Osmium Recovery as Membrane Nanomaterials through 10–Undecenoic Acid Reduction Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
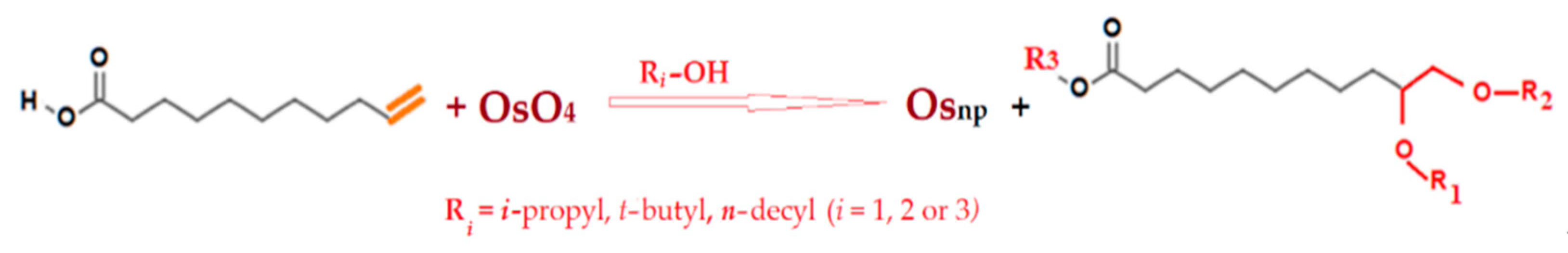
2.2. Procedures
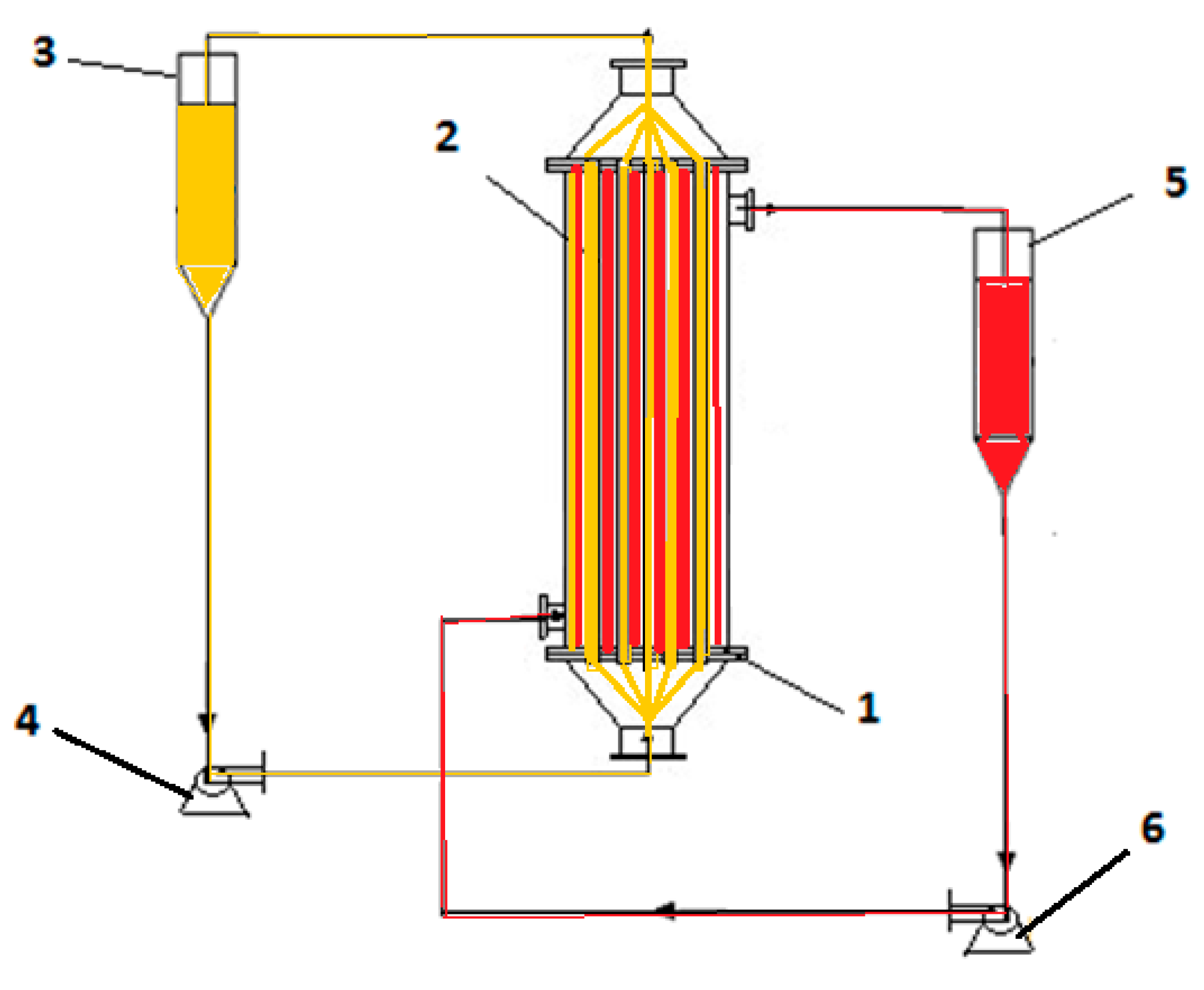
2.2.1. Obtaining Composite Membranes
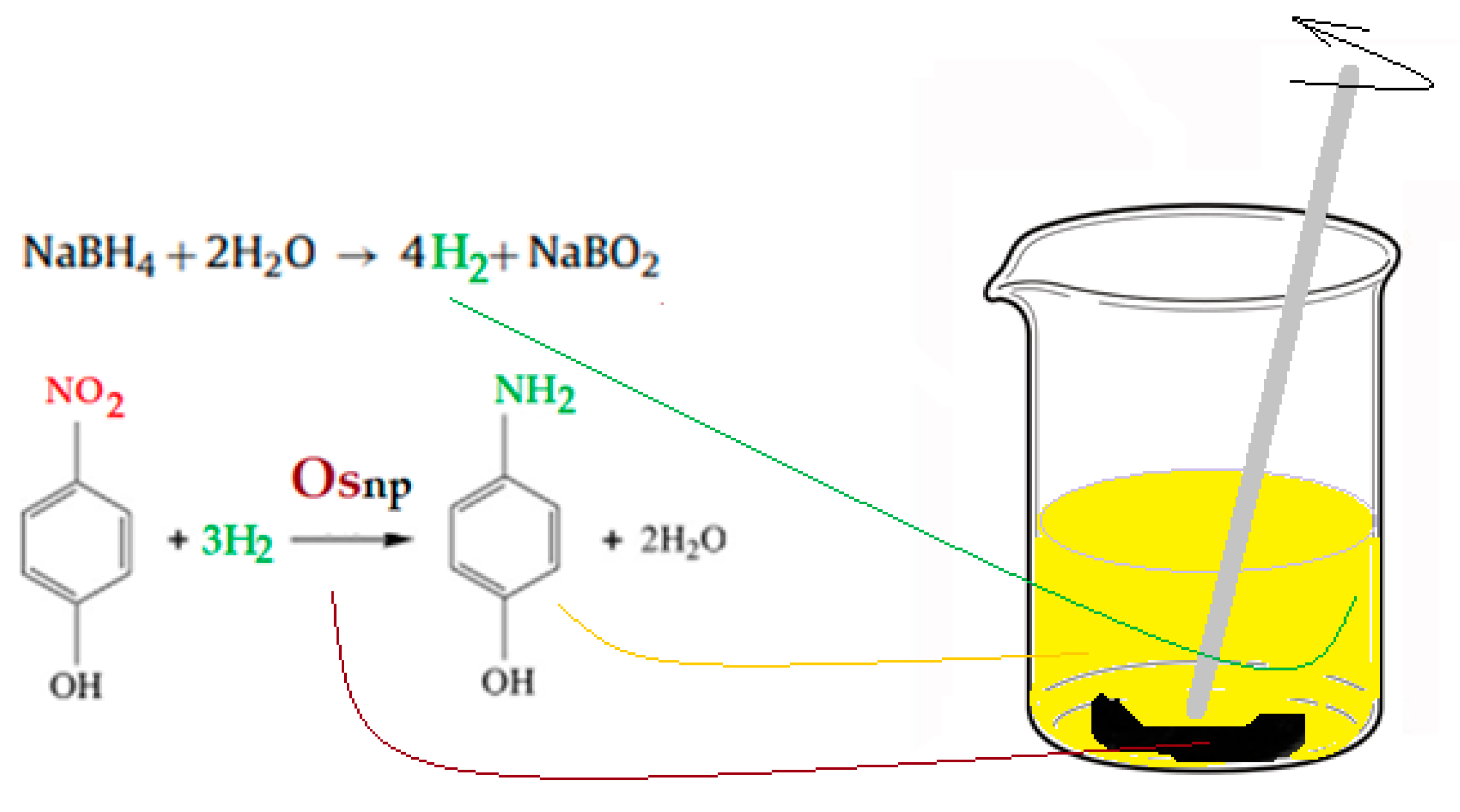
2.2.2. The Reduction Process of p–Nitrophenol to p–Aminophenol on Membrane Contactor
2.3. Apparatus and Instruments
3. Results and Discussions
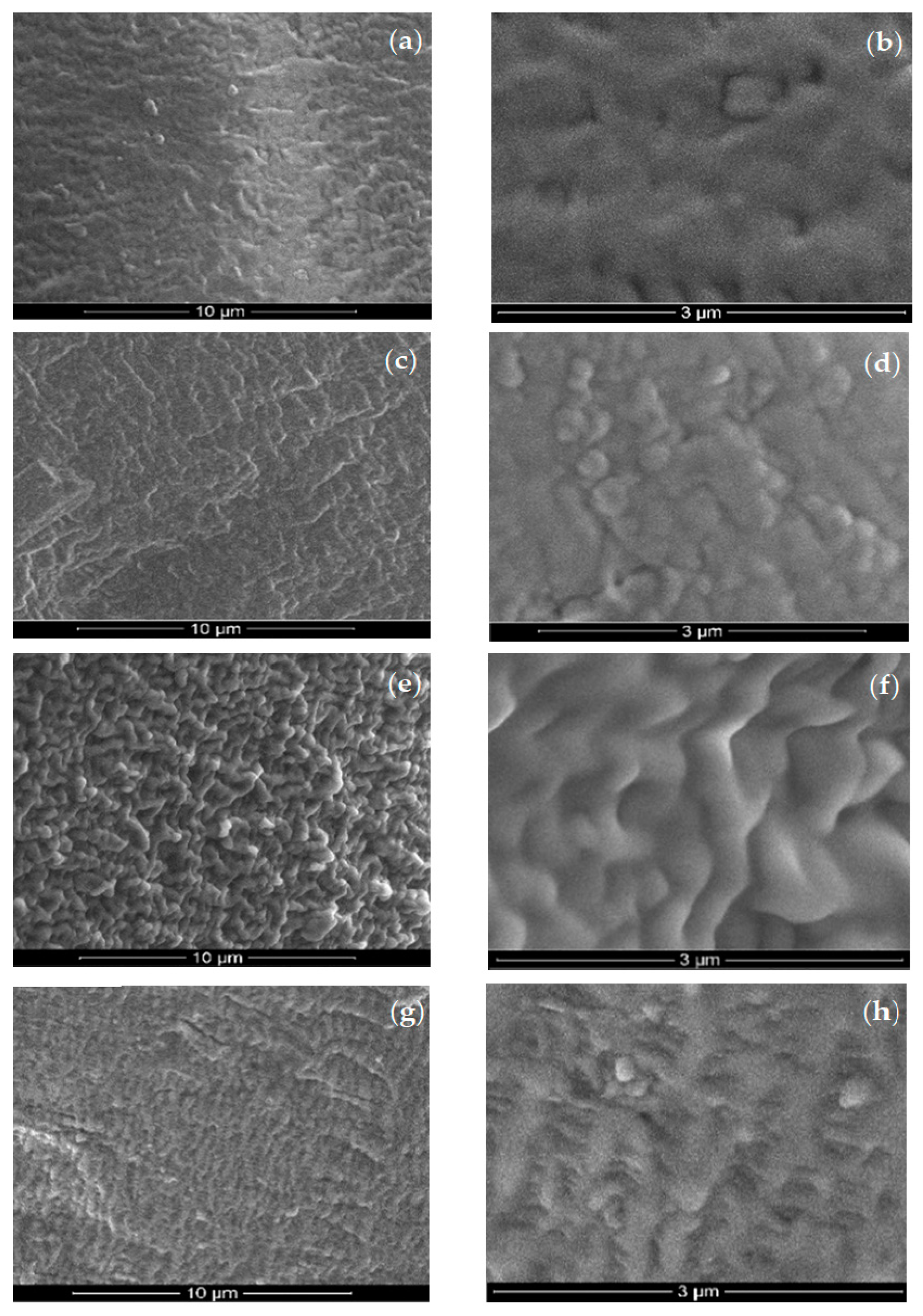
3.1. Characterization of Obtained Composite Membranes (Os–A–PPM)
3.2. Performances of the Composite Membranes (Os–A–PPM) in the Process of Reducing P-Nitrophenol
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liao, Z.; Zhu, J.; Li, X.; Van der Bruggen, B. Regulating composition and structure of nanofillers in thin film nanocomposite (TFN) membranes for enhanced separation performance: A critical review. Sep. Purif. Technol. 2021, 266, 118567. [Google Scholar] [CrossRef]
- Zhu, J.; Hou, J.; Uliana, A.; Zhang, Y.; Tian, M.; Van der Bruggen, B. The rapid emergence of two-dimensional nanomaterials for high-performance separation membranes. J. Mater. Chem. A 2018, 6, 3773–3792. [Google Scholar] [CrossRef]
- Lai, G.S.; Lau, W.J.; Goh, P.S.; Ismail, A.F.; Tan, Y.H.; Chong, C.Y.; Krause-Rehberg, R.; Awad, S. Tailor-made thin film nanocomposite membrane incorporated with graphene oxide using novel interfacial polymerization technique for enhanced water separation. Chem. Eng. J. 2018, 344, 524–534. [Google Scholar] [CrossRef]
- Mulder, M. The Use of Membrane Processes in Environmental Problems. An Introduction. In Membrane Processes in Separation and Purification. NATO ASI Series; Crespo, J.G., Böddeker, K.W., Eds.; Series E: Applied Sciences; Springer: Dordrecht, The Netherlands, 1994; Volume 272. [Google Scholar] [CrossRef]
- Drioli, E.; Stankiewicz, A.I.; Macedonio, F. Membrane engineering in process intensification—An overview. J. Membr. Sci. 2011, 380, 1–8. [Google Scholar] [CrossRef]
- Bernardoa, P.; Drioli, E. Membrane Gas Separation Progresses for Process Intensification Strategy in the Petrochemical Industry. Pet. Chem. 2010, 50, 271–282. [Google Scholar] [CrossRef]
- Firouzjaei, M.D.; Shamsabadi, A.A.; Aktij, S.A.; Seyedfour, S.F.; Sharifian Gh, M.; Rahimpour, A.; Esfahani Ulbricht, M.; Soroush, M. Exploiting synergetic effects of graphene oxide and a silver-based metal-organic M.R.; framework to enhance antifouling and anti-biofouling properties of thin-film nanocomposite membranes. ACS Appl. Mater. Interfaces 2018, 10, 42967–42978. [Google Scholar] [CrossRef] [PubMed]
- Abdelsamad, A.M.A.; Khalil, A.S.G.; Ulbricht, M. Influence of controlled functionalization of mesoporous silica nanoparticles as tailored fillers for thin-film nanocomposite membranes on desalination performance. J. Membr. Sci. 2018, 563, 149–161. [Google Scholar] [CrossRef]
- Ang, M.B.M.Y.; Pereira, J.M.; Trilles, C.A.; Aquino, R.R.; Huang, S.-H.; Lee, K.-R.; Lai, J.-Y. Performance and antifouling behavior of thin-film nanocomposite nanofiltration membranes with embedded silica spheres. Sep. Purif. Technol. 2019, 210, 521–529. [Google Scholar] [CrossRef]
- Yuan, Y.; Wu, H.; Bu, X.; Wu, Q.; Wang, X.; Han, C.; Li, X.; Wang, X.; Liu, W. Improving Ammonia Detecting Performance of Polyaniline Decorated rGO Composite Membrane with GO Doping. Materials 2021, 14, 2829. [Google Scholar] [CrossRef]
- Zhang, A.; Zhang, Y.; Pan, G.; Xu, J.; Yan, H.; Liu, Y. In situ formation of copper nanoparticles in carboxylated chitosan layer: Preparation and characterization of surface modified TFC membrane with protein fouling resistance and long-lasting antibacterial properties. Sep. Purif. Technol. 2017, 176, 164–172. [Google Scholar] [CrossRef]
- Rajakumaran, R.; Kumar, M.; Chetty, R. Morphological effect of ZnO nanostructures on desalination performance and antibacterial activity of thin-film nanocomposite (TFN) membrane. Desalination 2020, 495, 114673. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Zhu, J.; Zhang, Y.; Liu, J.; van der Bruggen, B. Construction of TiO2@graphene oxide incorporated antifouling nanofiltration membrane with elevated filtration performance. J. Membr. Sci. 2017, 533, 279–288. [Google Scholar] [CrossRef]
- Khalil, A.M.; Georgiadou, V.; Guerrouache, M.; Mahouche-Chergui, S.; Dendrinou-Samara, C.; Chehimi, M.M.; Carbonnier, B. Gold- decorated polymeric monoliths: In-situ vs. ex-situ immobilization strategies and flow through catalytic applications towards nitrophenols reduction. Polymer 2015, 77, 218–226. [Google Scholar] [CrossRef]
- Nechifor, G.; Păncescu, F.M.; Grosu, A.R.; Albu, P.C.; Oprea, O.; Tanczos, S.-K.; Bungău, C.; Grosu, V.-A.; Pîrțac, A.; Nechifor, A.C. Osmium Nanoparticles-Polypropylene Hollow Fiber Membranes Applied in Redox Processes. Nanomaterials 2021, 11, 2526. [Google Scholar] [CrossRef] [PubMed]
- Nechifor, A.C.; Goran, A.; Grosu, V.-A.; Pîrțac, A.; Albu, P.C.; Oprea, O.; Grosu, A.R.; Pașcu, D.; Păncescu, F.M.; Nechifor, G.; et al. Reactional Processes on Osmium–Polymeric Membranes for 5–Nitrobenzimidazole Reduction. Membranes 2021, 11, 633. [Google Scholar] [CrossRef] [PubMed]
- Chelucci, G.; Baldino, S.; Baratta, W. Recent Advances in Osmium-Catalyzed Hydrogenation and Dehydrogenation Reactions. Acc. Chem. Res. 2015, 48, 363–379. [Google Scholar] [CrossRef]
- Baratta, W.; Ballico, M.; Chelucci, G.; Siega, K.; Rigo, P. Osmium(II) CNN Pincer Complexes as Efficient Catalysts for Both Asymmetric Transfer and H2 Hydrogenation of Ketones. Angew. Chem. 2008, 120, 4434–4437. [Google Scholar] [CrossRef]
- Yoon, T.P.; Jacobsen, E.N. Privileged Chiral Catalysts. Science 2003, 299, 1691–1693. [Google Scholar] [CrossRef]
- Ma, L.; Abney, C.; Lin, W. Enantioselective catalysis with homochiral metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1248–1256. [Google Scholar] [CrossRef]
- Uribe-Godínez, J.; Castellanos, E.; Borja-Arco, R.H.; Altamirano-Gutiérrez, A.; Jiménez-Sandoval, O. Novel osmium-based electrocatalysts for oxygen reduction and hydrogen oxidation in acid conditions. J. Power Sources 2008, 177, 286–295. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, G.; Shi, L.; Ye, J. Single-Atom Catalysts: Emerging Multifunctional Materials in Heterogeneous Catalysis. Adv. Mat. 2018, 8, 1701343. [Google Scholar] [CrossRef]
- Sharpless, K.B.; Amberg, W.; Bennani, Y.L.; Crispino, G.A.; Hartung, J.; Jeong, K.S.; Kwong, H.L.; Morikawa, Z.M.; Wang, K. The osmium-catalyzed asymmetric dihydroxylation: A new ligand class and a process improvement. J. Org. Chem. 1992, 57, 2768–2771. [Google Scholar] [CrossRef]
- Kolb, H.C.; Van Nieuwenhze, M.S.; Sharpless, K.B. Catalytic Asymmetric Dihydroxylation. Chem. Rev. 1994, 94, 2483–2547. [Google Scholar] [CrossRef]
- Borja-Arco, E.; Castellanos, R.H.; Uribe-Godínez, J.; Altamirano-Gutiérrez, A.; Jiménez-Sandoval, O. Osmium–ruthenium carbonyl clusters as methanol tolerant electrocatalysts for oxygen reduction. J. Power Sources 2009, 188, 387–396. [Google Scholar] [CrossRef]
- Bolitho, E.M.; Coverdale, J.P.C.; Bridgewater, H.E.; Clarkson, G.J.; Quinn, P.D.; Sanchez-Cano, C.; Sadler, P.J. Tracking Reactions of Asymmetric Organo-Osmium Transfer Hydrogenation Catalysts in Cancer Cells. Angew. Chem. Int. Ed. 2021, 60, 6462–6472. [Google Scholar] [CrossRef]
- Vassilev, D.; Petkova, N.; Tumbarski, Y.; Koleva, M.; Denev, P. Application of the principles of “green chemistry” for the synthesis of 10-undecylenic aliphatic esters with antimicrobial activity. J. Renew. Mater. 2020, 8, 675–686. [Google Scholar] [CrossRef]
- Yasa, S.; Cheguru, S.; Krishnasamy, S.; Korlipara, P.; Rajak, A.; Penumarthy, V. Synthesis of 10-undecenoic acid based C22-dimer acid esters and their evaluation as potential lubricant basestocks. Ind. Crops Prod. 2017, 103, 141–151. [Google Scholar] [CrossRef]
- Raku, T.; Kitagawa, M.; Shimakawa, H.; Tokiwa, Y. Enzymatic synthesis of hydrophilic undecylenic acid sugar esters and their biodegradability. Biotechnol. Lett. 2003, 25, 161–166. [Google Scholar] [CrossRef]
- Cavalcante, I.M.; Rocha, N.R.D.C.; de Brito, D.H.A.; Schuller, A.P.D.; Câmara Neto, J.F.; de Morais, S.M.; de Luna, F.M.T.; Schanz, M.T.G.F.; Maier, M.E.; Ricardo, N.M.P.S. Synthesis and characterization of novel polyol esters of undecylenic acid as ecofriendly lubricants. J. Am. Oil Chem. Soc. 2019, 96, 75–82. [Google Scholar] [CrossRef] [Green Version]
- Huerta-Ángeles, G.; Brandejsová, M.; Kopecká, K.; Ondreáš, F.; Medek, T.; Židek, O.; Kulhánek, J.; Vagnerová, H.; Velebný, V. Synthesis and Physicochemical Characterization of Undecylenic Acid Grafted to Hyaluronan for Encapsulation of Antioxidants and Chemical Crosslinking. Polymers 2020, 12, 35. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, L.M.; Del Bel Cury, A.A.; Sartoratto, A.; Garcia Rehder, V.L.; Silva, W.J. Effects of Undecylenic Acid Released from Denture Liner on Candida Biofilms. J. Dent. Res. 2012, 91, 985–989. [Google Scholar] [CrossRef] [PubMed]
- Petrović, M.; Bonvin, D.; Hofmann, H.; Mionić Ebersold, M. Fungicidal PMMA-Undecylenic Acid Composites. Int. J. Mol. Sci. 2018, 19, 184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLain, N.; Ascanio, R.; Baker, C.; Strohaver, R.A.; Dolan, J.W. Undecylenic Acid Inhibits Morphogenesis of Candida albicans. Antimicrob. Agents Chemother. 2000, 44, 2873–2875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nechifor, A.C.; Stoian, M.G.; Voicu, S.I.; Nechifor, G. Modified Fe3O4 colloidal dispersed magnetic particles as carrier in liquid membranes. Optoelectron. Adv. Mater.-Rapid Commun. 2010, 4, 1118–1123. [Google Scholar]
- Ghimpusan, M.; Nechifor, G.; Din, I.S.; Nechifor, A.C.; Passeri, P. Application of Hollow Fiber Membrane Bioreactor Instead of Granular Activated Carbon Filtration for Treatment of Wastewater from Car Dismantler Activity. Mat. Plast. 2016, 53, 578–584. [Google Scholar]
- Ghimpusan, M.; Nechifor, G.; Nechifor, A.C.; Dima, S.O.; Passeri, P. Case studies on the physical-chemical parameters’ variation during three different purification approaches destined to treat wastewaters from food industry. J. Environ. Manag. 2017, 203, 811–816. [Google Scholar] [CrossRef]
- Din, I.S.; Cimbru, A.M.; Rikabi, A.A.K.K.; Tanczos, S.K.; Ticu (Cotorcea), S.; Nechifor, G. Iono-molecular Separation with Composite Membranes VI. Nitro-phenol separation through sulfonated polyether ether ketone on capillary polypropylene membranes. Rev. Chim. 2018, 69, 1603–1607. [Google Scholar] [CrossRef]
- Nechifor, A.C.; Cotorcea, S.; Bungău, C.; Albu, P.C.; Pașcu, D.; Oprea, O.; Grosu, A.R.; Pîrțac, A.; Nechifor, G. Removing of the Sulfur Compounds by Impregnated Polypropylene Fibers with Silver Nanoparticles-Cellulose Derivatives for Air Odor Correction. Membranes 2021, 11, 256. [Google Scholar] [CrossRef]
- Dimulescu, I.A.; Nechifor, A.C.; Bǎrdacǎ, C.; Oprea, O.; Paşcu, D.; Totu, E.E.; Albu, P.C.; Nechifor, G.; Bungău, S.G. Accessible Silver-Iron Oxide Nanoparticles as a Nanomaterial for Supported Liquid Membranes. Nanomaterials 2021, 11, 1204. [Google Scholar] [CrossRef]
- Grosu, A.R.; Nafliu, I.M.; Din, I.S.; Cimbru, A.M.; Nechifor, G. Neutralization with simultaneous separation of aluminum and copper ions from condensed water through capillary polypropylene and cellulose. UPB Sci. Bull. Ser. B Chem. Mater. Sci. 2020, 82, 25–34. [Google Scholar]
- Szczepański, P.; Diaconu, I. Transport of p-nitrophenol through an agitated bulk liquid membrane. Sep. Sci. Technol. 2012, 47, 1725–1732. [Google Scholar] [CrossRef]
- Kakoi, T.; Goto, M.; Natsukawa, S.; Lkemizu, K.; Nakashio, F. Recovery of phenols using liquid surfactant membranes prepared with newly synthesized surfactants. Sep. Sci. Technol. 1996, 31, 107–124. [Google Scholar] [CrossRef]
- Sirkar, K.K.; Shanbhag, P.V.; Kovvali, A.S. Membrane in a Reactor: A Functional Perspective. Ind. Eng. Chem. Res. 1999, 38, 3715–3737. [Google Scholar] [CrossRef]
- Koter, S.; Szczepański, P.; Mateescu, M.; Nechifor, G.; Badalau, L.; Koter, I. Modeling of the cadmium transport through a bulk liquid membrane. Sep. Purif. Technol. 2013, 107, 135–143. [Google Scholar] [CrossRef]
- Nechifor, A.C.; Goran, A.; Grosu, V.-A.; Bungău, C.; Albu, P.C.; Grosu, A.R.; Oprea, O.; Păncescu, F.M.; Nechifor, G. Improving the Performance of Composite Hollow Fiber Membranes with Magnetic Field Generated Convection Application on pH Correction. Membranes 2021, 11, 445. [Google Scholar] [CrossRef]
- Nafliu, I.M.; Al-Ani, H.N.A.; Grosu (Miron), A.R.; Albu, P.C.; Nechifor, G. Iono-molecular separation with composite membranes. viii. recuperative aluminium ions separation on capilary polypropylene S-EPDM composite membranes. Mat. Plast. 2019, 56, 32–36. [Google Scholar] [CrossRef]
- Harish, S.; Mathiyarasu, J.; Phani, K.L.N.; Yegnaraman, V. Synthesis of conducting polymer supported Pd nanoparticles in aqueous medium and catalytic activity towards 4-nitrophenol reduction. Catal. Lett. 2009, 128, 197–202. [Google Scholar] [CrossRef]
- Kuroda, K.; Ishida, T.; Haruta, M. Reduction of 4-nitrophenol to 4-aminophenol over Au nanoparticles deposited on PMMA. J. Mol. Catal. A Chem. 2009, 298, 7–11. [Google Scholar] [CrossRef]
- Koga, H.; Kitaoka, T. One-step synthesis of gold nanocatalysts on a micro-structured paper matrix for the reduction of 4-nitrophenol. Chem. Eng. J. 2011, 168, 420–425. [Google Scholar] [CrossRef]
- VanRheenen, V.; Kelly, R.C.; Cha, D.Y. An improved catalytic OsO4 oxidation of olefins to cis-1, 2-glycols using tertiary amine oxides as the oxidant. Tetrahedron Lett. 1976, 17, 1973–1976. [Google Scholar] [CrossRef]
- Brückner, C.; Dolphin, D. 2, 3-vic-Dihydroxy-meso-tetraphenylchlorins from the osmium tetroxide oxidation of meso-tetraphenylporphyrin. Tetrahedron Lett. 1995, 36, 3295–3298. [Google Scholar] [CrossRef]
- De Champdoré, M.; Lasalvia, M.; Piccialli, V. OsO4-catalyzed oxidative cyclization of geranyl and neryl acetate to cis-2, 5-bis (hydroxymethyl) tetrahydrofurans. Tetrahedron Lett. 1998, 39, 9781–9784. [Google Scholar] [CrossRef]
- Yu, W.; Mei, Y.; Kang, Y.; Hua, Z.; Jin, Z. Improved procedure for the oxidative cleavage of olefins by OsO4−NaIO4. Org. Lett. 2004, 6, 3217–3219. [Google Scholar] [CrossRef]
- Santiago, E.I.; Ticianelli, E.A. The performance of carbon-supported PtOs electrocatalysts for the hydrogen oxidation in the presence of CO. Int. J. Hydrogen Energy 2005, 30, 159–165. [Google Scholar] [CrossRef]
- Kujawski, W.; Warszawski, A.; Ratajczak, W.; Porebski, T.; Capala, W.; Ostrowska, I. Removal of phenol from wastewater by different separation techiniques. Desalination 2004, 163, 287–296. [Google Scholar] [CrossRef]
- Diaconu, I.; Nechifor, G.; Nechifor, A.C.; Ruse, E.; Totu, E.E. Membranary techniques used at the separation of some phenolic compounds from aqueous media. UPB Sci. Bull. Ser. B Chem. Mater. Sci. 2009, 71, 39–46. [Google Scholar]
- Szczepański, P.; Szidonia, T.K.; Ghindeanu, D.L.; Wódzki, R. Transport of p-nitrophenol in an agitated bulk liquid membrane system–Experimental and theoretical study by network analysis. Sep. Pur. Technol. 2014, 132, 616–626. [Google Scholar] [CrossRef]
- Macanás, J.; Ouyang, L.; Bruening, M.L.; Muñoz, M.; Remigy, J.-C.; Lahitte, J.-F. Development of polymeric hollow fiber membranes containing catalytic metal nanoparticles. Catal. Today 2010, 156, 181–186. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Dong, Z.; Na, C. Hierarchical carbon nanotube membrane-supported gold nanoparticles for rapid catalytic reduction of p-nitrophenol. ACS Sustain. Chem. Eng. 2013, 1, 746–752. [Google Scholar] [CrossRef]
- Wu, W.; Liu, G.; Liang, S.; Chen, Y.; Shen, L.; Zheng, H.; Yuan, R.; Hou, Y.; Wu, L. Efficient visible-light-induced photocatalytic reduction of 4-nitroaniline to p-phenylenediamine over nanocrystalline PbBi2Nb2O9. J. Catal. 2012, 290, 13–17. [Google Scholar] [CrossRef]
- Le, X.; Dong, Z.; Li, X.; Zhang, W.; Le, M.; Ma, J. Fibrous nano-silica supported palladium nanoparticles: An efficient catalyst for the reduction of 4-nitrophenol and hydrodechlorination of 4-chlorophenol under mild conditions. Catal. Commun. 2015, 59, 21–25. [Google Scholar] [CrossRef]
- Guan, H.; Chao, C.; Kong, W.; Hu, Z.; Zhao, Y.; Yuan, S.; Zhang, B. Magnetic porous PtNi/SiO2 nanofibers for catalytic hydrogenation of p-nitrophenol. Nanopart Res. 2017, 19, 187. [Google Scholar] [CrossRef]
- Feng, J.; Wang, Q.; Fan, D.; Ma, L.; Jiang, D.; Xie, J.; Zhu, J. Nickel-based xerogel catalysts: Synthesis via fast sol-gel method and application in catalytic hydrogenation of p-nitrophenol to p-aminophenol. Appl. Surf. Sci. 2016, 382, 135–143. [Google Scholar] [CrossRef]
- Liu, K.; Wang, Y.; Chen, P.; Zhong, W.; Liu, Q.; Li, M.; Wang, Y.; Wang, W.; Lu, Z.; Wang, D. Noncrystalline nickel phosphide decorated poly (vinyl alcohol-co-ethylene) nanofibrous membrane for catalytic hydrogenation of p-nitrophenol. Appl. Catal. B Environ. 2016, 196, 223–231. [Google Scholar] [CrossRef]
- Dong, Z.; Le, X.; Dong, C.; Zhang, W.; Li, X.; Ma, J. Ni@Pd core-shell nanoparticles modified fibrous silica nanospheres as highly efficient and recoverable catalyst for reduction of 4-nitrophenol and hydrodechlorination of 4-chlorophenol. Appl. Catal. B Environ. 2015, 162, 372–380. [Google Scholar] [CrossRef]


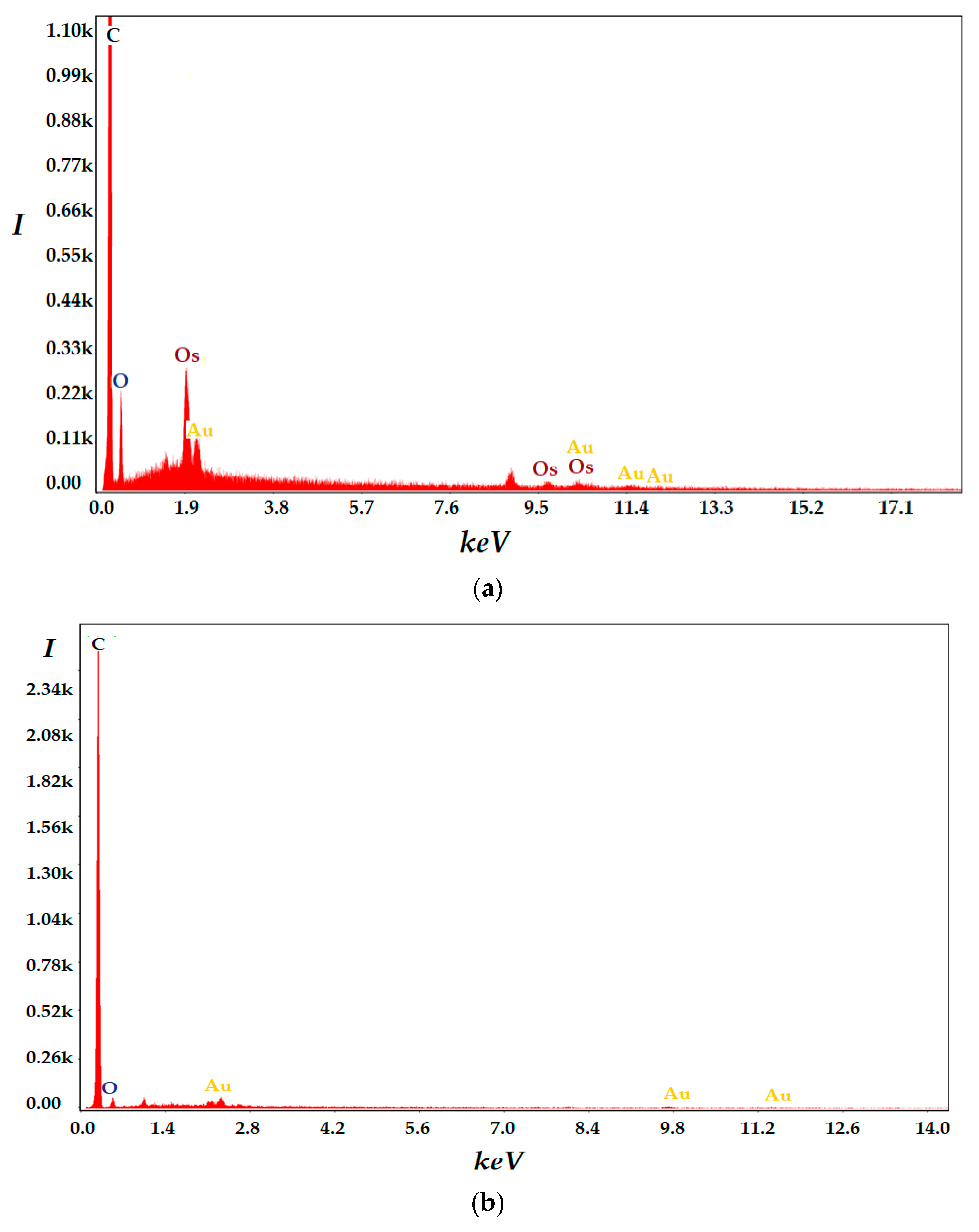
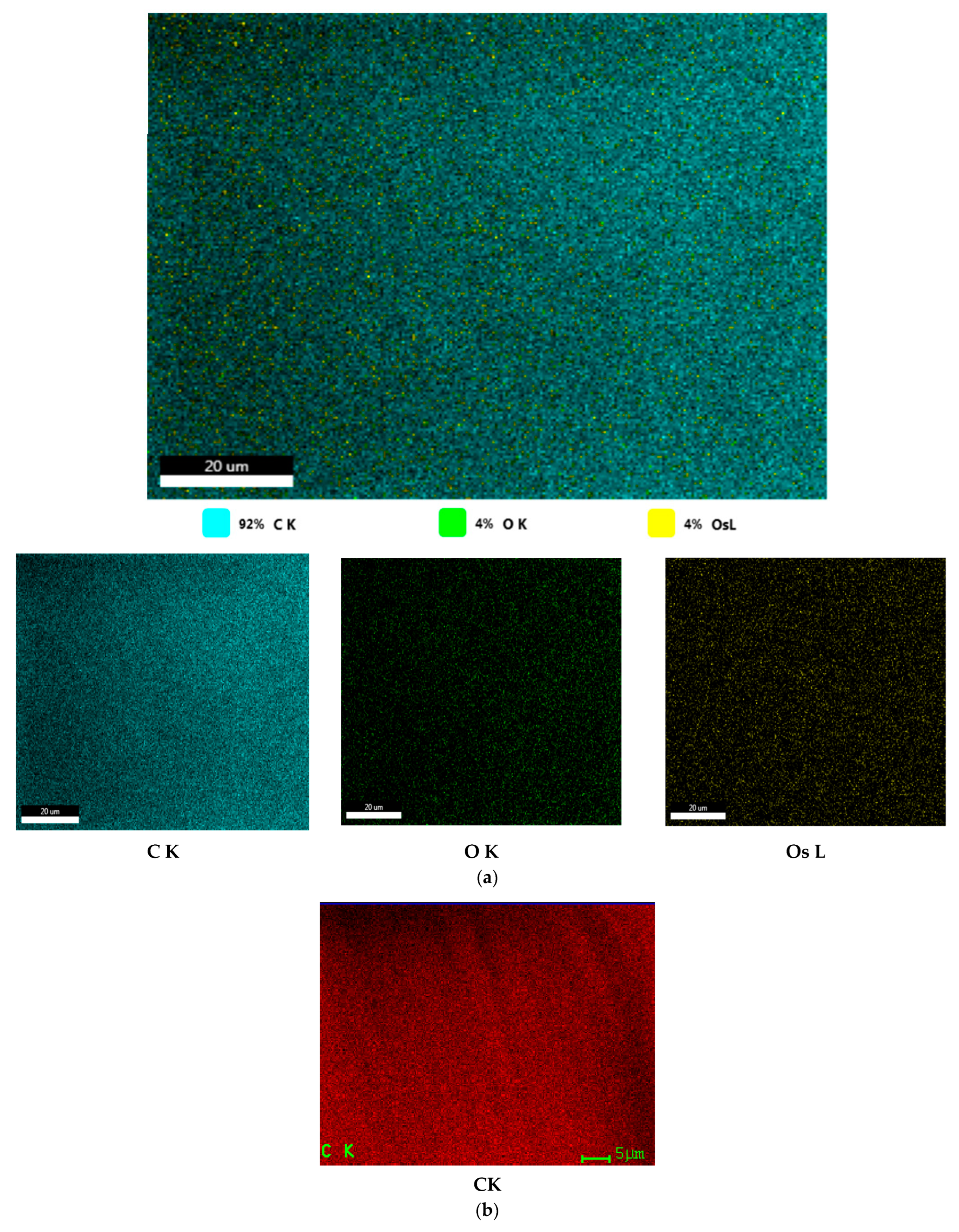
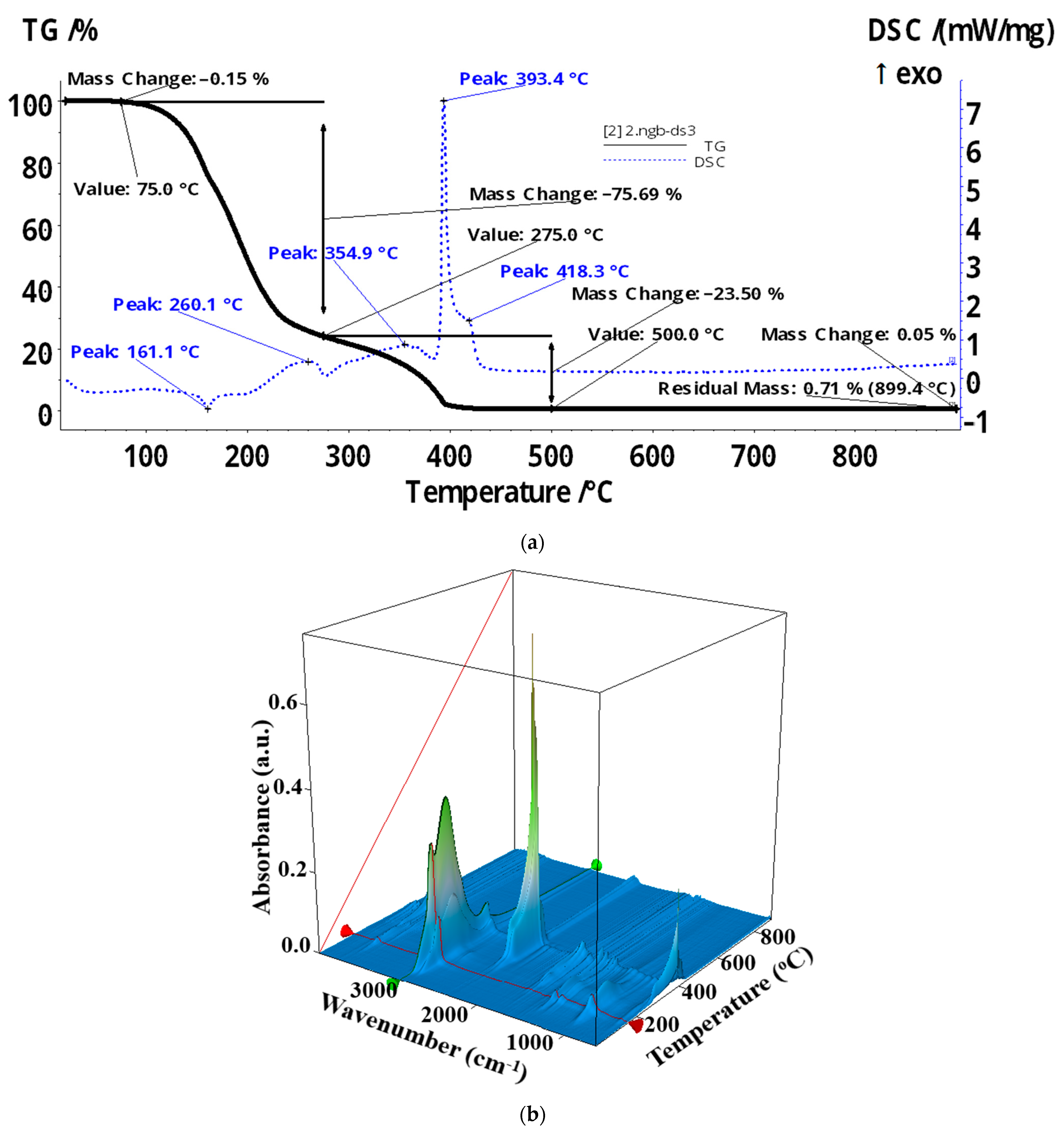
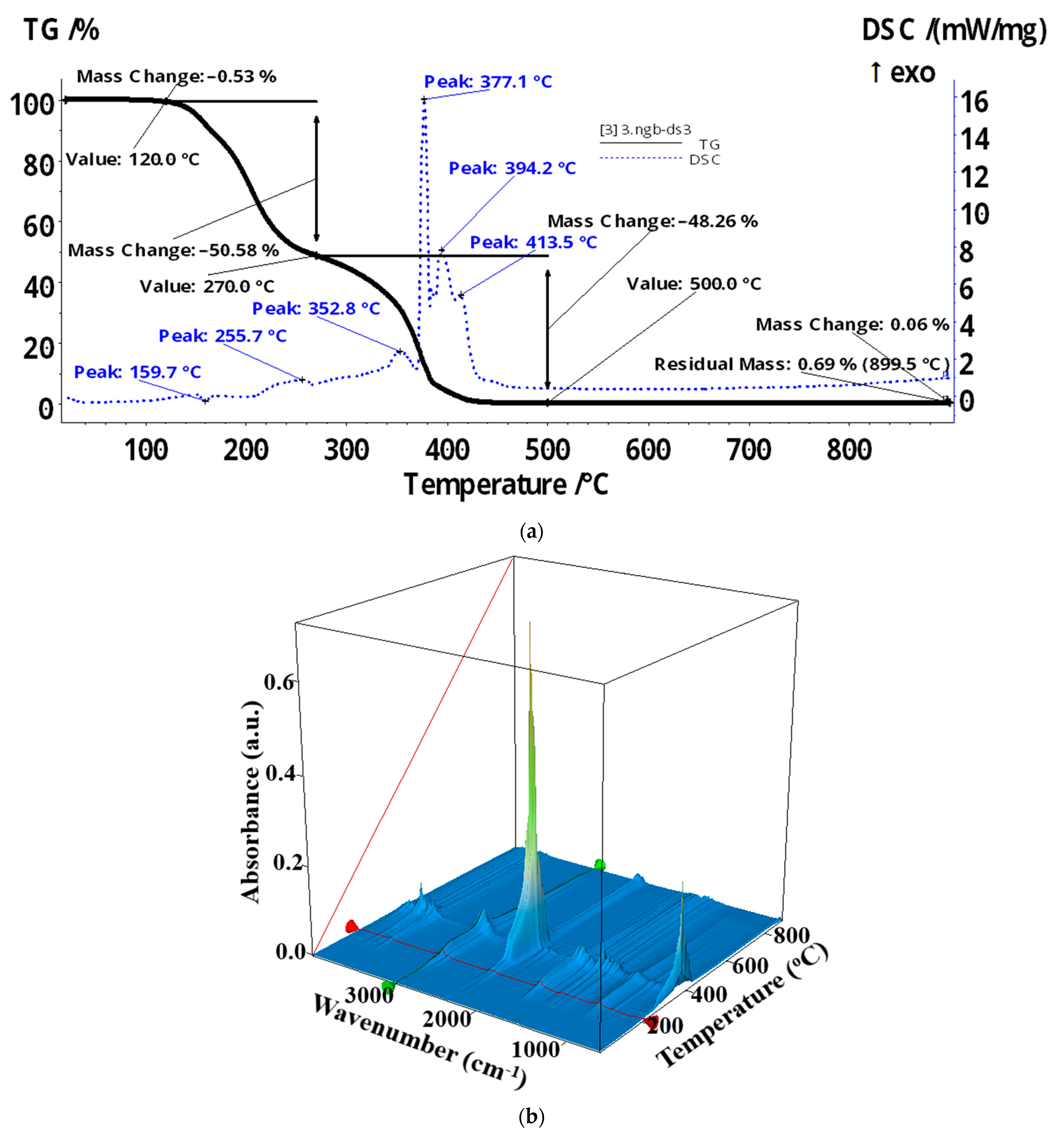
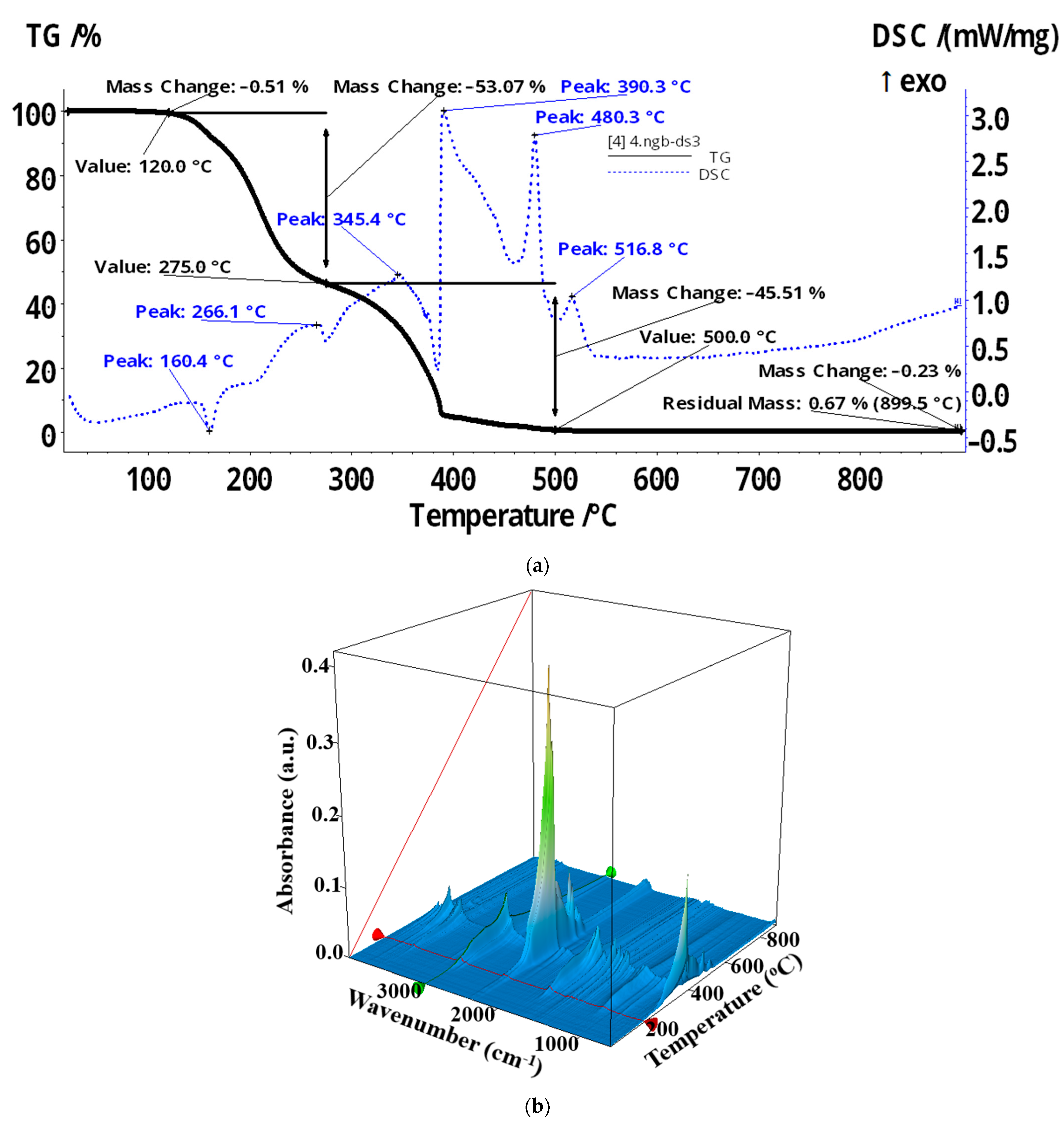
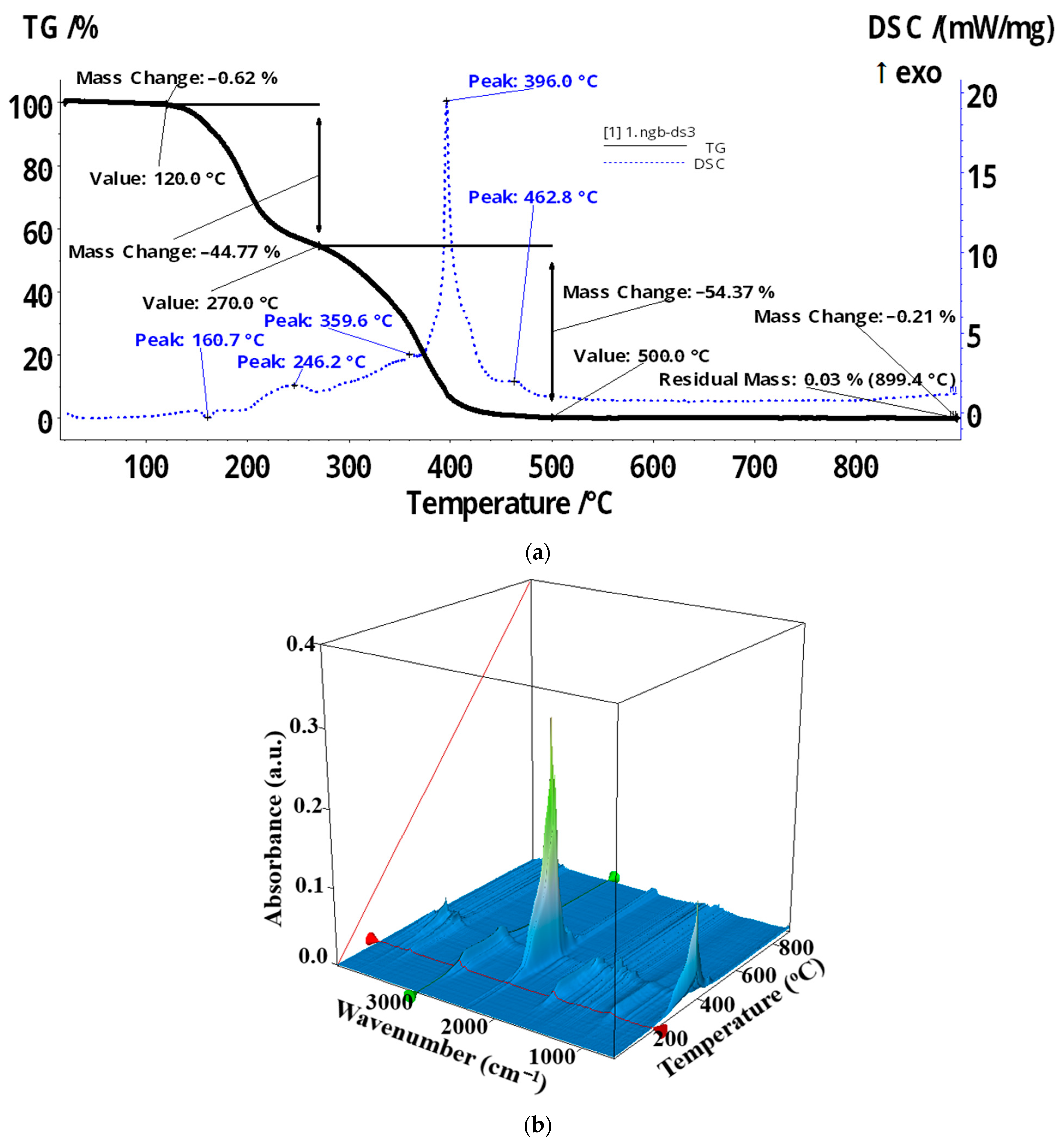
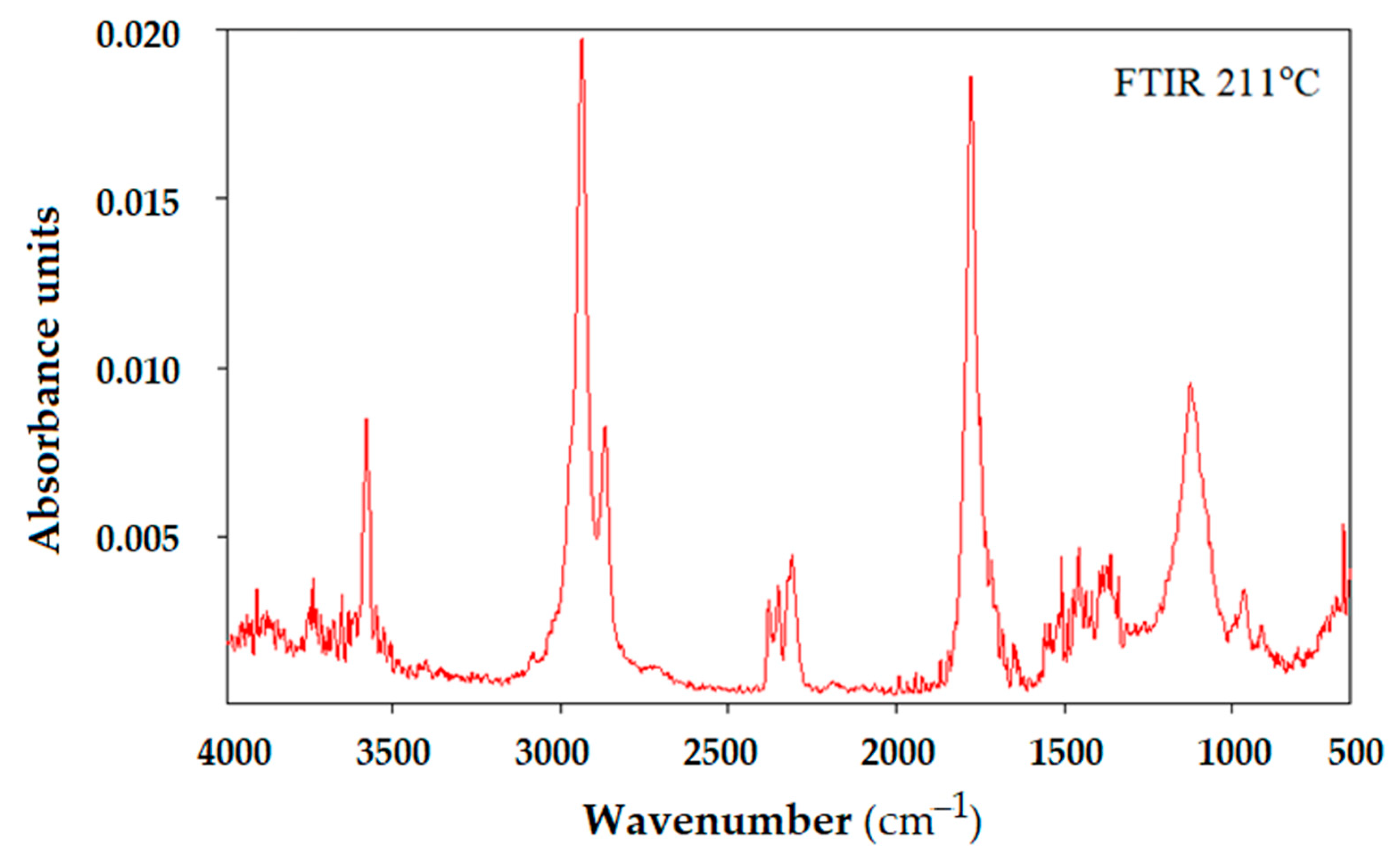



| Membrane | Element | Weight % | Atomic % | Net Int. | Error % | K Ratio | Z | A | F |
|---|---|---|---|---|---|---|---|---|---|
| Os–PPMi | C K | 89.64 | 93.09 | 946.60 | 5.45 | 0.7571 | 1.0075 | 0.8383 | 1.0000 |
| O K | 8.72 | 6.80 | 27.90 | 52.16 | 0.0074 | 0.9696 | 0.0873 | 1.0000 | |
| OsL | 1.64 | 0.11 | 25.70 | 60.43 | 0.0120 | 0.5837 | 1.2096 | 1.0361 | |
| Os–PPMt | C K | 84.89 | 89.65 | 788.70 | 8.56 | 0.6724 | 1.0107 | 0.7837 | 1.0000 |
| O K | 12.86 | 10.20 | 40.70 | 54.86 | 0.0115 | 0.9729 | 0.0918 | 1.0000 | |
| OsL | 2.25 | 0.15 | 34.30 | 65.33 | 0.0165 | 0.5862 | 1.2157 | 1.0306 | |
| Os–PPMn | C K | 83.97 | 90.02 | 811.30 | 5.28 | 0.6410 | 1.0152 | 0.7519 | 1.0000 |
| O K | 12.06 | 9.71 | 41.10 | 31.83 | 0.0108 | 0.9778 | 0.0913 | 1.0000 | |
| OsL | 3.97 | 0.27 | 61.40 | 44.29 | 0.0287 | 0.5905 | 1.2014 | 1.0214 | |
| Os–PPMp | C K | 92.10 | 94.22 | 2502.90 | 1.85 | 0.8207 | 1.0038 | 0.8877 | 1.0000 |
| O K | 7.49 | 5.75 | 57.20 | 12.51 | 0.0062 | 0.9656 | 0.0859 | 1.0000 | |
| OsL | 0.42 | 0.03 | 16.10 | 20.37 | 0.0031 | 0.5802 | 1.2122 | 1.0550 | |
| PPM support | C K | 94.79 | 96.94 | 3.09 | 652.16 | 0.8250 | 1.0027 | 0.8679 | 1.000 |
| O K | 3.78 | 2.90 | 18.00 | 25.03 | 0.0042 | 0.9887 | 0.1117 | 1.000 | |
| OsL | - | - | - | - | - | - | - | - |
| Catalytic Material | k (mmol·s−1) | Reference |
|---|---|---|
| Os–PPMi | 2.04 × 10−4 | This study |
| Os–PPMt | 2.89 × 10−4 | |
| Os–PPMn | 8.05 × 10−4 | |
| Os–PPMp | 1.01 × 10−4 | |
| Nanofibers PtNi/SiO2 | 434 × 10−3 | [63] |
| Nanofibers Ni/SiO2 | 18 × 10−3 | |
| Nanofibers Pt/SiO2 | 55 × 10−3 | |
| Ni–Ca–Al2O3 | 2.85 × 10−3 | [64] |
| Ni catalysts | 1.02 × 10−3 | |
| Ni–Al2O3 | 1.42 × 10−3 | |
| Nanofibers Ni–P 0.25/NFM 4.55 | 18.04 × 10−3–26.84 × 10−3 | [65] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albu, P.C.; Ferencz, A.; Al-Ani, H.N.A.; Tanczos, S.-K.; Oprea, O.; Grosu, V.-A.; Nechifor, G.; Bungău, S.G.; Grosu, A.R.; Goran, A.; et al. Osmium Recovery as Membrane Nanomaterials through 10–Undecenoic Acid Reduction Method. Membranes 2022, 12, 51. https://doi.org/10.3390/membranes12010051
Albu PC, Ferencz A, Al-Ani HNA, Tanczos S-K, Oprea O, Grosu V-A, Nechifor G, Bungău SG, Grosu AR, Goran A, et al. Osmium Recovery as Membrane Nanomaterials through 10–Undecenoic Acid Reduction Method. Membranes. 2022; 12(1):51. https://doi.org/10.3390/membranes12010051
Chicago/Turabian StyleAlbu, Paul Constantin, Andreea Ferencz (Dinu), Hussam Nadum Abdalraheem Al-Ani, Szidonia-Katalin Tanczos, Ovidiu Oprea, Vlad-Alexandru Grosu, Gheorghe Nechifor, Simona Gabriela Bungău, Alexandra Raluca Grosu, Alexandru Goran, and et al. 2022. "Osmium Recovery as Membrane Nanomaterials through 10–Undecenoic Acid Reduction Method" Membranes 12, no. 1: 51. https://doi.org/10.3390/membranes12010051
APA StyleAlbu, P. C., Ferencz, A., Al-Ani, H. N. A., Tanczos, S.-K., Oprea, O., Grosu, V.-A., Nechifor, G., Bungău, S. G., Grosu, A. R., Goran, A., & Nechifor, A. C. (2022). Osmium Recovery as Membrane Nanomaterials through 10–Undecenoic Acid Reduction Method. Membranes, 12(1), 51. https://doi.org/10.3390/membranes12010051










