A Review on Polymer Precursors of Carbon Molecular Sieve Membranes for Olefin/Paraffin Separation
Abstract
:1. Introduction
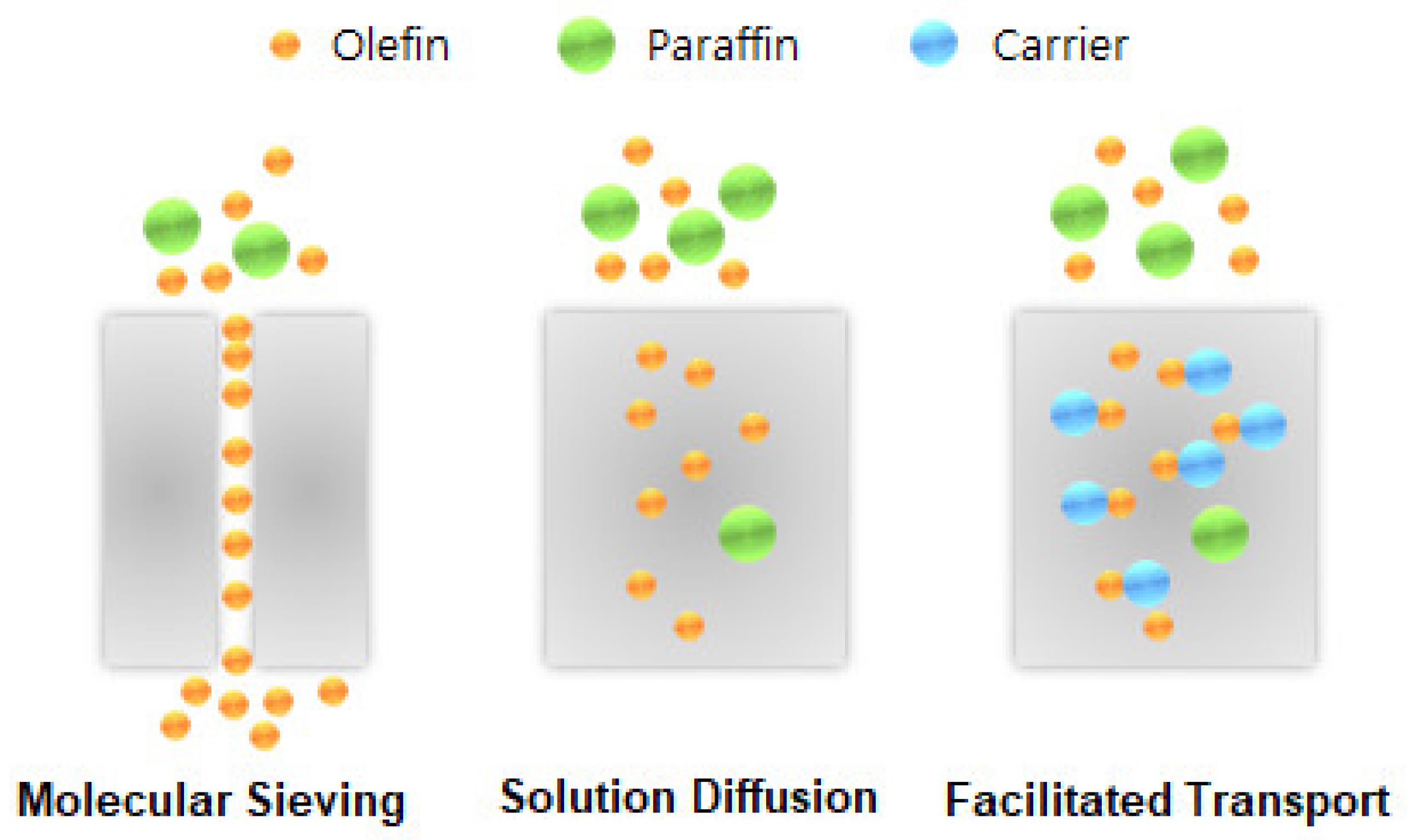
2. Structure and Gas Transport Mechanism of a CMS Membrane for Olefin/Paraffin Separation
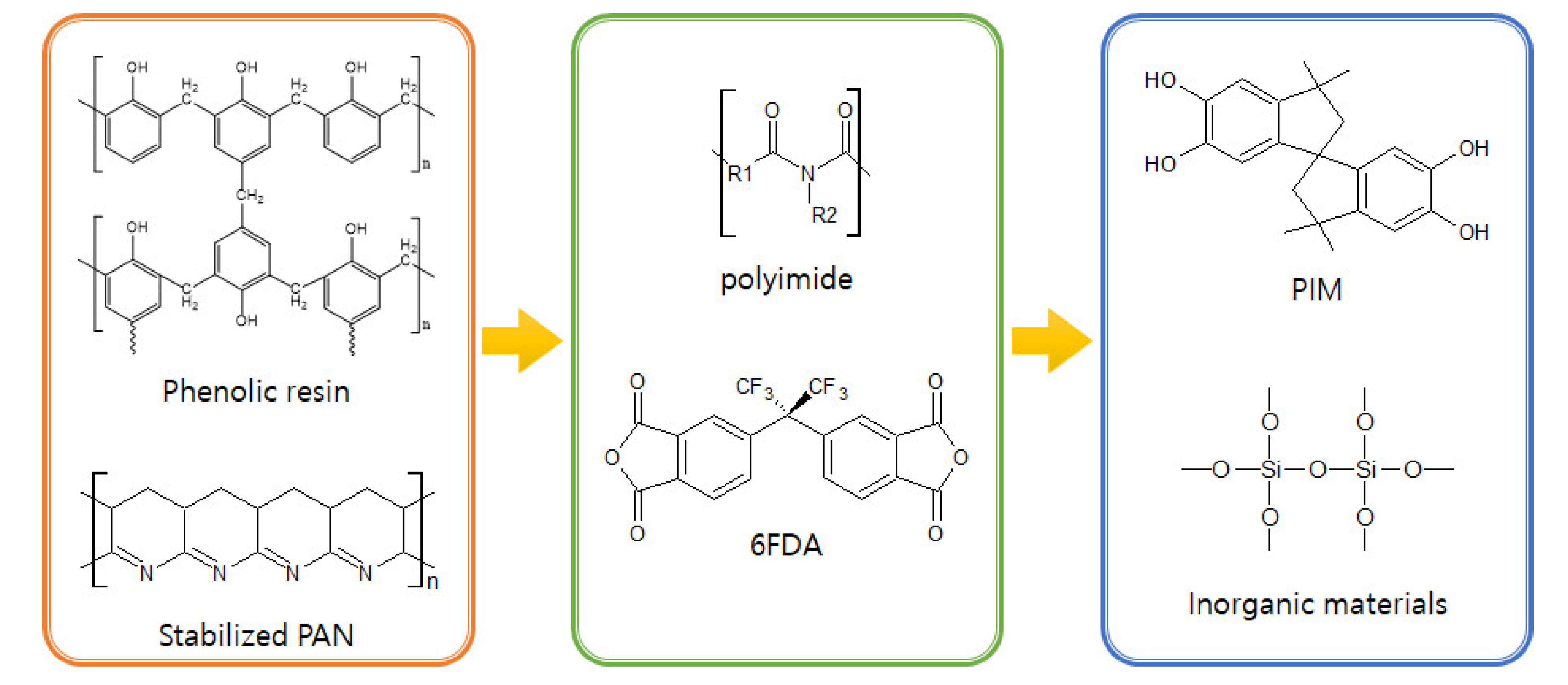
3. Polymer Precursors for CMS Membranes
3.1. Polyimide
3.1.1. Matrimid
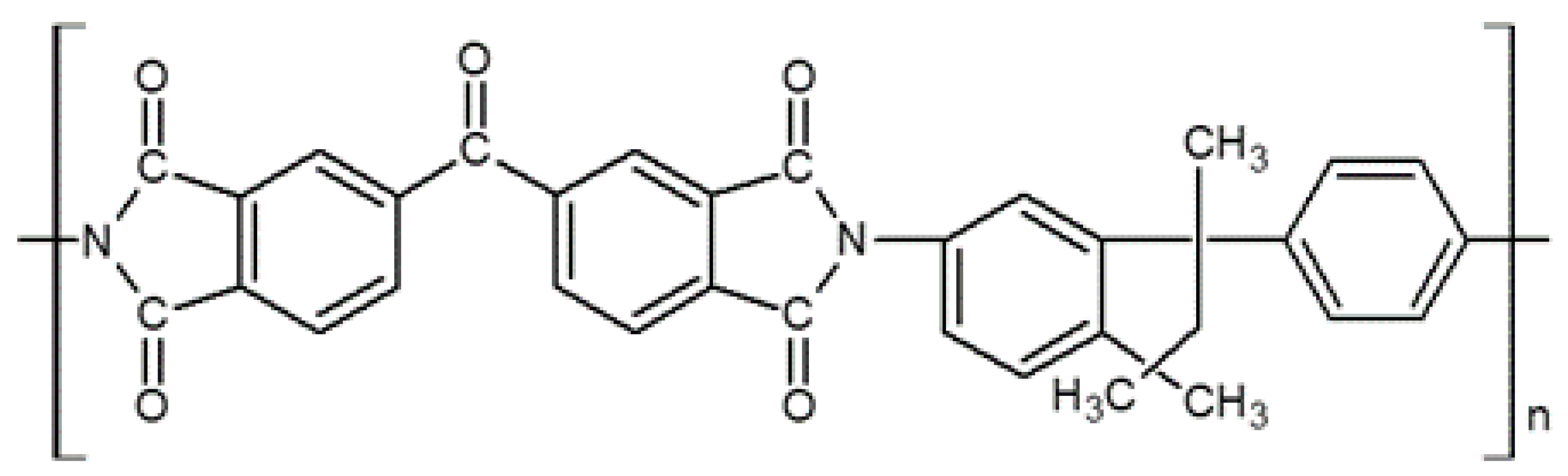
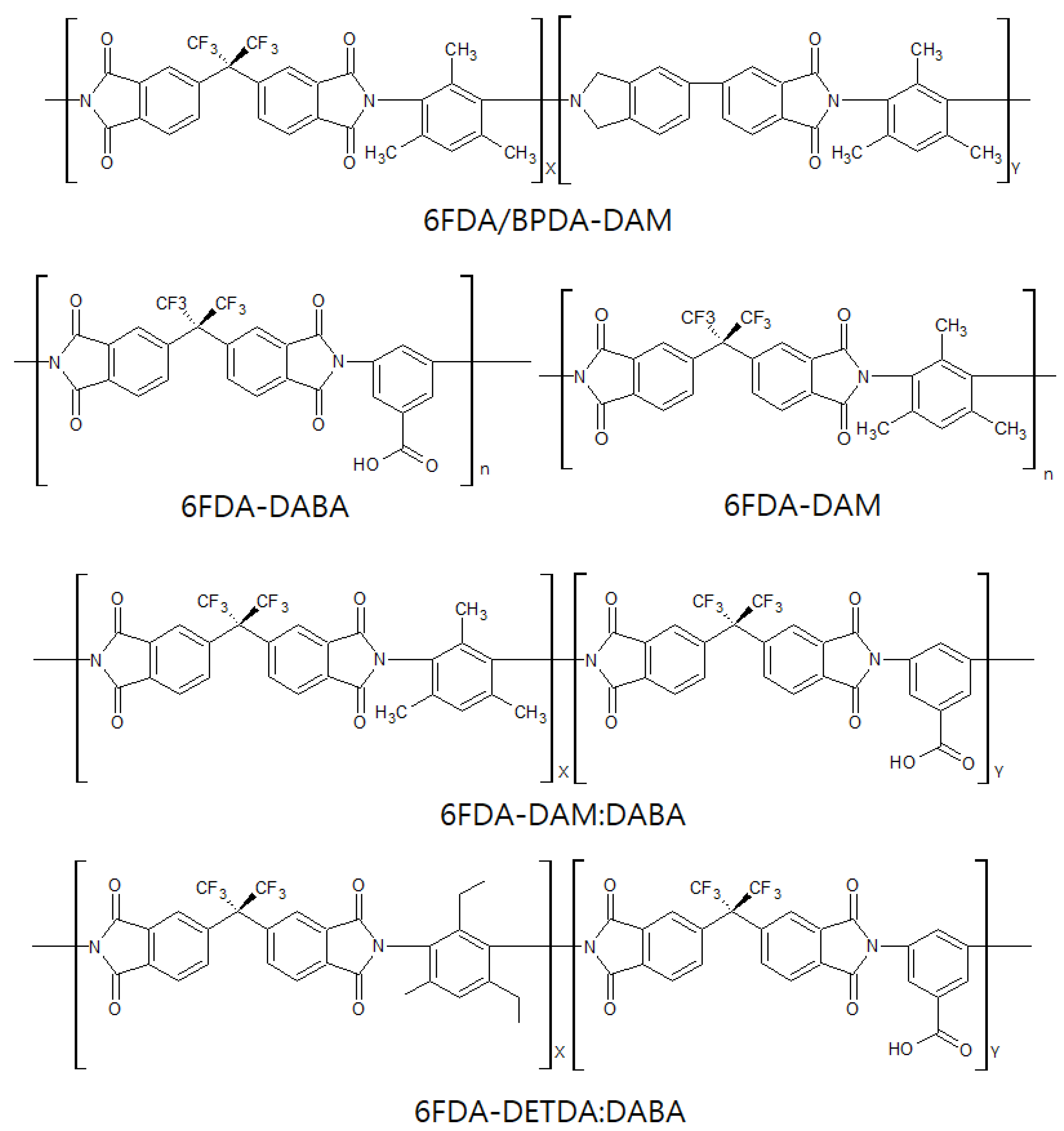
3.1.2. 6FDA-Based Polyimides
3.1.3. Other Polyimides
3.2. Phenolic Resin
3.3. Polymer of Intrinsic Microporosity (PIM)
3.4. Other Polymers
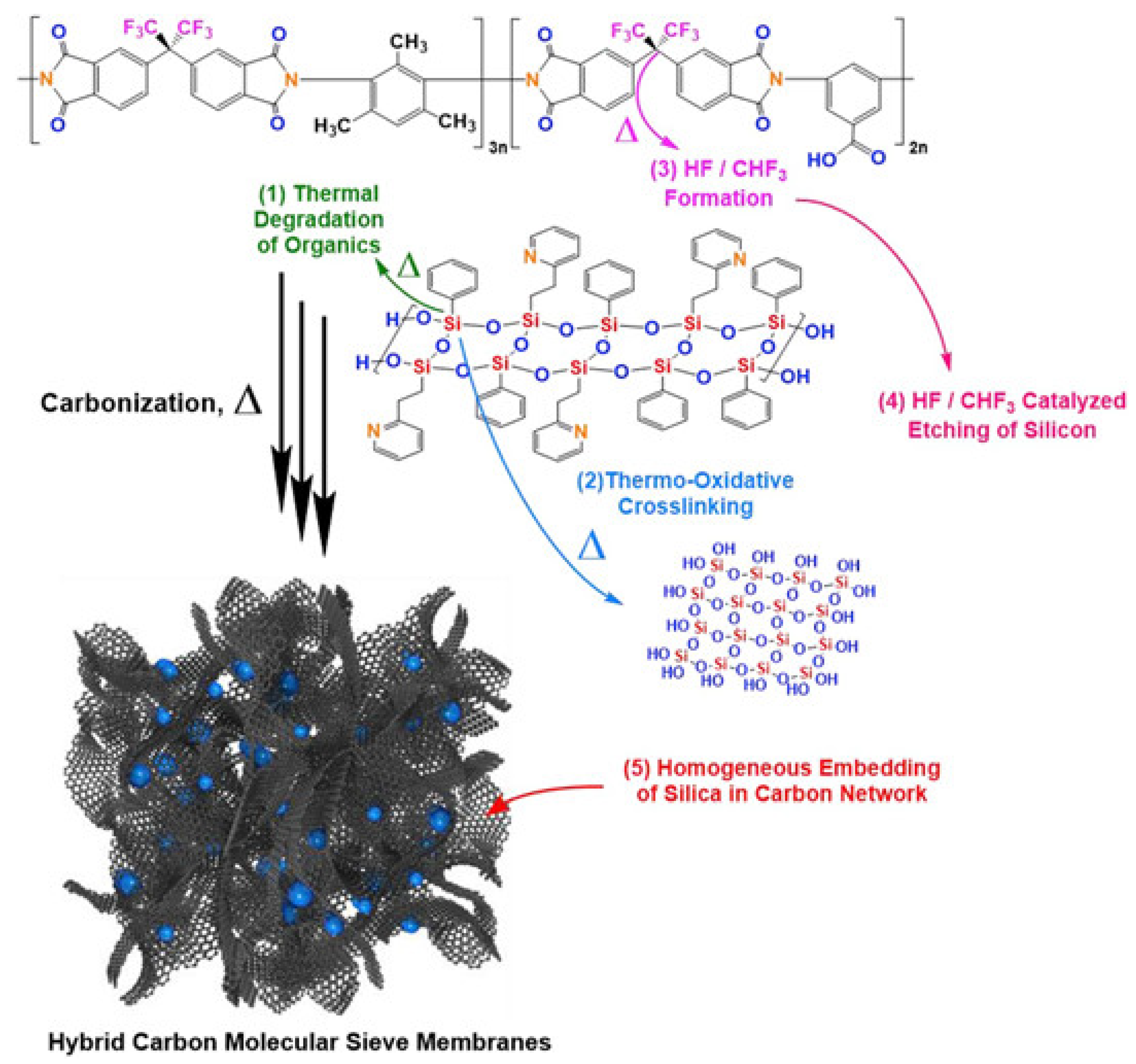
3.5. Inorganic-Containing Polymers
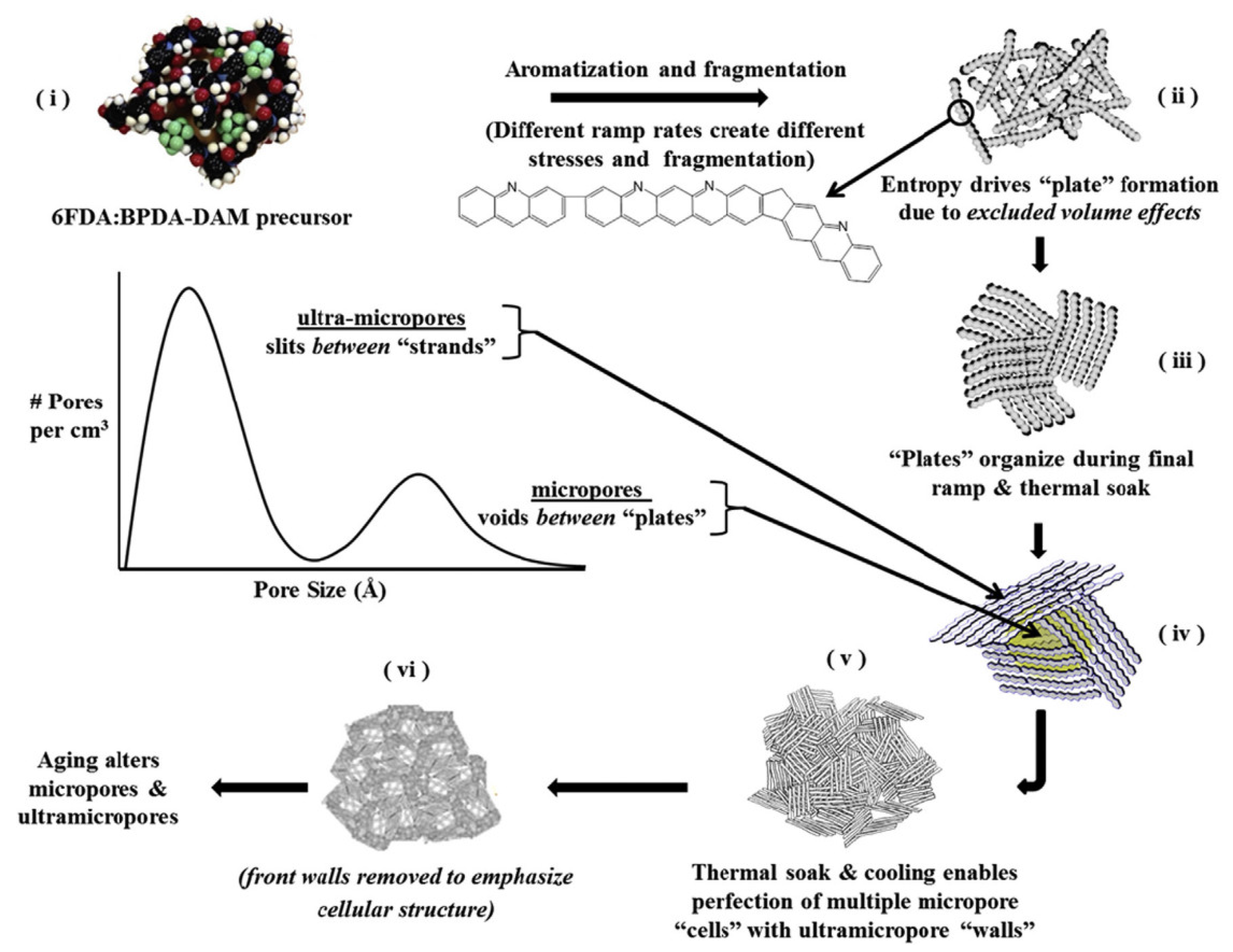
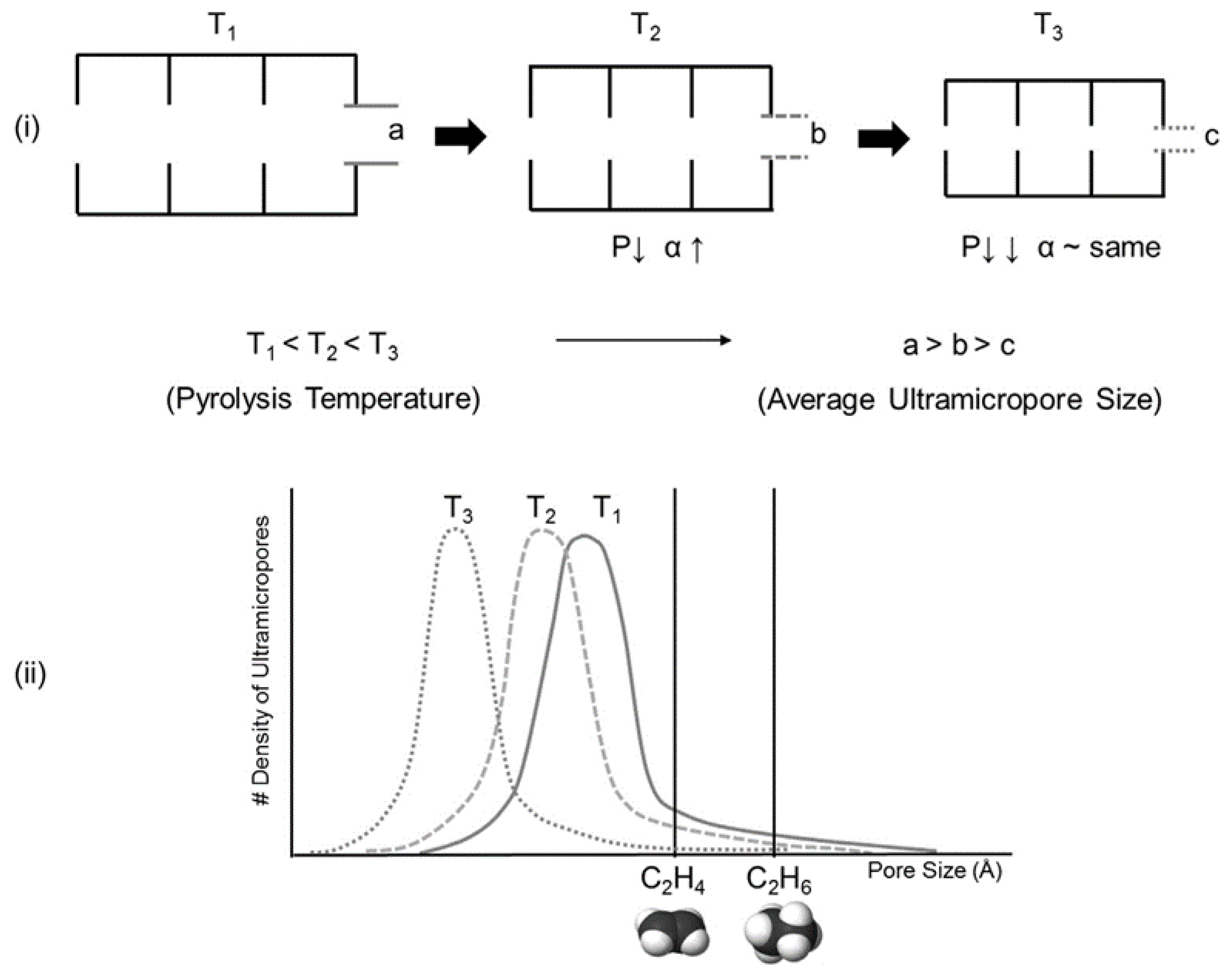
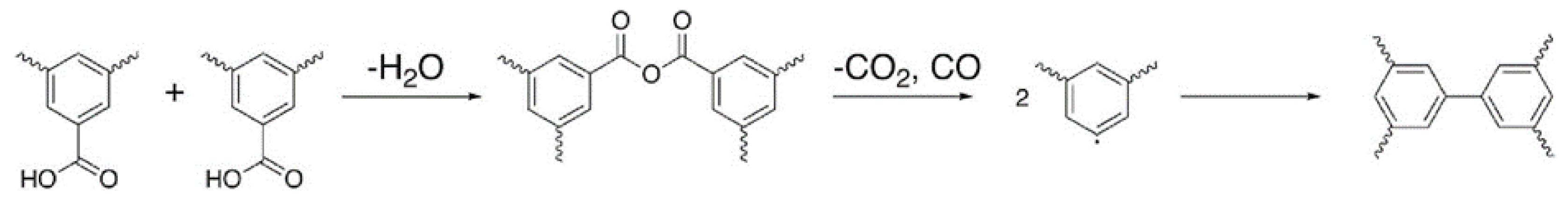
4. Pyrolysis Process
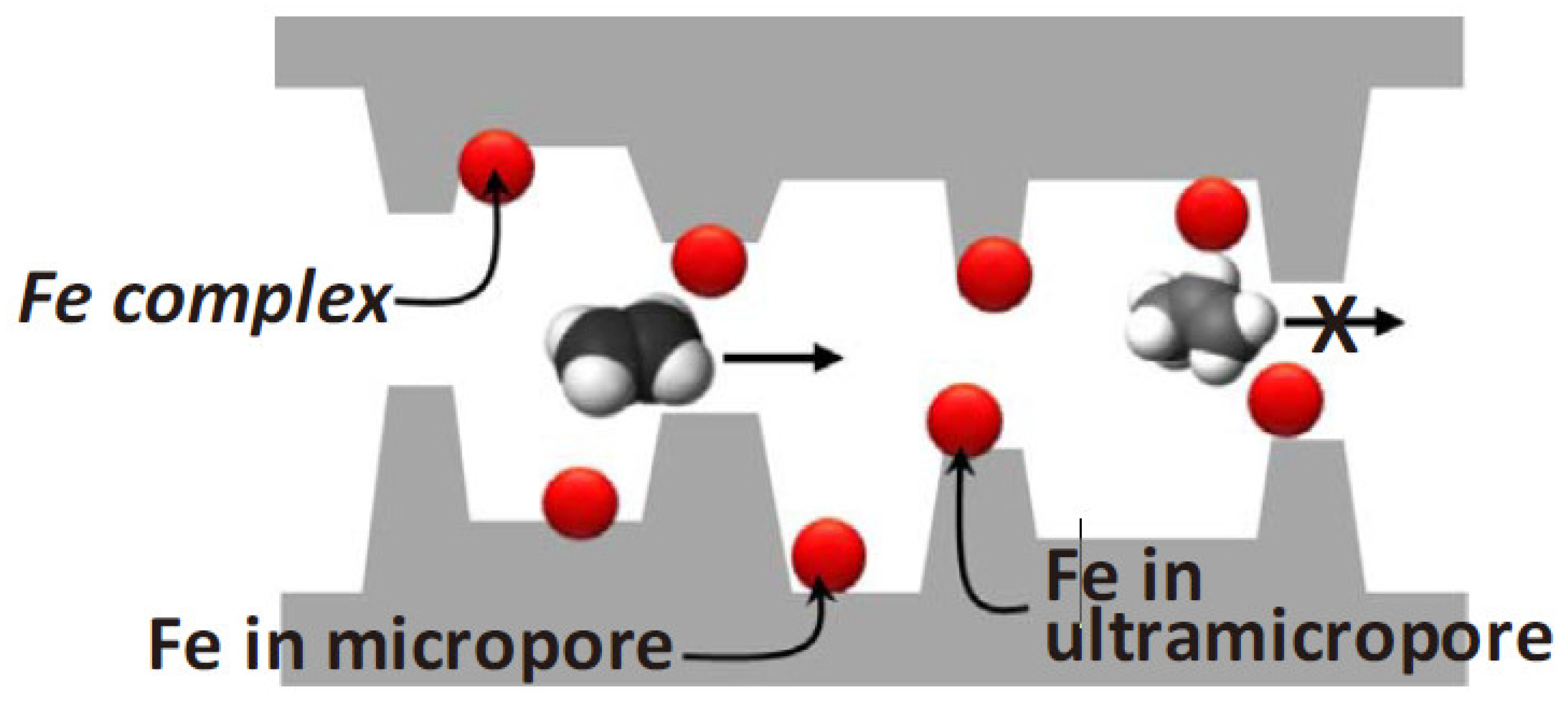
5. Aging and Stability Issues of CMS Membranes
5.1. Mechanical Stability
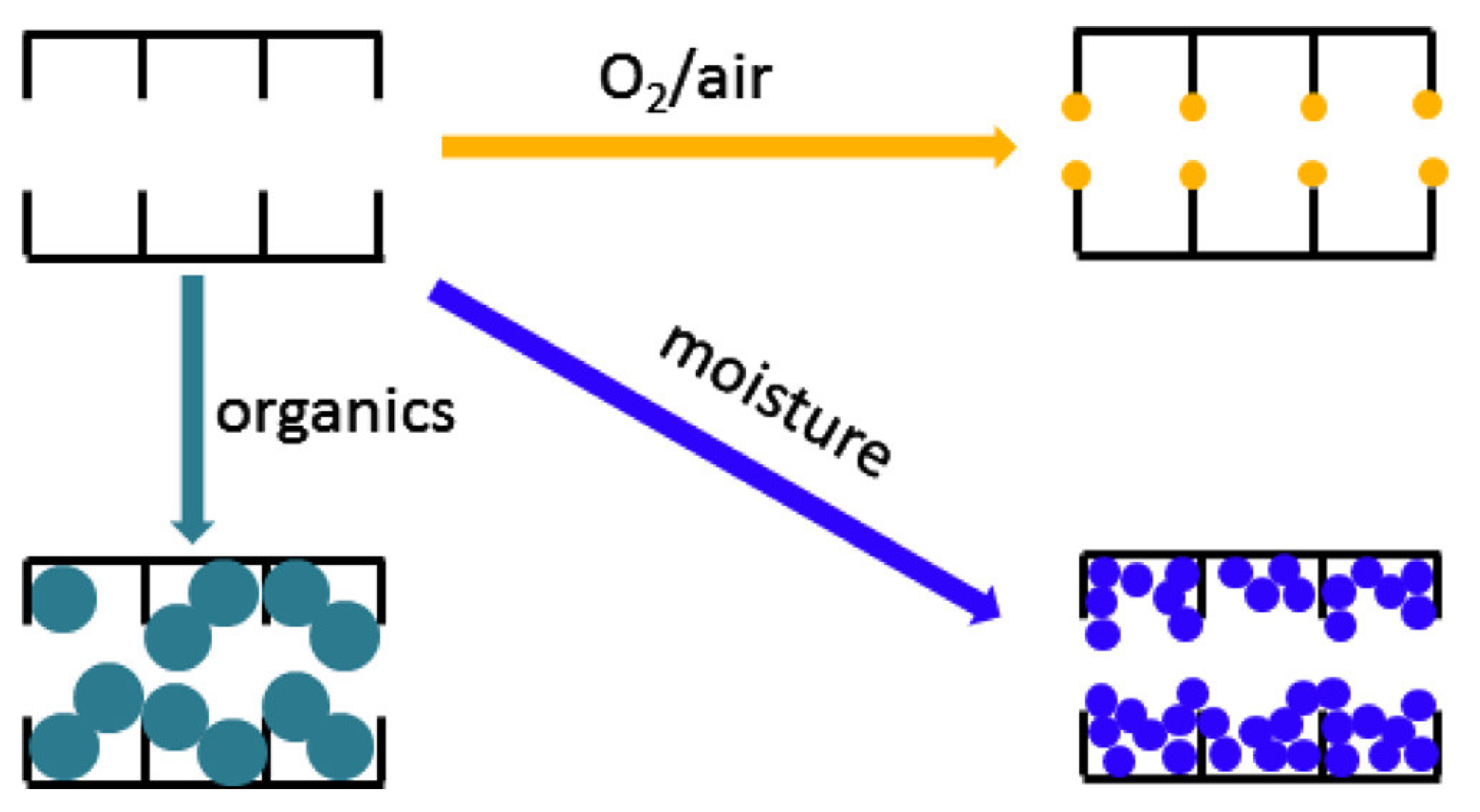
5.2. Chemical Aging
5.3. Physical Aging
6. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, W.-K.; Fevola, M.J.; Liable-Sands, L.M.; Rheingold, A.; Theopold, K. [(Ph)2nacnac]MCl2(THF)2(M = Ti, V, Cr): A New Class of Homogeneous Olefin Polymerization Catalysts Featuring β-Diiminate Ligands. Organometallics 1998, 17, 4541–4543. [Google Scholar] [CrossRef]
- Luo, Z.; Zheng, T.; Li, H.; Zhou, Q.; Wang, A.; Zhang, L.; Hu, Y. A Submicron Spherical Polypropylene Prepared by Heterogeneous Ziegler–Natta Catalyst. Ind. Eng. Chem. Res. 2015, 54, 11247–11250. [Google Scholar] [CrossRef]
- Kaminsky, W. Highly active metallocene catalysts for olefin polymerization. J. Chem. Soc. Dalton Trans. 1998, 1413–1418. [Google Scholar] [CrossRef]
- Jenke, D.R.; Jene, J.M.; Poss, M.; Story, J.; Tsilipetros, T.; Odufu, A.; Terbush, W. Accumulation of extractables in buffer solutions from a polyolefin plastic container. Int. J. Pharm. 2005, 297, 120–133. [Google Scholar] [CrossRef] [PubMed]
- Murugan, D.; Varughese, S.; Swaminathan, T. Recycled polyolefin-based plastic wastes for sound absorption. Polym. Plast. Technol. Eng. 2006, 45, 885–888. [Google Scholar] [CrossRef]
- Zhu, J.; Yuan, S.; Wang, J.; Zhang, Y.; Tian, M.; Van der Bruggen, B. Microporous organic polymer-based membranes for ultrafast molecular separations. Prog. Polym. Sci. 2020, 110, 101308. [Google Scholar] [CrossRef]
- Pellis, A.; Malinconico, M.; Guarneri, A.; Gardossi, L. Renewable polymers and plastics: Performance beyond the green. New Biotechnol. 2021, 60, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Xiang, D.; Yang, S.; Liu, X.; Mai, Z.; Qian, Y. Techno-economic performance of the coal-to-olefins process with CCS. Chem. Eng. J. 2014, 240, 45–54. [Google Scholar] [CrossRef]
- Chen, D.; Moljord, K.; Holmen, A. A methanol to olefins review: Diffusion, coke formation and deactivation on SAPO type catalysts. Microporous Mesoporous Mater. 2012, 164, 239–250. [Google Scholar] [CrossRef]
- Bobrov, V.S.; Digurov, N.G.; Skudin, V.V. Propane dehydrogenation using catalytic membrane. J. Membr. Sci. 2005, 253, 233–242. [Google Scholar] [CrossRef]
- Shi, M.; Lin, C.C.H.; Kuznicki, T.M.; Hashisho, Z.; Kuznicki, S.M. Separation of a binary mixture of ethylene and ethane by adsorption on Na-ETS-10. Chem. Eng. Sci. 2010, 65, 3494–3498. [Google Scholar] [CrossRef]
- Bachman, J.E.; Kapelewski, M.T.; Reed, D.A.; Gonzalez, M.I.; Long, J.R. M2 (m-dobdc)(M = Mn, Fe, Co, Ni) metal–organic frameworks as highly selective, high-capacity adsorbents for olefin/paraffin separations. J. Am. Chem. Soc. 2017, 139, 15363–15370. [Google Scholar] [CrossRef]
- Pinnau, I.; Toy, L.G. Solid polymer electrolyte composite membranes for olefin/paraffin separation. J. Membr. Sci. 2001, 184, 39–48. [Google Scholar] [CrossRef]
- Safarik, D.J.; Eldridge, R.B. Olefin/paraffin separations by reactive absorption: A review. Ind. Eng. Chem. Res. 1998, 37, 2571–2581. [Google Scholar] [CrossRef]
- Kroon, M.C.; Vega, L.F. Selective paraffin removal from ethane/ethylene mixtures by adsorption into aluminum methylphosphonate-α: A molecular simulation study. Langmuir 2009, 25, 2148–2152. [Google Scholar] [CrossRef] [PubMed]
- Amedi, H.R.; Aghajani, M. Economic Estimation of Various Membranes and Distillation for Propylene and Propane Separation. Ind. Eng. Chem. Res. 2018, 57, 4366–4376. [Google Scholar] [CrossRef]
- Kookos, I.K. Optimal design of membrane/distillation column hybrid processes. Ind. Eng. Chem. Res. 2003, 42, 1731–1738. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Y.; Zhou, X.; Xiao, J.; Xia, Q.; Wang, H.; Li, Z. Novel C-PDA adsorbents with high uptake and preferential adsorption of ethane over ethylene. Chem. Eng. Sci. 2016, 155, 338–347. [Google Scholar] [CrossRef]
- Eldridge, R.B. Olefin/paraffin separation technology: A review. Ind. Eng. Chem. Res. 1993, 32, 2208–2212. [Google Scholar] [CrossRef]
- Padin, J.; Rege, S.U.; Yang, R.T.; Cheng, L.S. Molecular sieve sorbents for kinetic separation of propane/propylene. Chem. Eng. Sci. 2000, 55, 4525–4535. [Google Scholar] [CrossRef]
- Jarvelin, H.; Fair, J.R. Adsorptive separation of propylene-propane mixtures. Ind. Eng. Chem. Res. 1993, 32, 2201–2207. [Google Scholar] [CrossRef]
- Martins, V.F.D.; Ribeiro, A.M.; Plaza, M.G.; Santos, J.C.; Loureiro, J.M.; Ferreira, A.F.P.; Rodrigues, A.E. Gas-phase simulated moving bed: Propane/propylene separation on 13X zeolite. J. Chromatogr. A 2015, 1423, 136–148. [Google Scholar] [CrossRef]
- Grande, C.A.; Poplow, F.; Rodrigues, A.E. Vacuum pressure swing adsorption to produce polymer-grade propylene. Sep. Sci. Technol. 2010, 45, 1252–1259. [Google Scholar] [CrossRef]
- Li, J.-R.; Kuppler, R.J.; Zhou, H.-C. Selective gas adsorption and separation in metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef]
- Campos, A.C.C.; Dos Reis, R.A.; Ortiz, A.; Gorri, D.; Ortiz, I. A Perspective of Solutions for Membrane Instabilities in Olefin/Paraffin Separations: A Review. Ind. Eng. Chem. Res. 2018, 57, 10071–10085. [Google Scholar] [CrossRef] [Green Version]
- Al-Muhtaseb, S.A. Role of catalyst type in the selective separation of olefinic and paraffinic hydrocarbons using xerogel-based adsorbents. Carbon 2008, 46, 1003–1009. [Google Scholar] [CrossRef]
- Sholl, D.S.; Lively, R.P. Seven chemical separations to change the world. Nat. News 2016, 532, 435. [Google Scholar] [CrossRef]
- Faiz, R.; Li, K. Olefin/paraffin separation using membrane based facilitated transport/chemical absorption techniques. Chem. Eng. Sci. 2012, 73, 261–284. [Google Scholar] [CrossRef]
- Faiz, R.; Li, K. Polymeric membranes for light olefin/paraffin separation. Desalination 2012, 287, 82–97. [Google Scholar] [CrossRef]
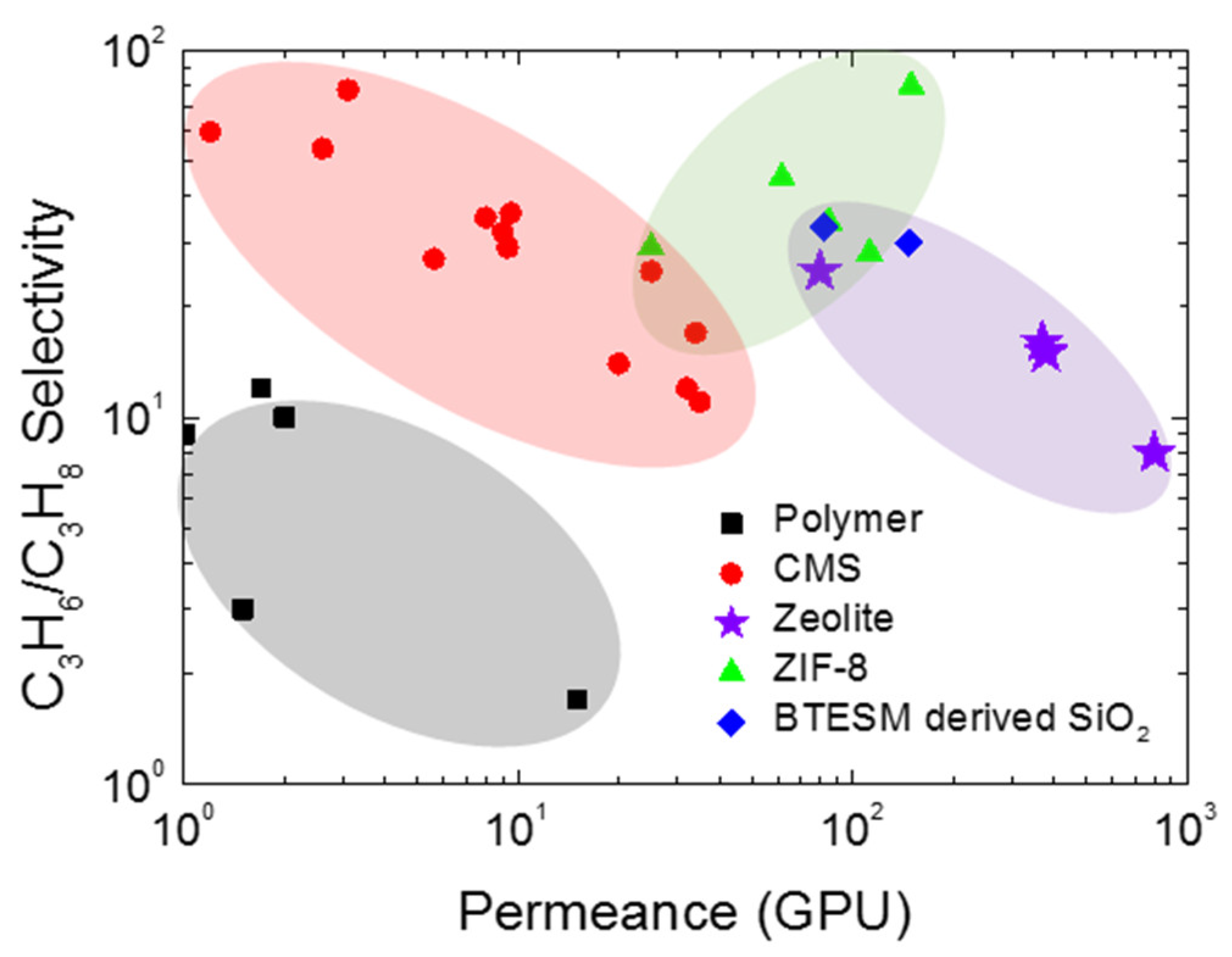
- Burns, R.L.; Koros, W.J. Defining the challenges for C3H6/C3H8 separation using polymeric membranes. J. Membr. Sci. 2003, 211, 299–309. [Google Scholar] [CrossRef]
- Pitsch, F.; Krull, F.F.; Agel, F.; Schulz, P.; Wasserscheid, P.; Melin, T.; Wessling, M. An Adaptive Self-Healing Ionic Liquid Nanocomposite Membrane for Olefin-Paraffin Separations. Adv. Mater. 2012, 24, 4306–4310. [Google Scholar] [CrossRef] [PubMed]
- Faiz, R.; Fallanza, M.; Ortiz, I.; Li, K. Separation of Olefin/Paraffin Gas Mixtures Using Ceramic Hollow Fiber Membrane Contactors. Ind. Eng. Chem. Res. 2013, 52, 7918–7929. [Google Scholar] [CrossRef]
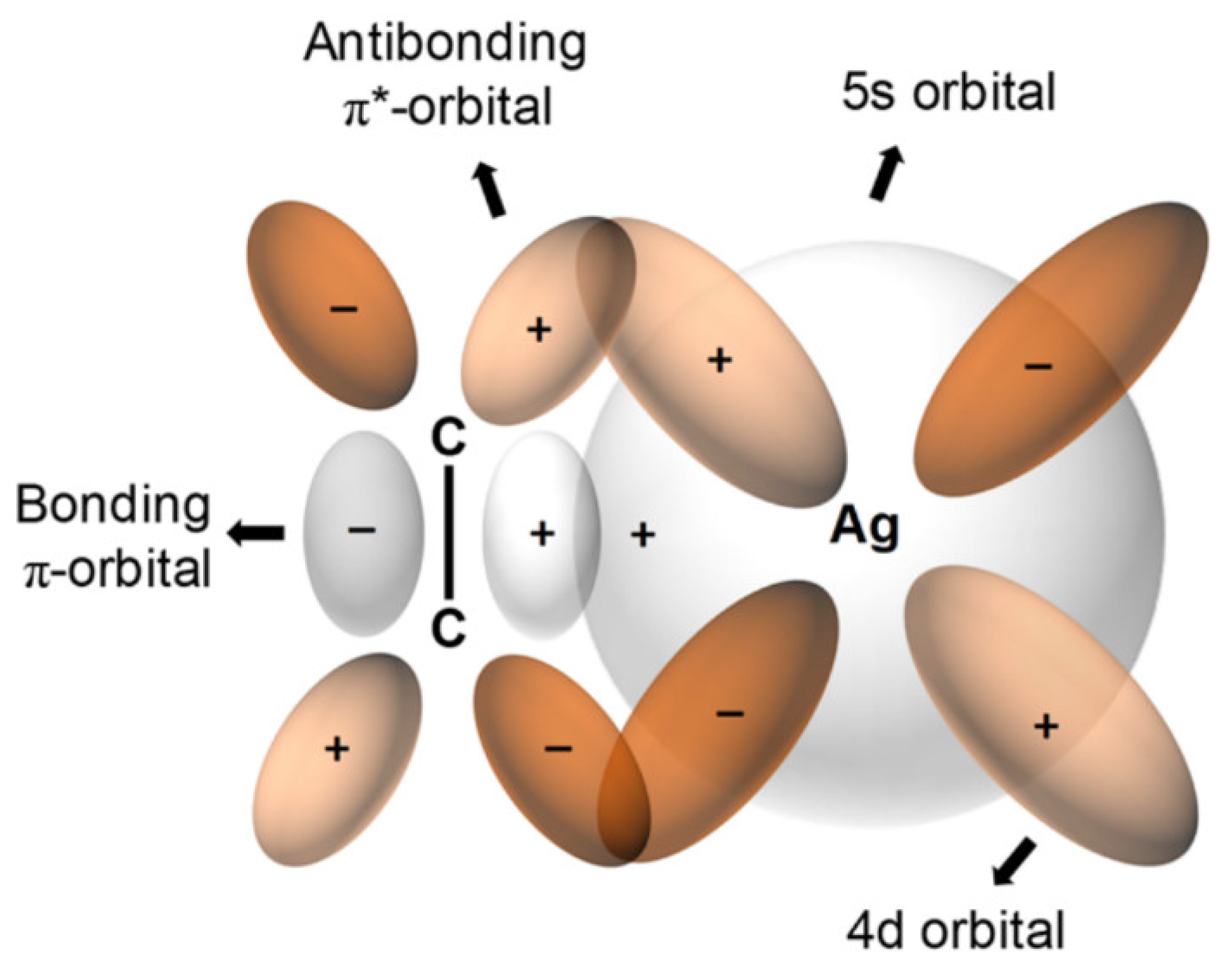
- Kang, S.W.; Char, K.; Kang, Y.S. Novel application of partially positively charged silver nanoparticles for facilitated transport in olefin/paraffin separation membranes. Chem. Mater. 2008, 20, 1308–1311. [Google Scholar] [CrossRef]
- Ho, W.S.; Dalrymple, D.C. Facilitated transport of olefins in Ag+-containing polymer membranes. J. Membr. Sci. 1994, 91, 13–25. [Google Scholar] [CrossRef]
- Huang, J.-F.; Luo, H.; Liang, C.; Jiang, D.-E.; Dai, S. Advanced liquid membranes based on novel ionic liquids for selective separation of olefin/paraffin via olefin-facilitated transport. Ind. Eng. Chem. Res. 2008, 47, 881–888. [Google Scholar] [CrossRef]
- Matulevicius, E.S.; Li, N.N. Facilitated transport through liquid membranes. Sep. Purif. Methods 1975, 4, 73–96. [Google Scholar] [CrossRef]
- De Miranda, D.M.V.; Dutra, L.D.S.; Way, D.; Amaral, N.; Wegenast, F.; Scaldaferri, M.C.; Jesus, N.; Pinto, J.C. A Bibliometric Survey of Paraffin/Olefin Separation Using Membranes. Membranes 2019, 9, 157. [Google Scholar] [CrossRef] [Green Version]
- Yoshino, M.; Nakamura, S.; Kita, H.; Okamoto, K.-I.; Tanihara, N.; Kusuki, Y. Olefin/paraffin separation performance of carbonized membranes derived from an asymmetric hollow fiber membrane of 6FDA/BPDA–DDBT copolyimide. J. Membr. Sci. 2003, 215, 169–183. [Google Scholar] [CrossRef]
- Islam, M.N.; Zhou, W.; Honda, T.; Tanaka, K.; Kita, H.; Okamoto, K.-I. Preparation and gas separation performance of flexible pyrolytic membranes by low-temperature pyrolysis of sulfonated polyimides. J. Membr. Sci. 2005, 261, 17–26. [Google Scholar] [CrossRef]
- Staudt-Bickel, C.; Koros, W.J. Olefin/paraffin gas separations with 6FDA-based polyimide membranes. J. Membr. Sci. 2000, 170, 205–214. [Google Scholar] [CrossRef]
- Das, M.; Koros, W.J. Performance of 6FDA–6FpDA polyimide for propylene/propane separations. J. Membr. Sci. 2010, 365, 399–408. [Google Scholar] [CrossRef]
- Ma, X.; Lin, B.K.; Wei, X.; Kniep, J.; Lin, Y.S. Gamma-Alumina Supported Carbon Molecular Sieve Membrane for Propylene/Propane Separation. Ind. Eng. Chem. Res. 2013, 52, 4297–4305. [Google Scholar] [CrossRef]
- Krol, J.J.; Boerrigter, M.; Koops, G.H. Polyimide hollow fiber gas separation membranes: Preparation and the suppression of plasticization in propane/propylene environments. J. Membr. Sci. 2001, 184, 275–286. [Google Scholar] [CrossRef]
- Bereciartua, P.J.; Cantín, Á.; Corma, A.; Jordá, J.L.; Palomino, M.; Rey, F.; Valencia, S.; Corcoran, E.W.; Kortunov, P.; Ravikovitch, P.I. Control of zeolite framework flexibility and pore topology for separation of ethane and ethylene. Science 2017, 358, 1068–1071. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Li, S.; Bai, Y.; Shao, L. Ultra-facile aqueous synthesis of nanoporous zeolitic imidazolate framework membranes for hydrogen purification and olefin/paraffin separation. J. Mater. Chem. A 2019, 7, 10898–10904. [Google Scholar] [CrossRef]
- Sakai, M.; Sasaki, Y.; Tomono, T.; Seshimo, M.; Matsukata, M. Olefin Selective Ag-Exchanged X-Type Zeolite Membrane for Propylene/Propane and Ethylene/Ethane Separation. ACS Appl. Mater. Interfaces 2019, 11, 4145–4151. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Zhang, X.; Li, L. Synthesis of zeolitic imidazolate framework-69 for adsorption separation of ethane and ethylene. J. Solid State Chem. 2017, 251, 198–203. [Google Scholar] [CrossRef]
- Zhou, M.; Korelskiy, D.; Ye, P.; Grahn, M.; Hedlund, J. A uniformly oriented MFI membrane for improved CO2 separation. Angew. Chem. Int. Ed. 2014, 53, 3492–3495. [Google Scholar] [CrossRef]
- Wang, C.; Liu, X.; Cui, R.; Zhang, B. In situ evaluation of defect size distribution for supported zeolite membranes. J. Membr. Sci. 2009, 330, 259–266. [Google Scholar] [CrossRef]
- Van Hoof, V.; Dotremont, C.; Buekenhoudt, A. Performance of Mitsui NaA type zeolite membranes for the dehydration of organic solvents in comparison with commercial polymeric pervaporation membranes. Sep. Purif. Technol. 2006, 48, 304–309. [Google Scholar] [CrossRef]
- Byun, S.C.; Jeong, Y.J.; Park, J.W.; Kim, S.D.; Ha, H.Y.; Kim, W.J. Effect of solvent and crystal size on the selectivity of ZSM-5/Nafion composite membranes fabricated by solution-casting method. Solid State Ion. 2006, 177, 3233–3243. [Google Scholar] [CrossRef]
- Cacho-Bailo, F.; Seoane, B.; Téllez, C.; Coronas, J. ZIF-8 continuous membrane on porous polysulfone for hydrogen separation. J. Membr. Sci. 2014, 464, 119–126. [Google Scholar] [CrossRef]
- Xu, G.; Yao, J.; Wang, K.; He, L.; Webley, P.A.; Chen, C.-S.; Wang, H. Preparation of ZIF-8 membranes supported on ceramic hollow fibers from a concentrated synthesis gel. J. Membr. Sci. 2011, 385, 187–193. [Google Scholar] [CrossRef]
- Li, L.; Yao, J.; Chen, R.; He, L.; Wang, K.; Wang, H. Infiltration of precursors into a porous alumina support for ZIF-8 membrane synthesis. Microporous Mesoporous Mater. 2013, 168, 15–18. [Google Scholar] [CrossRef]
- Nagano, T.; Fujisaki, S.; Sato, K.; Hataya, K.; Iwamoto, Y.; Nomura, M.; Nakao, S. Relationship between the Mesoporous Intermediate Layer Structure and the Gas Permeation Property of an Amorphous Silica Membrane Synthesized by Counter Diffusion Chemical Vapor Deposition. J. Am. Ceram. Soc. 2007, 91, 71–76. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Dinizdacosta, J. Hydrogen gas mixture separation by CVD silica membrane. J. Membr. Sci. 2008, 323, 144–147. [Google Scholar] [CrossRef]
- Kanezashi, M.; Fuchigami, D.; Yoshioka, T.; Tsuru, T. Control of Pd dispersion in sol–gel-derived amorphous silica membranes for hydrogen separation at high temperatures. J. Membr. Sci. 2013, 439, 78–86. [Google Scholar] [CrossRef]
- Kanezashi, M.; Sasaki, T.; Tawarayama, H.; Yoshioka, T.; Tsuru, T. Hydrogen Permeation Properties and Hydrothermal Stability of Sol-Gel-Derived Amorphous Silica Membranes Fabricated at High Temperatures. J. Am. Ceram. Soc. 2013, 96, 2950–2957. [Google Scholar] [CrossRef]
- Okamoto, K.-I.; Kawamura, S.; Yoshino, M.; Kita, H.; Hirayama, Y.; Tanihara, N.; Kusuki, Y. Olefin/paraffin separation through carbonized membranes derived from an asymmetric polyimide hollow fiber membrane. Ind. Eng. Chem. Res. 1999, 38, 4424–4432. [Google Scholar] [CrossRef]
- Rungta, M.; Xu, L.; Koros, W.J. Carbon molecular sieve dense film membranes derived from Matrimid® for ethylene/ethane separation. Carbon 2012, 50, 1488–1502. [Google Scholar] [CrossRef]
- Saufi, S.; Ismail, A. Fabrication of carbon membranes for gas separation—A review. Carbon 2004, 42, 241–259. [Google Scholar] [CrossRef]
- Xu, L.; Rungta, M.; Koros, W.J. Matrimid® derived carbon molecular sieve hollow fiber membranes for ethylene/ethane separation. J. Membr. Sci. 2011, 380, 138–147. [Google Scholar] [CrossRef]
- Lei, L.; Pan, F.; Lindbråthen, A.; Zhang, X.; Hillestad, M.; Nie, Y.; Bai, L.; He, X.; Guiver, M.D. Carbon hollow fiber membranes for a molecular sieve with precise-cutoff ultramicropores for superior hydrogen separation. Nat. Commun. 2021, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lagorsse, S. Carbon molecular sieve membranesSorption, kinetic and structural characterization. J. Membr. Sci. 2004, 241, 275–287. [Google Scholar] [CrossRef]
- Lagorsse, S.; Leite, A.; Magalhaes, F.D.; Bischofberger, N.; Rathenow, J.; Mendes, A. Novel carbon molecular sieve honeycomb membrane module: Configuration and membrane characterization. Carbon 2005, 43, 809–819. [Google Scholar] [CrossRef]
- Parsley, D.; Ciora, R.J.; Flowers, D.L.; Laukaitaus, J.; Chen, A.; Liu, P.K.; Yu, J.; Sahimi, M.; Bonsu, A.; Tsotsis, T.T. Field evaluation of carbon molecular sieve membranes for the separation and purification of hydrogen from coal- and biomass-derived syngas. J. Membr. Sci. 2014, 450, 81–92. [Google Scholar] [CrossRef]
- Haider, S.; Lindbråthen, A.; Lie, J.A.; Carstensen, P.V.; Johannessen, T.; Hägg, M.-B. Vehicle fuel from biogas with carbon membranes; a comparison between simulation predictions and actual field demonstration. Green Energy Environ. 2018, 3, 266–276. [Google Scholar] [CrossRef]
- Ismail, A.F.; Khulbe, K.C.; Matsuura, T. Gas Separation Membranes; Springer: Berlin, Germany, 2015. [Google Scholar]
- Hwang, Y.; Park, J.Y.; Lee, C.-S.; Kwon, O.S.; Park, S.-H.; Bae, J. Surface engineered poly(dimethylsiloxane)/carbon nanotube nanocomposite pad as a flexible platform for chemical sensors. Compos. Part A Appl. Sci. Manuf. 2018, 107, 55–60. [Google Scholar] [CrossRef]
- Salleh, W.N.W.; Ismail, A.F.; Matsuura, T.; Abdullah, M.S. Precursor selection and process conditions in the preparation of carbon membrane for gas separation: A review. Sep. Purif. Rev. 2011, 40, 261–311. [Google Scholar] [CrossRef]
- Ash, R.; Barrer, R.M.; Lowson, R.T. Diffusion of helium through a microporous carbon membrane. Surf. Sci. 1970, 21, 265–272. [Google Scholar] [CrossRef]
- Swaidan, R.; Ma, X.; Litwiller, E.; Pinnau, I. High pressure pure-and mixed-gas separation of CO2/CH4 by thermally-rearranged and carbon molecular sieve membranes derived from a polyimide of intrinsic microporosity. J. Membr. Sci. 2013, 447, 387–394. [Google Scholar] [CrossRef]
- Tanaka, K.; Taguchi, A.; Hao, J.; Kita, H.; Okamoto, K. Permeation and separation properties of polyimide membranes to olefins and paraffins. J. Membr. Sci. 1996, 121, 197–207. [Google Scholar] [CrossRef]
- Fuertes, A.B.; Menendez, I. Separation of hydrocarbon gas mixtures using phenolic resin-based carbon membranes. Sep. Purif. Technol. 2002, 28, 29–41. [Google Scholar] [CrossRef]
- Ma, X.; Williams, S.; Wei, X.; Kniep, J.; Lin, Y.S. Propylene/propane mixture separation characteristics and stability of carbon molecular sieve membranes. Ind. Eng. Chem. Res. 2015, 54, 9824–9831. [Google Scholar] [CrossRef]
- Richter, H.; Voss, H.; Kaltenborn, N.; Kämnitz, S.; Wollbrink, A.; Feldhoff, A.; Caro, J.; Roitsch, S.; Voigt, I. High-flux carbon molecular sieve membranes for gas separation. Angew. Chem. Int. Ed. 2017, 56, 7760–7763. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Wenz, G.B.; Sanders, E.S.; Kulkarni, S.S.; Qiu, W.; Ma, C.; Koros, W.J. Effects of pyrolysis conditions on gas separation properties of 6FDA/DETDA: DABA (3: 2) derived carbon molecular sieve membranes. J. Membr. Sci. 2016, 520, 699–711. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.-J.; Liao, K.-S.; Hu, C.-C.; Lee, K.-R.; Lai, J.-Y. Development and characterization of micropores in carbon molecular sieve membrane for gas separation. Microporous Mesoporous Mater. 2011, 143, 78–86. [Google Scholar] [CrossRef]
- Rodrigues, S.C.; Whitley, R.; Mendes, A. Preparation and characterization of carbon molecular sieve membranes based on resorcinol–formaldehyde resin. J. Membr. Sci. 2014, 459, 207–216. [Google Scholar] [CrossRef] [Green Version]
- Ismail, A.F.; David, L.I.B. A review on the latest development of carbon membranes for gas separation. J. Membr. Sci. 2001, 193, 1–18. [Google Scholar] [CrossRef]
- Rungta, M.; Xu, L.; Koros, W.J. Structure–performance characterization for carbon molecular sieve membranes using molecular scale gas probes. Carbon 2015, 85, 429–442. [Google Scholar] [CrossRef]
- Fu, Y.-J.; Hu, C.-C.; Lin, D.-W.; Tsai, H.-A.; Huang, S.-H.; Hung, W.-S.; Lee, K.-R.; Lai, J.-Y. Adjustable microstructure carbon molecular sieve membranes derived from thermally stable polyetherimide/polyimide blends for gas separation. Carbon 2017, 113, 10–17. [Google Scholar] [CrossRef]
- Anderson, C.J.; Pas, S.J.; Arora, G.; Kentish, S.E.; Hill, A.J.; Sandler, S.I.; Stevens, G.W. Effect of pyrolysis temperature and operating temperature on the performance of nanoporous carbon membranes. J. Membr. Sci. 2008, 322, 19–27. [Google Scholar] [CrossRef]
- Rungta, M.; Zhang, C.; Koros, W.J.; Xu, L. Membrane-based ethylene/ethane separation: The upper bound and beyond. AIChE J. 2013, 59, 3475–3489. [Google Scholar] [CrossRef]
- Rungta, M.; Wenz, G.B.; Zhang, C.; Xu, L.; Qiu, W.; Adams, J.S.; Koros, W.J. Carbon molecular sieve structure development and membrane performance relationships. Carbon 2017, 115, 237–248. [Google Scholar] [CrossRef] [Green Version]
- Qin, G.; Cao, X.; Wen, H.; Wei, W.; da Costa, J.C.D. Fine ultra-micropore control using the intrinsic viscosity of precursors for high performance carbon molecular sieve membranes. Sep. Purif. Technol. 2017, 177, 129–134. [Google Scholar] [CrossRef] [Green Version]
- Geng, K.; Arumugam, V.; Xu, H.; Gao, Y.; Jiang, D. Covalent organic frameworks: Polymer chemistry and functional design. Prog. Polym. Sci. 2020, 108, 101288. [Google Scholar] [CrossRef]
- Steel, K.M.; Koros, W.J. An investigation of the effects of pyrolysis parameters on gas separation properties of carbon materials. Carbon 2005, 43, 1843–1856. [Google Scholar] [CrossRef]
- Steel, K.M.; Koros, W.J. Investigation of porosity of carbon materials and related effects on gas separation properties. Carbon 2003, 41, 253–266. [Google Scholar] [CrossRef]
- Xu, L.; Rungta, M.; Brayden, M.K.; Martinez, M.V.; Stears, B.A.; Barbay, G.A.; Koros, W.J. Olefins-selective asymmetric carbon molecular sieve hollow fiber membranes for hybrid membrane-distillation processes for olefin/paraffin separations. J. Membr. Sci. 2012, 423, 314–323. [Google Scholar] [CrossRef]
- Xu, L.; Rungta, M.; Hessler, J.; Qiu, W.; Brayden, M.; Martinez, M.; Barbay, G.; Koros, W.J. Physical aging in carbon molecular sieve membranes. Carbon 2014, 80, 155–166. [Google Scholar] [CrossRef]
- Kim, S.-J.; Lee, P.S.; Chang, J.-S.; Nam, S.-E.; Park, Y.-I. Preparation of carbon molecular sieve membranes on low-cost alumina hollow fibers for use in C3H6/C3H8 separation. Sep. Purif. Technol. 2018, 194, 443–450. [Google Scholar] [CrossRef]
- Karunaweera, C.; Musselman, I.H.; Balkus, K.J.; Ferraris, J.P. Fabrication and characterization of aging resistant carbon molecular sieve membranes for C3 separation using high molecular weight crosslinkable polyimide, 6FDA-DABA. J. Membr. Sci. 2019, 581, 430–438. [Google Scholar] [CrossRef]
- Shin, J.H.; Yu, H.J.; Park, J.; Lee, A.; Hwang, S.S.; Kim, S.-J.; Park, S.; Cho, K.Y.; Won, W.; Lee, J.S. Fluorine-containing polyimide/polysilsesquioxane carbon molecular sieve membranes and techno-economic evaluation thereof for C3H6/C3H8 separation. J. Membr. Sci. 2020, 598, 117660. [Google Scholar] [CrossRef]
- Chu, Y.-H.; Yancey, D.; Xu, L.; Martinez, M.; Brayden, M.; Koros, W. Iron-containing carbon molecular sieve membranes for advanced olefin/paraffin separations. J. Membr. Sci. 2018, 548, 609–620. [Google Scholar] [CrossRef]
- Ma, X.; Lin, Y.S.; Wei, X.; Kniep, J. Ultrathin carbon molecular sieve membrane for propylene/propane separation. AIChE J. 2016, 62, 491–499. [Google Scholar] [CrossRef]
- Suda, H.; Haraya, K. Alkene/alkane permselectivities of a carbon molecular sieve membrane. Chem. Commun. 1997, 93–94. [Google Scholar] [CrossRef]
- Yamamoto, M.; Kusakabe, K.; Hayashi, J.-i.; Morooka, S. Carbon molecular sieve membrane formed by oxidative carbonization of a copolyimide film coated on a porous support tube. J. Membr. Sci. 1997, 133, 195–205. [Google Scholar] [CrossRef]
- Kusakabe, K.; Yamamoto, M.; Morooka, S. Gas permeation and micropore structure of carbon molecular sieving membranes modified by oxidation. J. Membr. Sci. 1998, 149, 59–67. [Google Scholar] [CrossRef]
- Kusuki, Y. Gas permeation properties and characterization of asymmetric carbon membranes prepared by pyrolyzing asymmetric polyimide hollow fiber membrane. J. Membr. Sci. 1997, 134, 245–253. [Google Scholar] [CrossRef]
- Hayashi, J.-I.; Mizuta, H.; Yamamoto, M.; Kusakabe, A.K.; Morooka, S.; Suh, S.-H. Separation of Ethane/Ethylene and Propane/Propylene Systems with a Carbonized BPDA−pp‘ODA Polyimide Membrane. Ind. Eng. Chem. Res. 1996, 35, 4176–4181. [Google Scholar] [CrossRef]
- Fuertes, A.B. Adsorption-selective carbon membrane for gas separation. J. Membr. Sci. 2000, 177, 9–16. [Google Scholar] [CrossRef]
- Menendez, I.; Fuertes, A.B. Aging of carbon membranes under different environments. Carbon 2001, 39, 733–740. [Google Scholar] [CrossRef]
- Teixeira, M.; Rodrigues, S.C.; Campo, M.; Tanaka, D.A.P.; Tanco, M.A.L.; Madeira, L.M.; Sousa, J.M.; Mendes, A. Boehmite-phenolic resin carbon molecular sieve membranes—Permeation and adsorption studies. Chem. Eng. Res. Des. 2014, 92, 2668–2680. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, M.; Campo, M.C.; Tanaka, D.A.P.; Tanco, M.A.L.; Magen, C.; Mendes, A. Composite phenolic resin-based carbon molecular sieve membranes for gas separation. Carbon 2011, 49, 4348–4358. [Google Scholar] [CrossRef]
- Centeno, T.A.; Vilas, J.L.; Fuertes, A.B. Effects of phenolic resin pyrolysis conditions on carbon membrane performance for gas separation. J. Membr. Sci. 2004, 228, 45–54. [Google Scholar] [CrossRef]
- Fuertes, A.B. Preparation and characterization of adsorption-selective carbon membranes for gas separation. Adsorption 2001, 7, 117–129. [Google Scholar] [CrossRef]
- Liao, K.-S.; Japip, S.; Lai, J.-Y.; Chung, N.T.-S. Boron-embedded hydrolyzed PIM-1 carbon membranes for synergistic ethylene/ethane purification. J. Membr. Sci. 2017, 534, 92–99. [Google Scholar] [CrossRef]
- Salinas, O.; Ma, X.; Wang, Y.; Han, Y.; Pinnau, I. Carbon molecular sieve membrane from a microporous spirobisindane-based polyimide precursor with enhanced ethylene/ethane mixed-gas selectivity. RSC Adv. 2017, 7, 3265–3272. [Google Scholar] [CrossRef] [Green Version]
- Salinas, O.; Ma, X.; Litwiller, E.; Pinnau, I. Ethylene/ethane permeation, diffusion and gas sorption properties of carbon molecular sieve membranes derived from the prototype ladder polymer of intrinsic microporosity (PIM-1). J. Membr. Sci. 2016, 504, 133–140. [Google Scholar] [CrossRef] [Green Version]
- Salinas, O.; Ma, X.; Litwiller, E.; Pinnau, I. High-performance carbon molecular sieve membranes for ethylene/ethane separation derived from an intrinsically microporous polyimide. J. Membr. Sci. 2016, 500, 115–123. [Google Scholar] [CrossRef] [Green Version]
- Swaidan, R.J.; Ma, X.; Pinnau, I. Spirobisindane-based polyimide as efficient precursor of thermally-rearranged and carbon molecular sieve membranes for enhanced propylene/propane separation. J. Membr. Sci. 2016, 520, 983–989. [Google Scholar] [CrossRef]
- Chng, M.L.; Xiao, Y.; Chung, N.T.-S.; Toriida, M.; Tamai, S. Enhanced propylene/propane separation by carbonaceous membrane derived from poly (aryl ether ketone)/2,6-bis(4-azidobenzylidene)-4-methyl-cyclohexanone interpenetrating network. Carbon 2009, 47, 1857–1866. [Google Scholar] [CrossRef]
- Park, H.B.; Lee, Y.M. Fabrication and characterization of nanoporous carbon/silica membranes. Adv. Mater. 2005, 17, 477–483. [Google Scholar] [CrossRef]
- Cui, L.; Qiu, W.; Paul, D.R.; Koros, W.J. Physical aging of 6FDA-based polyimide membranes monitored by gas permeability. Polymer 2011, 52, 3374–3380. [Google Scholar] [CrossRef]
- Fu, S.; Sanders, E.S.; Kulkarni, S.; Chu, Y.-H.; Wenz, G.B.; Koros, W.J. The significance of entropic selectivity in carbon molecular sieve membranes derived from 6FDA/DETDA:DABA(3:2) polyimide. J. Membr. Sci. 2017, 539, 329–343. [Google Scholar] [CrossRef]
- Qiu, W.; Chen, C.-C.; Xu, L.; Cui, L.; Paul, D.R.; Koros, W.J. Sub-TgCross-Linking of a Polyimide Membrane for Enhanced CO2Plasticization Resistance for Natural Gas Separation. Macromolecules 2011, 44, 6046–6056. [Google Scholar] [CrossRef]
- Qiu, W.; Zhang, K.; Li, F.S.; Zhang, K.; Koros, W.J. Gas Separation Performance of Carbon Molecular Sieve Membranes Based on 6FDA-mPDA/DABA (3:2) Polyimide. ChemSusChem 2014, 7, 1186–1194. [Google Scholar] [CrossRef]
- Fuertes, A.B.; Nevskaia, D.M.; Centeno, T.A. Carbon composite membranes from Matrimid® and Kapton® polyimides for gas separation. Microporous Mesoporous Mater. 1999, 33, 115–125. [Google Scholar] [CrossRef]
- Lee, W.H.; Bae, J.Y.; Yushkin, A.; Efimov, M.; Jung, J.T.; Volkov, A.; Lee, Y.M. Energy and time efficient infrared (IR) irradiation treatment for preparing thermally rearranged (TR) and carbon molecular sieve (CMS) membranes for gas separation. J. Membr. Sci. 2020, 613, 118477. [Google Scholar] [CrossRef]
- Zhou, W.; Yoshino, M.; Kita, H.; Okamoto, K.-i. Carbon molecular sieve membranes derived from phenolic resin with a pendant sulfonic acid group. Ind. Eng. Chem. Res. 2001, 40, 4801–4807. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, Y.; Chung, T.-S. Flexible thermally treated 3D PIM-CD molecular sieve membranes exceeding the upper bound line for propylene/propane separation. J. Mater. Chem. A 2017, 5, 4583–4595. [Google Scholar] [CrossRef] [Green Version]
- Jones, C.W.; Koros, W.J. Carbon molecular sieve gas separation membranes-I. Preparation and characterization based on polyimide precursors. Carbon 1994, 32, 1419–1425. [Google Scholar] [CrossRef]
- Kim, Y.K.; Lee, J.M.; Park, H.B.; Lee, Y.M. The gas separation properties of carbon molecular sieve membranes derived from polyimides having carboxylic acid groups. J. Membr. Sci. 2004, 235, 139–146. [Google Scholar] [CrossRef]
- Suda, H.; Haraya, K. Gas Permeation through Micropores of Carbon Molecular Sieve Membranes Derived from Kapton Polyimide. J. Phys. Chem. B 1997, 101, 3988–3994. [Google Scholar] [CrossRef]
- Salleh, W.N.W.; Ismail, A.F. Effects of carbonization heating rate on CO2 separation of derived carbon membranes. Sep. Purif. Technol. 2012, 88, 174–183. [Google Scholar] [CrossRef]
- Kiyono, M.; Williams, P.J.; Koros, W.J. Effect of pyrolysis atmosphere on separation performance of carbon molecular sieve membranes. J. Membr. Sci. 2010, 359, 2–10. [Google Scholar] [CrossRef]
- Tanihara, N.; Shimazaki, H.; Hirayama, Y.; Nakanishi, S.; Yoshinaga, T.; Kusuki, Y. Gas permeation properties of asymmetric carbon hollow fiber membranes prepared from asymmetric polyimide hollow fiber. J. Membr. Sci. 1999, 160, 179–186. [Google Scholar] [CrossRef]
- Yao, J.; Yu, W. Tensile strength and its variation for PAN-based carbon fibers. II. Calibration of the variation from testing. J. Appl. Polym. Sci. 2007, 104, 2625–2632. [Google Scholar] [CrossRef]
- Yoshimune, M.; Haraya, K. Flexible carbon hollow fiber membranes derived from sulfonated poly (phenylene oxide). Sep. Purif. Technol. 2010, 75, 193–197. [Google Scholar] [CrossRef]
- Karvan, O.; Johnson, J.R.; Williams, P.J.; Koros, W.J. A pilot-scale system for carbon molecular sieve hollow fiber membrane manufacturing. Chem. Eng. Technol. 2013, 36, 53–61. [Google Scholar] [CrossRef]
- Basu, S.; Khan, A.L.; Cano-Odena, A.; Liu, C.; Vankelecom, I.F.J. Membrane-based technologies for biogas separations. Chem. Soc. Rev. 2010, 39, 750–768. [Google Scholar] [CrossRef] [PubMed]
- Koh, D.-Y.; McCool, B.A.; Deckman, H.W.; Lively, R.P. Reverse osmosis molecular differentiation of organic liquids using carbon molecular sieve membranes. Science 2016, 353, 804–807. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.W.; Koros, W.J. Carbon molecular sieve gas separation membranes-II. Regeneration following organic exposure. Carbon 1994, 32, 1427–1432. [Google Scholar] [CrossRef]
- Wenz, G.B.; Koros, W.J. Tuning carbon molecular sieves for natural gas separations: A diamine molecular approach. AIChE J. 2017, 63, 751–760. [Google Scholar] [CrossRef]
- Shin, J.H.; Yu, H.J.; An, H.; Lee, A.S.; Hwang, S.S.; Lee, S.Y.; Lee, J.S. Rigid double-stranded siloxane-induced high-flux carbon molecular sieve hollow fiber membranes for CO2/CH4 separation. J. Membr. Sci. 2019, 570, 504–512. [Google Scholar] [CrossRef]

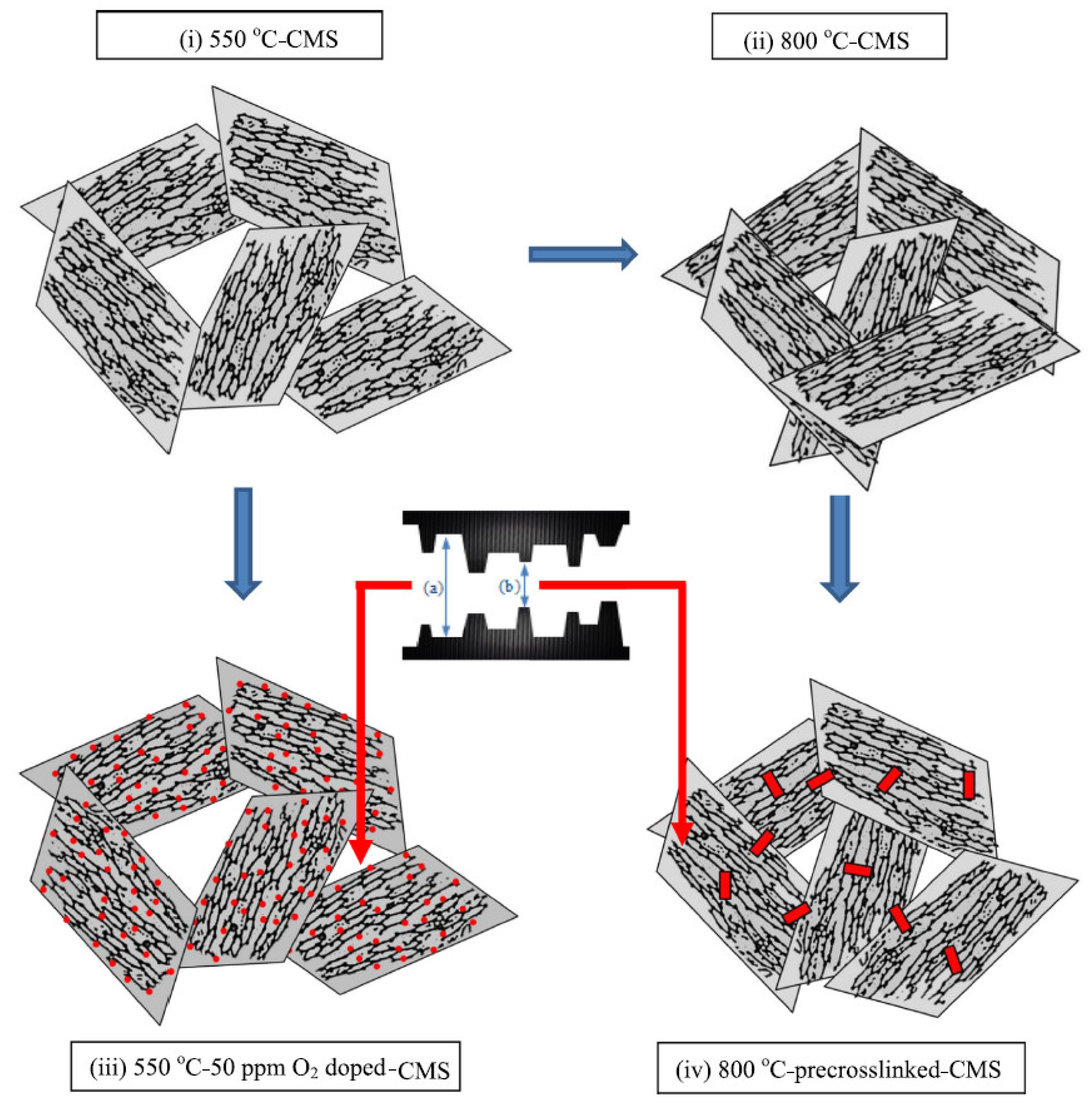

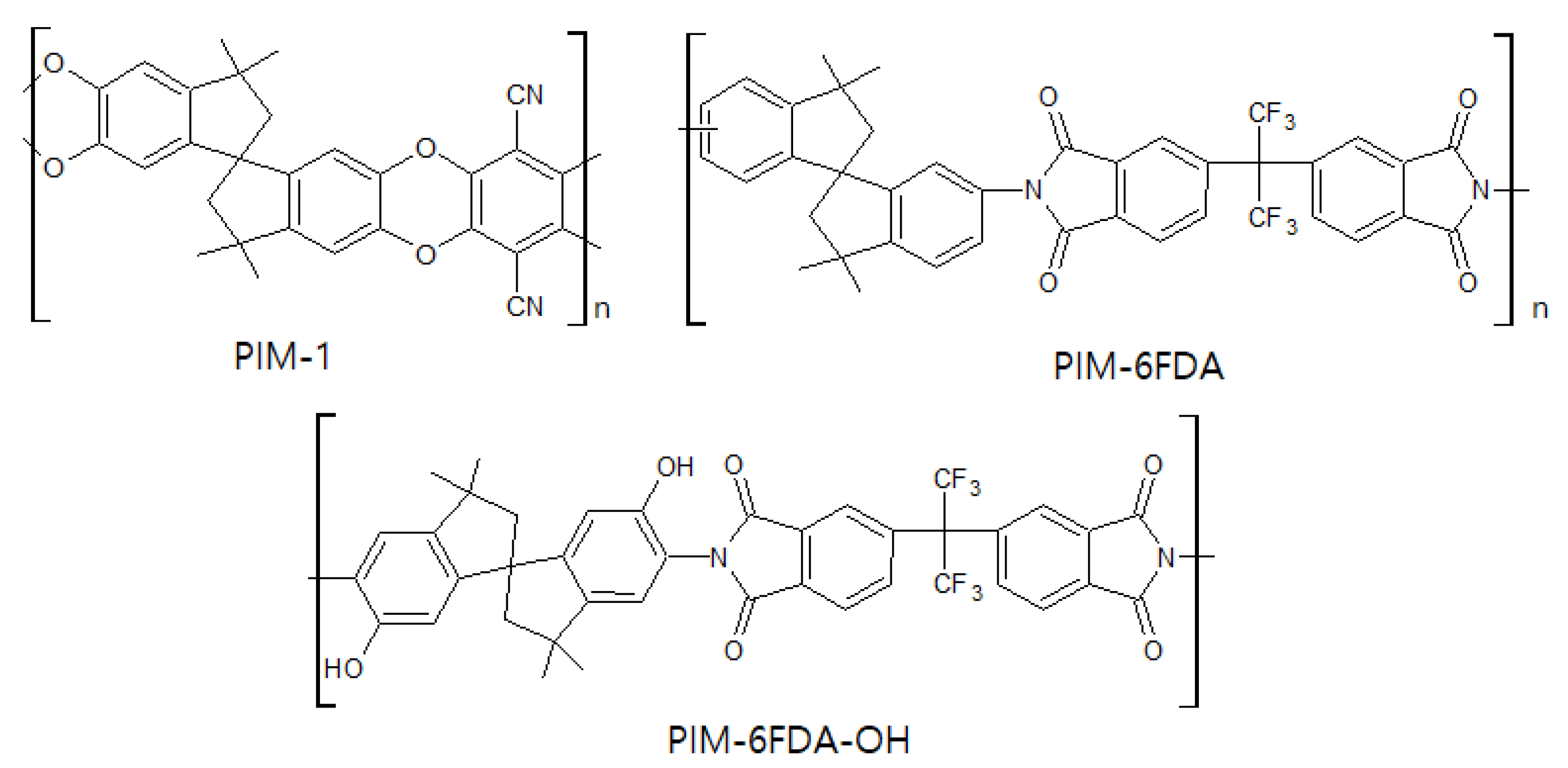
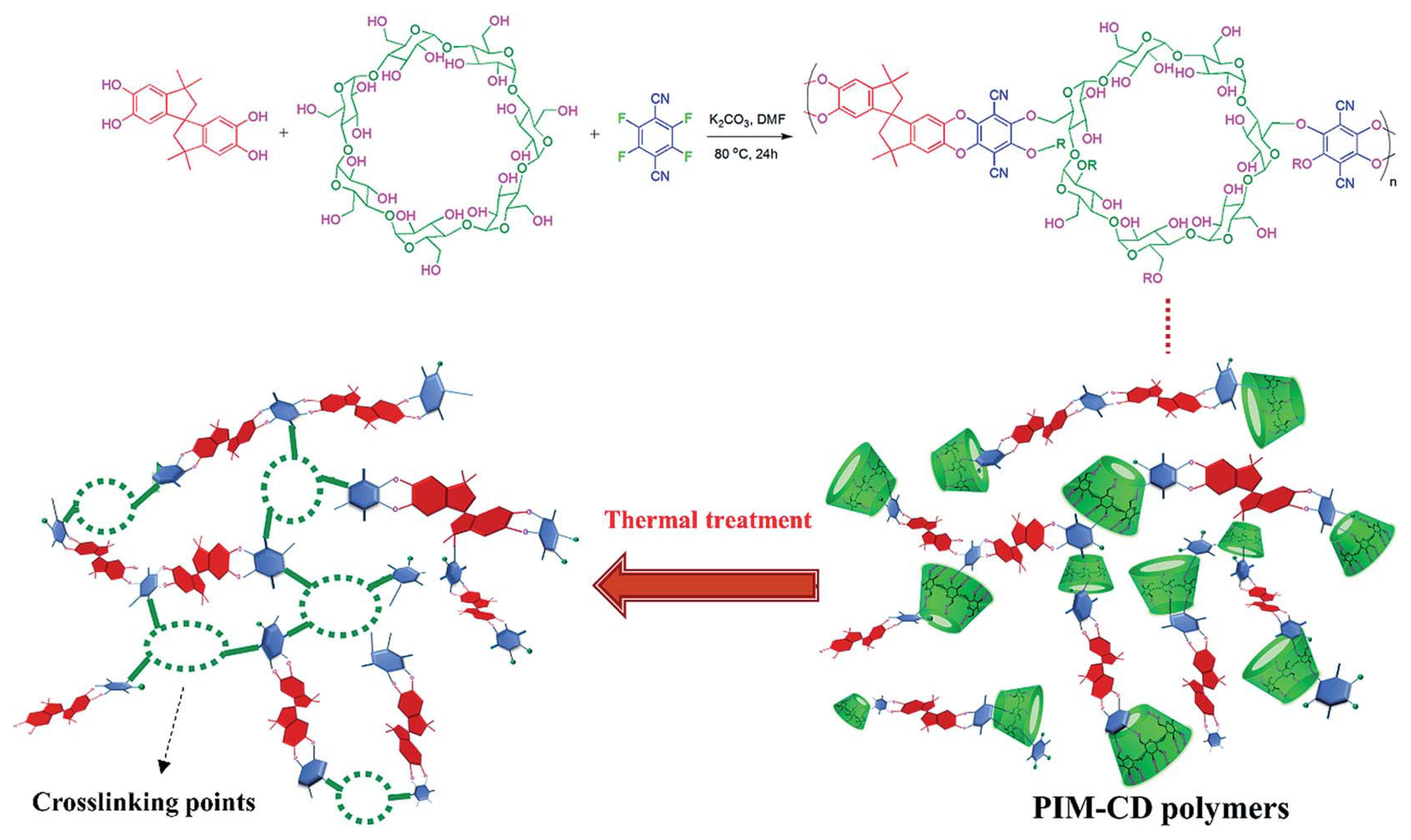
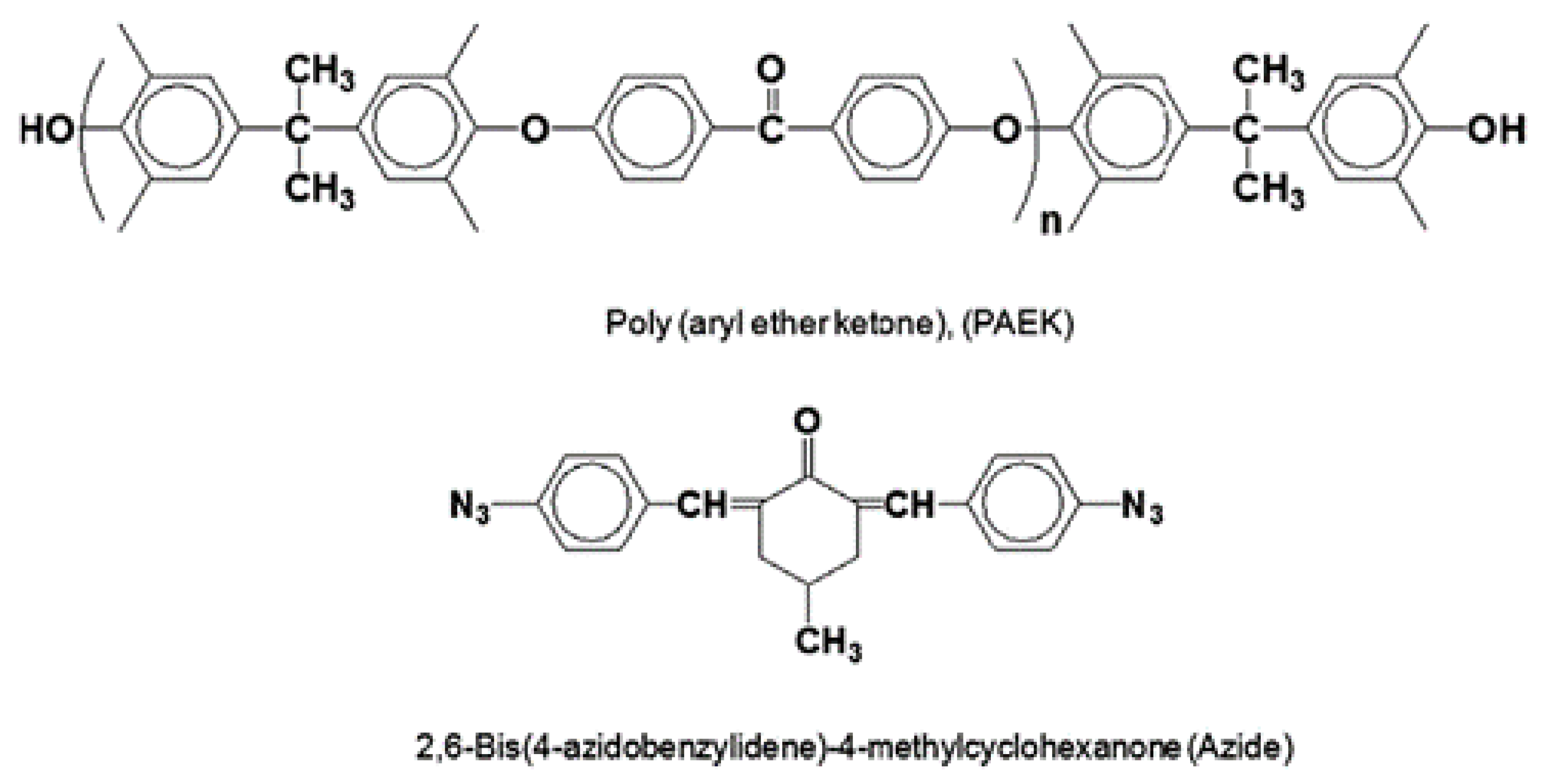


| Materials | Configuration | Pre-Treatment | Post-Treatment | Pyrolysis Temperature (°C) | Heating Rate (°C/min) | Soaking Time (h) | Atmosphere | Ref. |
|---|---|---|---|---|---|---|---|---|
| Matrimid | Film | 550, 800 | 4 | 2, 8 | Vacuum (<0.03 mmHg) | [88] | ||
| Matrimid | Film | 550–800 | 0.25–13.3 | 2 | Vacuum (<10 mtorr), Ar | [60] | ||
| Matrimid | Film | 675 | 0.25–13.3 | 2 | Ar | [85] | ||
| Matrimid | Film | 550–800 | 4 | Vacuum (0.03 mmHg) | [89] | |||
| Matrimid | Film, hollow fiber | 550–800 | 0.25–13.3 | 2 | Vacuum (<15 mtorr) | [62] | ||
| Matrimid | Hollow fiber | 550 | 0.25–13.3 | 2 | Ar (200 mL/min) | [90] | ||
| Matrimid | Hollow fiber | 550 | 0.25–13.3 | 2 | Ar (200 mL/min) | [91] | ||
| Matrimid | Alumina hollow fiber supported | 650 | 2.8 | 1 | He (100 mL/min) | [92] | ||
| Matrimid | Film | 500–800 | 0.25–13.3 | 2 | Vacuum, Ar | [81] | ||
| 6FDA/BPDA-DAM | Film | 550, 800 | 4 | 2, 8 | Vacuum (<0.03 mmHg) | [88] | ||
| 6FDA/BPDA-DAM | Film | 675 | 0.25–13.3 | 2 | Ar | [85] | ||
| 6FDA-DETDA/DABA | Film | O2 doping | 550–800 | 0.25–13.3 | 2 | Ar | [77] | |
| 6FDA-DETDA/DABA | Film | Pre-crosslinking at 370 °C for 1.5 h | 800 | 0.25–13.3 | 2 | Ar | [77] | |
| 6FDA-DABA | Film | Crosslinking at 350–450 °C for 2 h | 550 | 1 | 2 | N2 (200 mL/min) | [93] | |
| 6FDA-DAM/DABA | Film, hollow fiber | 675 | 0.25–10 | 2 | Ar (400 mL/min) | [94] | ||
| 6FDA-DAM/DABA | Alumina disc supported | 550–750 | 4 | 2 | Ar (100 mL/min) | [42] | ||
| 6FDA-DAM | Film | 550, 675 | 0.25–13.3 | 2 | Ar (200 mL/min) | [95] | ||
| 6FDA/BPDA-DAM | Hollow fiber | 550 | 0.25–13.3 | 2 | Ar (200 mL/min) | [90] | ||
| 6FDA-DAM | Hollow fiber | 550 | 0.25–13.3 | 2 | Ar (200 mL/min) | [90] | ||
| 6FDA/BPDA-DAM | Hollow fiber | 550 | 0.25–13.3 | 2 | Ar (200 mL/min) | [91] | ||
| 6FDA-based polyimide | Hollow fiber | 550 | 0.25–13.3 | 2 | Ar (200 mL/min) | [91] | ||
| 6FDA-based polyimide | Alumina disc supported | Pre-aging (oxidation) | 550 | 4 | 2 | Ar (100 mL/min) | [75] | |
| 6FDA-based polyimide | Alumina disc supported | 550 | 4 | 2 | Ar (100 mL/min) | [96] | ||
| Kapton | Film | Activation at 400 °C under Ar and He containing water vapor | 1000 | 10 | 2 | Vacuum (10−5 torr) | [97] | |
| BPDA-ODA/DAT | Alumina tubular supported | Oxidation in air at 400–500 °C | 500–700 | 5 | N2 (100 mL/min) | [98] | ||
| BPDA-pp’ODA | Alumina tubular supported | Imidizing at 300 °C for 1 h | Oxidation using O2/N2 mixture or pure O2 for 3 h | 600–900 | 5 | N2 | [99] | |
| BPDA_aromatic diamine | Hollow fiber | Thermostabilization at 400 °C for 30 min in air | 600–1000 | 15 | N2 (2000 mL/min) | [100] | ||
| BPDA-based | Hollow fiber | Thermostabilization in air at 400 °C for 0.5 h | 500–700 | 5 | N2 (100 mL/min) | [59] | ||
| BPDA-pp’ODA | Film | 370–450 | 5 | 1.5 | N2 (100 mL/min) | [39] | ||
| NTDA-BDSA/BAPF | Film | 700 | 5 | Ar | [101] | |||
| Phenolic resin | Ceramic tubular supported | Curing at 150 °C for 2 h | Oxidizing with air at 300–400 °C for 30 min | 700 | Vacuum (<0.01 mbar) | [102] | ||
| Phenolic resin | Ceramic tubular supported | Curing at 150 °C for 2 h | Storage under air, N2, and C3H6 | 700 | Vacuum (<0.01 mbar) | [103] | ||
| Phenolic resin | Alumina tubular supported | 550 | 274 | 2 | N2 (100 mL/min) | [104] | ||
| Phenolic resin | Alumina tubular supported | 550 | 1 | 2 | N2 | [105] | ||
| Phenolic resin | Ceramic tubular supported | 700–1000 | 0.5–10 | 1–8 | Vacuum (<1 Pa), N2 (285 mL/min) | [106] | ||
| Phenolic resin | Alumina tubular supported | Air oxidative treatment at 250–400 °C | Air oxidative treatment at 300–400 °C | 700 | Vacuum (<0.01 mbar) | [107] | ||
| Phenolic resin | Alumina tubular supported | Curing at 150 °C for 2 h | Air oxidative treatment at 75–350 °C for 0.5 h | 700 | Vacuum (<0.01 mbar) | [74] | ||
| PIM-1 | Film | 500–900 | 1 | 1 | Vacuum (<0.006 torr) | [108] | ||
| PIM-6FDA | Film | 500–800 | 3 | 0.5 | N2 (1000 mL/min) | [109] | ||
| PIM | Film | Annealing at 250 °C for 24 h | 400–800 | 3 | 0.5 | N2 (1000 mL/min) | [110] | |
| PIM-CD | Film | 300–600 | 1 | 2 | Vacuum | [110] | ||
| PIM-6FDA-OH | Film | 500–800 | 3 | 0.5 | N2 (1000 mL/min) | [111] | ||
| PIM-6FDA-OH | Film | 600 | 3 | 0.5 | N2 | [112] | ||
| PAEK/Azide | Film | 450–650 | 0.2–2 | 2 | vacuum | [113] | ||
| Polyimide | Film | Imidization at 100–300 °C for 1 h and 450 °C for 10 min | 600 | 5 | 1 | Inert gas (150 mL/min) | [114] | |
| Polyester-resin | Alumina tubular supported | Oxidation at 300–400 °C for 0.5 h | 700–800 | 1 | 0.5–1 | Ar | [76] |
| Materials | Pyrolysis Temperature (°C) | Operating Temperature (°C) | Test Gas * | C2H4 Permeance ** | C2H4/C2H6 Selectivity | C3H6 Permeance * | C3H6/C3H8 Selectivity | C4H8 Permeance * | C4H8/C4H10 Selectivity | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Matrimid | 550 | 35 | S | 9.1 B | 5.8 | [88] | ||||
| Matrimid | 800 | 35 | S | 0.1 B | 4.4 | [88] | ||||
| Matrimid | 550 | 35 | M | 8.3 B | 7.7 | [60] | ||||
| Matrimid | 675 | 35 | M | 10.9 B | 12.3 | [60] | ||||
| Matrimid | 675 | 35 | S | ~18.0 B | ~11.25 | [85] | ||||
| Matrimid | 500 | 35 | S | 13 B | 40 | [89] | ||||
| Matrimid | 675 | 35 | S | 10 B | ~12 | [62] | ||||
| Matrimid | 700 | 35 | S | 0.25 G | ~12 | [62] | ||||
| Matrimid | 550 | 35 | S | 2.1 G | 4 | 0.76 G | 21 | [90] | ||
| Matrimid | 550 | 35 | M | 5.5 G | 3.1 | [91] | ||||
| Matrimid | 650 | 25 | M | 69.2 G | 18 | [92] | ||||
| Matrimid | 500 | 35 | S | ~78.9 B | ~3.96 | [81] | ||||
| Matrimid | 550 | 35 | S | ~18.7 B | ~6.31 | [81] | ||||
| Matrimid | 675 | 35 | S | ~17.7 B | ~10.4 | [81] | ||||
| 6FDA/BPDA-DAM | 550 | 35 | S | 196 B | 100 | [88] | ||||
| 6FDA/BPDA-DAM | 800 | 35 | S | 1.3 B | 7.9 | [88] | ||||
| 6FDA/BPDA-DAM | 675 | 35 | S | ~58.7 B | ~7.63 | [85] | ||||
| 6FDA/DETDA-DABA | 550 | 35 | M | 2444 B | 12.7 | [77] | ||||
| 6FDA/DETDA-DABA (O2 doping) | 550 | 35 | M | 941 B | 23.1 | [77] | ||||
| 6FDA/DETDA-DABA (O2 doping) | 550 | 35 | M | 101 B | 50.7 | [77] | ||||
| 6FDA/DETDA-DABA | 800 | 35 | M | 71 B | 63.7 | [77] | ||||
| 6FDA/DETDA-DABA (pre-crosslinking) | 800 | 35 | M | 200 B | 52 | [77] | ||||
| 6FDA-DABA | 550 | 35 | M | 257 B | 20 | [93] | ||||
| 6FDA-DAM/DABA (embedding silica) | 675 | 35 | M | ~67 B | ~52 | [94] | ||||
| 6FDA-DAM/DABA (embedding silica) | 675 | 35 | M | ~4.1 G | ~35 | [94] | ||||
| 6FDA-based polyimide | 550 | 25 | M | 9 G | 36 | [42] | ||||
| 6FDA-DAM/DABA (Fe loading) | 550 | 35 | M | 10 B | 11 | [95] | ||||
| 6FDA-DAM/DABA (Fe loading) | 550 | 35 | M | ~100 B | ~8.53 | [95] | ||||
| 6FDA-DAM/DABA (Fe loading) | 550 | 35 | S | ~45.6 B | ~304.9 | [95] | ||||
| 6FDA/BPDA-DAM | 550 | 35 | S | 15.9 G | 3.9 | 17.5 G | 3.9 | [90] | ||
| 6FDA-DAM | 675 | 35 | M | 16.1 G | ~4.8 | [91] | ||||
| 6FDA/BPDA-DAM | 550 | 35 | M | 8.8 G | 3.9 | [91] | ||||
| 6FDA-based polyimide | 550 | 120 | M | 25.6 G | ~13 | [75] | ||||
| 6FDA-based polyimide | 550 | 25 | M | ~9.94 G | ~34 | [75] | ||||
| 6FDA-based polyimide | 550 | RT | M | 29.8 G | ~31 | [96] | ||||
| BPDA-ODA/DAT | 600 | 35 | M | 4.179 G | 25 | [98] | ||||
| BPDA-ODA/DAT | 600 | 100 | M | 18.5 G | 18 | [98] | ||||
| BPDA-pp’ODA | 700 | 100 | S | ~13.2 G | ~19.1 | [99] | ||||
| BPDA-aromatic diamine | 700 | 50 | S | ~8.69 | ~3.30 | [100] | ||||
| BPDA-aromatic diamine | 850 | 80 | S | ~0.30 G | ~7.26 | [100] | ||||
| BPDA-DDBT/DABA | 600 | 100 | M | 51 G | 12 | [59] | ||||
| BPDA-DDBT/DABA | 600 | 100 | M | 110 G | 3.1 | [59] | ||||
| NTDA-BDSA/BAPF | 450 | 35 | S | 31 B | 4.2 | 15 B | 34 | [39] | ||
| NTDA-BDSA/BAPF | 450 | 35 | M | 9.3 B | 19 | [39] | ||||
| NTDA-BAHFDS | 450 | 35 | S | 66 B | 3.5 | 41 B | 26 | [39] | ||
| NTDA-BAHFDS | 450 | 35 | M | 30 B | 11 | [39] | ||||
| NTDA-BAHFDS/BAPF | 450 | 35 | S | 30 B | 3.4 | 15 B | 21 | [39] | ||
| NTDA-BDSA | 450 | 35 | S | 14 B | 4.8 | 6.4 B | 29 | [39] | ||
| BPDA-pp’ODA | 700 | 35 | M | 2.36 G | 46 | [101] | ||||
| BPDA-pp’ODA | 700 | 100 | M | 8.66 G | 33 | [101] | ||||
| BPDA-pp’ODA | 700 | S | ~40 B | ~7 | [101] | |||||
| Kapton | 400–1000 | 100 | S | ~55.5 B | ~5.82 | ~11.8 B | ~25.2 | [97] | ||
| Phenolic resin | 700 | 20 ± 1 | S | ~1.38 G | 14 | [102] | ||||
| Phenolic resin | 700 | 20 ± 1 | S | ~3027 G | 1.3 | ~3092 G | 1.45 | [102] | ||
| Phenolic resin | 700 | 20 ± 1 | S | ~49.85 G | 2.35 | ~45.0 G | 16.59 | [103] | ||
| Phenolic resin | 550 | 20 | M | ~286.6 B | ~12.8 | [104] | ||||
| Phenolic resin | 550 | 20 | M | ~392.6 B | ~7.92 | [104] | ||||
| Phenolic resin (boehmite composite) | 550 | 20 | S | ~154.1 B | 14.6 | [105] | ||||
| Phenolic resin | 800 | M | 16 B | 5.4 | 19 B | 35 | [106] | |||
| Phenolic resin | 700 | 20 | S | ~48.7 G | ~3.75 | ~52.9 G | ~33.1 | [107] | ||
| Phenolic resin | 700 | S | 8.65 G | 2.2 | 84.4 G | 11.4 | [74] | |||
| PIM (boron-doped) | 700 | 35 | M | ~13.6 B | ~9.69 | [108] | ||||
| PIM-6FDA | 800 | 35 | M | 3.02 B | 17.9 | [108] | ||||
| PIM-1 | 600 | 35 | S | 44 B | 6.29 | [110] | ||||
| PIM-1 | 800 | 35 | S | 1.3 B | 13 | [110] | ||||
| PIM-CD | 400 | 35 | S | 2093 B | 5.19 | [122] | ||||
| PIM-CD | 600 | 35 | S | 42 B | 8.4 | [122] | ||||
| PIM-6FDA-OH | 800 | 35 | S | 10 B | 17.5 | [111] | ||||
| PIM-6FDA-OH | 800 | 35 | M | ~10 B | 14 | [111] | ||||
| PIM-6FDA-OH | 600 | 35 | M | 31 B | 17 | [112] | ||||
| PAEK/Azide | 550 | 35 | M | 3.6 B | 32 | [113] | ||||
| PI (silica dispersion) | 600 | 25 | S | 40 B | 13.3 | 36 B | 51.4 | [114] | ||
| PI (silica dispersion) | 600 | 25 | S | 150 B | 8.3 | 143 B | 30.4 | [114] | ||
| PI (silica dispersion) | 600 | 25 | S | 280 B | 5.4 | 244 B | 20.3 | [114] | ||
| PI (silica dispersion) | 600 | 25 | S | 155 B | 7.8 | 147 B | 29.4 | [114] | ||
| PI (silica dispersion) | 600 | 25 | S | 398 B | 5.3 | 375 B | 25 | [114] | ||
| Polyester-resin | 800 | 150 | S | ~574.0 G | ~1.93 | [76] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-J.; Kwon, Y.; Kim, D.; Park, H.; Cho, Y.H.; Nam, S.-E.; Park, Y.-I. A Review on Polymer Precursors of Carbon Molecular Sieve Membranes for Olefin/Paraffin Separation. Membranes 2021, 11, 482. https://doi.org/10.3390/membranes11070482
Kim S-J, Kwon Y, Kim D, Park H, Cho YH, Nam S-E, Park Y-I. A Review on Polymer Precursors of Carbon Molecular Sieve Membranes for Olefin/Paraffin Separation. Membranes. 2021; 11(7):482. https://doi.org/10.3390/membranes11070482
Chicago/Turabian StyleKim, Seong-Joong, YongSung Kwon, DaeHun Kim, Hosik Park, Young Hoon Cho, Seung-Eun Nam, and You-In Park. 2021. "A Review on Polymer Precursors of Carbon Molecular Sieve Membranes for Olefin/Paraffin Separation" Membranes 11, no. 7: 482. https://doi.org/10.3390/membranes11070482
APA StyleKim, S.-J., Kwon, Y., Kim, D., Park, H., Cho, Y. H., Nam, S.-E., & Park, Y.-I. (2021). A Review on Polymer Precursors of Carbon Molecular Sieve Membranes for Olefin/Paraffin Separation. Membranes, 11(7), 482. https://doi.org/10.3390/membranes11070482








