Potent Acrylamide Determination in Food Products Using Ion-Selective Electrode Technique
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Products, Materials and Chemicals
2.2. Preparation of Stock Solutions
2.3. Sample Preparation for the Estimation of Acrylamide Cations
2.4. Fabrication of the Selective Sensor
2.5. Active Component of Liquid-Sensor Layer
2.6. The Potential Layer Conditioned
2.7. Measurements of EM F
3. Results
3.1. Calibration Curves
3.2. Interference Study
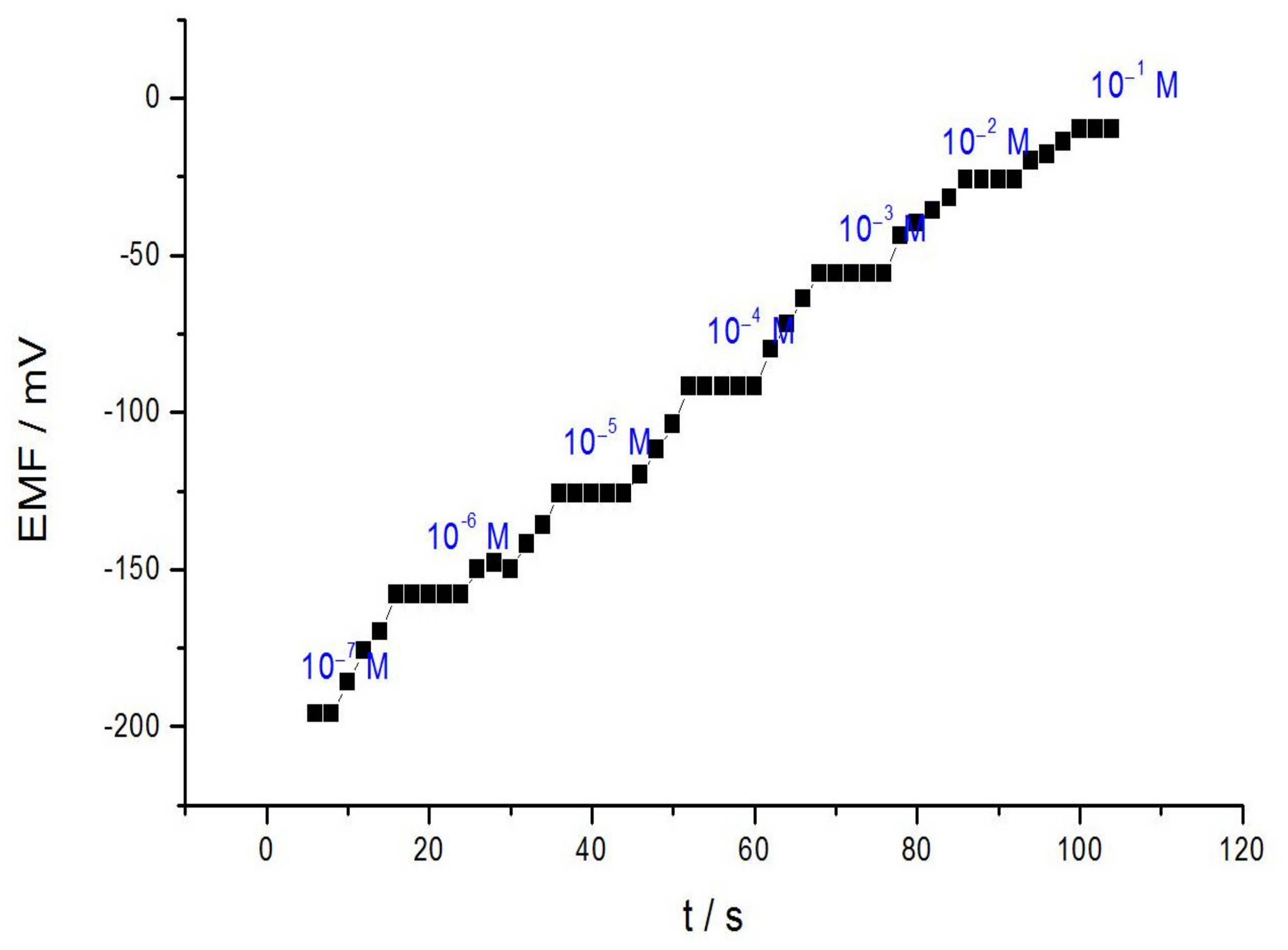
3.3. Dynamic Response Time of the Acrylamide Sensor
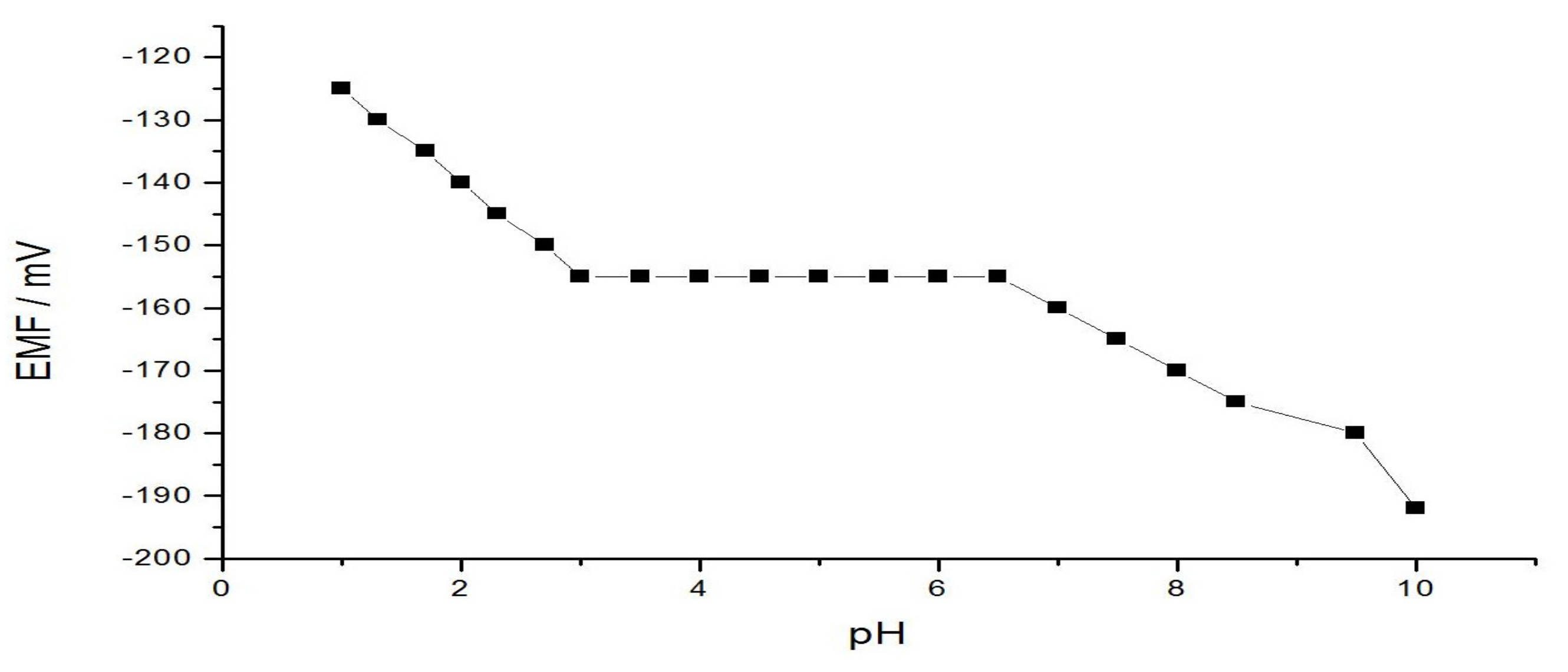
3.4. Influence of pH on the Sensor Potential
3.5. Lifespan of the AA Selective Electrode
3.6. Acrylamide Estimation in Food Product Specimen
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pundir, C.S.; Yadav, N.; Chhillar, A.K. Occurrence, synthesis, toxicity and detection methods for acrylamide determination in processed foods with special reference to biosensors. Trends Food Sci. Technol. 2019, 85, 211–225. [Google Scholar] [CrossRef]
- Omar, M.M.A.; Elbashir, A.A.; Schmitz, O.J. Capillary electrophoresis method with UV-detection for analysis of free amino acids concentrations in food. Food Chem. 2017, 214, 300–307. [Google Scholar] [CrossRef]
- Alpözen, E.; Guven, G.; Özdestan, Ö.; Üren, A. Determination of acrylamide in three different bread types by an in-house validated LC-MS/MS method. Acta Alimentaria 2015, 44, 211–220. [Google Scholar] [CrossRef]
- Beckett, J.M.; Ball, M.J. Effect of hereditary haemochromatosis genotypes and iron overload on other trace elements. Eur. J. Nutr. 2012, 52, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, F.; Zamani, H.A.; Joz-Yarmohammadi, F.; Ebrahimi, M.; Abedi, M.R. Potentiometric studies of a new solid-state contact iron (III)ion selective electrode. Bul. Chem. Commun. 2017, 49, 449–456. [Google Scholar]
- Mecheri, N.; Benounis, M.; Barhoumi, H. New modified selective platinum electrode based on poly (ethylene glycol) for Iron (III) detection in real water. Sens. Rev. 2017, 37, 436–443. [Google Scholar] [CrossRef]
- Takatsuki, S.; Nemoto, S.; Sasaki, K.; Maitani, T. Determination of acrylamide in processed foods by LC/MS using column switching. Shokuhin Eiseigaku Zasshi 2003, 44, 89–95. [Google Scholar] [CrossRef][Green Version]
- Omar, M.M.A.; Ibrahim, W.A.W.; Elbashir, A.A. Sol-gel hybrid methyl trimethoxysilane-tetraethoxysilane as a new dispersive solid-phase extraction material for acrylamide determination in food with direct gas chromatography-mass spectrometry analysis. Food Chem. 2014, 158, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.H.; Zhao, H.B.; Du, M.; Song, L.; Xu, X.B. Dispersive liquid–liquid microextraction for rapid and inexpensive determination of tetramethylpyrazine in vinegar. Food Chem. 2019, 286, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Brady, B.; Koerner, T.B.; Becalski, A.; Zhao, T.; Feng, S. Development of a highly sensitive competitive indirect enzyme-linked immunosorbent assay for detection of acrylamide in foods and water. Food Anal. Chem. 2014, 7, 1298–1304. [Google Scholar] [CrossRef]
- Alahari, A.; Trivelli, X.; Guérardel, Y.; Dover, L.G.; Besra, G.S.; Sacchettini, J.C.; Reynolds, R.C.; Coxon, G.D.; Kremer, L. Thia- cetazone, an antitubercular drug that inhibits cyclopropanation of cell wall mycolic acids in mycobacteria. PLoS ONE 2007, 2, e1343. [Google Scholar] [CrossRef] [PubMed]
- Safavi, A.; Maleki, N.; Farjami, E.; Mahyari, F.A. Simultaneous electrochemical determination of glutathione and glutathione disulfide at a nanoscale copper hydroxide composite carbon ionic liquid electrode. Anal. Chem. 2009, 81, 7538–7543. [Google Scholar] [CrossRef] [PubMed]
- Beitollahi, H.; Maleh, H.K.; Khabazzadeh, H. Nanomolar and selective determination of epinephrine in the presence of norepinephrine using carbon paste electrode modified with carbon nanotubes and novel 2-(4-oxo-3 -phenyl-3,4-dihydro-quinazolinyl)-N’-phenyl-hydrazinecarbothioamide. Anal. Chem. 2008, 80, 9848–9851. [Google Scholar] [CrossRef] [PubMed]
- Beitollahi, H.; Dourandish, Z.; Tajik, S.; Ganjali, M.R.; Norouzi, P.; Faridbod, F. Application of graphite screen printed electrode modified with dysprosium tungstate nanoparticles in voltammetric determination of epinephrine in the presence of acetylcholine. J. Rare Earths 2018, 36, 750–757. [Google Scholar] [CrossRef]
- Ganjali, M.R.; Beitollahi, H.; Zaimbashi, R.; Beitollahi, H.; Rezapour, M.; Larijani, B. Voltammetric determination of dopamine using glassy carbon electrode modified with ZnO/Al2O3 nanocomposite. Int. J. Electrochem. Sci. 2018, 13, 2519–2529. [Google Scholar] [CrossRef]
- Ganjali, M.R.; Dourandish, Z.; Beitollahi, H.; Tajik, S.; Hajiaghababaei, L.; Larijani, B. Highly sensitive determination of theophylline based on graphene quantum dots modified electrode. Int. J. Electrochem. Sci. 2018, 13, 2448–2461. [Google Scholar] [CrossRef]
- Motaghi, M.M.; Beitollahi, H.; Tajik, S.; Rahma, H. Nanostructure electrochemical sensor for voltammetric determination of vitamin C in the presence of vitamin B6: Application to real sample analysis. Int. J. Electrochem. Sci. 2016, 11, 7849–7860. [Google Scholar] [CrossRef]
- Soltani, H.; Beitollahi, H.; Mehrjardi, A.H.; Tajik, S.; Mahani, M.T. Voltammetric determination of glutathione using a modified single walled carbon nanotubes paste electrode. Anal. Bioanal. Electrochem. 2014, 6, 67–79. [Google Scholar]
- Shawish, H.M.A.; Ghalwa, N.A.; Saadeh, S.M.; Aeen, H.H. Development of novel potentiometric sensors for determination of tartrazine dye concentration in foodstuff products. Food Chem. 2012, 138, 126–132. [Google Scholar] [CrossRef]
- Shawish, H.M.A.; Habiby, M.; Aziz, H.S.A.; Saadeh, S.M.; Thaz, A. Determination of trihexyphenidyl hydrochloride drug in tablets and urine using a potententiometric carbon paste electrode. Sens. Actuators B Chem. 2016, 235, 18–26. [Google Scholar] [CrossRef]
- Saadeh, S.M.; Shawish, H.A.; Dalloul, H.M.; El-Halabi, N.M.; Daher, B.K. Lead(II) complexes with some SNO and ONO tridentate Schiff base ligands and their evaluation as lead(II) sensors. Mater. Sci. Eng. C 2012, 32, 619–624. [Google Scholar] [CrossRef]
- Vercelli, B.; Crotti, S.; Agostini, M. Voltammetric responses at modified electrodes and aggregation effects of two anticancer molecules. New J. Chem. 2020, 44, 18233–18241. [Google Scholar] [CrossRef]
- Shetti, N.B.; Mishra, A.; Basu, S.; Mascarenhas, R.J.; Kakarla, R.R.; Aminabhavi, T.M. Skin-Patchable electrodes for biosensor applications. ACS Biomater. Sci. Eng. 2020, 6, 1823–1835. [Google Scholar] [CrossRef]
- Bakker, E.; Buhlmann, P.; Pretsch, E. Carrier-based ion-selective electrodes and bulk optodes. Chem. Rev. 1997, 97, 3083–3132. [Google Scholar] [CrossRef]
- Baghbamidi, S.E.; Beitollahi, H.; Tajik, S.; Zadeh, R.H. Voltammetric sensor based on 1-benzyl-4-ferrocenyl-1H-[1,2,3]-triazole/carbon nanotube modified glassy carbon electrode; detection of hydrochlorothiazide in the presence of propranolol. Int. J. Electrochem. Sci. 2016, 11, 10874–10883. [Google Scholar] [CrossRef]
- Beitollahi, H.; Tajik, S. Construction of a nanostructure-based electrochemical sensor for voltammetric determination of bisphenol A. Environ. Monit. Assess. 2015, 187, 257–265. [Google Scholar] [CrossRef]
- Gupta, V.K.; Maleh, H.K.; Sedegh, R. Simultaneous determination of hydroxyl amine, phenol and sulfite in water and waste water samples using a voltammetric nano sensor. Int. J. Electrochem. Sci. 2015, 10, 303–316. [Google Scholar]
- Sekar, K.; Gupta, V.K.; Ramasamy, B.; Titus, A. A new approach for the degradation of high concentration of aromatic amine by hetero catalytic Fenton oxidation: Kinetic and spectroscopic studies. J. Mol. Liq. 2012, 173, 153–163. [Google Scholar]
- Gupta, V.K.; Margu, N.; Kumawat, L.K.; Singh, A.K. Selective naked-eye detection of magnesium (II) ions using a coumerin-derived fluorescent probe. Sens. Actuators B Chem. 2015, 207, 216–223. [Google Scholar] [CrossRef]
- Goyal, R.N.; Gupta, V.K.; Sangal, A.; Bachheti, N. Voltammetric determination of uric acid at a fullerence-C60-modified glassy carbon electrode. Electroanalysis 2005, 17, 2217–2223. [Google Scholar] [CrossRef]
- Gupta, V.K.; Margu, N.; Kumawat, L.K.; Singh, A.K. A reverseible fluorescence “off-on-off” sensor for sequential detection of aluminum and acetate/fluoride ions. Talanta 2015, 144, 80–89. [Google Scholar] [CrossRef]
- Gupta, V.K.; Singh, A.K.; Kumawat, L.K. Thiazole Schiff base turn-on fluorescent chemosensor for Al3+ ion. Sens. Actuators B Chem. 2014, 195, 98–108. [Google Scholar] [CrossRef]
- Xia, L.; Lu, G.M.; Yuan, L.W.; Xing, B.S.; Yan, L.; Yang, Y. Electrochemical sensor based on imprinted Sol-Gel polymer on Au NPs-MWCNTs-CS modified electrode for the determination of acrylamide. Food Anal. Methods 2016, 9, 114–121. [Google Scholar]
- Batra, B.; Lata, S.; Pundir, C.S. Construction of an improved amperometric acrylamide biosensor based on hemoglobin immobilized onto carboxylated multi-walled carbon nanotubes/iron oxide nanoparticles/chitosan composite film. Bioprocess Biosyst. Eng. 2013, 36, 1591–1599. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.; Chhillar, A.K.; Pundir, C.S. Characterization and application of haemoglobin nanoparticles for detection of acrylamide in processed foods. Int. J. Biol. Macromol. 2018, 107, 1000–1013. [Google Scholar] [CrossRef]
- Fuentes, F.J.G.; Manriquez, J.M.; Godínez, L.A.; Escarpa, A.; Mendoza, S. Electrochemical analysis of acrylamide using screen-printed carboxylated single-walled carbon nanotube electrodes. Electroanalysis 2014, 26, 1039–1044. [Google Scholar] [CrossRef]
- Huang, S.; Lu, S.; Huang, C.; Sheng, J.; Zhang, L.; Su, W. An electrochemical biosensor based on single-stranded DNA modified gold electrode for acrylamide determination. Sens. Actuators B Chem. 2016, 224, 22–30. [Google Scholar] [CrossRef]
- Garabagiu, S.; Mihailescu, J. Simple hemoglobin–gold nanoparticles modified electrode for the amperometric detection of acrylamide. J. Electroanal. Chem. 2011, 659, 196–200. [Google Scholar] [CrossRef]
- Batra, B.; Lata, S.; Sharma, S.; Pundir, C.S. An acrylamide biosensor based on immobilization of hemoglobin onto multi-walled carbon nanotube/copper nanoparticles/polyaniline hybrid film. Anal. Biochem. 2013, 433, 210–217. [Google Scholar] [CrossRef]
- Li, N.; Liu, X.; Zhu, J.; Zhou, B.; Jing, J.; Wang, A.; Xu, R.; Wen, Z.; Shi, X.; Guo, S. Simple and sensitive detection of acrylamide based on hemoglobin immobilization in carbon ionic liquid paste electrode. Food Control 2020, 109. [Google Scholar] [CrossRef]
- Gupta, V.K.; Singh, L.P.; Sing, B. A novel copper (II) selective sensor based on dimethyl(o-phenylene) bis(3-thioalloophanate) in PVC matrix. J. Mol. Liq. 2012, 174, 11–16. [Google Scholar] [CrossRef]
- Gupta, V.K.; Kumar, S.; Singh, L.P.; Shoora, S.; Sethi, B.K. Cadmium (II) ion sensing through p-tert-butyl calix[6] arene based potentiometric sensor. J. Mol. Liq. 2014, 195, 65–68. [Google Scholar] [CrossRef]
- Yola, M.L.; Gupta, V.K.; Eren, T.; Sen, A.E.; Atar, N. A novel electroanalytical nano sensor based on graphene oxide/silver nanoparticles for simultaneous determination of quercetin and morin. Electrochimica Acta 2014, 120, 204–211. [Google Scholar] [CrossRef]
- Alil, I.; Gupta, V.K.; Khan, T.A.; Asim, M. Removal of arsenate from aqueous solution by electro-coagulation method using Al-Fe electrodes. Int. J. Electrochem. Sci. 2012, 7, 1898–1907. [Google Scholar]
- Ahmed, F.A. Spectrophotometric determination of cobalt in biological and environmental samples using 2, 6-pyridinedicarboxaldehyde thiosemicarbazone. Int. J. Anal. Pharm. Bio. Sci. 2013, 2, 6–12. [Google Scholar]
- Basha, V.S.; Chowdary, P.G.; Renuka, M. Non-Extractive Spectrophotometric Determination of Cobalt (II) using 2-Acetylthiophene Isonico tinoylhydrazone in Environmental and Pharmaceutical Samples. J. Pharma. Drug Res. 2019, 2, 102–109. [Google Scholar]
- Sulaiman, S.T.; Hamoudi, T.A. Spectrophotometric Determination of Cobalt(II) with Mordant Blue 9-Application to Vitamin B12 (Injections and Powder). Raf. J. Sci. 2018, 27, 93–100. [Google Scholar]
- Kuliyev, K.A.; Verdizadeh, N.A.; Suleymanova, G.S. Spectrophotometric Determination of Cobalt (II) with 2, 6-Dithiolphenol and Its Derivatives in the Presence of Hydrophobic Amines. Am. J. Chem. 2016, 6, 95–101. [Google Scholar]
- Makino, T.; Sainto, M.; Horiguchi, D.; Kina, K.; Wilairat, P. A highly sensitive colorimetric determination of serium zinc using water-soluble pyridylazo dye. Clin. Chim. Acta 1982, 120, 127–133. [Google Scholar] [PubMed]
- Pengyan, L.; Zhang, L.; Liu, L. Determination of acrylamide using high performance liquid chromatographic method. Chem. J. Internet 2008, 10, 9–16. [Google Scholar]
- Khalil, S.; Harthi, S.S.A. Ion-selective Membrane Sensor for Magnesium Determination in Pharmaceutical Formulations. Int. J. Electrochem. Sci. 2020, 15, 9223–9231. [Google Scholar] [CrossRef]
- Gadzekpo, V.Y.P.; Christian, G.D. Determination of selectivity coefficients of ion-selective electrodes. Anal. Chim. Acta 1984, 164, 279–282. [Google Scholar] [CrossRef]
- Panggabean, A.S. Preparation and characterization ion selective electrode Cd(ii) based on chitosan in PVC membrane. Indones. J. Chem. 2011, 11, 285–289. [Google Scholar] [CrossRef]
- Khalil, M.M.; Issa, Y.M.; Zayed, S.I.M.; Mohamed, N.A. Improved determination of pinaverium bromide in pharmaceutical preparations and human urine utilizing a coated wire electrode. Int. J. Adv. Res. 2015, 6, 638–652. [Google Scholar]

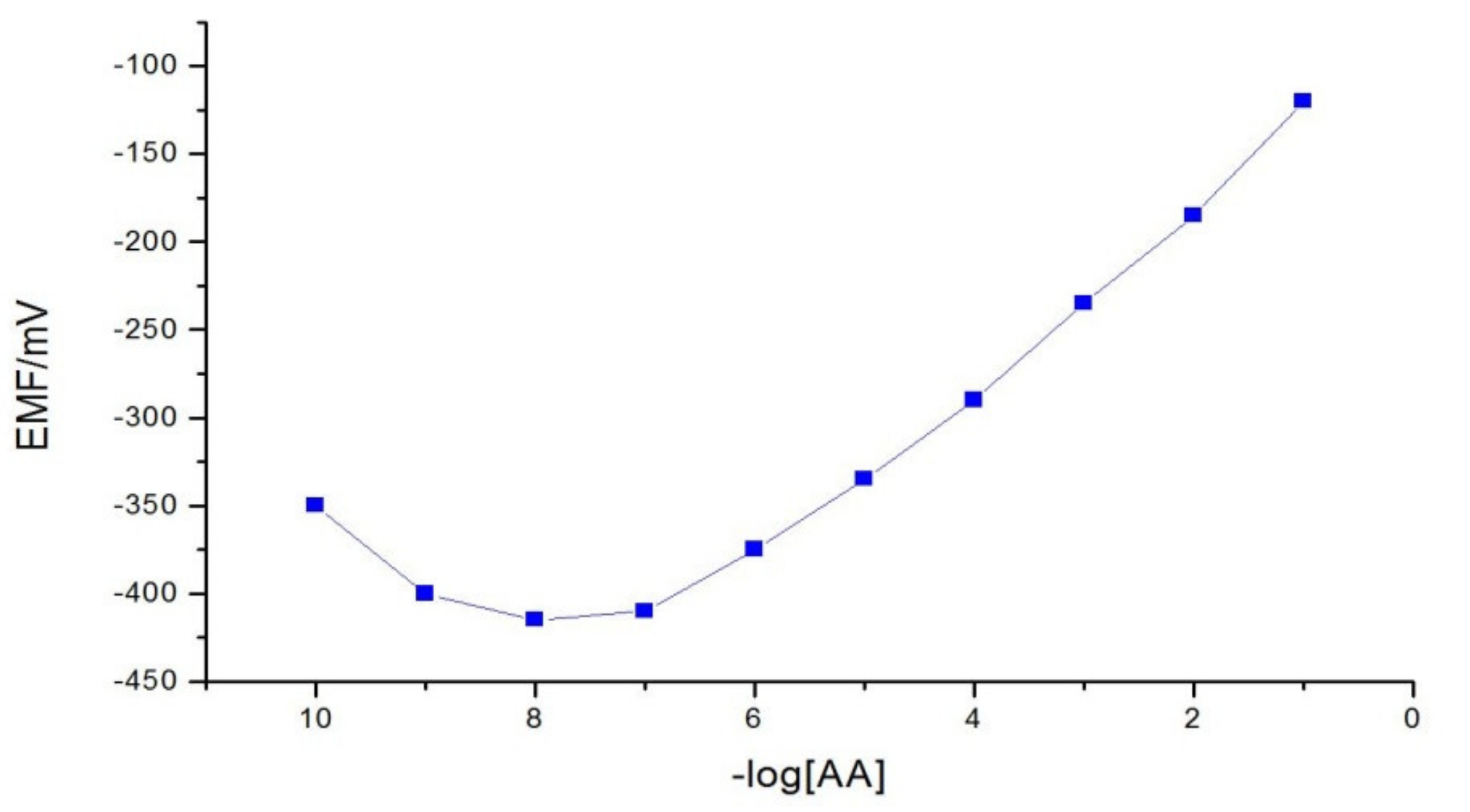
| Specific Slope, mV/decade | 52.33 |
| Intercept/mV | −46.40 ± 0.30 |
| Limit of detection/mol dm−3 | 1.60 × 10−8 |
| Linearity range/mol dm−3 | 1.0 × 10−7–1.0 × 10−1 |
| Response time/s | 8 |
| Lifetime/d | 120 |
| pH working range | 3.0–6.5 |
| K | Ei = Ej | ai = aj | MPM |
|---|---|---|---|
| Ca2+ | 0.211 ± 0.0031 | 0.272 ± 0.020 | 0.226 ± 0.0130 |
| Mg2+ | 0.234 ± 0.0011 | 0.211 ± 0.002 | 0.215 ± 0.0310 |
| Cu2+ | 0.274 ± 0.0032 | 0.268 ± 0.041 | 0.265 ± 0.0023 |
| L-Alanine | ----- | ----- | 0.046 ± 0.0031 |
| Arginine | ----- | ----- | 0.022 ± 0.0013 |
| L-Cystine | ----- | ----- | 0.024 ± 0.0023 |
| Glucose | ----- | ----- | 0.123 ± 0.0012 |
| D-Fructose | ----- | ----- | 0.156 ± 0.0041 |
| Glycin | ------ | ------ | 0.135 + 0.0032 |
| Foodstuff Sample | AA Added mg Kg−1 | HPLC Technique | Calibration Curve Method | Standard Addition Method | ||||
|---|---|---|---|---|---|---|---|---|
| Recovery ± SD a | AA Found mg Kg−1 | Relative Error % | Recovery ± SD a | AA Found mg Kg−1 | Relative Error % | Recovery ± SD a | ||
| Roast Coffee (dry) | 200 | 99.55 ± 0.25 | 199.25 | 0.75 | 99.63 ± 0.26 | 195.15 | 4.85 | 97.57 ± 0.35 |
| Instant(soluble) coffee | 350 | 99.65 ± 0.16 | 349.48 | 0.52 | 99.85 ± 0.32 | 346.03 | 3.97 | 98.86 ± 0.65 |
| Potato chips | 375 | 99.75 ± 0.23 | 374.45 | 0.55 | 99.85 ± 0.35 | 371.06 | 3.94 | 98.94 ± 0.54 |
| Crisp bread | 225 | 99.66 ± 0.34 | 224.55 | 0.45 | 99.80 ± 0.56 | 221.02 | 3.98 | 98.23 ± 0.72 |
| French fries | 120 | 99.45 ± 0.36 | 119.12 | 0.82 | 99.26 ± 0.45 | 116.15 | 3.85 | 96.79 ± 0.85 |
| Toast | 150 | 99.72 ± 0.55 | 149.54 | 0.46 | 99.69 ± 0.35 | 146.14 | 3.86 | 97.42 ± 0.66 |
| Cearls based foods | 175 | 99.80 ± 0.45 | 174.35 | 0.65 | 99.62 ± 0.31 | 171.25 | 3.75 | 97.85 ± 0.89 |
| Ref. | Linear Range (M) | Response Time (s) | Longlife (Day) | Detection Limit (M) | pH Range |
|---|---|---|---|---|---|
| This work data | 1.0 × 10−7–1.0 × 10−1 | 8 | 120 | 1.6 × 10−8 | 3.0–6.5 |
| 33 | 7.0 × 10−7–7.0 × 10−5 | ------ | 9 | 3.9 × 10−5 | 7.4 |
| 34 | 5.0 × 10−4–7.0 × 10−4 | <2 | 100 | 2.0 × 10−3 | 5.5 |
| 35 | 1.0 × 10−5–1.0 × 10−4 | <2 | 120 | 1.0 × 10−5 | 5.0 |
| 36 | 1.0 × 10−5–2.0 × 10−4 | 10 | ------ | 3.0 × 10−4 | ------- |
| 37 | 4.0 × 10−5–2.0 × 10−4 | 12 | 60 | 8.0 × 10−9 | 4.5 |
| 38 | 1.0 × 10−8–1.0 × 10−5 | 10 | 60 | 4.0 × 10−8 | --------- |
| 39 | 5.0 × 10−9–7.5 × 10−4 | 20 | ------- | 2.0 × 10−8 | 505 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalil, S.; El-Beltagy, A.; El-Sharnouby, M. Potent Acrylamide Determination in Food Products Using Ion-Selective Electrode Technique. Membranes 2021, 11, 645. https://doi.org/10.3390/membranes11080645
Khalil S, El-Beltagy A, El-Sharnouby M. Potent Acrylamide Determination in Food Products Using Ion-Selective Electrode Technique. Membranes. 2021; 11(8):645. https://doi.org/10.3390/membranes11080645
Chicago/Turabian StyleKhalil, Sabry, Alaa El-Beltagy, and Mohamed El-Sharnouby. 2021. "Potent Acrylamide Determination in Food Products Using Ion-Selective Electrode Technique" Membranes 11, no. 8: 645. https://doi.org/10.3390/membranes11080645
APA StyleKhalil, S., El-Beltagy, A., & El-Sharnouby, M. (2021). Potent Acrylamide Determination in Food Products Using Ion-Selective Electrode Technique. Membranes, 11(8), 645. https://doi.org/10.3390/membranes11080645





