Enhancing Absorption Performance of CO2 by Amine Solution through the Spiral Wired Channel in Concentric Circular Membrane Contactors
Abstract
:1. Introduction
2. Theoretical Formulation
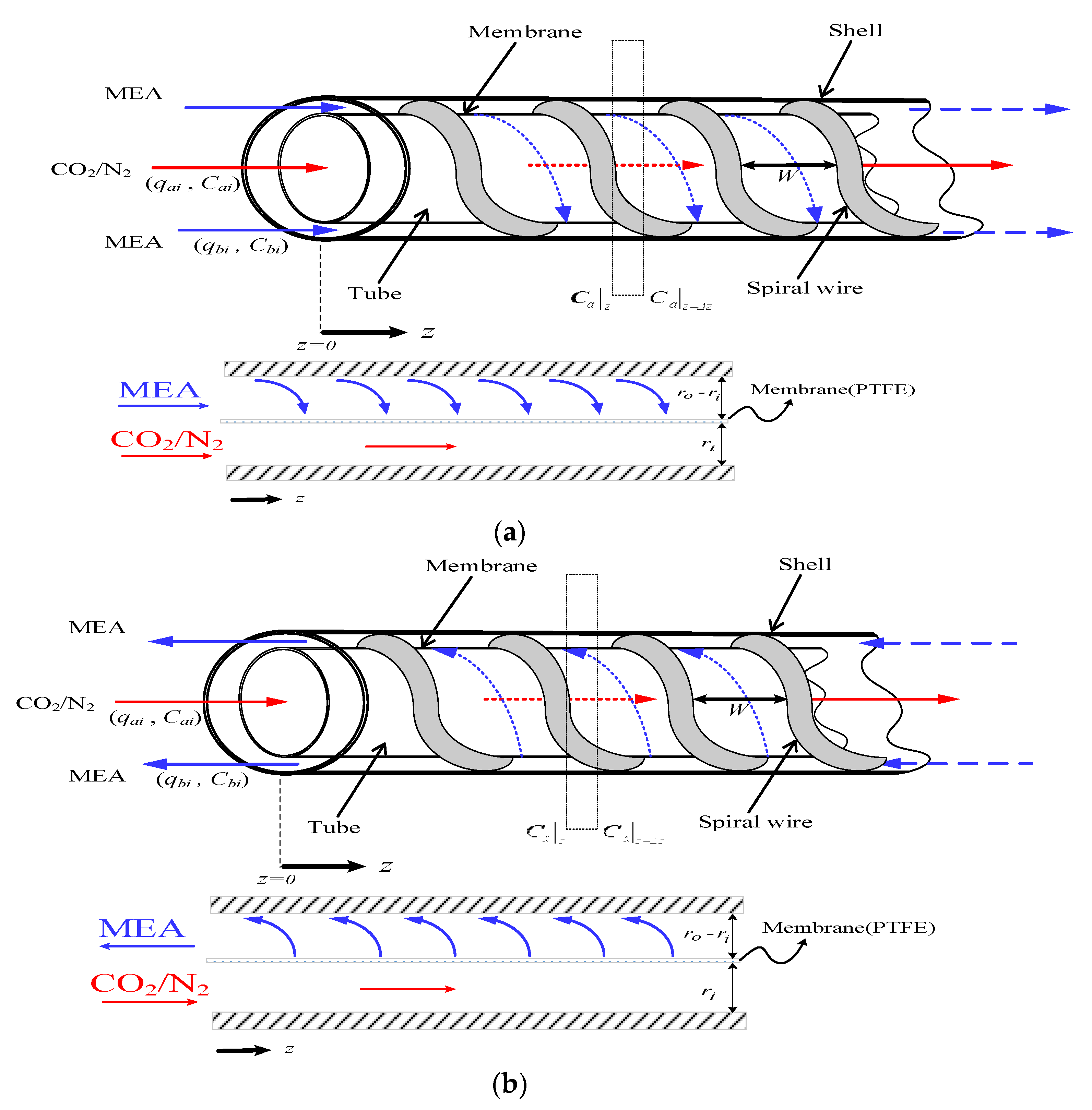
2.1. Mass Transfer
- (a)
- The system is operated at steady-state and normal pressure conditions;
- (b)
- The porous hydrophobic membrane is not wetted by the MEA solution;
- (c)
- The membrane material does not react with the MEA solution;
- (d)
- Henry’s law applies to the interface between the gas phase and the liquid phase.
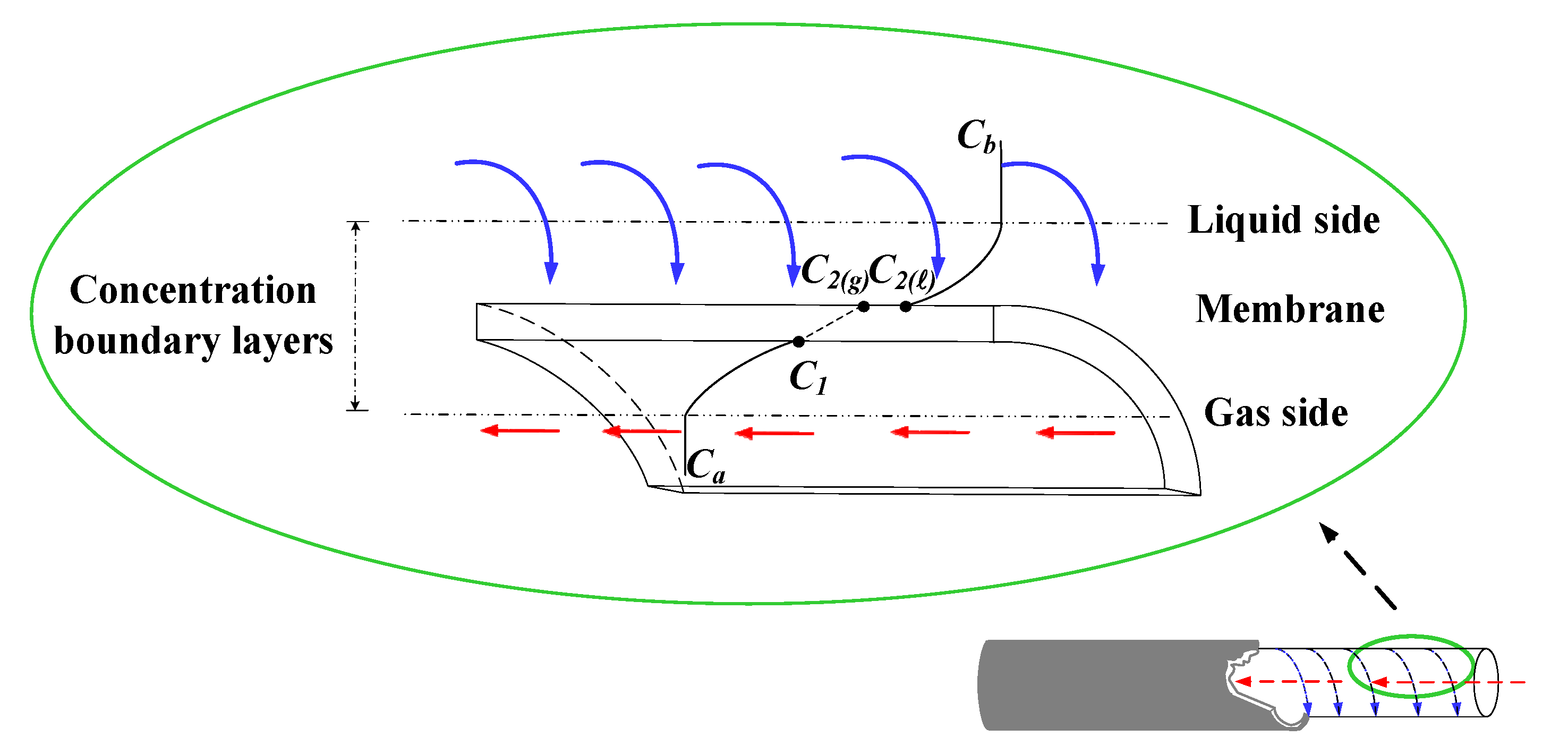
2.2. Concentration Polarization
2.3. Mass-Transfer Nhancement Factor
2.4. Absorption Flux Improvement
2.5. Power Consumption Increment
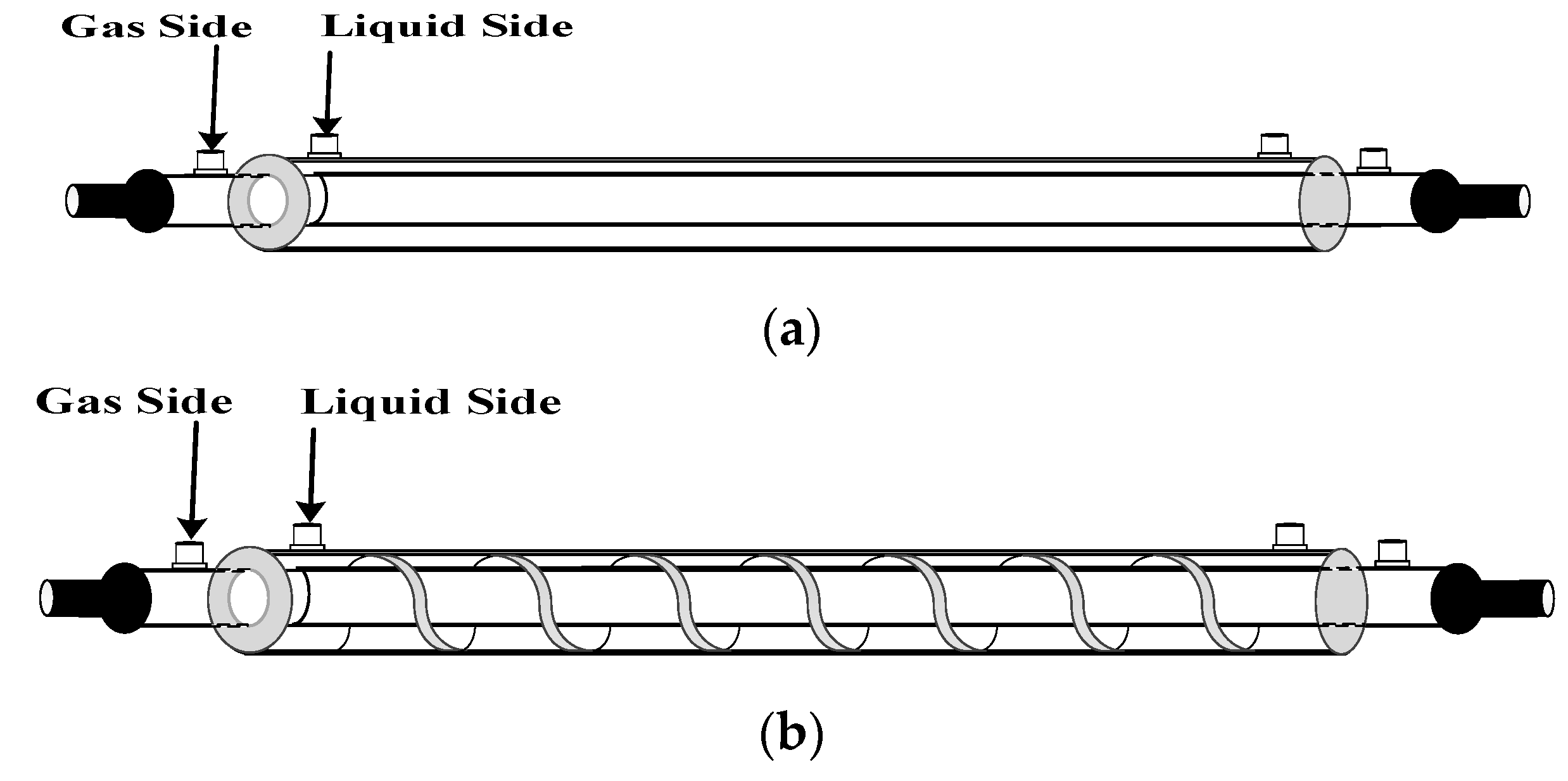
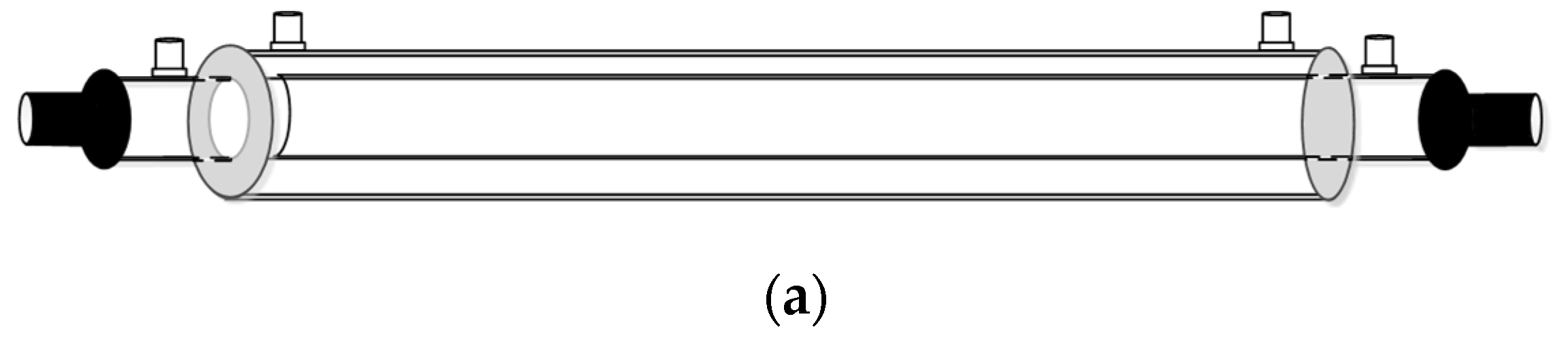
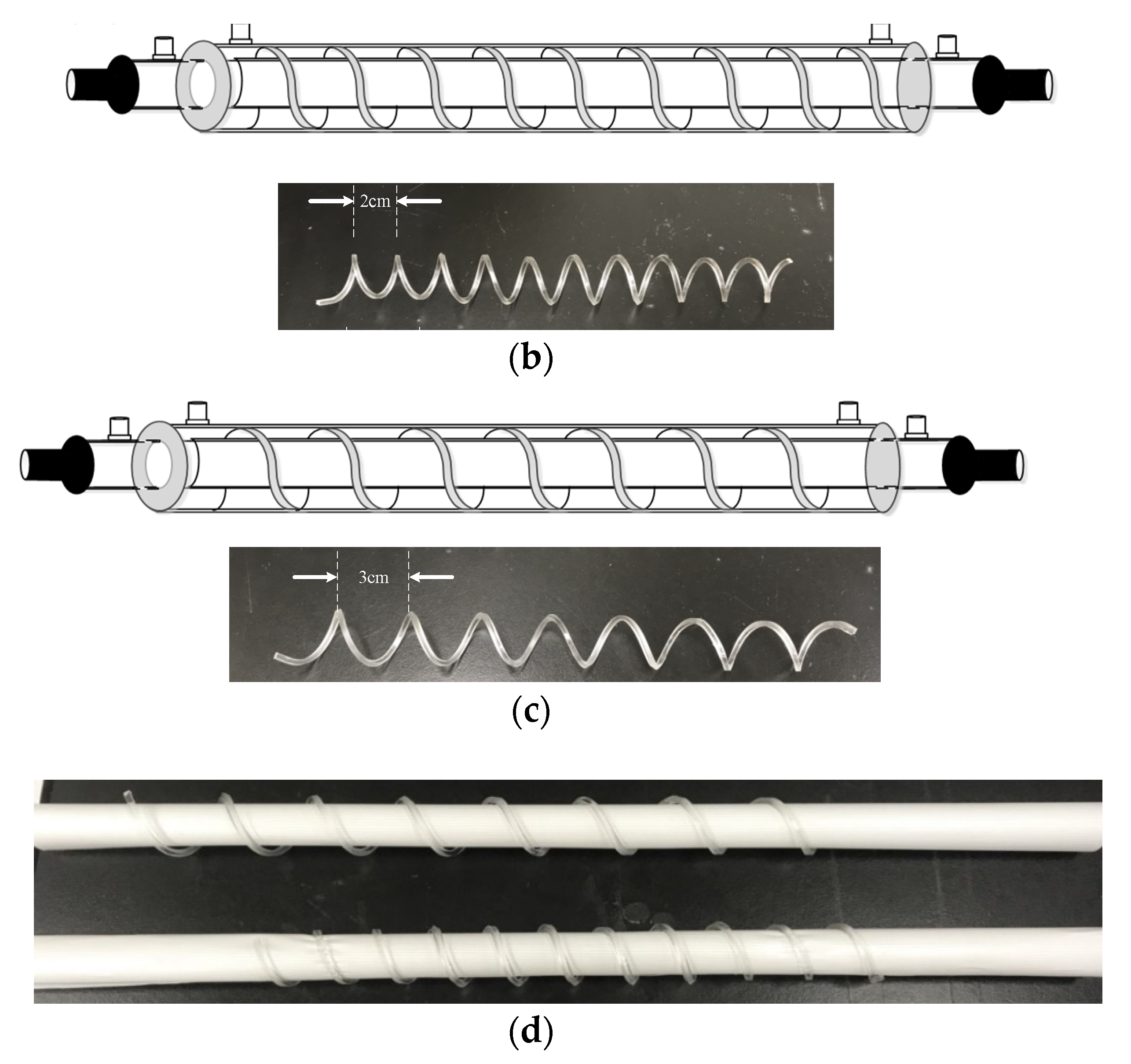
2.6. The Design of Spiral Wired Annulus Channel
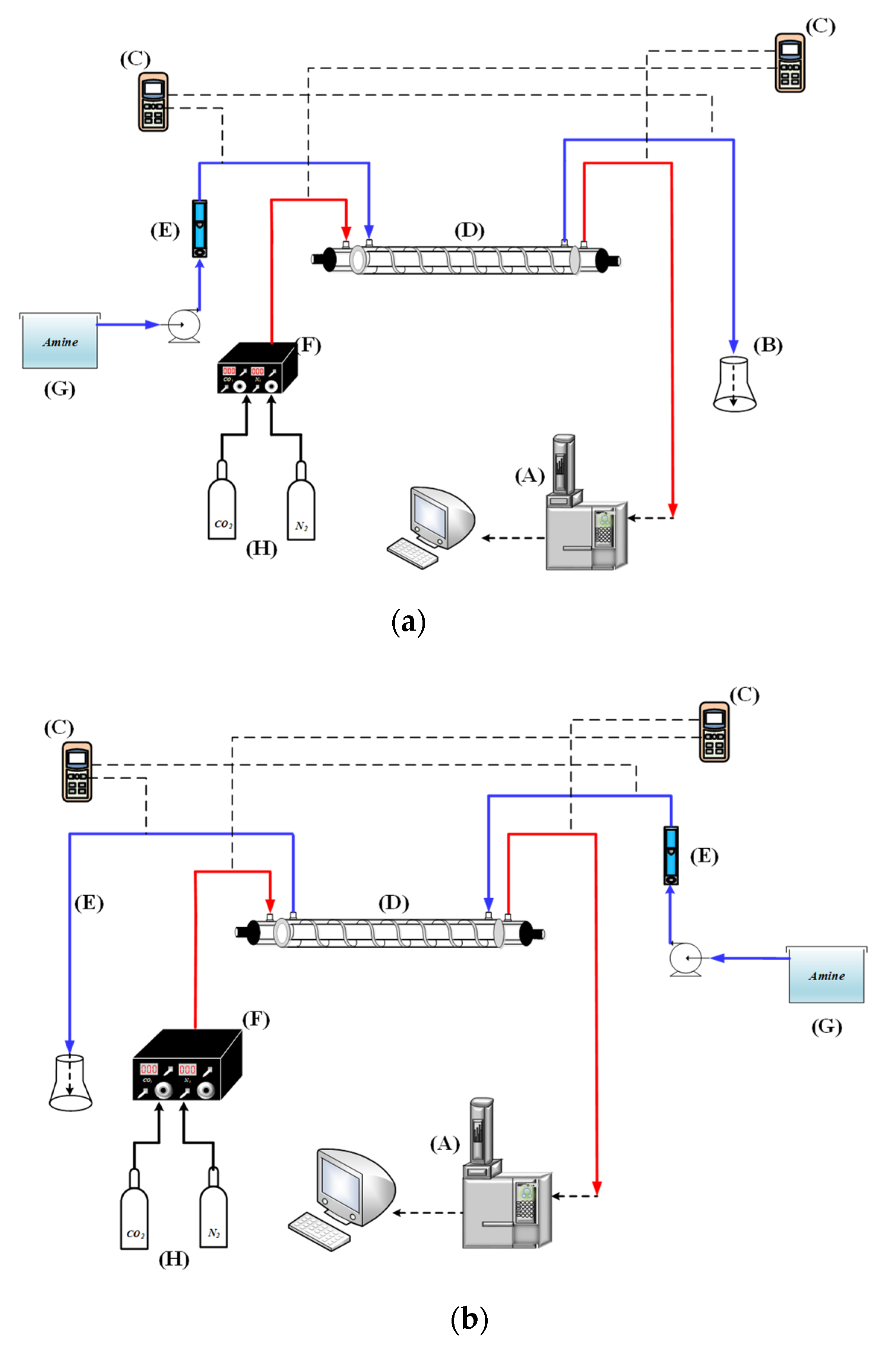
3. Experimental Study
4. Results and Discussions
4.1. Correlated Sherwood Numbers
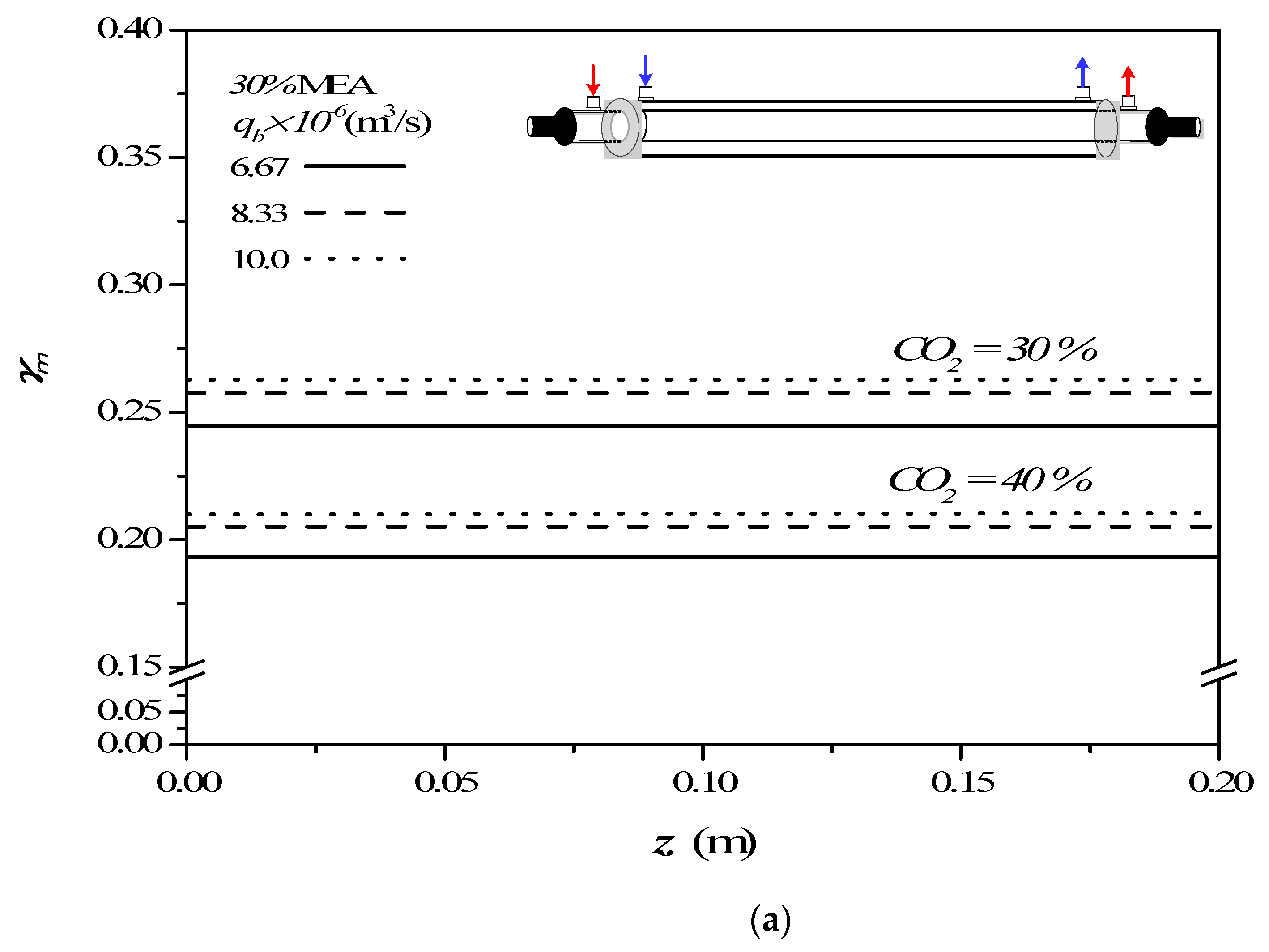
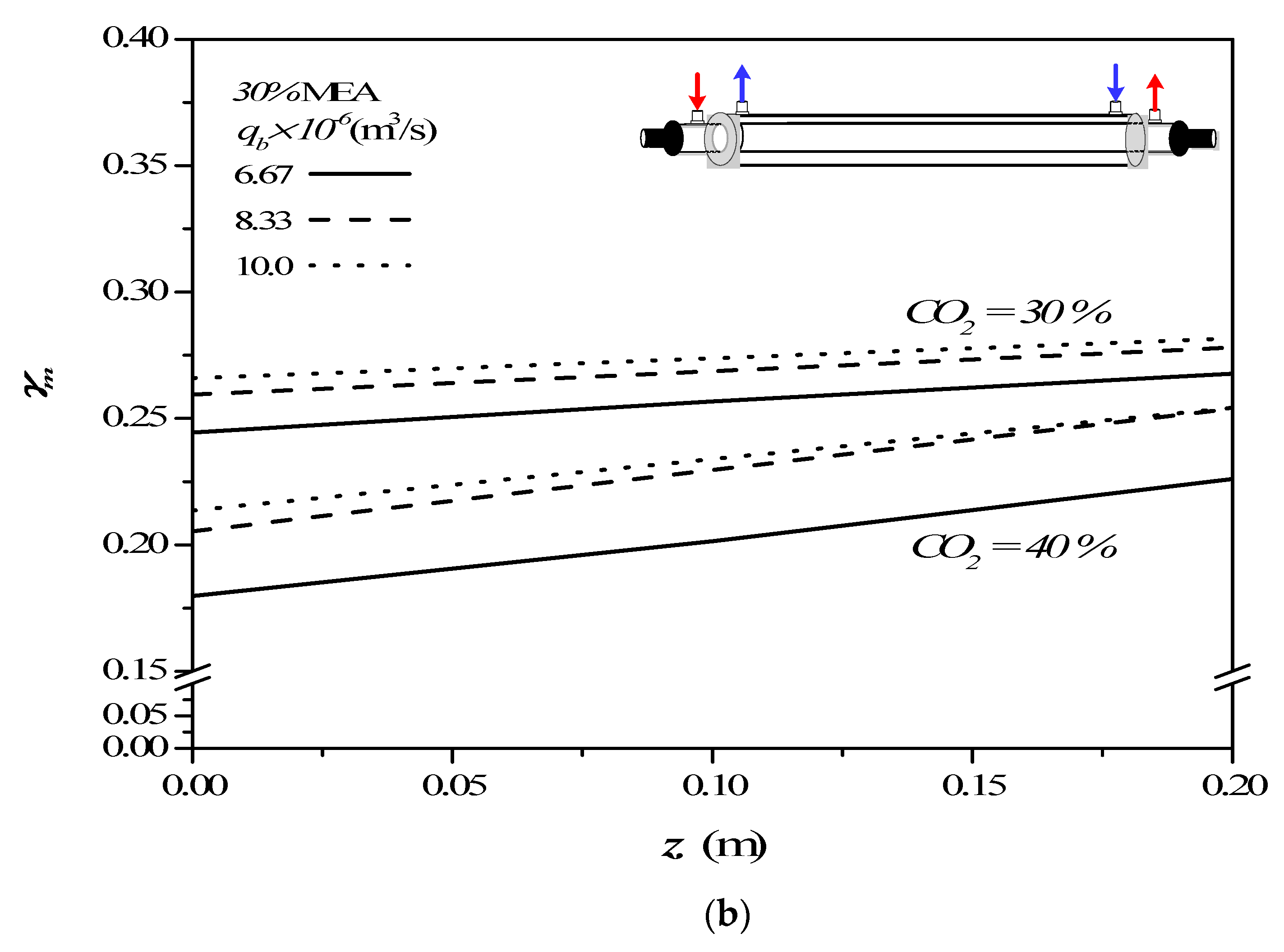
4.2. Effects of Device Parameters and Operating Conditions on Concentration Polarization
4.3. CO2 Absorption Flux Enhancement by Embedding Spiral Wires
4.4. Further CO2 Absorption Flux Enhancement
4.5. Power Consumption Increment
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Concentration (mol m−3) | |
| Mean value of (mol m−3) | |
| Membrane coefficient based on the Knudsen diffusion model (mol m−2 Pa−1 s−1) | |
| Membrane coefficient based on the molecular diffusion model (kg m−2 Pa−1 s−1) | |
| Membrane permeation coefficient (mol m−2 Pa−1 s−1) | |
| Diffusion coefficient of CO2 in MEA (m2 s−1) | |
| Equivalent hydraulic diameter of channel (m), i = spiral, empty | |
| Accuracy deviation of experimental results from the theoretical predictions | |
| absorption flux enhancement | |
| Fanning friction factor | |
| Dimensionless Henry’s constant | |
| Hydraulic dissipate energy (J kg−1), i = spiral, empty | |
| Absorption flux enhancement | |
| Power consumption relative index | |
| Molar flux (mol m−2 s−1) | |
| Mass-transfer coefficient in the gas feed stream (m s−1) | |
| Mass-transfer coefficient in the liquid absorbent side (m s−1) | |
| Equilibrium constant | |
| Reduced equilibrium constant | |
| Overall mass-transfer coefficient of membrane (m s−1) | |
| Friction loss (J kg−1), j = CO2, MEA | |
| Channel length (m) | |
| Length of spiral wired channel (m) | |
| Average molecular weight of CO2 and N2 gas mixture (kg mol−1) | |
| Nexp | Number of experimental measurements |
| Saturation vapor pressure in the gas feed flow side (Pa) | |
| Saturation vapor pressure in the liquid absorbent flow side (Pa) | |
| Volumetric flow rate of the gas feed stream (m3 s−1) | |
| Volumetric flow rate of the MEA absorbent side (m3 s−1) | |
| Gas constant (8.314 J mol−1 K−1) | |
| Re | Reynolds number |
| Radius of inner tube (m) | |
| Radius of shell (m) | |
| rp | Membrane pore radius (m) |
| Sc | Dimensionless Schmidt number |
| ShS | Enhanced dimensionless Sherwood number |
| Dimensionless Sherwood number for laminar flow | |
| Wp | Pitch width (m) |
| Absorption flux (mol m−2 s−1) | |
| Natural log mean CO2 mole fraction in the membrane | |
| Axial coordinate along the flow direction (m) | |
| Greek letters | |
| Mass-transfer enhancement factor | |
| Aspect ratio of the channel | |
| Thickness of membrane (µm) | |
| Membrane porosity | |
| Average velocity (m s−1) | |
| Density (kg m−3), i = CO2, MEA | |
| Concentration polarization coefficients | |
| Subscripts | |
| 1 | Membrane surface on MEA side |
| Liquid phase on membrane surface on MEA side | |
| Gas phase on membrane surface on MEA side | |
| a | The gas feed flow channel |
| b | The liquid absorbent flow channel |
| cal | Calculated results |
| spiral | Inserting spiral wires as promoters |
| empty | Empty channel |
| exp | Experimental results |
| in | Inlet |
| out | Outlet |
| theo | Theoretical predictions |
| Superscripts | |
| con | Concurrent-flow operations |
| counter | Countercurrent-flow operations |
References
- Herzog, H.; Eliasson, B.; Kaarstad, O. Capturing greenhouse gases. Sci. Am. 2000, 182, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Mangalapally, H.P.; Notz, R.; Hoch, S.; Asprion, N.; Sieder, G.; Garcia, H.; Hasse, H. Pilot plant experimental studies of post combustion CO2 capture by reactive absorption with MEA and new solvents. Energy Procedia 2009, 1, 963–970. [Google Scholar] [CrossRef] [Green Version]
- Sayari, A.; Belmabkhout, Y.; Serna-Guerrero, R. Flue gas treatment via CO2 adsorption. Chem. Eng. J. 2011, 171, 760–774. [Google Scholar] [CrossRef]
- Li, J.L.; Chen, B.-H. Review of CO2 absorption using chemical solvents in hollow fiber membrane contactors. Sep. Purif. Technol. 2005, 41, 109–122. [Google Scholar] [CrossRef]
- Eide-Haugmo, I.; Lepaumier, H.; Einbu, A.; Vernstad, K.; da Silva, E.F.; Svendsen, H.F. Chemical stability and biodegradability of new solvents for CO2 capture. Energy Procedia 2011, 4, 1631–1636. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.E.; Yan, Y.F.; Zhang, L.; Ju, S.X.; Chen, Y.X.; Ran, J.Y.; Pu, G.; Qin, C.L. Theoretical Study on CO2 Absorption from Biogas by Membrane Contactors: Effect of Operating Parameters. Ind. Eng. Chem. Res. 2014, 53, 14075–14083. [Google Scholar] [CrossRef]
- Ramakula, P.; Prapasawada, T.; Pancharoena, U.; Pattaveekongkab, W. Separation of radioactive metal ions by hollow fiber-supported liquid membrane and permeability analysis. J. Chin. Inst. Chem. Eng. 2007, 38, 489–494. [Google Scholar] [CrossRef]
- Sea, B.; Park, Y.I. Comparison of porous hollow fibers as a membrane contactor for carbon dioxide absorption. J. Ind. Eng. Chem. 2002, 8, 290–296. [Google Scholar]
- Rezakazemi, M.; Sadrzadeh, M.; Matsuura, T. Thermally stable polymers for advanced high-performance gas separation membranes. Prog. Energy Combust. Sci. 2018, 66, 1–41. [Google Scholar] [CrossRef]
- Yang, Y.; Chuah, C.Y.; Bae, T.-H. Polyamine-appended porous organic polymers for efficient post-combustion CO2 capture. Chem. Eng. J. 2019, 358, 1227–1234. [Google Scholar] [CrossRef]
- Zhang, X.M.; Tu, Z.H.; Li, H.; Li, L.; Wu, Y.T.; Hu, X.B. Supported protic-ionic-liquid membranes with facilitated transport mechanism for the selective separation of CO2. J. Membr. Sci. 2017, 527, 60–67. [Google Scholar] [CrossRef]
- Lin, Y.-F.; Ko, C.-C.; Chen, C.-H.; Tung, K.-L.; Chang, K.-S.; Chung, T.-W. Sol–gel preparation of polymethylsilsesquioxane aerogel membranes for CO2 absorption fluxes in membrane contactors. Appl. Energy 2014, 129, 25–31. [Google Scholar] [CrossRef]
- Lin, Y.F.; Kuo, J.W. Mesoporous bis(trimethoxysilyl)hexane (BTMSH)/tetraethyl orthosilicate (TEOS)-based hybrid silica aerogel membranes for CO2 capture. Chem. Eng. J. 2016, 300, 29–35. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, Y.; Chew, N.G.P.; Malde, C.; Wang, R. Biocatalytic PVDF composite hollow fiber membranes for CO2 removal in gas-liquid membrane contactor. J. Membr. Sci. 2019, 572, 532–544. [Google Scholar] [CrossRef]
- Zhang, C.-Y.; Hu, H.-C.; Chai, X.-S.; Pan, L.; Xiao, X.-M. A novel method for the determination of adsorption partition coefficients of minor gases in a shale sample by headspace gas chromatography. J. Chromatogr. A 2013, 1310, 121–125. [Google Scholar] [CrossRef]
- Wang, W.P.; Lin, H.T.; Ho, C.D. An analytical study of laminar co-current flow gas absorption through a parallel-plate gas–liquid membrane contactor. J. Membr. Sci. 2006, 278, 181–189. [Google Scholar] [CrossRef]
- Chuah, C.Y.; Kim, K.; Lee, J.; Koh, D.-Y.; Bae, T.-H. CO2 Absorption Using Membrane Contactors: Recent Progress and Future Perspective. Ind. Eng. Chem. Res. 2020, 59, 6773–6794. [Google Scholar] [CrossRef]
- Kianfar, E.; Pirouzfar, V.; Sakhaeinia, H. An experimental study on absorption/stripping CO2 using mono-ethanol amine hollow fiber membrane contactor. J. Taiwan Inst. Chem. Eng. 2017, 80, 954–962. [Google Scholar] [CrossRef]
- Belaissaoui, B.; Favre, E. Evaluation of a dense skin hollow fiber gas-liquid membrane contactor for high pressure removal of CO2 from syngas using Selexol as the absorbent. Chem. Eng. Sci. 2018, 184, 186–199. [Google Scholar] [CrossRef]
- Yang, Y.; Chuah, C.Y.; Gong, H.; Bae, T.-H. Robust microporous organic copolymers containing triphenylamine for high pressure CO2 capture application. J. CO2 Util. 2017, 19, 214–220. [Google Scholar] [CrossRef]
- Karoor, S.; Sirkar, K.K. Gas absorption studies in microporous hollow fiber membrane modules. Ind. Eng. Chem. Res. 1993, 32, 674–684. [Google Scholar] [CrossRef]
- Bakhshali, N.; Tahery, R.; Banazadeh, H. Modelling and simulation of mass transfer in tubular gas-liquid membrane contactors for turbulent flow conditions and comparison of results with laminar flow conditions. Middle-East J. Sci. Res. 2013, 10, 1419–1430. [Google Scholar]
- Bandini, S.; Gostoli, C.; Sarti, G.C. Role of heat and mass transfer in membrane distillation process. Desalination 1991, 81, 91–106. [Google Scholar] [CrossRef]
- Lawson, K.W.; Lloyd, D.R. Membrane distillation II: Direct contact membrane distillation. J. Membr. Sci. 1996, 120, 123–133. [Google Scholar] [CrossRef]
- Schofield, R.W.; Fane, A.G.; Fell, C.J.D. Heat and mass transfer in membrane distillation. J. Membr. Sci. 1987, 33, 299–313. [Google Scholar] [CrossRef]
- Phattaranawik, J.; Jiraratananon, R.; Fane, A. Effects of net-type spacers on heat and mass transfer in direct contact membrane distillation and comparison with ultrafiltration studies. J. Membr. Sci. 2003, 217, 193–206. [Google Scholar] [CrossRef]
- Srisurichan, S.; Jiraratananon, R.; Fane, A. Mass transfer mechanisms and transport resistances in direct contact membrane distillation process. J. Membr. Sci. 2006, 277, 186–194. [Google Scholar] [CrossRef]
- Afza, K.N.; Hashemifard, S.; Abbasi, M. Modelling of CO2 absorption via hollow fiber membrane contactors: Comparison of pore gas diffusivity models. Chem. Eng. Sci. 2018, 190, 110–121. [Google Scholar] [CrossRef]
- Hosseinzadeh, A.; Hosseinzadeh, M.; Vatani, A.; Mohammadi, T. Mathematical modeling for the simultaneous absorption of CO2 and SO2 using MEA in hollow fiber membrane contactors. Chem. Eng. Process. Process. Intensif. 2017, 111, 35–45. [Google Scholar] [CrossRef]
- Wang, M.; Lawal, A.; Stephenson, P.; Sidders, J.; Ramshaw, C. Post-combustion CO2 capture with chemical absorption: A state-of-the-art review. Chem. Eng. Res. Des. 2011, 89, 1609–1624. [Google Scholar] [CrossRef] [Green Version]
- Rezzadori, K.; Penha, F.M.; Proner, M.C.; Zin, G.; Petrus, J.C.C.; Di Luccio, M. Impact of Organic Solvents on Physicochemical Properties of Nanofiltration and Reverse-Osmosis Membranes. Chem. Eng. Technol. 2019, 42, 2700–2708. [Google Scholar] [CrossRef]
- Panahinia, F.; Safdari, J.; Ghannadi-Maragheh, M.; Amani, P.; Mallah, M.H. Modeling and simulation of a horizontal pulsed sieve-plate extraction column using axial dispersion model. Sep. Sci. Technol. 2017, 52, 1–16. [Google Scholar] [CrossRef]
- Meng, J.; Li, P.; Cao, B. High-Flux Direct-Contact Pervaporation Membranes for Desalination. ACS Appl. Mater. Interfaces 2019, 11, 28461–28468. [Google Scholar] [CrossRef] [PubMed]
- Legallais, C.; Catapano, G.; Harten, B.; Baurmeister, U. A theoretical model to predict the in vitro performance of hemodialfilters. J. Membr. Sci. 2000, 168, 3–15. [Google Scholar] [CrossRef]
- Santos, J.; Geraldes, V.; Velizarov, S.; Crespo, J. Investigation of flow patterns and mass transfer in membrane module channels filled with flow-aligned spacers using computational fluid dynamics (CFD). J. Membr. Sci. 2007, 305, 103–117. [Google Scholar] [CrossRef]
- Lou, Y.; Gogar, R.; Hao, P.; Lipscomb, G.; Amo, K.; Kniep, J. Simulation of net spacers in membrane modules for carbon dioxide capture. Sep. Sci. Technol. 2017, 52, 168–185. [Google Scholar] [CrossRef]
- Ho, C.-D.; Chen, L.; Huang, M.-C.; Lai, J.-Y.; Chen, Y.-A. Distillate flux enhancement in the air gap membrane distillation with inserting carbon-fiber spacers. Sep. Sci. Technol. 2017, 52, 2817–2828. [Google Scholar] [CrossRef]
- Shakaib, M.; Hasani, S.; Mahmood, M. CFD modeling for flow and mass transfer in spacer-obstructed membrane feed channels. J. Membr. Sci. 2009, 326, 270–284. [Google Scholar] [CrossRef]
- Hamimour, N.; Sandall, O.C. Absorption of carbon dioxide into aqueous methyldiethanolamine. Chem. Eng. Sci. 1984, 39, 1791–1796. [Google Scholar] [CrossRef]
- Yeh, H.-M. Enrichment of heavy water in rotated wired concentric-tube thermal diffusion columns. Sep. Purif. Technol. 2004, 40, 321–325. [Google Scholar] [CrossRef]
- Rongwong, W.; Boributh, S.; Assabumrungrat, S.; Laosiripojana, N.; Jiraratananon, R. Simultaneous absorption of CO2 and H2S from biogas by capillary membrane contactor. J. Membr. Sci. 2012, 392–393, 38–47. [Google Scholar] [CrossRef]
- Lee, H.J.; Park, Y.G.; Kim, M.K.; Lee, S.H.; Park, J.H. Study on CO2 absorption performance of lab-scale ceramic hollow fiber membrane contactor by gas/liquid flow direction and module design. Sep. Purif. Technol. 2019, 220, 189–196. [Google Scholar] [CrossRef]
- Ansaloni, L.; Hartono, A.; Awais, M.; Knuutila, H.K.; Deng, L. CO2 capture using highly viscous amine blends in non-porous membrane contactors. Chem. Eng. J. 2019, 359, 1581–1591. [Google Scholar] [CrossRef]
- Ding, Z.W.; Ma, R.Y.; Fane, A.G. A new model for mass transfer in direct contact membrane distillation. Desalination 2003, 151, 217–227. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Hwang, S.T. Concentration polarization, separation factor, and Peclet number in membrane processes. J. Membr. Sci. 1997, 132, 73–90. [Google Scholar] [CrossRef]
- Zheng, Q.; Dong, L.; Chen, J.; Gao, G.; Fei, W. Absorption solubility calculation and process simulation for CO2 capture. J. Chem. Ind. Eng. 2010, 61, 1740–1746. [Google Scholar]
- Lawson, K.W.; Lloyd, D.R. Membrane distillation. J. Membr. Sci. 1997, 124, 1–25. [Google Scholar] [CrossRef]
- Iversen, S.B.; Bhatia, V.K.; Dam-Jpohansen, K.; Jonsson, G. Characterization of microporous membranes for use in membrane contactors. J. Membr. Sci. 1997, 130, 205–217. [Google Scholar] [CrossRef]
- Lin, S.H.; Tung, K.L.; Chang, H.W.; Lee, K.R. Influence of Fluorocarbon Fat-Membrane Hydrophobicity on Carbon Dioxide Recovery. Chemosphere 2009, 75, 1410–1416. [Google Scholar] [CrossRef] [PubMed]
- Welty, J.R.; Wicks, C.E.; Wilson, R.E. Fundamentals of Momentum, Heat, and Mass Transfer, 3rd ed.; John Wiley & Sons: New York, NY, USA, 1984. [Google Scholar]
- Moffat, R.J. Describing the uncertainties in experimental results. Exp. Therm. Fluid Sci. 1988, 1, 3–17. [Google Scholar] [CrossRef] [Green Version]




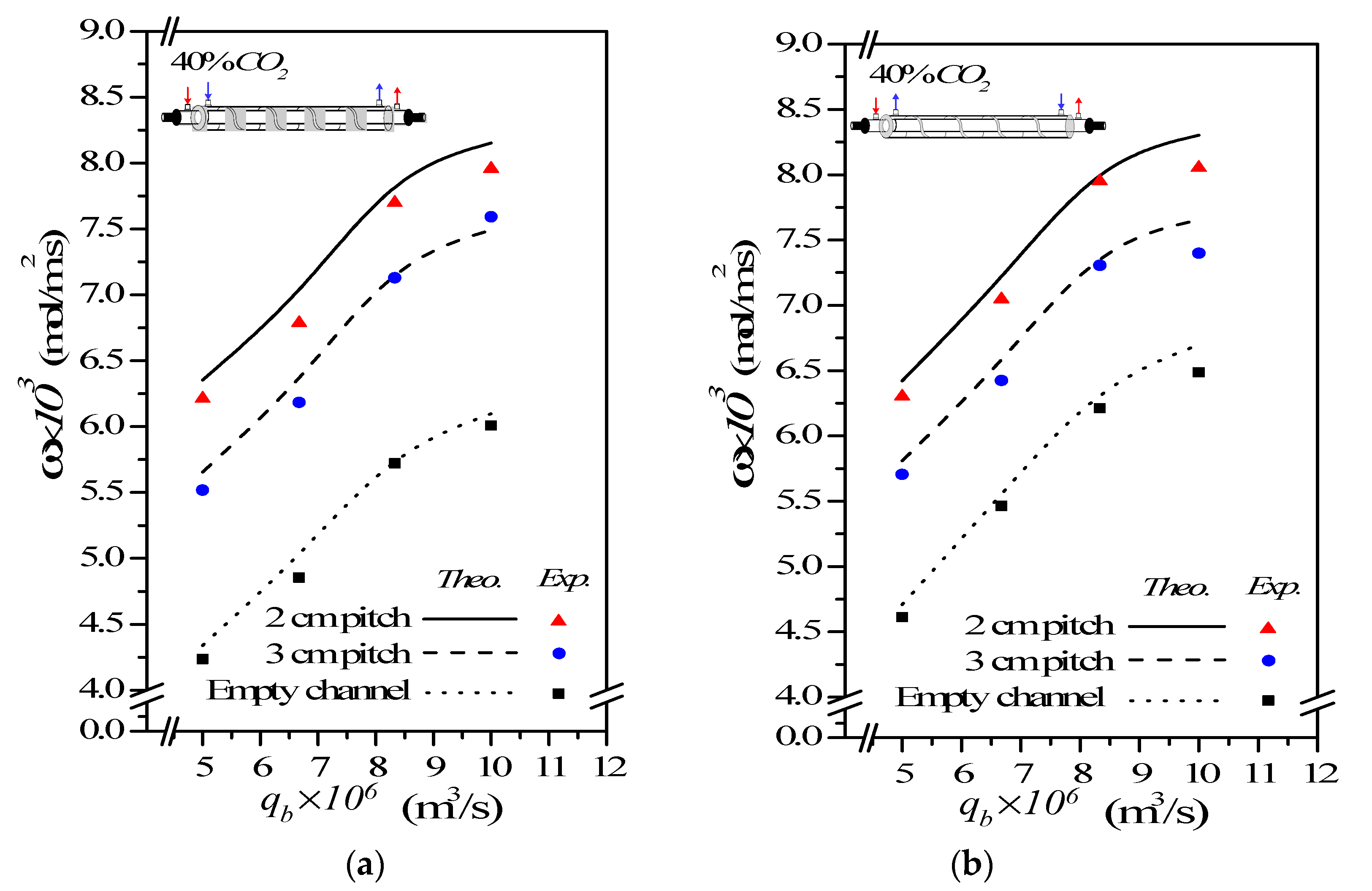
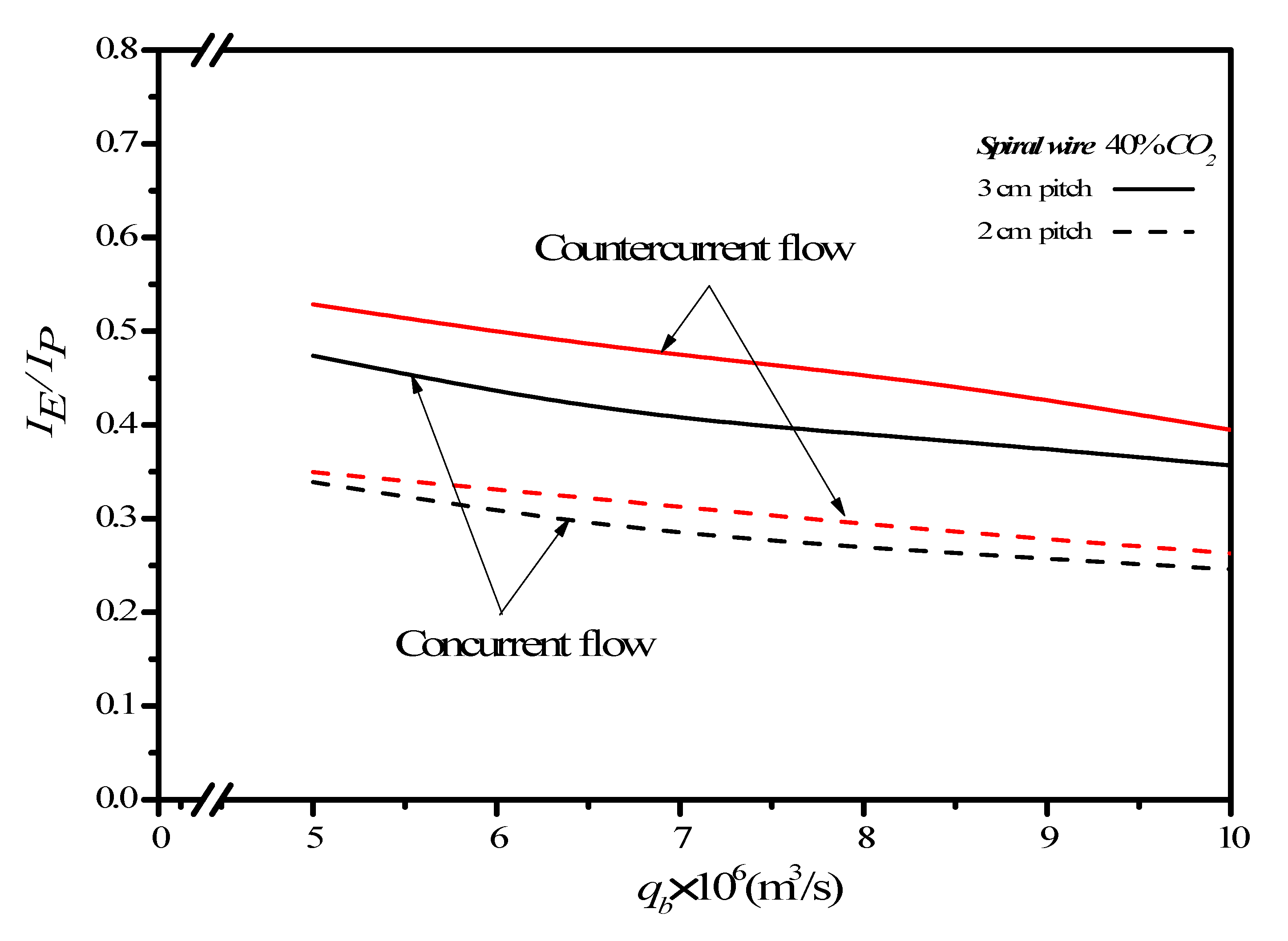
(%) m3/s | Concurrent Flow | Countercurrent Flow | |||||
|---|---|---|---|---|---|---|---|
| 30 | 5.0 | 5.23 | 5.31 | 1.53 | 5.35 | 5.45 | 1.83 |
| 6.67 | 5.83 | 5.92 | 1.51 | 5.92 | 6.05 | 2.14 | |
| 8.33 | 6.21 | 6.37 | 2.50 | 6.33 | 6.51 | 2.78 | |
| 10.0 | 6.63 | 6.53 | 2.08 | 6.46 | 6.65 | 1.60 | |
| 40 | 5.0 | 5.58 | 5.70 | 2.10 | 5.76 | 5.87 | 1.87 |
| 6.67 | 6.31 | 6.40 | 1.41 | 6.47 | 6.61 | 2.12 | |
| 8.33 | 6.98 | 7.22 | 3.33 | 7.27 | 7.37 | 1.28 | |
| 10.0 | 7.19 | 7.39 | 2.75 | 7.36 | 7.54 | 2.29 | |
| 45 | 5.0 | 6.21 | 6.35 | 2.22 | 6.30 | 6.42 | 1.88 |
| 6.67 | 6.79 | 6.99 | 2.86 | 7.05 | 7.20 | 2.06 | |
| 8.33 | 7.70 | 7.94 | 3.02 | 7.95 | 8.12 | 2.05 | |
| 10.0 | 7.96 | 8.15 | 2.40 | 8.05 | 8.30 | 3.03 | |
(%) (m3 s−1) | Concurrent-Flow Operations (mol m−2 s−1) | |||||
|---|---|---|---|---|---|---|
| Empty Channel | 2 cm Spiral Wire | 3 cm Spiral Wire | ||||
| (%) | (%) | |||||
| 30 | 5.0 | 3.93 | 5.31 | 35.18 | 4.77 | 21.61 |
| 6.67 | 4.46 | 5.92 | 32.84 | 5.30 | 18.89 | |
| 8.33 | 4.90 | 6.37 | 30.06 | 5.67 | 15.78 | |
| 10.0 | 5.07 | 6.53 | 28.80 | 5.87 | 15.71 | |
| 35 | 5.0 | 4.08 | 5.70 | 39.87 | 5.11 | 25.41 |
| 6.67 | 4.68 | 6.40 | 36.76 | 5.79 | 23.62 | |
| 8.33 | 5.42 | 7.22 | 33.09 | 6.59 | 21.43 | |
| 10.0 | 5.62 | 7.39 | 31.60 | 6.77 | 20.42 | |
| 40 | 5.0 | 4.34 | 6.35 | 46.45 | 5.66 | 30.35 |
| 6.67 | 5.01 | 6.99 | 39.42 | 6.33 | 26.22 | |
| 8.33 | 5.82 | 7.94 | 36.31 | 7.26 | 24.74 | |
| 10.0 | 6.10 | 8.15 | 33.71 | 7.49 | 22.87 | |
(%) (m3 s−1) | Countercurrent-Flow Operations (mol m−2 s−1) | |||||
|---|---|---|---|---|---|---|
| Empty Channel | 2 cm Spiral Wire | 3 cm Spiral Wire | ||||
| (%) | (%) | |||||
| 30 | 5.0 | 4.12 | 5.45 | 38.68 | 4.92 | 25.19 |
| 6.67 | 4.67 | 6.05 | 35.65 | 5.43 | 21.75 | |
| 8.33 | 5.15 | 6.51 | 32.86 | 5.79 | 18.16 | |
| 10.0 | 5.28 | 6.65 | 31.16 | 5.92 | 16.77 | |
| 35 | 5.0 | 4.38 | 5.87 | 43.87 | 5.27 | 29.17 |
| 6.67 | 5.15 | 6.61 | 41.24 | 6.00 | 28.21 | |
| 8.33 | 5.86 | 7.37 | 35.98 | 6.76 | 23.06 | |
| 10.0 | 6.06 | 7.54 | 34.16 | 6.95 | 21.57 | |
| 40 | 5.0 | 4.71 | 6.42 | 47.93 | 5.81 | 33.87 |
| 6.67 | 5.55 | 7.20 | 43.71 | 6.55 | 30.74 | |
| 8.33 | 6.40 | 8.12 | 39.52 | 7.48 | 28.52 | |
| 10.0 | 6.70 | 8.30 | 36.07 | 7.65 | 25.41 | |
(%) m3/s | Countercurrent-Flow Operations | |||||
|---|---|---|---|---|---|---|
| Empty Channel | 2 cm Spiral Wire | 3 cm Spiral Wire | ||||
| 30 | 5.0 | 4.85 | 32.27 | 38.68 | 20.24 | 25.19 |
| 6.67 | 4.71 | 29.27 | 35.65 | 16.27 | 21.75 | |
| 8.33 | 5.10 | 26.41 | 32.86 | 12.43 | 18.16 | |
| 10.0 | 4.14 | 25.95 | 31.16 | 12.13 | 16.77 | |
| 35 | 5.0 | 7.35 | 34.01 | 43.87 | 20.33 | 29.17 |
| 6.67 | 10.04 | 28.35 | 41.24 | 16.51 | 28.21 | |
| 8.33 | 8.19 | 25.69 | 35.98 | 13.75 | 23.06 | |
| 10.0 | 7.83 | 24.42 | 34.16 | 12.74 | 21.57 | |
| 40 | 5.0 | 8.53 | 36.30 | 47.93 | 24.07 | 33.87 |
| 6.67 | 10.78 | 29.73 | 43.71 | 19.61 | 30.74 | |
| 8.33 | 9.97 | 26.87 | 39.52 | 16.87 | 28.52 | |
| 10.0 | 9.84 | 23.88 | 36.07 | 14.18 | 25.41 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, C.-D.; Chang, H.; Lin, G.-H.; Chew, T.L. Enhancing Absorption Performance of CO2 by Amine Solution through the Spiral Wired Channel in Concentric Circular Membrane Contactors. Membranes 2022, 12, 4. https://doi.org/10.3390/membranes12010004
Ho C-D, Chang H, Lin G-H, Chew TL. Enhancing Absorption Performance of CO2 by Amine Solution through the Spiral Wired Channel in Concentric Circular Membrane Contactors. Membranes. 2022; 12(1):4. https://doi.org/10.3390/membranes12010004
Chicago/Turabian StyleHo, Chii-Dong, Hsuan Chang, Guan-Hong Lin, and Thiam Leng Chew. 2022. "Enhancing Absorption Performance of CO2 by Amine Solution through the Spiral Wired Channel in Concentric Circular Membrane Contactors" Membranes 12, no. 1: 4. https://doi.org/10.3390/membranes12010004
APA StyleHo, C.-D., Chang, H., Lin, G.-H., & Chew, T. L. (2022). Enhancing Absorption Performance of CO2 by Amine Solution through the Spiral Wired Channel in Concentric Circular Membrane Contactors. Membranes, 12(1), 4. https://doi.org/10.3390/membranes12010004







