Abstract
Demand for nickel and cobalt sulfate is expected to increase due to the rapidly growing Li-battery industry needed for the electrification of automobiles. This has led to an increase in the production of sodium sulfate as a waste effluent that needs to be processed to meet discharge guidelines. Using bipolar membrane electrodialysis (BPED), acids and bases can be effectively produced from corresponding salts found in these waste effluents. However, the efficiency and environmental sustainability of the overall BPED process depends upon several factors, including the properties of the ion exchange membranes employed, effluent type, and temperature which affects the viscosity and conductivity of feed effluent, and the overpotentials. This work focuses on the recycling of Na2SO4 rich waste effluent, through a feed and bleed BPED process. A high ion-exchange capacity and ionic conductivity with excellent stability up to 41 °C is observed during the proposed BPED process, with this temperature increase also leading to improved current efficiency. Five and ten repeating units were tested to determine the effect on BPED stack performance, as well as the effect of temperature and current density on the stack voltage and current efficiency. Furthermore, the concentration and maximum purity (>96.5%) of the products were determined. Using the experimental data, both the capital expense (CAPEX) and operating expense (OPEX) for a theoretical plant capacity of 100 m3 h−1 of Na2SO4 at 110 g L−1 was calculated, yielding CAPEX values of 20 M EUR, and OPEX at 14.2 M EUR/year with a payback time of 11 years, however, the payback time is sensitive to chemical and electricity prices.
1. Introduction
The mining and metallurgical industries produce large amounts of waste during the processing and neutralization of acidic streams [1]. Sulfuric acid is commonly used for leaching metals and their oxides [2,3] and sodium hydroxide is subsequently used for their precipitation and acid neutralization, as well as for pH control of other processes [4]. As a result, undesirable sodium sulfate (Na2SO4) solution is produced [5]. The rapidly growing battery industry is one of the sectors in which such solutions are produced. For example, typical production processes for nickel, manganese and cobalt (NMC) battery cathode materials comprising of leaching and co-precipitation stages produce liquid streams rich in Na2SO4 [6,7,8]. The stream can be considered as a potential source for by-product(s) if revenue can be generated from it [9], or a waste stream if discharged or disposed, with approximately half of the world production of commercially traded Na2SO4 generated as a by-product [10].
Na2SO4 is not a direct threat to the environment, as sulfate is a chemically inert, non-volatile and nontoxic compound [11]. On the other hand, despite being one of the key nutrients [12], sulfate increases the salinity level of surface water [13], affecting the cycling of other nutrients, binding of metals, and formation of toxic substances in aquatic systems [12]. In high levels, it is harmful to freshwater biota. However, the contribution of sulfate-rich emissions from anthropogenic activities is poorly known [12]. In European water legislation, sulfate is not listed as a polluting substance to be taken into account for setting emission limit values [12,14]. Na2SO4 can be crystallized and, to a certain extent, sold or diluted and discharged into waterways, provided that it complies with environmental legislation. However, discharging of sulfate waste streams should preferably be controlled and monitored systematically [12] or avoided when possible [15], although not strictly regulated in most countries. Due to increasing pressure related to water sustainability issues [16], it is expected that environmental regulations for wastewater treatment could become stricter. Should regulations become stricter, the processing of Na2SO4 effluents could become a requirement despite not having direct financial incentives.
Modifications in processes may eliminate the formation of Na2SO4 emissions [7]. If this is not an option, a variety of options for the valorization of Na2SO4 exist. Van der Bruggen et al. [17] explored whether thermal processes, such as evaporation and distillation, can be suitable for the reduction in the waste fraction of brine. Atia et al. recovered Na2SO4 with a purity of 96% by evaporation–crystallization. Sodium sulfate meeting commercial specifications could be used as a raw material in, for example, the detergent or pulp and paper industry [10]. However, these methods are not as applicable if the salinity of the effluent is below 5000 ppm [18]. In cases where the salinity is lower than that, more commonly membrane separation processes are being employed, such as electrodialysis (ED), bipolar membrane electrodialysis (BPED) and reverse osmosis (RO). [18] These techniques have long been utilized for desalination, softening, and contaminant removal from waste effluents. Except for RO which relies on porous membranes, both ED and BPED processes rely on cation and anion exchange membranes to selectively transport ions [11,19,20,21] upon application of an electric voltage over an ED or BPED stack, of which multiple configurations are shown in Figure 1. Thus, a combination of different configurations of ion exchange membranes allows either dilution of waste salt streams or recovery into corresponding acid and base. The recovery of acid and base from its corresponding salt solution is achieved through bipolar membranes splitting water into protons and hydroxides [15]. Therefore, in the BPED of Na2SO4, recombination of protons with sulfate ions, and hydroxide ions with sodium ions leads to the formation of H2SO4 and NaOH solutions [5]. Recovered acid and base solutions can be reused for leaching and precipitation of metals or in other process streams.

Figure 1.
Different configurations of membrane electrodialysis process: (a) BCA configuration, (b) standard ED configuration, (c) BCAA configuration and (d) BCCA configuration.
According to Bazinet and Geoffrey, the worldwide market for ED equipment reached USD 318 million in 2019 and is expected to grow to market values of USD 458 million with annual growth between 5.5–5.8% up to 2025 [22]. In addition to this, the number of companies providing ED equipment to market, totals 45 in 2020, from a mere 6 companies in 1980 [22]. The ED-based techniques are receiving increased attention for the treatment of waste water, as they offer promising prospects for the recovery of selected ions in the form of concentrated streams [23] or reuse valuable compounds from saline streams by using bipolar membranes [24]. Water dissociates into protons and hydroxyl ions at 0.83 V across the BPM, and combining them with salt constituents produces acid and base in the two product steams. Kroupa et al. (2014) have treated a Na2SO4 effluent coming from uranium processing through electrodialysis with bipolar membranes [25]. The aim of their lab-scale experiment was to study the effect of various process parameters on energy consumption, and to achieve the target concentration of 5.5 wt% (0.56 M) for H2SO4 and 6.5 wt% (1.63 M) for NaOH. The purity of the products was in the range of 71.9–83.3% for H2SO4 and 97.0–98.3% for NaOH. Kroupa et al. (2016) additionally investigated the BPED of sodium sulfate in four different stack configurations [26] and compared the results with the standard ED process. After running BPED (BCA configuration Figure 1a) of 35 g/L Na2SO4 (0.25 M) solution for 8 h, acid concentration of 60 g/L (0.6122 M) and base concentration of 46 g/L (1.15 M) were reached. From ED of either 25 g/L H2SO4 (0.255 M) or 25 g/L NaOH (0.625 M) solution in a three-compartment configuration (Figure 1b), acid concentration of 105 g/L (1.07 M) and base concentration of 98 g/L (2.45 M) were reached in 16 h. The same feed solution was used also in a four-compartment configuration (BCAA and BCCA configuration Figure 1c,d), but no significant effect was noticed on the acid concentration. However, the NaOH concentration was raised to 58 g/L (1.45 M) in the four-compartment configuration (BCCA configuration, Figure 1d). Kinčl et al. (2017) carried out BPED of pure Na2SO4 solution at pilot scale using heterogeneous membranes [27]. Their aim was to scale up their pilot trials results to an industrial scale. They achieved maximum product concentrations were 1.5% (0.16 M) for H2SO4 and 4% (1.0 M) for NaOH at a current density of 350 A m−2. The purity of their products was ca. 85% with the current efficiency of 62%. A recent experimental study was carried out by Bruinsma et al. (2021) [5] concerning BPED of (0.7 M) Na2SO4 in combination with reverse osmosis (RO). They operated BPED in BCA configuration (Figure 1a) with 10 repeat units (RUs) in a batch mode at a constant voltage of 30 V. Both the current density and salt splitting were observed 25% higher on increasing the temperature of the BPED from 25 to 35 °C. In contrast, cation exchange membrane (CEM) current efficiency is drastically dropped from 100% to 83% due to an increase in proton leakage. Furthermore, they achieved purity values of 99% for NaOH and 90% for H2SO4.
The overall BPED efficiency strongly depends on the temperature which affects the ionic conductivity of ion-exchange membranes (IEM). The temperature has a strong effect on diffusion coefficients, as defined by the Stokes–Einstein equation [28,29], and via the viscosity, the fluid velocity. Furthermore, the selectivity and permeability of ions through IEMs can also be affected as the hydration of ions decreases with an increase in temperature. In general, however, increased ionic fluxes [30] as well as lower membrane resistances [31] are expected at higher temperatures, which improves the energy efficiency of BPED. Thus, the temperature study is crucial in defining the optimal performance of the BPED process, which is needed in the design of industrial plants, considering capital costs and energy consumption. Degradation of membranes in terms of selectivity of ions [32], capacity loss, and lifetime depletion of the ion exchange resin are the major drawbacks of higher temperature operations [33].
In the last few years, the performance, stability and operating window of AEMs, CEMs and BPMs have improved significantly. Older generations of membranes typically showed low stability, mainly in caustic and strong acidic environments [21], and produced low purity products due to low selectivity and relatively low current efficiencies. With newer homogeneous membranes such as those offered by SUEZ Water Technologies and Solutions [34], higher purity products can be produced at elevated temperatures and at higher current density. Hence, we tested these membranes in order to determine the commercial viability of the process.
In this present paper, BPED of an industrial effluent is carried out in a feed and bleed mode which can be utilized in scaling up to an industrial-sized system. The aim of the study is to check the performance of the SUEZ membranes in a BPED stack with 5 and 10 repeating units of a BCA (BPM-CEM-AEM) configuration at different temperatures and current densities. Furthermore, the current efficiency was evaluated as a function of temperature, current density, and product concentrations. The capital and operational expenditures of an industrial-sized system are also discussed briefly.
2. Materials and Methods
All experiments were conducted with the Suez laboratory/bench scale MkI ED stack with an active membrane area of 280 cm2. Different sets of experiments were carried out with 5 and 10 repeating units (Figure 2), and conductivities, inlet stack pressures and flow rates were monitored for all flows by Indumax CLS50D, Cerabar M PMC51, and Promag 53H (Endress Hauser, Germany). In addition, pH and temperature for the feed effluent were measured by Orbisint CPS11D Memosens (Endress Hauser, Germany). The anode consisted of platinum-coated titanium and the cathode was made of stainless steel. The properties of the SUEZ membranes used in the tests are listed in Table 1 and contain manufacturer specified data on the water uptake (WU) and ion exchange capacity (IEC) whereas the membrane fixed charge was calculated, Equation 1. The composition of the effluent is given inTable 2, and the conductivity of the effluent was determined to be 85 mS cm−1.
where is the membrane fixed charge (M), the ion exchange capacity (meq g−1), the density of a hydrated membrane and X its water content, whereas the BPM used was manufactured through lamination of CR61P and the treated AR103P membrane.

Figure 2.
Schematic of BPED process with BCA as a repeating unit (RU).

Table 1.
Membrane’s characteristics [34].

Table 2.
Effluent composition.
The experiments were conducted in a batch feed and bleed configuration, which is depicted in Figure 3. When the conductivity of either the acid or base reaches its target value, 20% of its volume is replaced with DI-distilled water. Similarly, 30% of the feed compartment is replaced with fresh feed solution after reaching the target conductivity. These steps were repeated continuously, with the final product concentrations being determined through titration and the purity of the products were assessed using ICP-OES (SFS-EN ISO 11885:2009).

Figure 3.
Schematic representation of the batch feed and bleed operation of the BPED process.
3. Result and Discussion
In this section, BPED results are presented and discussed for three temperatures (24 °C, 34 °C, and 41 °C). Generally, membrane stability tends to decrease above these temperatures, and for that reason, it was decided to keep 41 °C as the maximum temperature. At these temperatures three current densities were tested (300, 400 and 500 A m−2), which were well below the limiting current density, with the current efficiency being determined for each counterion.
3.1. Stack Voltage
Figure 4a,b show the stack voltage variation with current density and temperature. Raising the current density raises the cell voltage because its major part comes from ohmic losses in the membranes and the solutions. It also accelerates mass transfer at the solution-membrane interface and electro-osmosis in the membrane and raises temperature via Joule heat. Stack voltage is found to increase linearly with the current density at a fixed temperature (Figure 4a), whereas it decreases on increasing temperature at a fixed current density (Figure 4b) due to the higher conductivity of the solutions and lower membrane resistance [35] Energy efficiency should be improved by operating at 41 °C, which can be maintained with heat exchangers, additionally retaining heat produced by the operation of the BPED stack. At 41 °C and 500 A m−2 the voltage per stack is 2.6 V, whereas Kinčl et al. [27] operated at 3 V per stack for optimal performance using heterogeneous membranes at 350 A m−2.

Figure 4.
Cell voltage variation in Stack of 10 repeating units (a) at different current densities, and (b) at different temperature.
3.2. Current Efficiency
In this part, the effect of current density and temperature on current efficiency is discussed. Current efficiencies of acid and base production were calculated from Equations (2) and (3) given below:
where (mol s−1) and (mol s−1) are the accumulation rates of protons in acid and hydroxide ions in base, number of repeating units, I electric current (A), F Faraday constant (As mol−1), (L s−1) and (L s−1) volumetric flow rates of acid and base products, and proton (M) and hydroxide ion (M) concentrations in acid and base. The current efficiency of the acid and base production was studied at varying temperatures with a fixed current density of 300 A m−2; the results are shown in Figure 5. A noticeably higher current efficiency is found at 41 °C compared with 23 °C for both acid and base production. It is not clear why this should happen, as the process is run under constant current conditions, although increased conductivities [35,36] and water splitting rate [36] have an effect on the stack voltage.
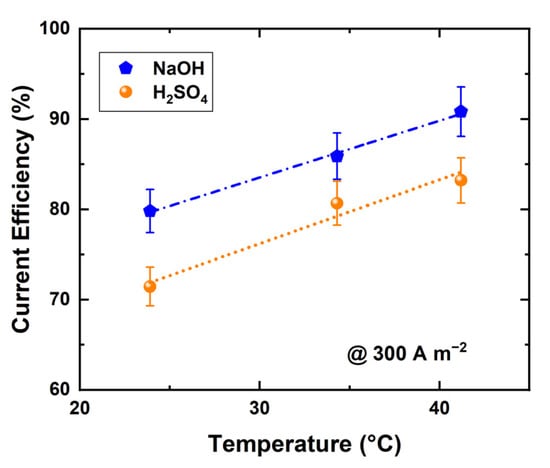
Figure 5.
Current efficiency of BPED products in a stack of 10 RUs at different temperaTable 300. A m−2 (products concentrations were 0.5 ± 0.02 N).
The impact of current density on the current efficiency of both acid and base production was also determined (see Figure 6a,b). It appears that the current efficiency is not greatly affected by an increase in current density, which implies that the process is not under mass transport control. Mass transfer limitation can reduce the current efficiency through the onset of unwanted side-reactions.
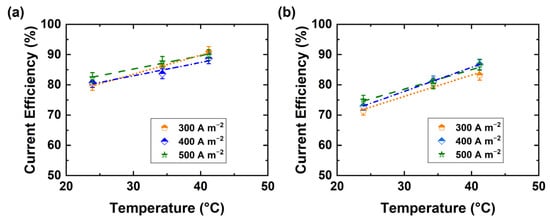
Figure 6.
Current efficiency of BPED products in a stack of 10 repeating units at different current densities of (a) NaOH (0.5 ± 0.01 M), and (b) H2SO4 (0.25 ± 0.01 M).
Current efficiency was also investigated as a function of BPED products concentration (Figure 7). At low product concentrations current efficiencies above 85% are observed, and on increasing product concentrations efficiencies drop to 55% for H2SO4 and 70% for NaOH, indicating higher leakage of co-ions. The lower H2SO4 efficiency compared with NaOH indicates that the proton leakage through the AEM is significantly higher than the hydroxyl leakage through the CEM when the concentrations of protons and hydroxide ions are equal in the acid and base, respectively. Furthermore, the same conditions of products were conducted with pure Na2SO4 (0.5 M) solution as feed solution. Surprisingly, there were no significant differences noticed, although the concentration of Na2SO4 in waste effluent was 110 g L−1 (0.775 M), Table 2. The minor variations in current efficiency can possibly be attributed to the effluent impurities either slightly fouling the membrane and or other side reactions occurring. In addition, these current efficiency results are comparable with our single membranes simulations and experimental studies [37].
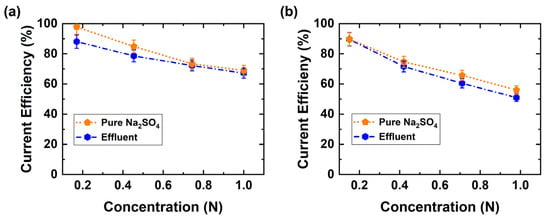
Figure 7.
Current efficiency variation as a function of BPED products using pure Na2SO4 or the industrial effluent. (a) Base, (b) Acid (300 A m−2 at room temperature).
The previous experiments were conducted with 10 repeating units. A single test was made with a stack containing five repeating units only, to study the effect of the number of repeating units on current efficiency (Figure 8a,b). Theoretically, current leaking through the liquid manifolds could increase as the number of RUs increases. The current efficiency of base and acid is evaluated as a function of current density in both cases. The addition of five repeating units does not greatly affect the current efficiency. Hence, it appears that with the present cell design and implementation (5 vs. 10 RU) there is no significant current leakage. However, the current leakage effects may become more significant at a higher number of RU.
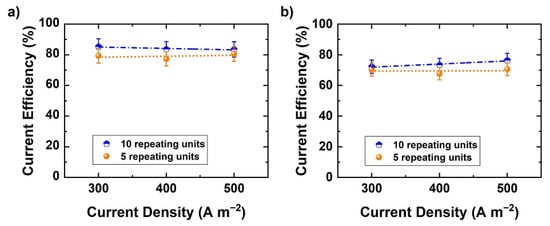
Figure 8.
Current efficiency variation as a function of number of RUs at different current densities: (a) NaOH and (b) H2SO4.
Purities of the products are defined as
where is the mass concentration (g L−1) of sulfuric acid and concentration of sodium sulfate in the acid product; is the mass concentration of sodium hydroxide and the mass concentration of sodium sulfate in the base product.
In our experiments, both H2SO4 and NaOH are produced with a purity of over 96% (Figure 9), which is higher than the purity achieved by Kroupa et al. [26] and Kinčl et al. [27]. The only impurity in the acid compartment is sodium ions, whereas sulfate ions appear in the base compartment because a BPM can also leak small amounts of these ions [38]. As seen in Figure 9 below, high purity (>96%) of products was reached with the homogeneous SUEZ membranes. In contrast, Kinčl et al. could reach only 80% purity when using heterogeneous membranes [27].
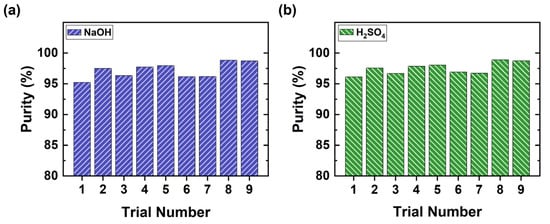
Figure 9.
BPED of the effluent; purity of products (a) NaOH and (b) H2SO4.
3.3. Capital and Operational Expense Calculations
Based on the results above and the conditions assumed shown in Table 3, the CAPEX and OPEX for a 100 m3 h−1 BPED were calculated for a stream of 110 g L−1 Na2SO4. For projected acid production of 144 m3 h−1 at 1.0 N, and base production of 144 m3 h−1 at 1.0 N, an estimated 256 industrial BPED stacks (12,000 m2 total active membrane surface) would be required, operating at 1.4 kWh kg−1 of treated Na2SO4.

Table 3.
Operating condition.
The CAPEX (These values might be varied in range ± 20%) comes to approximately EUR 20 M for all BPED equipment, excluding feed/product tanks, pre/post treatment and integration cost. The OPEX cost breakdown can be noted in the table below (Table 4) and assumes 8000 working hours per year, and maintenance includes membrane, stack and equipment maintenance. In this case, the costs for electricity make up almost half of all the operational costs. The total OPEX (These values might be varied in range ± 20%) comes to 14.2 M EUR/year, assuming pricing of 0.05 EUR/kWh for electricity and EUR 2 m−3 of DI-H2O. At 300 EUR/ton for 98% NaOH and 100 EUR/ton for 95% H2SO4 however, chemical consumption cost can be reduced by approximately 16.1 M EUR/year at production rates of 5.0 ton h−1 and 5.3 ton h−1 for NaOH and H2SO4, respectively, if the chemicals are reused at the site. Taking this into account, the process can be operated at a margin of approximately 1.8 M EUR/year. This cost off-set leads to an estimated payback time of 11 years.

Table 4.
OPEX estimations for operation of the BPED process.
3.4. Sensitivity Analysis
Depending on market conditions and location, the average price for utilities and chemicals varies significantly and impacts OPEX values. For instance, an increase in electricity pricing from 0.05 to 0.09 EUR/kWh results in an increase from 14.2 to 19.2 M EUR/year for the OPEX, which in turn decreases the margin. Alternatively, higher chemical prices, i.e., NaOH costs from 300 to 400 EUR/ton, increase the cost-reduction margin by 24.5% from 16.1 to 20.1 M EUR/year and reduce the payback time to 3.4 years. Depending on the effluent stream process, membrane lifetime can vary significantly and will subsequently increase or decrease OPEX with regard to membrane maintenance. As stated previously, CAPEX and OPEX calculations are required for individual application cases.
The environmental footprint of the BPED should also be considered. The process itself does not cause any direct greenhouse gas emissions. However, the BPED technology is a highly intensive energy consumer leading into indirect effects on the environment from energy production [39]. The electricity should preferably be generated from renewable energy sources with a low CO2 footprint. Other environmental aspects can also be taken into consideration. The emissions arising from the transportation of purchased chemicals to the site can be avoided if the produced NaOH and H2SO4 can be used on site. The application of BPED is also potentially a cost-effective approach. The operational costs for purchased raw materials could be decreased and industrial sites currently disposing the stream could partially diminish disposal costs [40]. At the same time, environmental risks related to the disposal of Na2SO4 are mitigated.
Both the study and the CAPEX/OPEX show promising results; for this reason, integration studies can be performed on BPED, e.g., into the leaching process of Ni during the refining process, one possibility being if integrated together with evaporators and reverse osmosis (RO) units, a zero liquid discharge setup can be achieved. In this theoretical process, the water recovered through both the RO and evaporator processes is used to dilute and maintain liquid levels for both the acid and base products. While RO and evaporators have relatively high operating costs (approximately EUR 5 m−3 for RO and EUR 30 m−3 for evaporators), their cost can be offset through government grants aimed at incentivizing zero liquid discharge operations. Similar flow diagrams could be integrated to other processes, such as battery material production. Recently, the potential of integrating BPED to treat Na2SO4-rich streams at an industrial level has been recognized in the battery sector [8].
4. Conclusions
We have shown that the BPED process can be used to effectively treat waste sodium sulfate effluent streams. Using state-of-the-art homogeneous membranes operating at 2.6 V per cell, 500 A m−2 and 41 °C, acid and base purity values exceeding 95% can be reached. These performance values are superior to the earlier performance reported for heterogeneous membranes. The primary impurities in the acid and base streams are sodium and sulfate ions, respectively, due to ion leakage through the BPM. Additionally, it was observed that the temperature increase led to an improvment in current efficiency. Varying the number of RUs from 5 to 10 had a negligible effect on the current efficiency, indicating that the shunt current in the short stack was insignificant. As increasing temperature improves both the voltage and current efficiency of the process, it is desirable to operate the system at the maximum temperature that the membrane can endure. During operation, the base production stream showed higher current efficiencies compared with the acid stream, which is attributed to the higher proton leakage through the AEM than hydroxide leakage through the CEM. BPED might serve in addition or as a replacement to existing processes such as evaporators and RO, with BPED showing specific energy consumption of 1.4 kWh kg−1 Na2SO4 treated and costing approximately 0.215 EUR/kg Na2SO4 treated, depending on conditions.
Overcoming some technical or economic barriers for the recovery of acid and base streams from effluent streams using BPED, including but not limited to both the BPED performance and pre-treatment, is required for a successful scale-up. For instance, effective pre-treatment of effluent streams to remove impurities can ensure long membrane lifetime, reducing the cost of operation. In addition, additional purification and concentration of the products might be required, depending on the given case. Further improvements regarding membrane performance and operating parameters can lead to decreased energy consumption and emphasize the techno-economic perspective. Future work would see the integration of a BPED system into an industrially relevant process, to determine the effects and issues associated with impurity build-up due to stream recycling in a closed loop. Furthermore, the integration of BPED with processes such as evaporators or RO should be investigated to determine whether a zero liquid discharge configuration is feasible.
The feasibility of integrating the BPED process to a given chemical process should always be considered case by case in detail, as the feed streams vary from site to site and prices of, e.g., chemicals and utilities depend on the location. Tailor-made solutions are often needed; however, the capital and operational expenditure calculations in this study give a good overview of the potential cost-effectiveness of the BPED process. With ever-increasing legislation, regulations regarding wastewater discharge and migration to zero liquid discharge processes, the demand for BPED technology is expected to increase.
Author Contributions
Conceptualization, K.; data curation, K. and R.R.; formal analysis, K., W.D.B. and H.P.; funding acquisition, L.M.; investigation, K., W.D.B. and R.R.; methodology, K., H.P. and P.M.; project administration, P.K. and L.M.; resources, P.K. and H.P.; supervision, P.K. and L.M.; visualization, K. and W.D.B.; writing—original draft, K.; writing—review and editing, K., W.D.B., P.K., H.P., R.R. and L.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work has received funding from the European Institute of Innovation and Technology (EIT), a body of the European Union, under the Horizon 2020, the EU Framework Programme for Research and Innovation. Project Name and Number: Credit -18243. SUEZ Water Technologies and Solutions is acknowledged for the supply of the membranes.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Matinde, E. Mining and metallurgical wastes: A review of recycling and re-use practices. J. S. Afr. Inst. Min. Metall. 2018, 118. [Google Scholar] [CrossRef]
- Khalid, M.K.; Hamuyuni, J.; Agarwal, V.; Pihlasalo, J.; Haapalainen, M.; Lundström, M. Sulfuric acid leaching for capturing value from copper rich converter slag. J. Clean. Prod. 2019, 215, 1005–1013. [Google Scholar] [CrossRef]
- Ramachandra Rao, S. Resource Recovery from Process Wastes. Resource Recovery and Recycling from Metallurgical Wastes; Elsevier: Amsterdam, The Netherlands, 2006; pp. 375–457. ISBN 9780080451312. [Google Scholar]
- Rodriguez, J.; Stopić, S.; Krause, G.; Friedrich, B. Feasibility assessment of electrocoagulation towards a new sustainable wastewater treatment. Environ. Sci. Pollut. Res. Int. 2007, 14, 477–482. [Google Scholar] [CrossRef]
- Bruinsma, O.; Branken, D.J.; Lemmer, T.N.; van der Westhuizen, L.; Rossouw, S. Sodium sulfate splitting as zero brine process in a base metal refinery: Screening and optimization in batch mode. Desalination 2021, 511, 115096. [Google Scholar] [CrossRef]
- Ahmed, S.; Nelson, P.A.; Gallagher, K.G.; Susarla, N.; Dees, D.W. Cost and energy demand of producing nickel manganese cobalt cathode material for lithium ion batteries. J. Power Sources 2017, 342, 733–740. [Google Scholar] [CrossRef] [Green Version]
- Zang, G.; Zhang, J.; Xu, S.; Xing, Y. Techno-economic analysis of cathode material production using flame-assisted spray pyrolysis. Energy 2021, 218, 119504. [Google Scholar] [CrossRef]
- Korkiakoski, J.; Leppäkoski, E.; Ahonen, S.; Hakala, K.; Happo, M. Environmental Impact Assessment of Battery Material Production. No. 1510051473, Finnish Battery Chemicals Oy, Finland, 2021. Available online: https://www.ymparisto.fi/fi-fi/Asiointi_luvat_ja_ymparistovaikutusten_arviointi/Ymparistovaikutusten_arviointi/YVAhankkeet/Akkumateriaalin_tuotanto_Finnish_Battery_Chemicals_Oy__Kotka_Hamina_Kokkola_ja_Vaasa (accessed on 18 September 2021).
- Atia, T.A.; Elia, G.; Hahn, R.; Altimari, P.; Pagnanelli, F. Closed-loop hydrometallurgical treatment of end-of-life lithium ion batteries: Towards zero-waste process and metal recycling in advanced batteries. J. Energy Chem. 2019, 35, 220–227. [Google Scholar] [CrossRef] [Green Version]
- Carla Lupi, A.P. Treatment of some liquid waste associated with lead battery recycling. In Proceedings of the 2008 Global Symposium on Recycling, Waste Treatment and Clean Technology (REWAS 2008), UK, October 2008. [Google Scholar]
- Shin, H.S.; Jung, J.Y.; Bae, B.U.; Paik, B.C. Phase-separated anaerobic toxicity assays for sulfate and sulfide. Water Environ. Res. 1995, 67, 802–806. [Google Scholar] [CrossRef]
- Ekholm, P.; Lehtoranta, J.; Taka, M.; Sallantaus, T.; Riihimäki, J. Diffuse sources dominate the sulfate load into Finnish surface waters. Sci. Total Environ. 2020, 748, 141297. [Google Scholar] [CrossRef]
- Mark, A.R. A new process for sulfate removal from industrial waters. ASMR 1999, 1999, 546–550. [Google Scholar] [CrossRef]
- Thomas, B.; German, G.S.; Hande, Y.; Serge, R.; Luis, D.S. Best Available Techniques (BAT) Reference Document for Com-mon Waste Water and Waste Gas Treatment/Management Systems in the Chemical Sector: Industrial Emissions Directive 2010/75/EU (Integrated Pollution Prevention And Control); Publications Office, 2016. Available online: https://www.semanticscholar.org/paper/Best-Available-Techniques-(BAT)-Reference-Document-Thomas-Santonja/b278de5e3cd33ba7daf2c3ef58d231b2e5442401 (accessed on 18 September 2021).
- Kroupa, J.; Kinčl, J.; Cakl, J. Recovery of H2SO4 and NaOH from Na2SO4 by electrodialysis with heterogeneous bipolar membrane. Desalin. Water Treat. 2014, 56, 3238–3246. [Google Scholar] [CrossRef]
- Cipolletta, G.; Ozbayram, E.G.; Eusebi, A.L.; Akyol, Ç.; Malamis, S.; Mino, E.; Fatone, F. Policy and legislative barriers to close water-related loops in innovative small water and wastewater systems in Europe: A critical analysis. J. Clean. Prod. 2021, 288, 125604. [Google Scholar] [CrossRef]
- van der Bruggen, B.; Lejon, L.; Vandecasteele, C. Reuse, treatment, and discharge of the concentrate of pressure-driven membrane processes. Environ. Sci. Technol. 2003, 37, 3733–3738. [Google Scholar] [CrossRef]
- Strathmann, H. Membrane separation processes. J. Membr. Sci. 1981, 9, 121–189. [Google Scholar] [CrossRef]
- Juda, W.; McRae, W.A. Coherent ion-exchange gels and membranes. J. Am. Chem. Soc. 1950, 72, 1044. [Google Scholar] [CrossRef]
- Kim, D.H. A review of desalting process techniques and economic analysis of the recovery of salts from retentates. Desalination 2011, 270, 1–8. [Google Scholar] [CrossRef]
- Strathmann, H. Ion-Exchange Membrane Separation Processes, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2004; ISBN 9780444502360. [Google Scholar]
- Bazinet, L.; Geoffroy, T.R. Electrodialytic Processes: Market Overview, Membrane Phenomena, Recent Developments and Sustainable Strategies. Membranes 2020, 10, 221. [Google Scholar] [CrossRef] [PubMed]
- Korngold, E.; Aronov, L.; Daltrophe, N. Electrodialysis of brine solutions discharged from an RO plant. Desalination 2009, 242, 215–227. [Google Scholar] [CrossRef]
- Koter, S.; Warszawski, A. Electromembrane processes in environment protection. Pol. J. Environ. Stud. 2000, 9, 45–56. [Google Scholar]
- Kroupa, J.; Cakl, J.; Kinčl, J.; Toman, F. Integration of electrodialysis with bipolar membrane into technology of treatment of extra wastewater containing sodium sulfate. Innov. Remediat. Technol. Exp. 2014, 7. [Google Scholar]
- Kroupa, J.; Cakl, J.; Kinčl, J. Increase the Concentration of Products from Electrodialysis with Heterogeneous Bipolar Membrane. Available online: http://www.ekomonitor.eu/sites/default/files/soubory/2015/kroupa_ft.pdf (accessed on 18 September 2021).
- Kinčl, J.; Jiříček, T.; Neděla, D.; Toman, F.; Cakl, J. Electromembrane Processes in Mine Water Treatment. 2017. Available online: https://www.imwa.info/docs/imwa_2017/IMWA2017_Kincl_1154.pdf (accessed on 18 September 2021).
- Galitskaya, E.; Privalov, A.F.; Weigler, M.; Vogel, M.; Kashin, A.; Ryzhkin, M.; Sinitsyn, V. NMR diffusion studies of proton-exchange membranes in wide temperature range. J. Membr. Sci. 2020, 596, 117691. [Google Scholar] [CrossRef]
- Zhang, H.; Davison, W. Performance Characteristics of Diffusion Gradients in Thin Films for the in Situ Measurement of Trace Metals in Aqueous Solution. Anal. Chem. 1995, 67, 3391–3400. [Google Scholar] [CrossRef]
- Tseng, S.; Li, Y.-M.; Lin, C.-Y.; Hsu, J.-P. Salinity gradient power: Influences of temperature and nanopore size. Nanoscale 2016, 8, 2350–2357. [Google Scholar] [CrossRef]
- Fontananova, E.; Zhang, W.; Nicotera, I.; Simari, C.; van Baak, W.; Di Profio, G.; Curcio, E.; Drioli, E. Probing membrane and interface properties in concentrated electrolyte solutions. J. Membr. Sci. 2014, 459, 177–189. [Google Scholar] [CrossRef]
- Aittola, J.P.; Chyssler, J.; Ringberg, H. Thermal Stability of Ion-Exchange Regins; Studsvik Energiteknik AB: Nyköping, Sweden, 1982. [Google Scholar]
- DuPont. Available online: https://www.dupont.com/content/dam/dupont/amer/us/en/water-solutions/public/documents/en/45-D01456-en.pdf (accessed on 18 September 2021).
- SUEZ Water Technologies, Ionics CR61P & Ionics AR103P. Available online: https://my.suezwatertechnologies.com/WTSCustomerPortal/s/content-downloadDN=FSelIXMembranes.pdf (accessed on 18 September 2021).
- Benneker, A.M.; Klomp, J.; Lammertink, R.G.; Wood, J.A. Influence of temperature gradients on mono- and divalent ion transport in electrodialysis at limiting currents. Desalination 2018, 443, 62–69. [Google Scholar] [CrossRef]
- Benson, G.C.; Gordon, A.R. A Reinvestigation of the Conductance of Aqueous Solutions of Potassium Chloride, Sodium Chloride, and Potassium Bromide at Temperatures from 15° to 45 °C. J. Chem. Phys. 1945, 13, 473–474. [Google Scholar] [CrossRef]
- Kuldeep; Kauranen, P.; Pajari, H.; Pajarre, R.; Murtomäki, L. Electrodiffusion of ions in ion exchange membranes: Finite element simulations and experiments. Chem. Eng. J. Adv. 2021, 8, 100169. [Google Scholar] [CrossRef]
- Raucq, D.; Pourcelly, G.; Gavach, C. Production of sulphuric acid and caustic soda from sodium sulphate by electromembrane processes. Comparison between electro-electrodialysis and electrodialysis on bipolar membrane. Desalination 1993, 91, 163–175. [Google Scholar] [CrossRef]
- Herrero-Gonzalez, M.; Admon, N.; Dominguez-Ramos, A.; Ibañez, R.; Wolfson, A.; Irabien, A. Environmental sustainability assessment of seawater reverse osmosis brine valorization by means of electrodialysis with bipolar membranes. Environ. Sci. Pollut. Res. Int. 2020, 27, 1256–1266. [Google Scholar] [CrossRef]
- Fernandez-Gonzalez, C.; Dominguez-Ramos, A.; Ibañez, R.; Irabien, A. Electrodialysis with Bipolar Membranes for Valorization of Brines. Sep. Purif. Rev. 2016, 45, 275–287. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).