND | 72.9 ± 1.3 ND |
| 9 | V(V) Mo(VI) Al(III) Co(II) Cu(II) Fe(III) Mn(II) Ni(II) | 50.4 ± 1.3 99.3 ± 2.6 ND ND ND ND ND ND | 49.6 ± 0.5 19.8 ± 2.1 ND ND ND ND ND ND |
| Ionic Species | pH 1.1 (Step I) | pH 2.5 (Step II) | ||
|---|---|---|---|---|
| Extraction (%) | Back-Extraction (%) | Extraction (%) | Back-Extraction (%) | |
| V(V) | ND | ND | 74.2 ± 1.9 | 73.7 ± 1.4 |
| Mo(VI) | 96.4 ± 1.2 | 19.6 ± 2.6 | ND | ND |
| Al(III) | ND | ND | ND | ND |
| Co(II) | ND | ND | ND | ND |
| Cu(II) | ND | ND | ND | ND |
| Fe(III) | ND | ND | ND | ND |
| Mn(II) | ND | ND | ND | ND |
| Ni(II) | ND | ND | ND | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bahrami, S.; Dolatyari, L.; Shayani-Jam, H.; Yaftian, M.R.; Kolev, S.D. On the Potential of a Poly(vinylidenefluoride-co-hexafluoropropylene) Polymer Inclusion Membrane Containing Aliquat® 336 and Dibutyl Phthalate for V(V) Extraction from Sulfate Solutions. Membranes 2022, 12, 90. https://doi.org/10.3390/membranes12010090
Bahrami S, Dolatyari L, Shayani-Jam H, Yaftian MR, Kolev SD. On the Potential of a Poly(vinylidenefluoride-co-hexafluoropropylene) Polymer Inclusion Membrane Containing Aliquat® 336 and Dibutyl Phthalate for V(V) Extraction from Sulfate Solutions. Membranes. 2022; 12(1):90. https://doi.org/10.3390/membranes12010090
Chicago/Turabian StyleBahrami, Salar, Leila Dolatyari, Hassan Shayani-Jam, Mohammad Reza Yaftian, and Spas D. Kolev. 2022. "On the Potential of a Poly(vinylidenefluoride-co-hexafluoropropylene) Polymer Inclusion Membrane Containing Aliquat® 336 and Dibutyl Phthalate for V(V) Extraction from Sulfate Solutions" Membranes 12, no. 1: 90. https://doi.org/10.3390/membranes12010090
APA StyleBahrami, S., Dolatyari, L., Shayani-Jam, H., Yaftian, M. R., & Kolev, S. D. (2022). On the Potential of a Poly(vinylidenefluoride-co-hexafluoropropylene) Polymer Inclusion Membrane Containing Aliquat® 336 and Dibutyl Phthalate for V(V) Extraction from Sulfate Solutions. Membranes, 12(1), 90. https://doi.org/10.3390/membranes12010090







