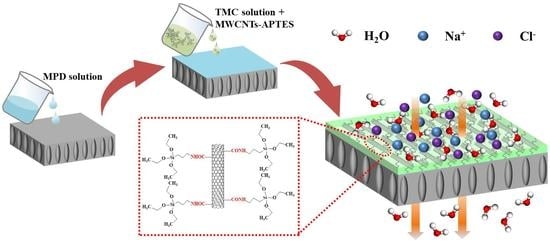
Novel Thin Film Nanocomposite Forward Osmosis Membranes Prepared by Organic Phase Controlled Interfacial Polymerization with Functional Multi-Walled Carbon Nanotubes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
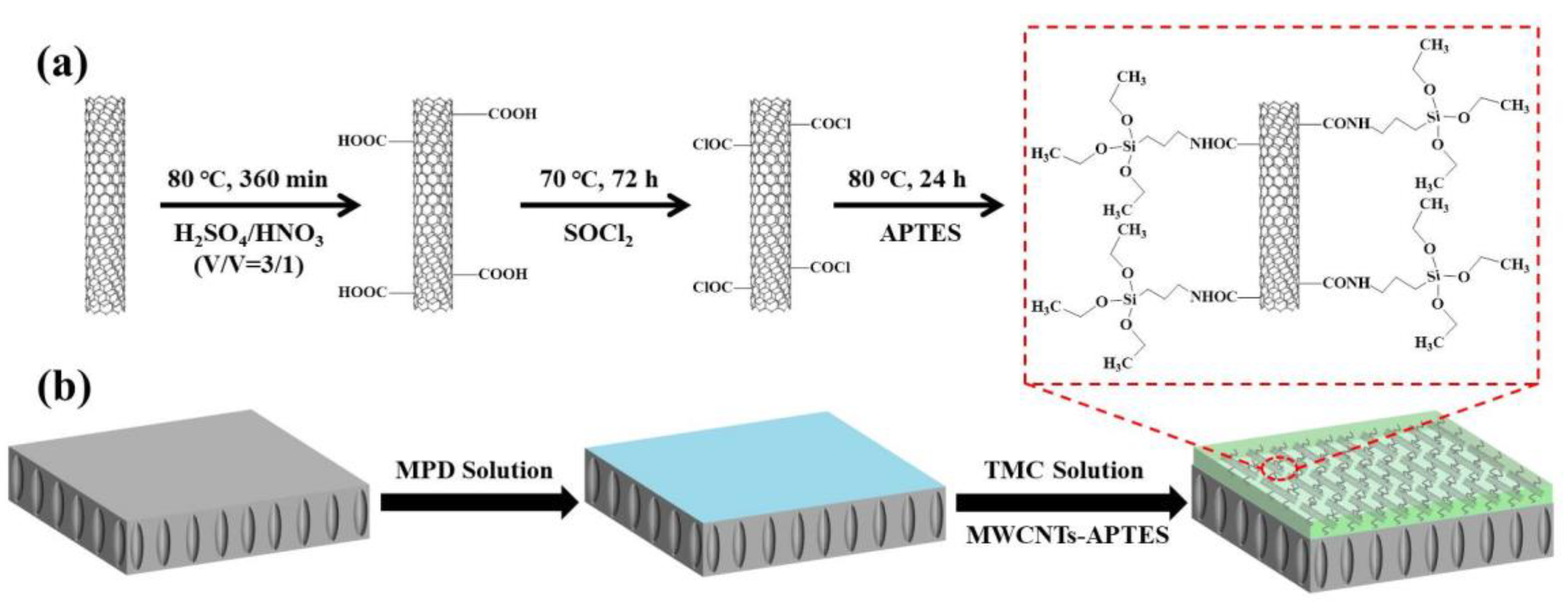
2.2. Preparation of MWCNTs-APTES
2.3. Preparation of the PSF Support Layer
2.4. Fabrication of TFC and TFN FO Membranes
2.5. Characterizations
2.6. Determination of Membranes Intrinsic Separation Performance
2.7. Estimation of TFC and TFN Membranes’ FO Performance
3. Results and Discussion
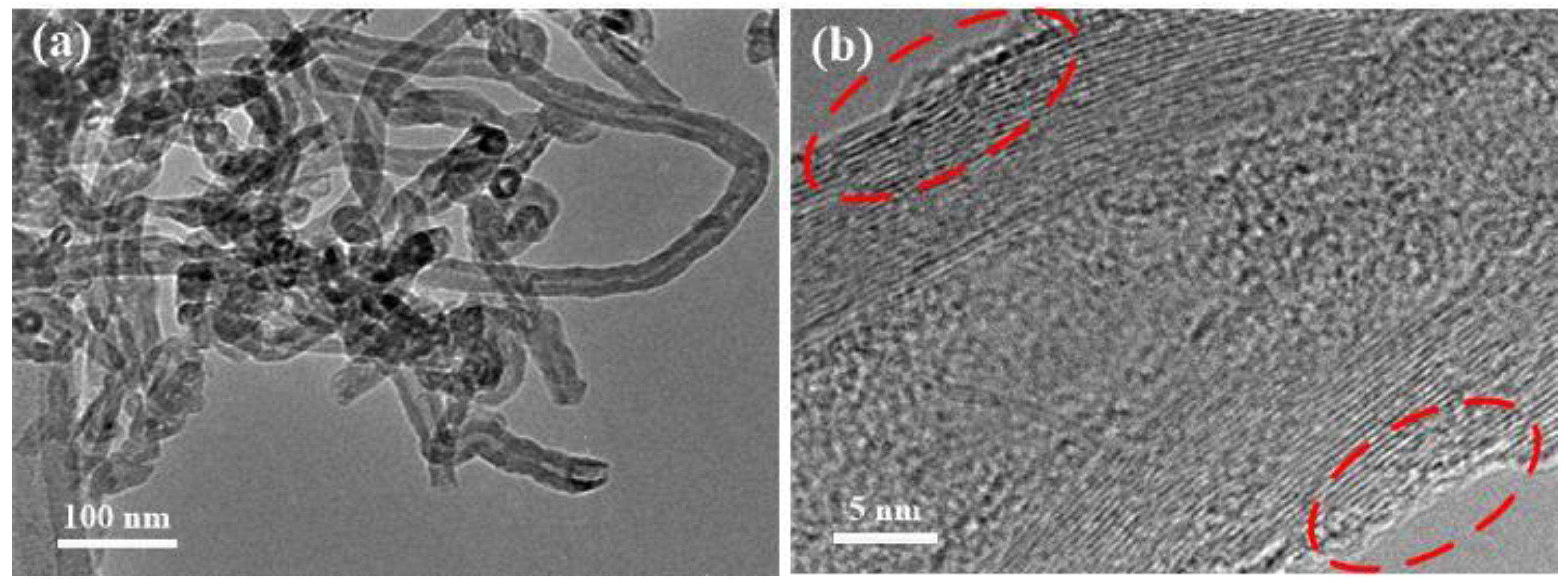
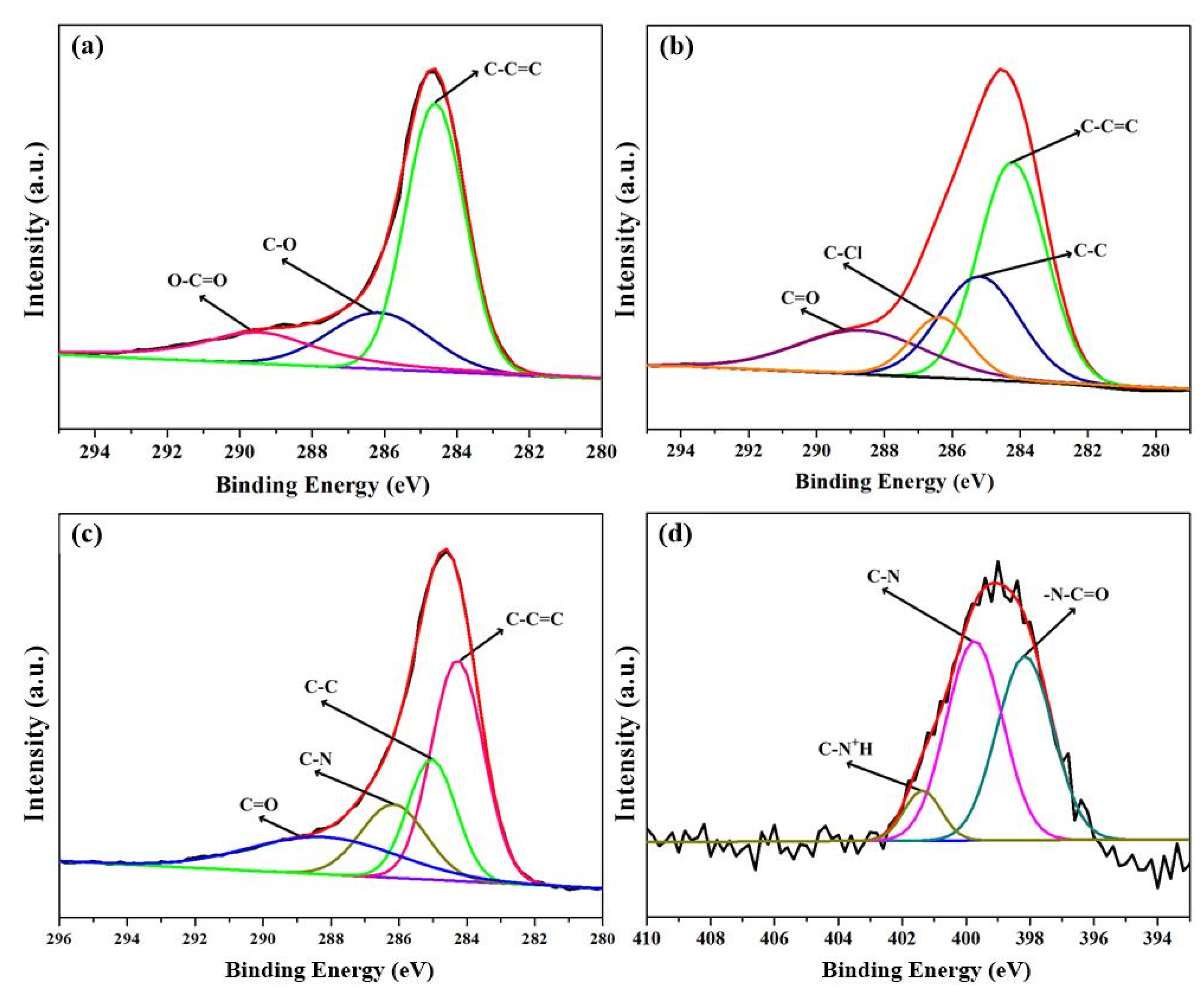
3.1. Characterization of MWCNTs-APTES
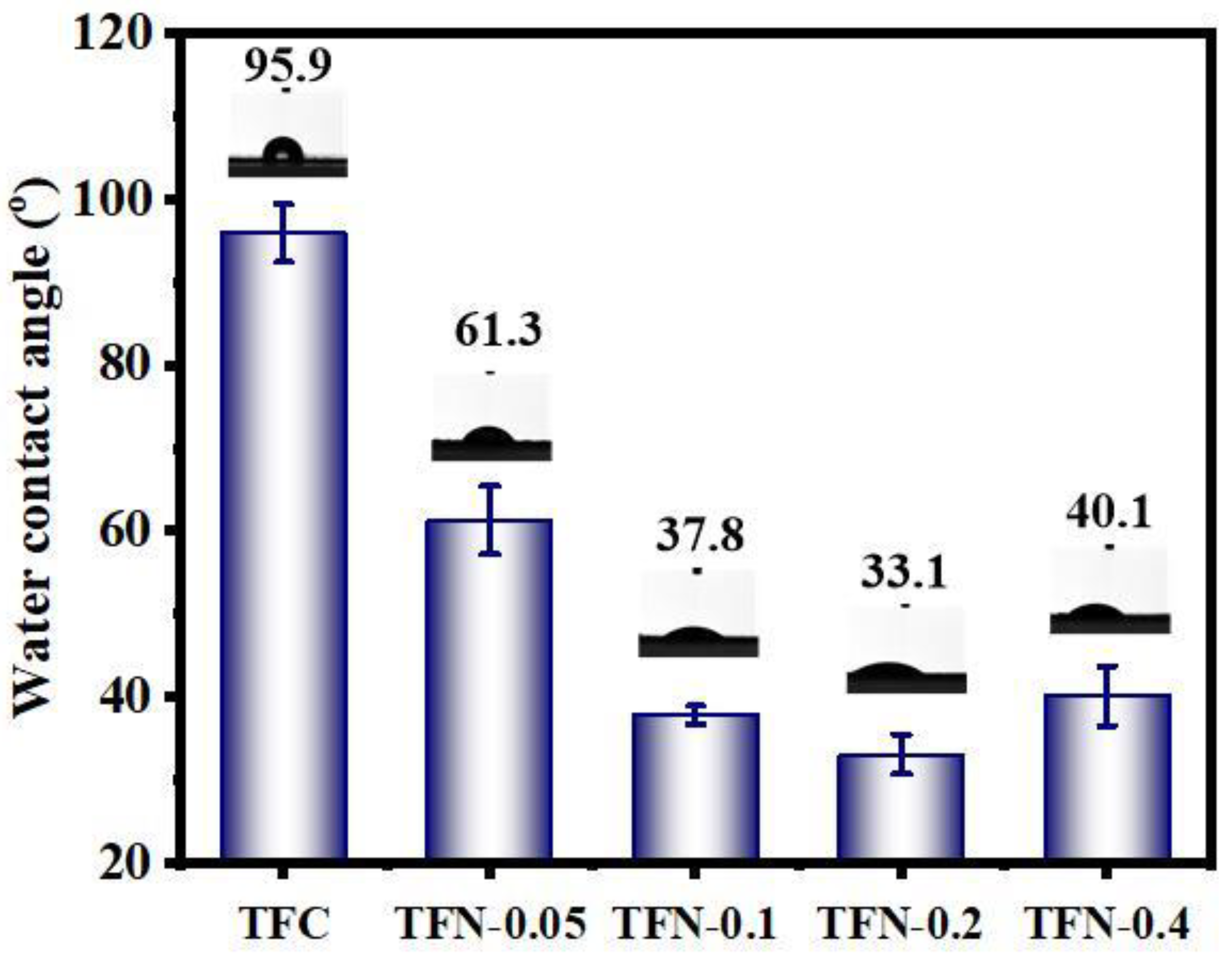
3.2. Characterization of TFC and TFN Membranes
3.3. Intrinsic Separation Properties of TFC and TFN Membranes
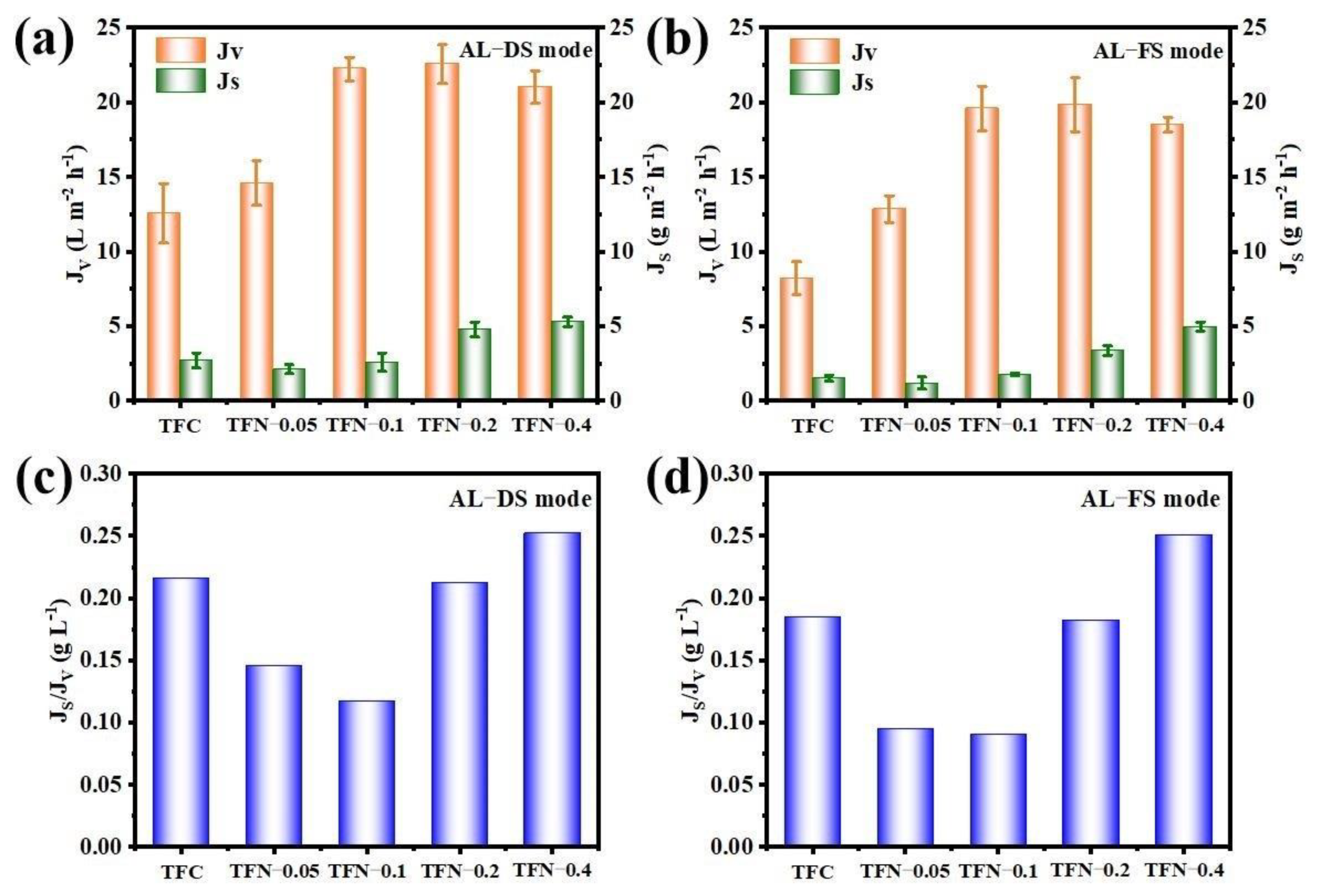
3.4. FO Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Marinas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef]
- Elimelech, M.; Phillip, W.A. The future of seawater desalination: Energy, technology, and the environment. Science 2011, 333, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Werber, J.R.; Osuji, C.O.; Elimelech, M. Materials for next-generation desalination and water purification membranes. Nat. Rev. Mater. 2016, 1, 16018–16033. [Google Scholar] [CrossRef]
- Zhang, R.N.; Liu, Y.N.; He, M.R.; Su, Y.L.; Zhao, X.T.; Elimelech, M.; Jiang, Z.Y. Antifouling membranes for sustainable water purification: Strategies and mechanisms. Chem. Soc. Rev. 2016, 45, 5888–5924. [Google Scholar] [CrossRef]
- Fane, A.G.; Wang, R.; Hu, M.X. Synthetic membranes for water purification: Status and future. Angew. Chem. Int. Ed. 2015, 54, 3368–3386. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Xu, Z.H.; Zhang, T.T.; Meng, Q.; Zhang, X.; Shen, C.; Lu, Y.H.; Zhang, G.; Gao, C.J. Self-assembly of robust graphene oxide membranes with chirality for highly stable and selective molecular separation. J. Mater. Chem. A 2020, 8, 16985–16993. [Google Scholar] [CrossRef]
- Liao, F.Q.; Xu, Z.H.; Fan, Z.X.; Meng, Q.; Lv, B.S.; Ye, X.W.; Shen, C.; Zhang, G. Confined assembly of ultrathin dual-functionalized Z-MXene nanosheet intercalated GO nanofilms with controlled structure for size-selective permeation. J. Mater. Chem. A 2021, 9, 12236–12243. [Google Scholar] [CrossRef]
- Suwaileh, W.; Pathak, N.; Shon, H.; Hilal, N. Forward osmosis membranes and processes: A comprehensive review of research trends and future outlook. Desalination 2020, 485, 114455. [Google Scholar] [CrossRef]
- Ke, X.X.; Wang, T.Y.; Wu, X.Q.; Chen, J.P.; Zhao, Q.B.; Zheng, Y.M. Alleviation of reverse salt leakage across nanofiber supported thin-film composite forward osmosis membrane via heat-curing in hot water. Membranes 2021, 11, 237. [Google Scholar] [CrossRef]
- Qasim, M.; Darwish, N.A.; Sarp, S.; Hilal, N. Water desalination by forward (direct) osmosis phenomenon: A comprehensive review. Desalination 2015, 374, 47–69. [Google Scholar] [CrossRef]
- Liu, T.Y.; Yuan, H.G.; Liu, Y.Y.; Ren, D.; Su, Y.C.; Wang, X. Metal-organic framework nanocomposite thin films with interfacial bindings and self-standing robustness for high water flux and enhanced ion selectivity. ACS Nano 2018, 12, 9253–9265. [Google Scholar] [CrossRef] [PubMed]
- Klaysom, C.; Cath, T.Y.; Depuydt, T.; Vankelecom, I.F.J. Forward and pressure retarded osmosis: Potential solutions for global challenges in energy and water supply. Chem. Soc. Rev. 2013, 42, 6959–6989. [Google Scholar] [CrossRef] [PubMed]
- Alihemati, Z.; Hashemifard, S.A.; Matsuura, T.; Ismail, A.F.; Hilal, N. Current status and challenges of fabricating thin film composite forward osmosis membrane: A comprehensive roadmap. Desalination 2020, 491, 114557. [Google Scholar] [CrossRef]
- Xu, L.N.; Yang, T.T.; Li, M.D.; Chang, J.Q.; Xu, J. Thin-film nanocomposite membrane doped with carboxylated covalent organic frameworks for efficient forward osmosis desalination. J. Membr. Sci. 2020, 610, 118111. [Google Scholar] [CrossRef]
- Park, M.J.; Phuntsho, S.; He, T.; Nisola, G.M.; Tijing, L.D.; Li, X.M.; Chen, G.; Chung, W.J.; Shon, H.K. Graphene oxide incorporated polysulfone substrate for the fabrication of flat-sheet thin-film composite forward osmosis membranes. J. Membr. Sci. 2015, 493, 496–507. [Google Scholar] [CrossRef]
- Xu, G.R.; Xu, J.M.; Feng, H.J.; Zhao, H.L.; Wu, S.B. Tailoring structures and performance of polyamide thin film composite (PA-TFC) desalination membranes via sublayers adjustment-a review. Desalination 2017, 417, 19–35. [Google Scholar] [CrossRef]
- Suzaimi, N.D.; Goh, P.S.; Ismail, A.F.; Mamah, S.C.; Malek, N.; Lim, J.W.; Wong, K.C.; Hilal, N. Strategies in forward osmosis membrane substrate fabrication and modification: A review. Membranes 2020, 10, 332. [Google Scholar] [CrossRef]
- Ma, D.C.; Peh, S.B.; Han, G.; Chen, S.B. Thin-film nanocomposite (TFN) membranes incorporated with super-hydrophilic metal-organic framework (MOF) UiO-66: Toward enhancement of water flux and salt rejection. ACS Appl. Mater. Interfaces 2017, 9, 7523–7534. [Google Scholar] [CrossRef]
- Tang, Y.Y.; Yu, H.Y.; Xing, Y.L.; Gao, C.J.; Xu, J. Microstructure and desalination performance of polyamide membranes interfacially regulated via single-side post-modified CNTs networks. Desalination 2020, 482, 114408. [Google Scholar] [CrossRef]
- Wu, X.; Ding, M.M.; Xu, H.; Yang, W.; Zhang, K.S.; Tian, H.L.; Wang, H.T.; Xie, Z.L. Scalable Ti3C2Tx MXene interlayered forward osmosis membranes for enhanced water purification and organic solvent recovery. ACS Nano 2020, 14, 9125–9135. [Google Scholar] [CrossRef] [PubMed]
- He, M.L.; Wang, L.; Lv, Y.T.; Wang, X.D.; Zhu, J.N.; Zhang, Y.; Liu, T.T. Novel polydopamine/metal organic framework thin film nanocomposite forward osmosis membrane for salt rejection and heavy metal removal. Chem. Eng. J. 2020, 389, 124452. [Google Scholar] [CrossRef]
- Akther, N.; Phuntsho, S.; Chen, Y.; Ghaffour, N.; Shon, H.K. Recent advances in nanomaterial-modified polyamide thin-film composite membranes for forward osmosis processes. J. Membr. Sci. 2019, 584, 20–45. [Google Scholar] [CrossRef]
- Seyedpour, S.F.; Dadashi Firouzjaei, M.; Rahimpour, A.; Zolghadr, E.; Arabi Shamsabadi, A.; Das, P.; Akbari Afkhami, F.; Sadrzadeh, M.; Tiraferri, A.; Elliott, M. Toward sustainable tackling of biofouling implications and improved performance of TFC FO membranes modified by Ag-MOF nanorods. ACS Appl. Mater. Interfaces 2020, 12, 38285–38298. [Google Scholar] [CrossRef]
- Huang, M.; Chen, Y.; Huang, C.H.; Sun, P.; Crittenden, J. Rejection and adsorption of trace pharmaceuticals by coating a forward osmosis membrane with TiO2. Chem. Eng. J. 2015, 279, 904–911. [Google Scholar] [CrossRef]
- Li, J.Q.; Wang, Q.; Deng, L.Y.; Kou, X.H.; Tang, Q.C.; Hu, Y.X. Fabrication and characterization of carbon nanotubes-based porous composite forward osmosis membrane: Flux performance, separation mechanism, and potential application. J. Membr. Sci. 2020, 604, 118050. [Google Scholar] [CrossRef]
- Wu, X.; Shaibani, M.; Smith, S.J.D.; Konstas, K.; Hill, M.R.; Wang, H.T.; Zhang, K.S.; Xie, Z.L. Microporous carbon from fullerene impregnated porous aromatic frameworks for improving the desalination performance of thin film composite forward osmosis membranes. J. Mater. Chem. A 2018, 6, 11327–11336. [Google Scholar] [CrossRef]
- Wei, X.; Kong, X.; Yang, J.; Zhang, G.; Chen, J.; Wang, J. Structure influence of hyperbranched polyester on structure and properties of synthesized nanofiltration membranes. J. Membr. Sci. 2013, 440, 67–76. [Google Scholar] [CrossRef]
- Yadav, S.; Saleem, H.; Ibrar, I.; Naji, O.; Hawari, A.A.; Alanezi, A.A.; Zaidi, S.J.; Altaee, A.; Zhou, J. Recent developments in forward osmosis membranes using carbon-based nanomaterials. Desalination 2020, 482, 114375. [Google Scholar] [CrossRef]
- Shan, M.J.; Kang, H.; Xu, Z.W.; Li, N.; Jing, M.L.; Hu, Y.L.; Teng, K.Y.; Qian, X.M.; Shi, J.; Liu, L.Y. Decreased cross-linking in interfacial polymerization and heteromorphic support between nanoparticles: Towards high-water and low-solute flux of hybrid forward osmosis membrane. J. Colloid Interfaces Sci. 2019, 548, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Song, X.J.; Wang, L.; Tang, C.Y.; Wang, Z.N.; Gao, C.J. Fabrication of carbon nanotubes incorporated double-skinned thin film nanocomposite membranes for enhanced separation performance and antifouling capability in forward osmosis process. Desalination 2015, 369, 1–9. [Google Scholar] [CrossRef]
- Gong, G.H.; Wang, P.; Zhou, Z.Y.; Hu, Y.X. New insights into the role of an interlayer for the fabrication of highly selective and permeable thin-film composite nanofiltration membrane. ACS Appl. Mater. Interfaces 2019, 11, 7349–7356. [Google Scholar] [CrossRef]
- Zhang, G.; Lu, S.; Zhang, L.; Meng, Q.; Shen, C.; Zhang, J. Novel polysulfone hybrid ultrafiltration membrane prepared with TiO2-g-HEMA and its antifouling characteristics. J. Membr. Sci. 2013, 436, 163–173. [Google Scholar] [CrossRef]
- Ni, L.H.; Liao, Z.P.; Chen, K.; Xie, J.; Li, Q.; Qi, J.W.; Sun, X.Y.; Wang, L.J.; Li, J.S. Defect-engineered UiO-66-NH2 modified thin film nanocomposite membrane with enhanced nanofiltration performance. Chem. Commun. 2020, 56, 8372–8375. [Google Scholar] [CrossRef]
- Amini, M.; Jahanshahi, M.; Rahimpour, A. Synthesis of novel thin film nanocomposite (TFN) forward osmosis membranes using functionalized multi-walled carbon nanotubes. J. Membr. Sci. 2013, 435, 233–241. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.F.; Wang, T.C.; Fan, Z.; Zhang, G. A thin film nanocomposite membrane with pre-immobilized UiO-66-NH2 toward enhanced nanofiltration performance. RSC Adv. 2019, 9, 24802–24810. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Zhang, Y.; Li, Q.; Zhang, G. Metal-organic framework composite membranes: Synthesis and separation applications. Chem. Eng. Sci. 2015, 135, 232–257. [Google Scholar] [CrossRef]
- Tian, M.; Wang, Y.N.; Wang, R. Synthesis and characterization of novel high-performance thin film nanocomposite (TFN) FO membranes with nanofibrous substrate reinforced by functionalized carbon nanotubes. Desalination 2015, 370, 79–86. [Google Scholar] [CrossRef]
- Loeb, S.; Titelman, L.; Korngold, E.; Freiman, J. Effect of porous support fabric on osmosis through a Loeb-Sourirajan type asymmetric membrane. J. Membr. Sci. 1997, 129, 243–249. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, L.; Guan, C.Y.; Liu, C.X.; Lang, W.Z.; Wang, Y. Construction of SiO2@MWNTs incorporated PVDF substrate for reducing internal concentration polarization in forward osmosis. J. Membr. Sci. 2018, 564, 328–341. [Google Scholar] [CrossRef]
- Yang, X.; He, Y.; Zeng, G.; Chen, X.; Shi, H.; Qing, D.; Li, F.; Chen, Q. Bio-inspired method for preparation of multiwall carbon nanotubes decorated superhydrophilic poly(vinylidene fluoride) membrane for oil/water emulsion separation. Chem. Eng. J. 2017, 321, 245–256. [Google Scholar] [CrossRef]
- Zhao, S.; Zou, L.; Tang, C.Y.; Mulcahy, D. Recent developments in forward osmosis: Opportunities and challenges. J. Membr. Sci. 2012, 396, 1–21. [Google Scholar] [CrossRef]
- Lutchmiah, K.; Verliefde, A.R.; Roest, K.; Rietveld, L.C.; Cornelissen, E.R. Forward osmosis for application in wastewater treatment: A review. Water Res. 2014, 58, 179–197. [Google Scholar] [CrossRef] [PubMed]



| Membrane | Rejection of NaCl (%) | A (L m−2 h−1 bar−1) | B (L m−2 h−1) | B/A (Bar) | S (µm) |
|---|---|---|---|---|---|
| TFC | 96.1 ± 0.76 | 1.56 ± 0.08 | 0.32 ± 0.02 | 0.205 ± 0.002 | 1555 ± 93 |
| TFN-0.05 | 97.3 ± 0.65 | 3.11 ± 0.03 | 0.40 ± 0.01 | 0.129 ± 0.001 | 1114 ± 64 |
| TFN-0.1 | 97.4 ± 1.16 | 3.59 ± 0.12 | 0.48 ± 0.02 | 0.134 ± 0.007 | 646 ± 18 |
| TFN-0.2 | 95.7 ± 0.84 | 4.10 ± 0.09 | 0.92 ± 0.07 | 0.224 ± 0.013 | 737 ± 47 |
| TFN-0.4 | 93.6 ± 0.53 | 3.40 ± 0.16 | 1.16 ± 0.05 | 0.341 ± 0.012 | 610 ± 32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zheng, J.; Xu, L.; Yin, M.; Zhang, G.; Zhao, W.; Zhang, Z.; Shen, C.; Meng, Q. Novel Thin Film Nanocomposite Forward Osmosis Membranes Prepared by Organic Phase Controlled Interfacial Polymerization with Functional Multi-Walled Carbon Nanotubes. Membranes 2021, 11, 476. https://doi.org/10.3390/membranes11070476
Zhang X, Zheng J, Xu L, Yin M, Zhang G, Zhao W, Zhang Z, Shen C, Meng Q. Novel Thin Film Nanocomposite Forward Osmosis Membranes Prepared by Organic Phase Controlled Interfacial Polymerization with Functional Multi-Walled Carbon Nanotubes. Membranes. 2021; 11(7):476. https://doi.org/10.3390/membranes11070476
Chicago/Turabian StyleZhang, Xu, Jiuhan Zheng, Lusheng Xu, Ming Yin, Guoliang Zhang, Wenqian Zhao, Zeyu Zhang, Chong Shen, and Qin Meng. 2021. "Novel Thin Film Nanocomposite Forward Osmosis Membranes Prepared by Organic Phase Controlled Interfacial Polymerization with Functional Multi-Walled Carbon Nanotubes" Membranes 11, no. 7: 476. https://doi.org/10.3390/membranes11070476
APA StyleZhang, X., Zheng, J., Xu, L., Yin, M., Zhang, G., Zhao, W., Zhang, Z., Shen, C., & Meng, Q. (2021). Novel Thin Film Nanocomposite Forward Osmosis Membranes Prepared by Organic Phase Controlled Interfacial Polymerization with Functional Multi-Walled Carbon Nanotubes. Membranes, 11(7), 476. https://doi.org/10.3390/membranes11070476