Rare Earth Elements Recovery Using Selective Membranes via Extraction and Rejection
Abstract
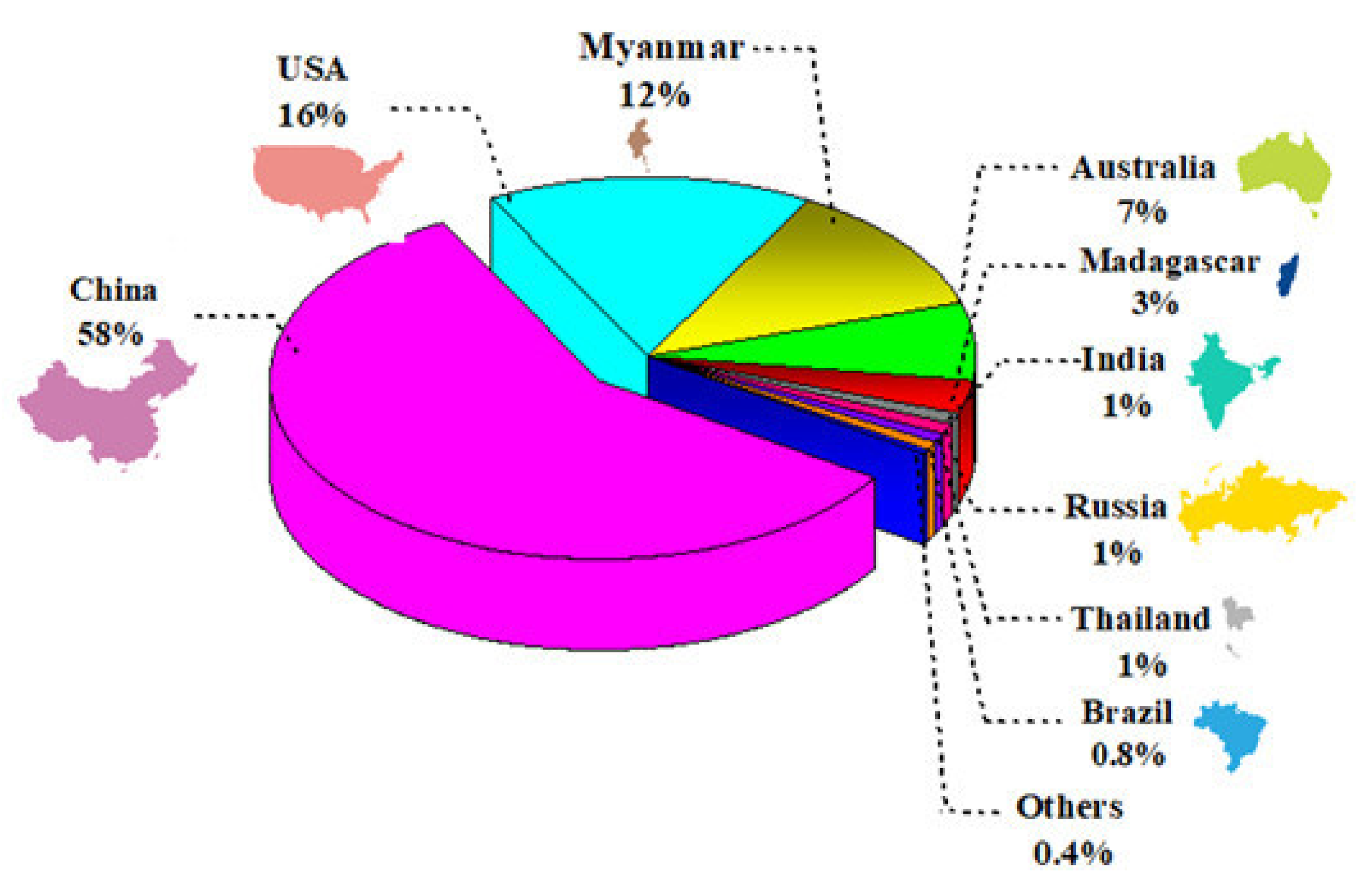
:1. Introduction
2. Membrane Separation Technology (MST) for Recovery of REEs
- Diffusion/permeation mechanism.
- Facilitated/retarded permeation mechanism.
- Rejection mechanism.
3. Diffusion/Permeation Mechanism
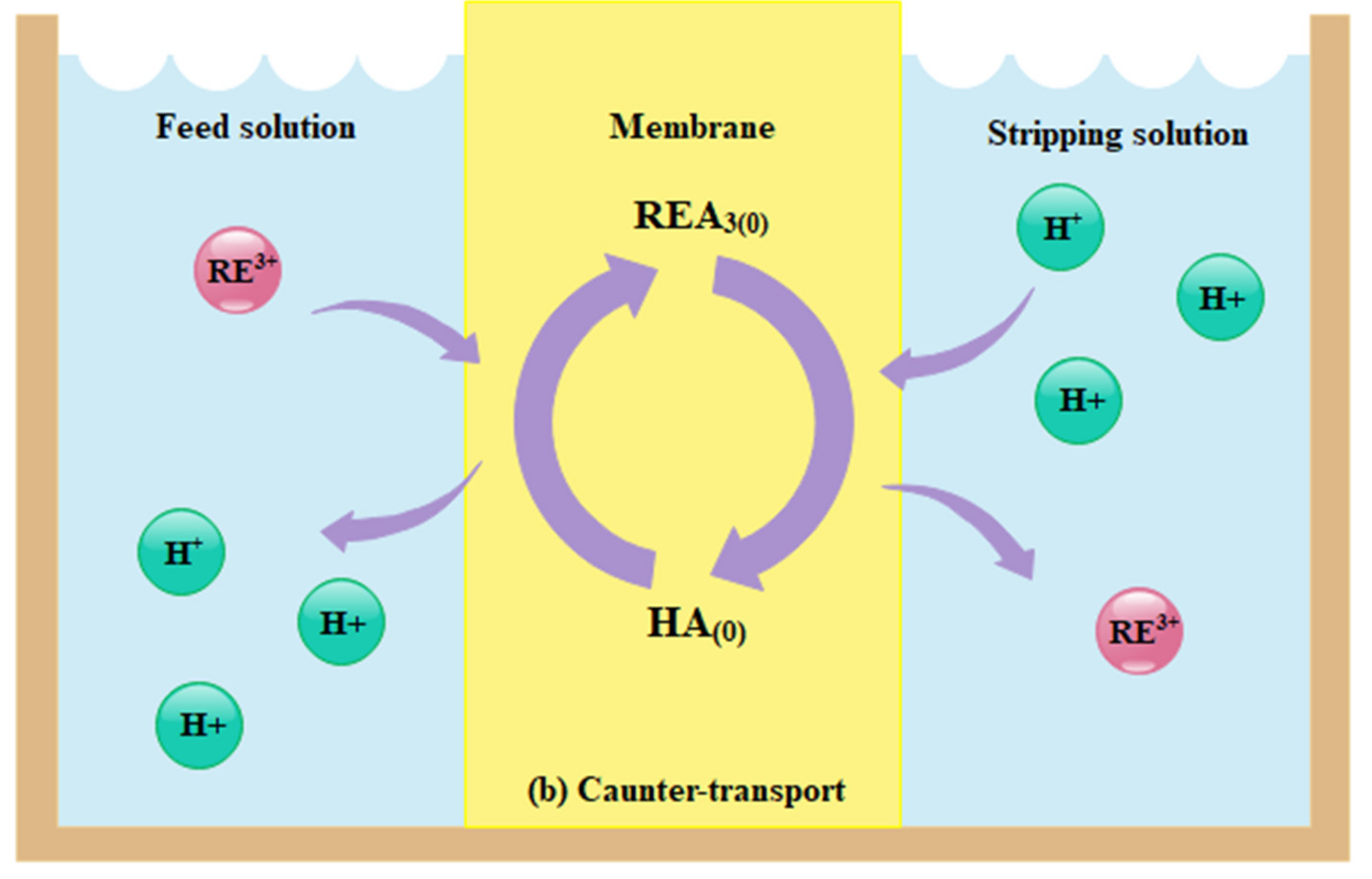
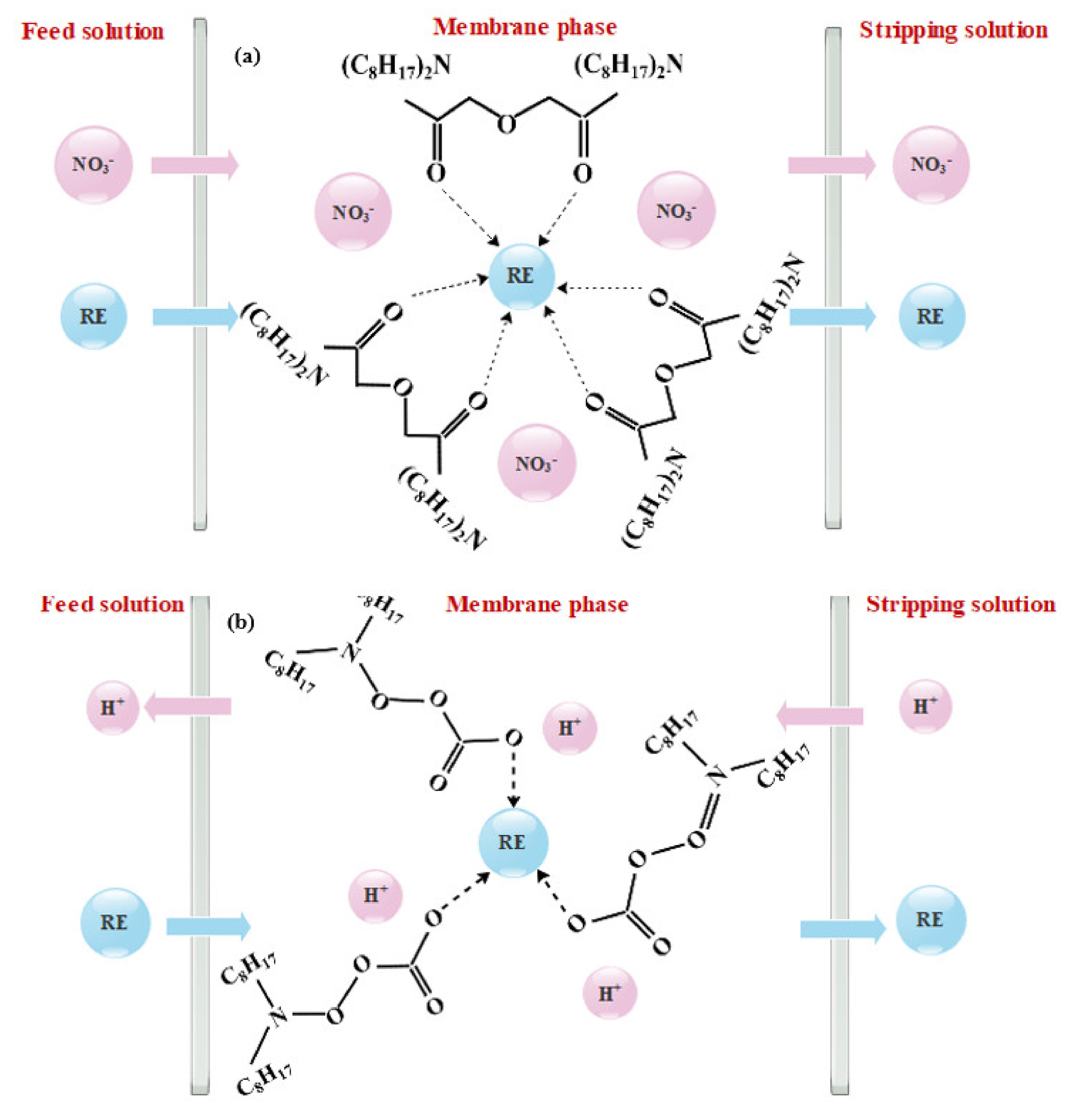
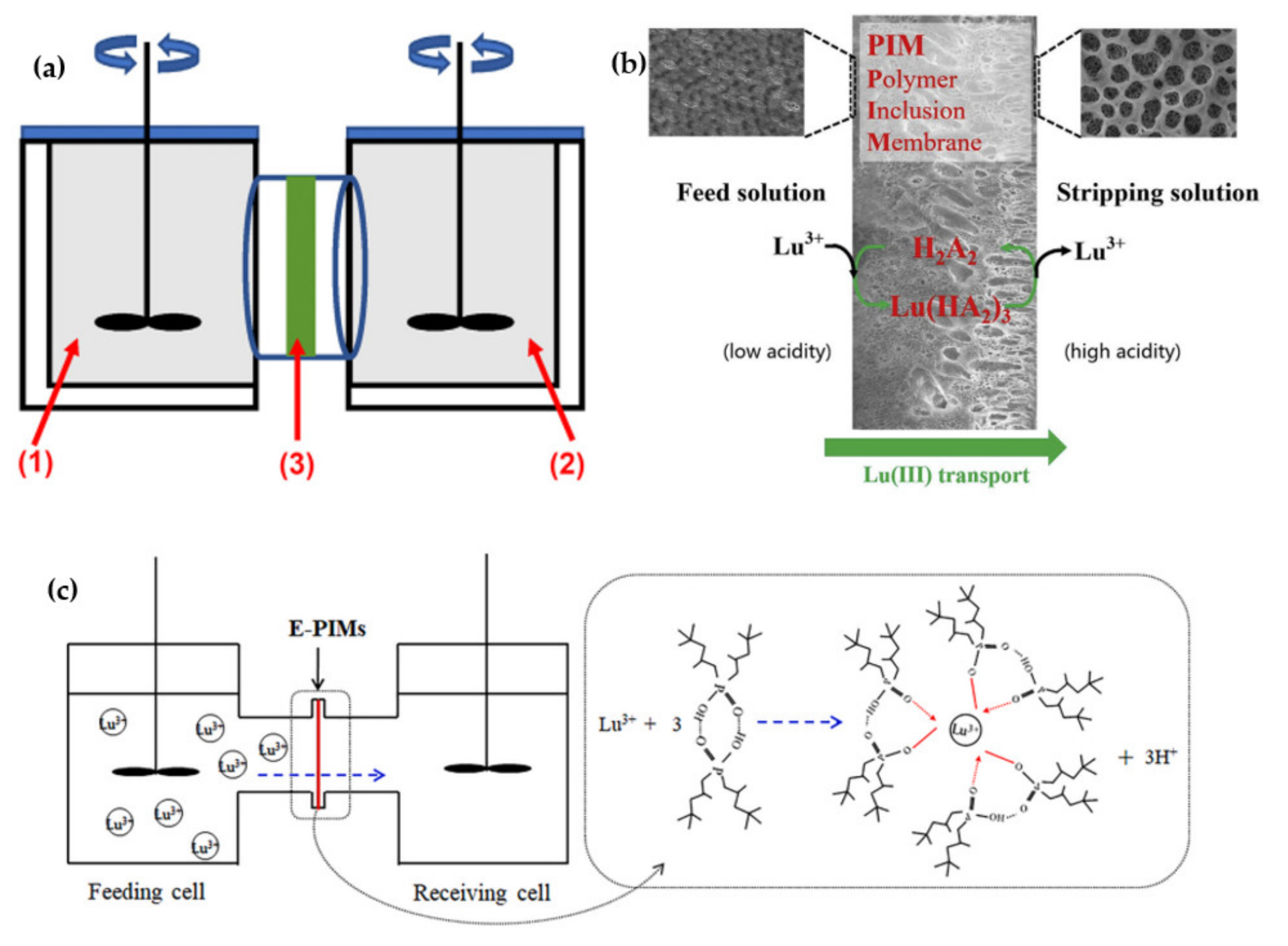
3.1. Supported Liquid Membranes (SLMs)
3.2. Polymer Inclusion Membranes (PIMs)
4. Facilitated/Retarded Permeation Mechanism
4.1. Ion Imprinted Membranes (IIMs)
4.2. Nanocomposite Membranes
5. Rejection Mechanism
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pramanik, B.K.; Nghiem, L.D.; Hai, F.I. Extraction of strategically important elements from brines: Constraints and opportunities. Water Res. 2020, 168, 115149. [Google Scholar] [CrossRef]
- Kim, D.; Powell, L.; Delmau, L.H.; Peterson, E.S.; Herchenroeder, J.; Bhave, R.R. A supported liquid membrane system for the selective recovery of rare earth elements from neodymium-based permanent magnets. Sep. Sci. Technol. 2016, 51, 1716–1726. [Google Scholar] [CrossRef]
- Ali, A.H.; Dakroury, G.A.; Hagag, M.S.; Abdo, S.M.; Allan, K.F. Sorption of Some Rare Earth Elements from Acidic Solution onto Poly (acrylic acid–co-acrylamide/16, 16-dimethylheptadecan-1-amine) Composite. J. Polym. Environ. 2021. [Google Scholar] [CrossRef]
- Chen, L.; Wu, Y.; Dong, H.; Meng, M.; Li, C.; Yan, Y.; Chen, J. An overview on membrane strategies for rare earths extraction and separation. Sep. Purif. Technol. 2018, 197, 70–85. [Google Scholar] [CrossRef]
- Li, F.; Yang, Z.; Weng, H.; Chen, G.; Lin, M.; Zhao, C. High efficient separation of U(VI) and Th(IV) from rare earth elements in strong acidic solution by selective sorption on phenanthroline diamide functionalized graphene oxide. Chem. Eng. J. 2018, 332, 340–350. [Google Scholar] [CrossRef]
- Balaram, V. Rare earth elements: A review of applications, occurrence, exploration, analysis, recycling, and environmental impact. Geosci. Front. 2019, 10, 1285–1303. [Google Scholar] [CrossRef]
- Huang, S.; Chen, J.; Zou, D. A preliminary study of polymer inclusion membrane for lutetium(III) separation and membrane regeneration. J. Rare Earths 2021, 39, 1256–1263. [Google Scholar] [CrossRef]
- Liu, J.; Martin, P.F.; Peter McGrail, B. Rare-earth element extraction from geothermal brine using magnetic core-shell nanoparticles-techno-economic analysis. Geothermics 2021, 89, 101938. [Google Scholar] [CrossRef]
- Hidayah, N.N.; Abidin, S.Z. The evolution of mineral processing in extraction of rare earth elements using solid-liquid extraction over liquid-liquid extraction: A review. Miner. Eng. 2017, 112, 103–113. [Google Scholar] [CrossRef] [Green Version]
- Elbashier, E.; Mussa, A.; Hafiz, M.; Hawari, A.H. Recovery of rare earth elements from waste streams using membrane processes: An overview. Hydrometallurgy 2021, 204, 105706. [Google Scholar] [CrossRef]
- Asadollahzadeh, M.; Torkaman, R.; Torab-Mostaedi, M. Extraction and Separation of Rare Earth Elements by Adsorption Approaches: Current Status and Future Trends. Sep. Purif. Rev. 2020, 50, 417–444. [Google Scholar] [CrossRef]
- Zhao, Z.; Qiu, Z.; Yang, J.; Lu, S.; Cao, L.; Zhang, W.; Xu, Y. Recovery of rare earth elements from spent fluid catalytic cracking catalysts using leaching and solvent extraction techniques. Hydrometallurgy 2017, 167, 183–188. [Google Scholar] [CrossRef]
- Kose Mutlu, B.; Cantoni, B.; Turolla, A.; Antonelli, M.; Hsu-Kim, H.; Wiesner, M.R. Application of nanofiltration for Rare Earth Elements recovery from coal fly ash leachate: Performance and cost evaluation. Chem. Eng. J. 2018, 349, 309–317. [Google Scholar] [CrossRef]
- Drobniak, A.; Mastalerz, M. Rare Earth Elements: A brief overview. Indiana J. Earth 2022, 4. [Google Scholar] [CrossRef]
- Hammache, Z.; Bensaadi, S.; Berbar, Y.; Audebrand, N.; Szymczyk, A.; Amara, M. Recovery of rare earth elements from electronic waste by diffusion dialysis. Sep. Purif. Technol. 2021, 254, 117641. [Google Scholar] [CrossRef]
- Pavón, S.; Fortuny, A.; Coll, M.T.; Sastre, A.M. Improved rare earth elements recovery from fluorescent lamp wastes applying supported liquid membranes to the leaching solutions. Sep. Purif. Technol. 2019, 224, 332–339. [Google Scholar] [CrossRef]
- Ni’am, A.C.; Wang, Y.-F.; Chen, S.-W.; Chang, G.-M.; You, S.-J. Simultaneous recovery of rare earth elements from waste permanent magnets (WPMs) leach liquor by solvent extraction and hollow fiber supported liquid membrane. Chem. Eng. Process.—Process Intensif. 2020, 148, 107831. [Google Scholar] [CrossRef]
- Dong, Z.; Mattocks, J.A.; Deblonde, G.J.P.; Hu, D.; Jiao, Y.; Cotruvo, J.A.; Park, D.M. Bridging Hydrometallurgy and Biochemistry: A Protein-Based Process for Recovery and Separation of Rare Earth Elements. ACS Cent. Sci. 2021, 7, 1798–1808. [Google Scholar] [CrossRef] [PubMed]
- Goode, J.R. Options for the separation of rare earth elements. In Proceedings of the IMPC 2016 28th International Mineral Processing Congress, Quebec City Convention Center, Quebec City, QC, Canada, 11–15 September 2016. [Google Scholar]
- Hassas, B.V.; Rezaee, M.; Pisupati, S.V. Effect of various ligands on the selective precipitation of critical and rare earth elements from acid mine drainage. Chemosphere 2021, 280, 130684. [Google Scholar] [CrossRef]
- Park, D.; Middleton, A.; Smith, R.; Deblonde, G.; Laudal, D.; Theaker, N.; Hsu-Kim, H.; Jiao, Y. A biosorption-based approach for selective extraction of rare earth elements from coal byproducts. Sep. Purif. Technol. 2020, 241, 116726. [Google Scholar] [CrossRef]
- Smith, R.C.; Taggart, R.K.; Hower, J.C.; Wiesner, M.R.; Hsu-Kim, H. Selective Recovery of Rare Earth Elements from Coal Fly Ash Leachates Using Liquid Membrane Processes. Environ. Sci. Technol. 2019, 53, 4490–4499. [Google Scholar] [CrossRef]
- Pramanik, B.K.; Shu, L.; Jegatheesan, J.; Shah, K.; Haque, N.; Bhuiyan, M.A. Rejection of rare earth elements from a simulated acid mine drainage using forward osmosis: The role of membrane orientation, solution pH, and temperature variation. Process Saf. Environ. Prot. 2019, 126, 53–59. [Google Scholar] [CrossRef]
- Oleinikova, M.; Muñoz, M. Transport of rare earth metal ions through activated composite membranes containing DEHPA. Solvent Extr. Ion Exch. 2000, 18, 401–419. [Google Scholar] [CrossRef]
- Tian, M.; Jia, Q.; Liao, W. Studies on Synergistic solvent extraction of rare earth elements from nitrate medium by mixtures of 8-hydroxyquinoline with Cyanex 301 or Cyanex 302. J. Rare Earths 2013, 31, 604–608. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, Y.; Guo, X.; Dong, Y.; Su, X.; Sun, X. The recovery of rare earth by a novel extraction and precipitation strategy using functional ionic liquids. J. Mol. Liq. 2018, 254, 414–420. [Google Scholar] [CrossRef]
- Kegl, T.; Košak, A.; Lobnik, A.; Novak, Z.; Kralj, A.K.; Ban, I. Adsorption of rare earth metals from wastewater by nanomaterials: A review. J. Hazard. Mater. 2020, 386, 121632. [Google Scholar] [CrossRef] [PubMed]
- Korenevsky, A.A.; Sorokin, V.V.; Karavaiko, G.I. Biosorption of rare earth elements. Process Metall. 1999, 9, 299–306. [Google Scholar] [CrossRef]
- Xin, W.; Lin, C.; Fu, L.; Kong, X.Y.; Yang, L.; Qian, Y.; Zhu, C.; Zhang, Q.; Jiang, L.; Wen, L. Nacre-like Mechanically Robust Heterojunction for Lithium-Ion Extraction. Matter 2021, 4, 737–754. [Google Scholar] [CrossRef]
- Brewer, A.; Chang, E.; Park, D.M.; Kou, T.; Li, Y.; Lammers, L.N.; Jiao, Y. Recovery of Rare Earth Elements from Geothermal Fluids through Bacterial Cell Surface Adsorption. Environ. Sci. Technol. 2019, 53, 7714–7723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Ramasamy, D.L.; Sillanpää, M.; Repo, E. Separation and concentration of rare earth elements from wastewater using electrodialysis technology. Sep. Purif. Technol. 2021, 254, 117442. [Google Scholar] [CrossRef]
- Xie, Z.; Chen, Q.; Zhao, L. Extraction of trace rare earth by liquid surfactant membranes from phosphate rock. Zhongguo Xitu Xuebao/J. Chin. Rare Earth Soc. 2013, 31, 269–274. [Google Scholar]
- Davoodi-Nasab, P.; Rahbar-Kelishami, A.; Safdari, J.; Abolghasemi, H. Application of emulsion nanofluids membrane for the extraction of gadolinium using response surface methodology. J. Mol. Liq. 2017, 244, 368–373. [Google Scholar] [CrossRef]
- Wang, J.; Dang, Y.; Fei, D.; Fan, W.; Dong, W. Recovery of trace lanthanum from phosphoric acid using liquid membrane extraction. Zhongguo Xitu Xuebao/J. Chin. Rare Earth Soc. 2012, 30, 13–20. [Google Scholar]
- Tang, J.; Wai, C.M. Transport of trivalent lanthanides through a surfactant membrane containing an ionizable macrocyclic polyether. J. Membr. Sci. 1989, 46, 349–356. [Google Scholar] [CrossRef]
- Yang, X.J.; Fane, A.G.; Soldenhoff, K. Comparison of liquid membrane processes for metal separations: Permeability, stability, and selectivity. Ind. Eng. Chem. Res. 2003, 42, 392–403. [Google Scholar] [CrossRef]
- Gaikwad, A.G.; Rajput, A.M. Transport of yttrium metal ions through fibers supported liquid membrane solvent extraction. J. Rare Earths 2010, 28, 1–6. [Google Scholar] [CrossRef]
- Pei, L.; Yao, B.; Zhang, C. Transport of Tm(III) through dispersion supported liquid membrane containing PC-88A in kerosene as the carrier. Sep. Purif. Technol. 2009, 65, 220–227. [Google Scholar] [CrossRef]
- Pei, L.; Wang, L.; Yu, G. Study on a novel flat renewal supported liquid membrane with D2EHPA and hydrogen nitrate for neodymium extraction. J. Rare Earths 2012, 30, 63–68. [Google Scholar] [CrossRef]
- Pei, L.; Wang, L.; Yu, G. Separation of Eu(III) with supported dispersion liquid membrane system containing D2EHPA as carrier and HNO3 solution as stripping solution. J. Rare Earths 2011, 29, 7–14. [Google Scholar] [CrossRef]
- Doležal, J.; Moreno, C.; Hrdlička, A.; Valiente, M. Selective transport of lanthanides through supported liquid membranes containing non-selective extractant, di-(2-ethylhexyl)phosphoric acid, as a carrier. J. Membr. Sci. 2000, 168, 175–181. [Google Scholar] [CrossRef]
- Pei, L.; Yao, B.; Fu, X. Study on transport of Dy(III) by dispersion supported liquid membrane. J. Rare Earths 2009, 27, 447–456. [Google Scholar] [CrossRef]
- Ambare, D.N.; Ansari, S.A.; Anitha, M.; Kandwal, P.; Singh, D.K.; Singh, H.; Mohapatra, P.K. Non-dispersive solvent extraction of neodymium using a hollow fiber contactor: Mass transfer and modeling studies. J. Membr. Sci. 2013, 446, 106–112. [Google Scholar] [CrossRef]
- Ramakul, P.; Mooncluen, U.; Yanachawakul, Y.; Leepipatpiboon, N. Mass transport modeling and analysis on the mutual separation of lanthanum(III) and cerium(IV) through a hollow fiber supported liquid membrane. J. Ind. Eng. Chem. 2012, 18, 1606–1611. [Google Scholar] [CrossRef]
- Yadav, K.K.; Anitha, M.; Singh, D.K.; Kain, V. NdFeB magnet recycling: Dysprosium recovery by non-dispersive solvent extraction employing hollow fibre membrane contactor. Sep. Purif. Technol. 2018, 194, 265–271. [Google Scholar] [CrossRef]
- Pei, L.; Wang, L.; Guo, W.; Zhao, N. Stripping dispersion hollow fiber liquid membrane containing PC-88A as carrier and HCl for transport behavior of trivalent dysprosium. J. Membr. Sci. 2011, 378, 520–530. [Google Scholar] [CrossRef]
- Geist, A.; Weigl, M.; Müllich, U.; Gompper, K. Application of novel extractants for actinide(III)/lanthanide(III) separation in hollow-fibre modules. Membr. Technol. 2003, 2003, 5–7. [Google Scholar] [CrossRef]
- Kubota, F.; Kakoi, T.; Goto, M.; Furusaki, S.; Nakashio, F.; Hano, T. Permeation behavior of rare earth metals with a calix[4]arene carboxyl derivative in a hollow-fiber membrane. J. Membr. Sci. 2000, 165, 149–158. [Google Scholar] [CrossRef]
- Martínez, J.; Rodríguez Varela, R.; Forsberg, K.; Rasmuson, Å. Factors influencing separation selectivity of rare earth elements in flat sheet supported liquid membranes. Chem. Eng. Sci. 2018, 191, 134–155. [Google Scholar] [CrossRef]
- Li, X.; Sun, Y. Progress in solid-liquid extraction resin for separation of rare earth elements. J. Rare Earths 2005, 23, 581–592. [Google Scholar]
- Nghiem, L.D.; Mornane, P.; Potter, I.D.; Perera, J.M.; Cattrall, R.W.; Kolev, S.D. Extraction and transport of metal ions and small organic compounds using polymer inclusion membranes (PIMs). J. Membr. Sci. 2006, 281, 7–41. [Google Scholar] [CrossRef]
- Zolfonoun, E.; Yousefi, S.R. Simultaneous Determination of Rare Earth Elements by ICP OES After On-Line Enrichment Using Multi-Walled Carbon Nanotubes Coated Cellulose Acetate Membrane. J. Braz. Chem. Soc. 2016, 27, 2348–2353. [Google Scholar] [CrossRef]
- Croft, C.F.; Almeida, M.I.G.S.; Cattrall, R.W.; Kolev, S.D. Separation of lanthanum(III), gadolinium(III) and ytterbium(III) from sulfuric acid solutions by using a polymer inclusion membrane. J. Membr. Sci. 2018, 545, 259–265. [Google Scholar] [CrossRef]
- Yusoff, M.M.; Mostapa, N.R.N.; Sarkar, M.S.; Biswas, T.K.; Rahman, M.L.; Arshad, S.E.; Sarjadi, M.S.; Kulkarni, A.D. Synthesis of ion imprinted polymers for selective recognition and separation of rare earth metals. J. Rare Earths 2017, 35, 177–186. [Google Scholar] [CrossRef]
- Kala, R.; Biju, V.M.; Rao, T.P. Synthesis, characterization, and analytical applications of erbium(III) ion imprinted polymer particles prepared via γ-irradiation with different functional and crosslinking monomers. Anal. Chim. Acta 2005, 549, 51–58. [Google Scholar] [CrossRef]
- Zhang, N.; Hu, B.; Huang, C. A new ion-imprinted silica gel sorbent for on-line selective solid-phase extraction of dysprosium(III) with detection by inductively coupled plasma-atomic emission spectrometry. Anal. Chim. Acta 2007, 597, 12–18. [Google Scholar] [CrossRef]
- Wang, J.; Wei, J.; Li, J. Straw-supported ion imprinted polymer sorbent prepared by surface imprinting technique combined with AGET ATRP for selective adsorption of La3+ ions. Chem. Eng. J. 2016, 293, 24–33. [Google Scholar] [CrossRef]
- Hou, H.; Jing, Y.; Wang, Y.; Wang, Y.; Xu, J.; Chen, J. Solvent extraction performance of Ce(III) from chloride acidic solution with 2-ethylhexyl phosphoric acid-2-ethylhexyl ester (EHEHPA) by using membrane dispersion micro-extractor. J. Rare Earths 2015, 33, 1114–1121. [Google Scholar] [CrossRef]
- Hou, H.; Wang, Y.; Xu, J.; Chen, J. Solvent extraction of La(III) with 2-ethylhexyl phosphoric acid-2-ethylhexyl ester (EHEHPA) by membrane dispersion micro-extractor. J. Rare Earths 2013, 31, 1114–1118. [Google Scholar] [CrossRef]
- Qiu, S.; Xue, M.; Zhu, G. Metal–organic framework membranes: From synthesis to separation application. Chem. Soc. Rev. 2014, 43, 6116–6140. [Google Scholar] [CrossRef] [PubMed]
- Carboni, M.; Abney, C.W.; Liu, S.; Lin, W. Highly porous and stable metal–organic frameworks for uranium extraction. Chem. Sci. 2013, 4, 2396–2402. [Google Scholar] [CrossRef]
- Xu, D.; Shah, Z.; Sun, G.; Peng, X.; Cui, Y. Recovery of rare earths from phosphate ores through supported liquid membrane using N, N, N′, N′-tetraoctyl diglycol amid. Miner. Eng. 2019, 139, 105681. [Google Scholar] [CrossRef]
- Baba, Y.; Kubota, F.; Kamiya, N.; Goto, M. Selective recovery of dysprosium and neodymium ions by a supported liquid membrane based on ionic liquids. Solvent Extr. Res. Dev. 2011, 18, 193–198. [Google Scholar] [CrossRef] [Green Version]
- Asadollahzadeh, M.; Torkaman, R.; Torab-Mostaedi, M.; Hemmati, A.; Ghaemi, A. Efficient recovery of neodymium and praseodymium from NdFeB magnet-leaching phase with and without ionic liquid as a carrier in the supported liquid membrane. Chem. Pap. 2020, 74, 4193–4201. [Google Scholar] [CrossRef]
- Mohammad Tehrani, B.; Rahbar-Kelishami, A. A novel mass transfer coefficient correlation for improved rare earth metals extraction via supported nanoliquids membrane. Chem. Eng. Process.–Process Intensif. 2019, 143, 107635. [Google Scholar] [CrossRef]
- Asadollahzadeh, M.; Torkaman, R.; Torab-Mostaedi, M.; Ghaemi, A.; Hemmati, A. Green imidazolium ionic liquid selectively facilitates Ce(III) ion transport through supported liquid membrane. Int. J. Environ. Anal. Chem. 2020, 1–16. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Mohapatra, P.K.; Hassan, P.A.; Manchanda, V.K. Studies on the selective Am3+ transport, irradiation stability and surface morphology of polymer inclusion membranes containing Cyanex-301 as carrier extractant. J. Hazard. Mater. 2011, 192, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Baczyńska, M.; Waszak, M.; Nowicki, M.; Przadka, D.; Borysiak, S.; Regel-Rosocka, M. Characterization of Polymer Inclusion Membranes (PIMs) Containing Phosphonium Ionic Liquids as Zn(II) Carriers. Ind. Eng. Chem. Res. 2018, 57, 5070–5082. [Google Scholar] [CrossRef]
- Huang, S.; Chen, J.; Chen, L.; Zou, D.; Liu, C. A polymer inclusion membrane functionalized by di(2-ethylhexyl) phosphinic acid with hierarchically ordered porous structure for Lutetium(III) transport. J. Membr. Sci. 2020, 593, 117458. [Google Scholar] [CrossRef]
- Chen, L.; Dong, H.; Pan, W.; Dai, J.; Dai, X.; Pan, J. Poly (vinyl alcohol-co-ethylene) (EVOH) modified polymer inclusion membrane in heavy rare earths separation with advanced hydrophilicity and separation property. Chem. Eng. J. 2021, 426, 131305. [Google Scholar] [CrossRef]
- Ansari, S.A.; Mohapatra, P.K.; Manchanda, V.K. Cation transport across plasticized polymeric membranes containing N, N, N′, N′-tetraoctyl-3-oxapentanediamide (TODGA) as the carrier. Desalination 2010, 262, 196–201. [Google Scholar] [CrossRef]
- Chen, L.; Chen, J. Asymmetric Membrane Containing Ionic Liquid [A336][P507] for the Preconcentration and Separation of Heavy Rare Earth Lutetium. ACS Sustain. Chem. Eng. 2016, 4, 2644–2650. [Google Scholar] [CrossRef]
- Lozano, L.J.; Godínez, C.; Ríos, A.P.d.l.; Hernández-Fernández, F.J.; Sánchez-Segado, S.; Alguacil, F.J. Recent advances in supported ionic liquid membrane technology. Membr. Sci. 2011, 376, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Chen, J. Extraction and separation of heavy rare earth elements: A review. Sep. Purif. Technol. 2021, 276, 119263. [Google Scholar] [CrossRef]
- Makowka, A.; Pospiech, B. Synthesis of Polymer Inclusion Membranes based on Cellulose Triacetate for Recovery of Lanthanum(III) from Aqueous Solutions. Autex Res. J. 2019, 19, 288–292. [Google Scholar] [CrossRef] [Green Version]
- Kusumocahyo, S.P.; Kanamori, T.; Sumaru, K.; Aomatsu, S.; Matsuyama, H.; Teramoto, M.; Shinbo, T. Development of polymer inclusion membranes based on cellulose triacetate: Carrier-mediated transport of cerium(III). J. Membr. Sci. 2004, 244, 251–257. [Google Scholar] [CrossRef]
- Hiratani, K.; Kusumocahyo, S.P.; Kameta, N.; Sugaya, K.; Shinbo, T.; Kanamori, T. Synthesis of noncyclic carriers for cerium ion transport through polymer inclusion membrane. Chem. Lett. 2005, 34, 1636–1637. [Google Scholar] [CrossRef]
- Guo, W.; Ngo, H.H.; Li, J. A mini-review on membrane fouling. Bioresour. Technol. 2012, 122, 27–34. [Google Scholar] [CrossRef]
- Kojiro, S.; Iori, F.; Kiyoshi, F.; Hiroyuki, O.; Tsuyoshi, S.; Tatsuya, O.; Yoshinari, B.; Hirochika, N. Extraction behavior of Rare-earth elements using a Mono-alkylated Diglycolamic acid extractant. Solvent Extr. Res. Dev. Jpn. 2016, 23, 151–159. [Google Scholar]
- Abdollahi, H.; Maleki, S.; Sayahi, H.; Gharabaghi, M.; Darvanjooghi, M.H.K.; Magdouli, S.; Brar, S.K. Superadsorbent Fe3O4-coated carbon black nanocomposite for separation of light rare earth elements from aqueous solution: GMDH-based Neural Network and sensitivity analysis. J. Hazard. Mater. 2021, 416, 125665. [Google Scholar] [CrossRef]
- Liu, E.; Xu, X.; Zheng, X.; Zhang, F.; Liu, E.; Li, C. An ion imprinted macroporous chitosan membrane for efficiently selective adsorption of dysprosium. Sep. Purif. Technol. 2017, 189, 288–295. [Google Scholar] [CrossRef]
- Lu, J.; Wu, Y.; Lin, X.; Gao, J.; Dong, H.; Chen, L.; Qin, Y.; Wang, L.; Yan, Y. Anti-fouling and thermosensitive ion-imprinted nanocomposite membranes based on grapheme oxide and silicon dioxide for selectively separating europium ions. Hazard. Mater. 2018, 353, 244–253. [Google Scholar] [CrossRef]
- Cui, K.; Gao, B.; Tai, M.; Su, B. A facile bionic strategy towards Gd(III)-imprinted membranes via interlaced stacking of one-dimensional/two-dimensional nanocomposite materials. J. Taiwan Inst. Chem. Eng. 2019, 95, 652–659. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, Y.; Zhang, F.; Li, Z.; Yan, Y. Dual-template docking oriented ionic imprinted bilayer mesoporous films with efficient recovery of neodymium and dysprosium. J. Hazard. Mater. 2018, 353, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Lu, J.; Xing, W.; Ma, F.; Gao, J.; Lin, X.; Yu, C.; Yan, M. Double-layer-based molecularly imprinted membranes for template-dependent recognition and separation: An imitated core-shell-based synergistic integration design. Chem. Eng. J. 2020, 397, 125371. [Google Scholar] [CrossRef]
- Wu, Y.; Lin, R.; Ma, F.; Xing, W.; Pan, J. Three-dimensional macroporous wood-based selective separation membranes decorated with well-designed Nd(III)-imprinted domains: A high-efficiency recovery system for rare earth element. J. Colloid Interface Sci. 2021, 587, 703–714. [Google Scholar] [CrossRef]
- Lai, X.; Hu, Y.; Fu, Y.; Wang, L.; Xiong, J. Synthesis and Characterization of Lu(III) Ion Imprinted Polymer. Inorg. Organomet. Polym. Mater. 2012, 22, 112–118. [Google Scholar] [CrossRef]
- Comandella, D.; Bonani, W.; Ciscar, J.B.; Ponti, J.; Cologna, M.; Popa, K.; Gilliland, D. Recovery of rare earth elements by nanometric CeO2embedded into electrospun PVA nanofibres. RSC Adv. 2021, 11, 19351–19362. [Google Scholar] [CrossRef]
- Ouda, M.; Hai, A.; Krishnamoorthy, R.; Govindan, B.; Othman, I.; Kui, C.C.; Choi, M.Y.; Hasan, S.W.; Banat, F. Surface tuned polyethersulfone membrane using an iron oxide functionalized halloysite nanocomposite for enhanced humic acid removal. Environ. Res. 2022, 204, 112113. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Chakraborty, S. Nanocomposite polymeric membrane a new trend of water and wastewater treatment: A short review. Groundw. Sustain. Dev. 2021, 12, 100533. [Google Scholar] [CrossRef]
- Ng, L.Y.; Chua, H.S.; Ng, C.Y. Incorporation of graphene oxide-based nanocomposite in the polymeric membrane for water and wastewater treatment: A review on recent development. J. Environ. Chem. Eng. 2021, 9, 105994. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Seo, K.-S.; Choi, S.-H. Polymeric nanocomposite proton exchange membranes prepared by radiation-induced polymerization for direct methanol fuel cell. Radiat. Phys. Chem. 2016, 118, 35–41. [Google Scholar] [CrossRef]
- Liang, B.; He, X.; Hou, J.; Li, L.; Tang, Z. Membrane Separation in Organic Liquid: Technologies, Achievements, and Opportunities. Adv. Mater. 2019, 31, e1806090. [Google Scholar] [CrossRef] [PubMed]
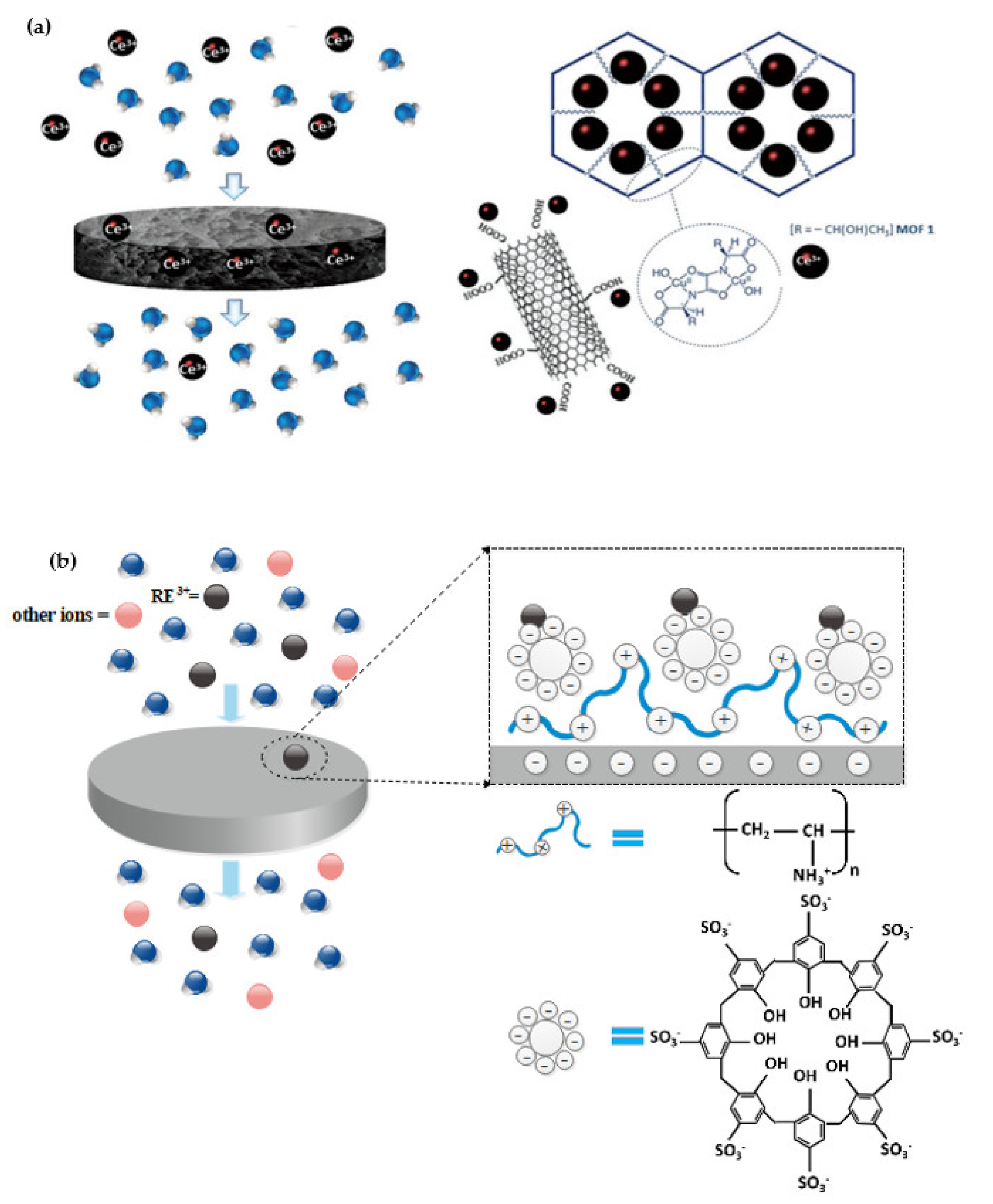
- Tursi, A.; Mastropietro, T.F.; Bruno, R.; Baratta, M.; Ferrando-Soria, J.; Mashin, A.I.; Nicoletta, F.P.; Pardo, E.; De Filpo, G.; Armentano, D. Synthesis and Enhanced Capture Properties of a New BioMOF@SWCNT-BP: Recovery of the Endangered Rare-Earth Elements from Aqueous Systems. Adv. Mater. Interfaces 2021, 8, 2100730. [Google Scholar] [CrossRef]
- Toutianoush, A.; El-Hashani, A.; Schnepf, J.; Tieke, B. Multilayer membranes of p-sulfonato-calix[8]arene and polyvinylamine and their use for selective enrichment of rare earth metal ions. Appl. Surf. Sci. 2005, 246, 430–436. [Google Scholar] [CrossRef]
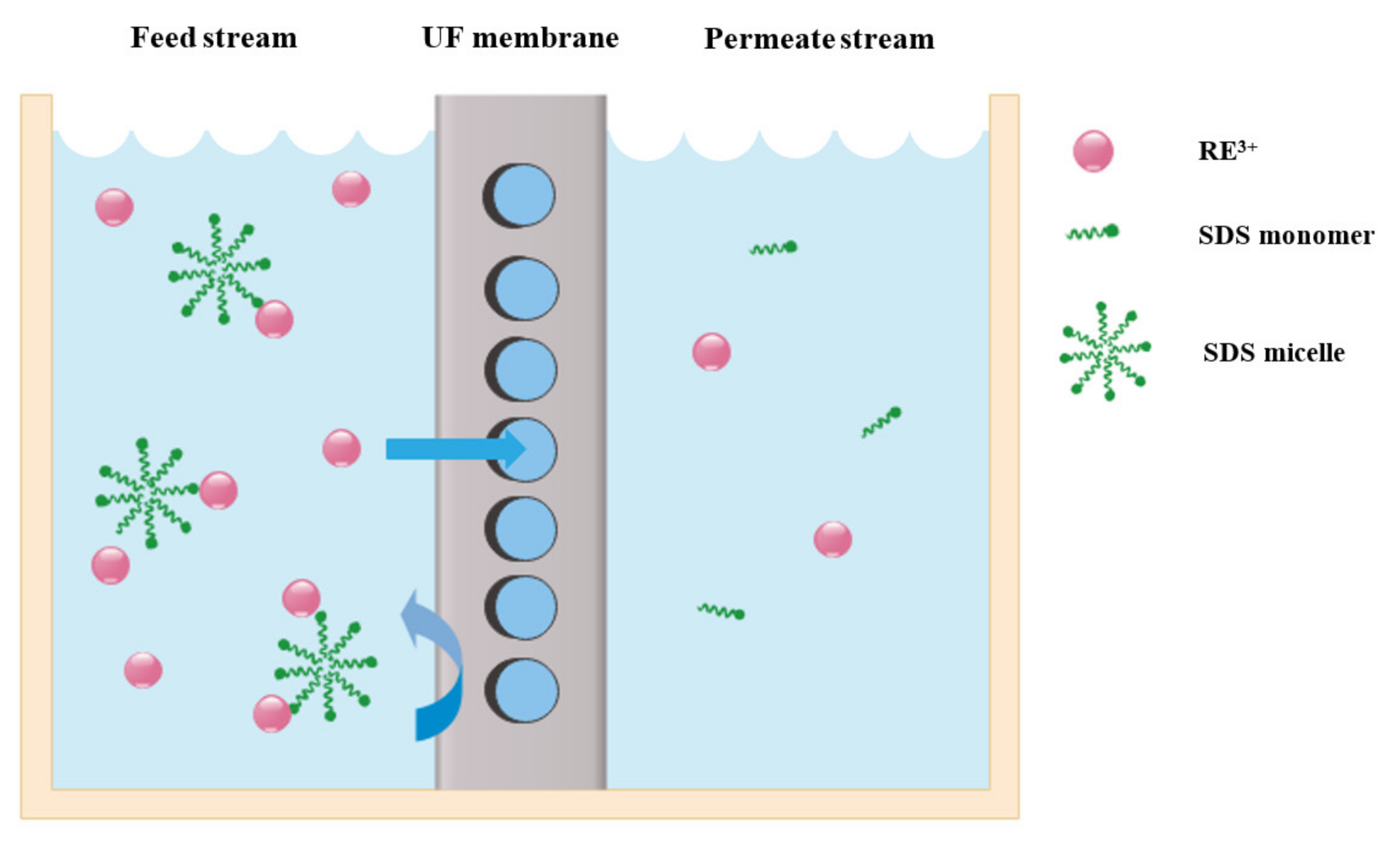
- Kose-Mutlu, B.; Hsu-Kim, H.; Wiesner, M.R. Separation of rare earth elements from mixed-metal feedstocks by micelle enhanced ultrafiltration with sodium dodecyl sulfate. Environ. Technol. 2020, 1–13. [Google Scholar] [CrossRef]
- Sorin, A.; Favre-Reguillon, A.; Pellet-Rostaing, S.; Sba, M.; Szymczyk, A.; Fievet, P.; Lemaire, M. Rejection of Gd(III) by nanofiltration assisted by complexation on charged organic membrane: Influences of pH, pressure, flux, ionic strength and temperature. Membr. Sci. 2005, 267, 41–49. [Google Scholar] [CrossRef]
- Murthy, Z.V.P.; Choudhary, A. Application of nanofiltration to treat rare earth element (neodymium) containing water. J. Rare Earths 2011, 29, 974–978. [Google Scholar] [CrossRef]
- Duan, H.; Lin, J.; Gong, Z.; Huang, J.; Yang, S. Removal of high-salinity matrices through polymer-complexation–ultrafiltration for the detection of trace levels of REEs using inductively coupled plasma mass spectrometry. Talanta 2015, 143, 287–293. [Google Scholar] [CrossRef]
- Innocenzi, V.; Prisciandaro, M.; Tortora, F.; Mazziotti di Celso, G.; Vegliò, F. Treatment of WEEE industrial wastewaters: Removal of yttrium and zinc by means of micellar enhanced ultra filtration. Waste Manag. 2018, 74, 393–403. [Google Scholar] [CrossRef]
- López, J.; Reig, M.; Vecino, X.; Gibert, O.; Cortina, J.L. Comparison of acid-resistant ceramic and polymeric nanofiltration membranes for acid mine waters treatment. Chem. Eng. J. 2020, 382, 122786. [Google Scholar] [CrossRef]
- Ağtaş, M.; Dilaver, M.; Koyuncu, İ. Ceramic membrane overview and applications in textile industry: A review. Water Sci. Technol. 2021, 84, 1059–1078. [Google Scholar] [CrossRef] [PubMed]
- López, J.; Reig, M.; Gibert, O.; Cortina, J.L. Recovery of sulphuric acid and added value metals (Zn, Cu and rare earths) from acidic mine waters using nanofiltration membranes. Sep. Purif. Technol. 2019, 212, 180–190. [Google Scholar] [CrossRef]


| Membrane Matrix | Carrier | Solvent | RE3+ | Description | References |
|---|---|---|---|---|---|
| - | D2EHPA 2 | - | Y3+ Nd3+ Dy3+ | Development of a mathematical transient model and utilization of a new extraction selectivity definition to study the extraction and separation of REE ion mixtures with the FSHSLM process | [49] |
| PTFE 1 | Aliquat-336 | - | Gd3+ Nd3+ | Improvement of 28.3% and 49.5% permeability coefficient for Gd3+ and Nd3+ extraction using hydrophobic nanoparticle SiO2 | [65] |
| PVDF | TODGA 3 | n-Octane | La3+ Ce3+ Pr3+ Nd3+ | Very fast permeation and collection of more than 95.0% of REE ions using 0.1 M TODGA as carrier | [62] |
| polyvinylidene fluoride | D2EHPA | Kerosene | Nd3+ | Development of a novel flat renewal supported liquid membrane (FRSLM) and obtaining an extraction percentage of 92.9 for Nd3+ in 75 min | [39] |
| Polypropylene (PP) | TODGA Cyanex 923 TBP 4 | IsoparTM L | Nd3+ Pr3+ Dy3+ | Use the SLM coupled with hollow supported fiber membrane for extraction of REE ions from permanent magnets wastes in the presence of non-REEs | [2] |
| - | [C6MIM][NTf2] TOPO 5 TPB | Kerosene | Nd3+ Pr3+ | Achievement of the highest permeability coefficients with the synergistic extractant containing [C6MIM][NTf2] ionic liquid, TOPO and TPB carriers. | [64] |
| PTFE | combination of ionic liquids TBP D2EHPA | - | Ce3+ | Observation of maximum permeation coefficient of 30%, 20% and 10% v/v for D2EHPA, TBP and C6MIM.NTF2 extractant | [66] |
| PVDF | DODGAA 6 | [C8mim][Tf2N] | Nd3+ Dy3+ | More than 99% extraction and purification of REEs from Fe ions with [C8mim][Tf2N] containing 10 mM DODGAA | [63] |
| Membrane Matrix | Carrier | Plasticizer | RE3+ | Description | References |
|---|---|---|---|---|---|
| CTA 1 | D2EHPA and TBP 4 | NPOE 8 | La3+ Ce3+ | Selectivity order (Ce/La) for TBP carrier = 1.4 Selectivity order (Ce/La) for D2EHPA carrier = 2.8 | [75] |
| CTA | CMPO 5 and TODGA 6 | - | Ce3+ | Use of combined carriers with high effective transport of cerium ions | [76] |
| PVDF 2 | P277 | - | Lu3+ | Selective separation of Lu3+ from La3+ and Sm3+ dependent on the PH of the feed solution | [7] |
| CTA | Noncyclic ionophores | - | Ce3+ | A new combination of carriers for excellent separation of cerium ions | [77] |
| CTA | TODGA | NPOE | La3+ Eu3+ Lu3+ | High transport percentage of La3+ = 60.4%, Eu3+ = 91.2% and Lu3+ = 98.0% | [71] |
| PVC 3 | D2EHPA | - | La3+ Yb3+ Gd3+ | Selective extraction of Yb3+ with thermodynamic extraction constants of Yb3+ = 92,700, Gd3+ = 85.5 and La3+ = 0.896 dependent on PH of the feed solution | [53] |
| PVDF | [A336][P507] | - | Lu3+ | Use of bifunctional ionic liquid with good permeation coefficient of 2.80 µm/s compared to liquid-liquid extraction | [72] |
| EVOH 7 | Cyanex272 | - | Yb3+ Lu3+ | High permeability coefficients of Lu3+ = 114.82 μm/s and Yb3+ = 156 μm/s | [69] |
| Target Ion | Membrane Substance | PH of Adsorption | Initial Concentration (ppm) | Adsorption Capacity (mg/g) | Competitive Ions | References |
|---|---|---|---|---|---|---|
| Dy3+ | chitosan membrane | 7 | 50 | 23.3 | Nd3+ Pr3+ Tb3+ Fe3+ | [81] |
| Eu3+ | Grapheme oxide (GO)/modified silicon dioxide (kSiO2) on PDA-modified substrate | 7 | 50 | 101.14 | La3+ Gd3+ Sm3+ | [82] |
| Gd3+ | Polydopamine/modified graphene oxide (PDA@GO) and carbon nanotubes (GICNTs) on cellulose membrane | 7 | 60 | - | La3+ Eu3+ | [83] |
| Nd3+ | polydopamine (PDA)-modified on basswood surfaces | 7 | 90 | 120.87 | Tb3+ Fe3+ Dy3+ | [86] |
| Lu3+ | 4-vinylpyridine–acetylacetone/EDMA | 5.5 | 20 | 64.2 | Fe3+ −Mg2+ Ca2+ −Al3+ La3+ −Nd3+ Y3+ −Gd3+ Dy3+ −Tm3+ Lu3+ | [87] |
| Membrane Type | Active Layer | Organic Chelate | RE3+ | MWCO (Da) | PH | Rejection (%) | References |
|---|---|---|---|---|---|---|---|
| NF270 | Polyamide on a thin flm composite | - | La3+ Pr3+ Nd3+ Sm3+ Dy3+ Yb3+ | 180 ± 20 | 1 | >98 | [103] |
| Amicon Ultra-15 centrifugal filter units | - | PAA | La3+ Sm3+ | 30 | 7.5 | >89 | [99] |
| NF-300 | Polyamide | EDTA SDS | Ce3+ Nd3+ | 300 | 2–10 | >90 | [98] |
| Single tube MembraloxO Tl-70 ceramic membrane | - | SDS | Y3+ | 1 | 5–6 | 99 | [100] |
| UP020 | - | SDS | Tb3+ Nd3+ Eu3+ Er3+ Y3+ Dy3+ | 20 | 3.5 | >97 | [96] |
| TiO2 supported Al2O3 ceramic membrane | TiO2 | - | REE ions Al3+ Zn2+ Cu2+ | 200 | - | 60 | [101] |
| Membrane Type | Functional PH | Selectivity | Life Time | Price |
|---|---|---|---|---|
| Supported liquid membrane (SLM) | Acidic and neutral conditions | Higher for LREEs | Low due to pore blockage | Low for continuous extraction process, few consuption chemical materials and easily operation |
| Polymer inclusion membrane (PIM) | Acidic and neutral conditions | Higher for HREEs | Low due to fouling, low thermal, and chemical stability | Low for continuous extraction process, few consuption chemical materials and easily operation |
| Ion imprinted membrane (IIM) | 5–7 | High | Low due to loss of active sites after a period | High for production cost and needed post treatment |
| Nanocomposite membrane | Acidic and neutral conditions | low | Long for high chemical and thermal stability | High for production cost |
| Ultrafiltration | More suitable for high acidic conditions | Low | Long for high chemical and mechanical stability | High for production cost and needed pre-treatment |
| Nanofiltration | More suitable for high acidic conditions | Low | Long for high chemical and mechanical stability | High for production cost, needed pre-treatment and consumption of chelating agent |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bashiri, A.; Nikzad, A.; Maleki, R.; Asadnia, M.; Razmjou, A. Rare Earth Elements Recovery Using Selective Membranes via Extraction and Rejection. Membranes 2022, 12, 80. https://doi.org/10.3390/membranes12010080
Bashiri A, Nikzad A, Maleki R, Asadnia M, Razmjou A. Rare Earth Elements Recovery Using Selective Membranes via Extraction and Rejection. Membranes. 2022; 12(1):80. https://doi.org/10.3390/membranes12010080
Chicago/Turabian StyleBashiri, Atiyeh, Arash Nikzad, Reza Maleki, Mohsen Asadnia, and Amir Razmjou. 2022. "Rare Earth Elements Recovery Using Selective Membranes via Extraction and Rejection" Membranes 12, no. 1: 80. https://doi.org/10.3390/membranes12010080
APA StyleBashiri, A., Nikzad, A., Maleki, R., Asadnia, M., & Razmjou, A. (2022). Rare Earth Elements Recovery Using Selective Membranes via Extraction and Rejection. Membranes, 12(1), 80. https://doi.org/10.3390/membranes12010080








