Analysis of Polyvinylidene Fluoride Membranes Fabricated for Membrane Distillation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of PVDF Membranes
2.3. Contact Angle (CA) Measurement
2.4. Measurement of Liquid Entry Pressure
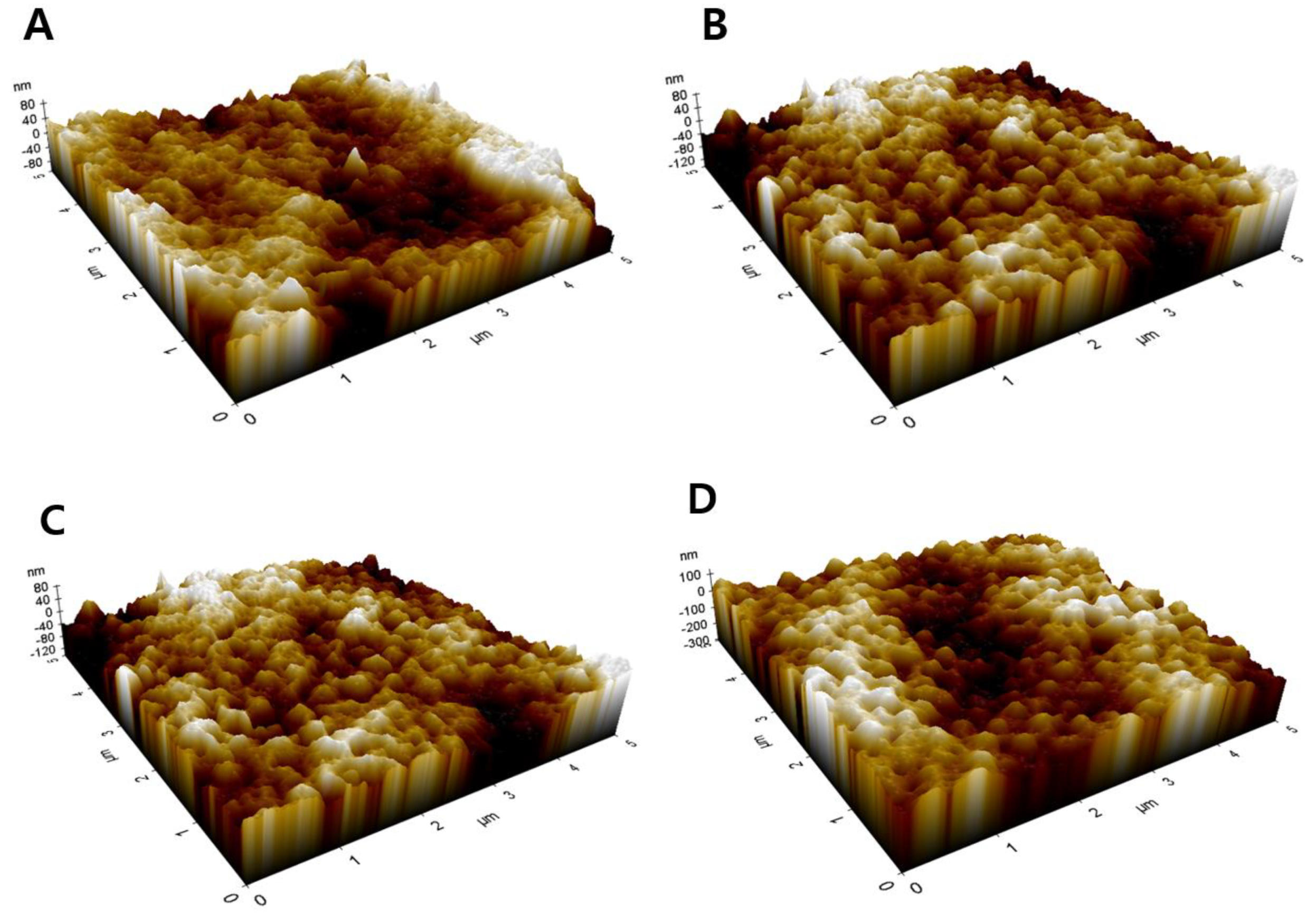
2.5. Analysis of Membrane Morphology
2.6. Analysis of Pore Size Distribution and Thickness Measurement
2.7. Measurement of Porosity
2.8. Measurement of Flux and Rejection
2.9. Calculation of Additional Membrane Properties
2.9.1. Calculation of Water Vapor Permeability (Bw)
2.9.2. Estimation of Salt Leakage Ratio (Lw)
2.9.3. Determination of Fiber Radius (Rf)
3. Results and Discussion
3.1. Characterization of Fabricated Membranes
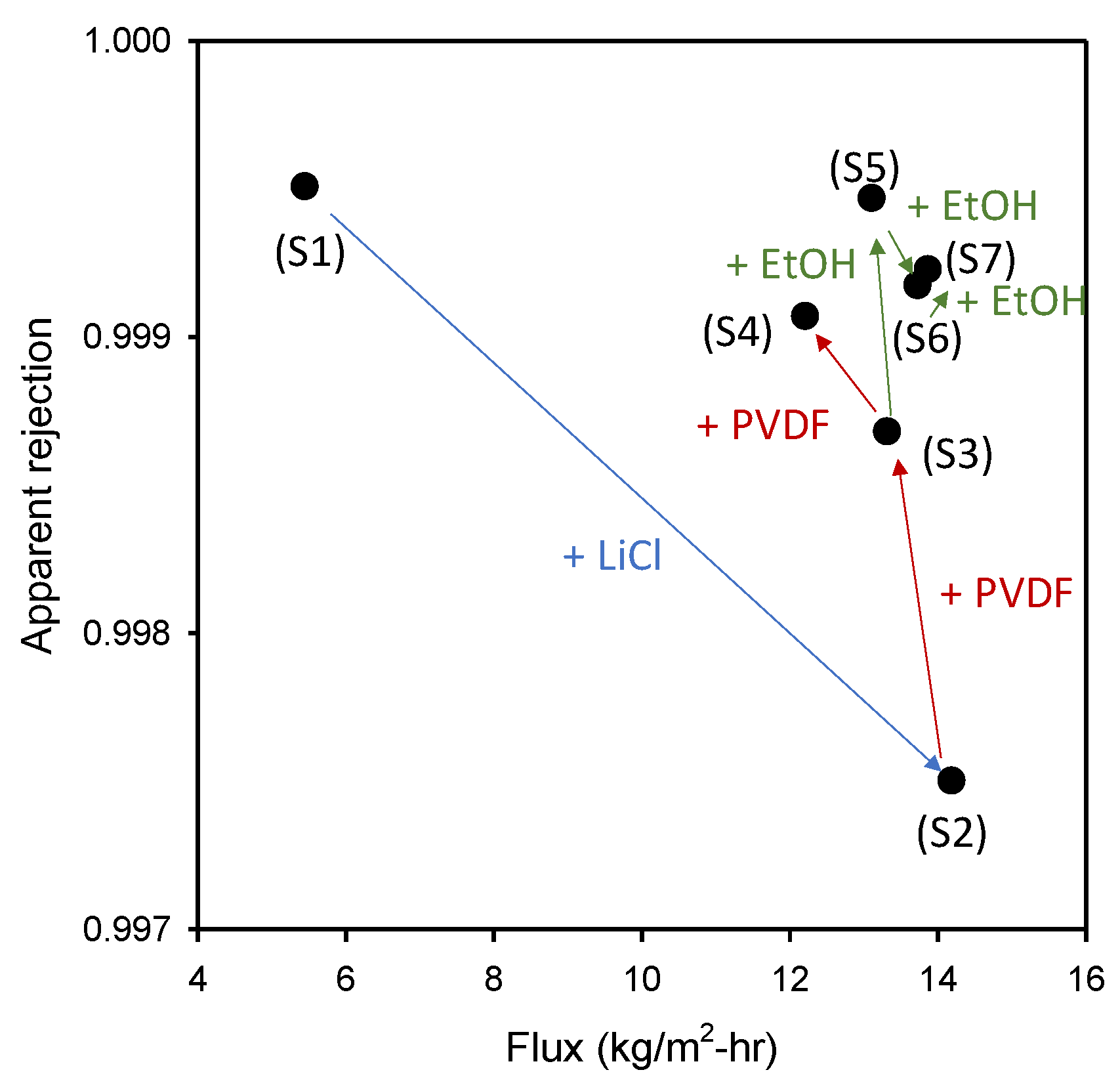
3.2. DCMD Performance
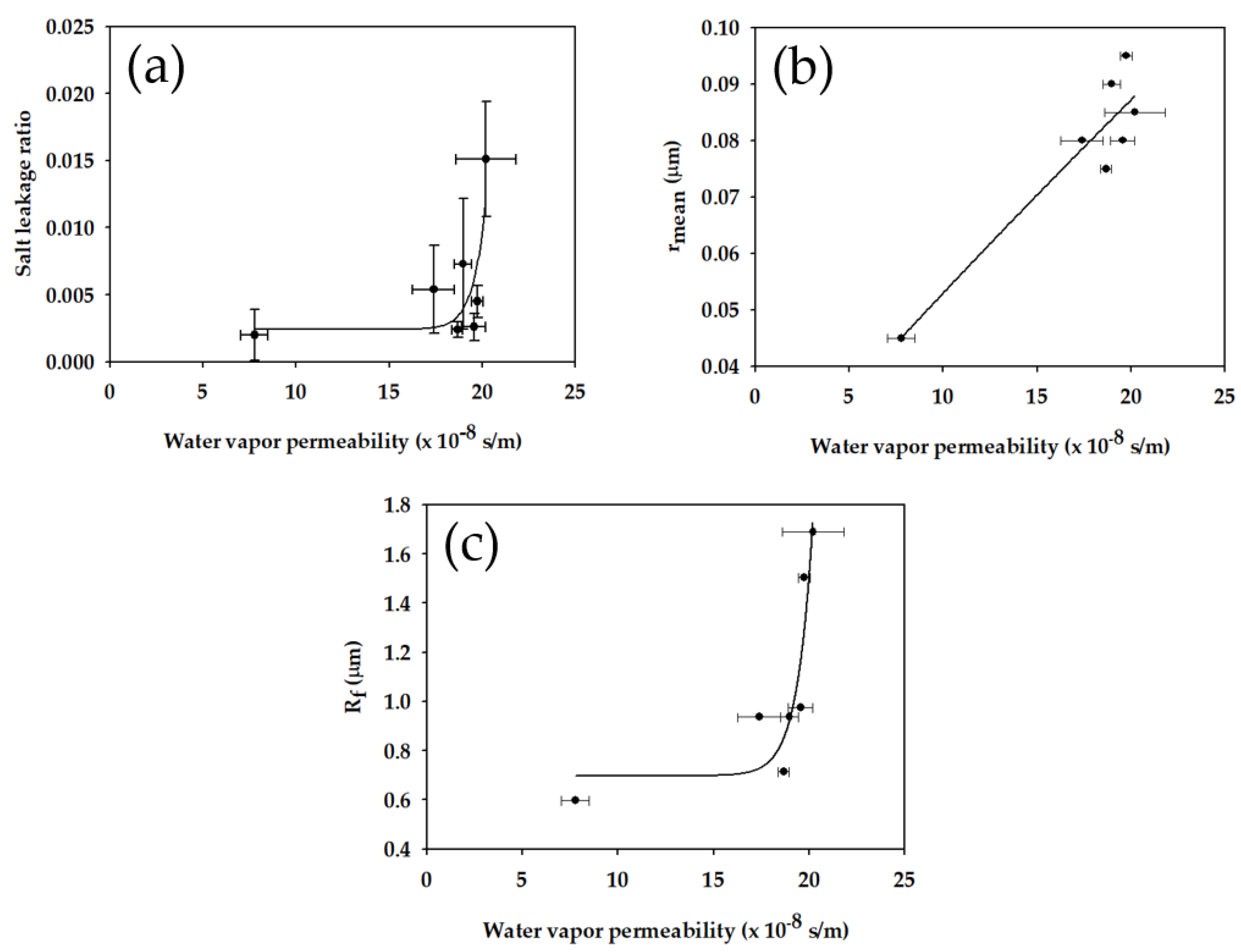
3.3. In-Depth Analysis of Membrane Properties
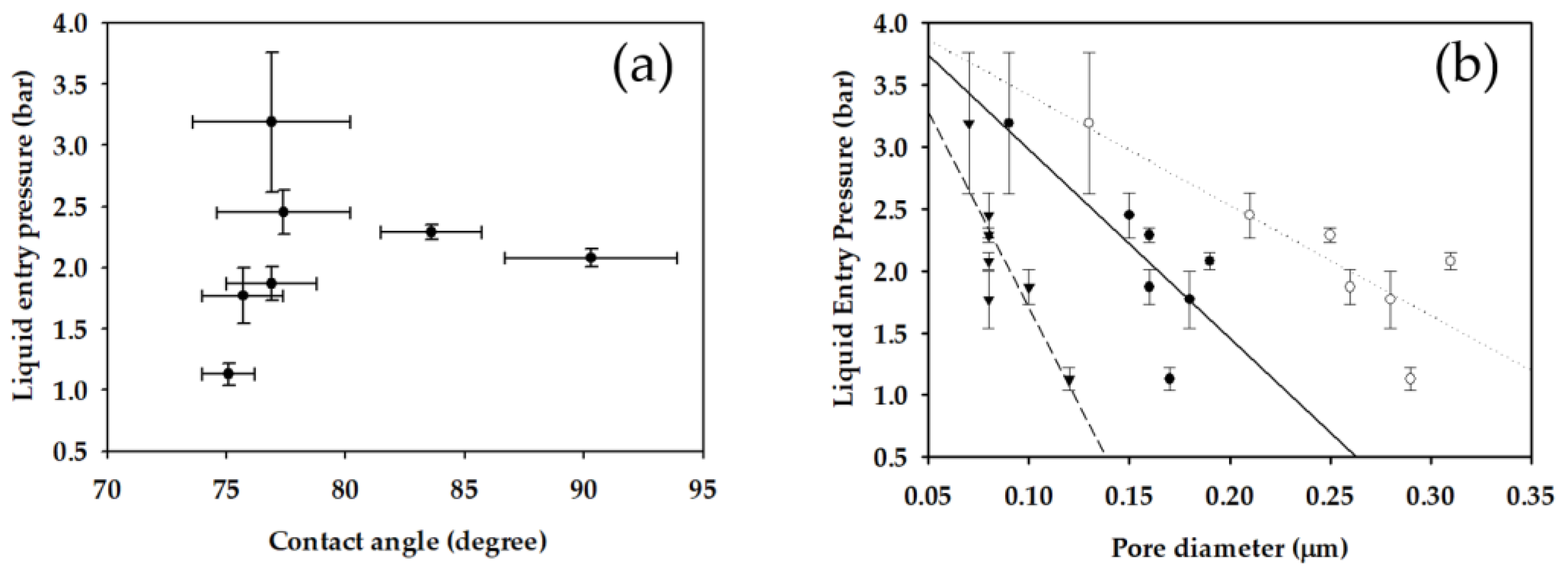
3.4. Correlations among Different Properties
4. Conclusions
- Depending on the fabrication conditions, membranes with flux, rejection, contact angle (CA), liquid entry pressure (LEP), and pore sizes were obtained. Without LiCl, a membrane with small pore size, high LEP and low flux was prepared. When LiCl was used, an increase in PVDF concentration led to the formation of denser membranes. The flux and rejection were further adjusted by controlling the EtOH concentration in the non-solvent.
- Using the equations derived in this work, Bw, Lw, and Rf were calculated. It was found that Bw and Lw ranged from 7.76 × 10−8 s/m to 20.19 × 10−8 s/m and from 0.0020 to 0.0151, respectively. An increase in Bw resulted in an increased Lw, indicating a trade-off between the two properties. This is attributed to the fact that the membranes with higher Bw values have larger pore sizes.
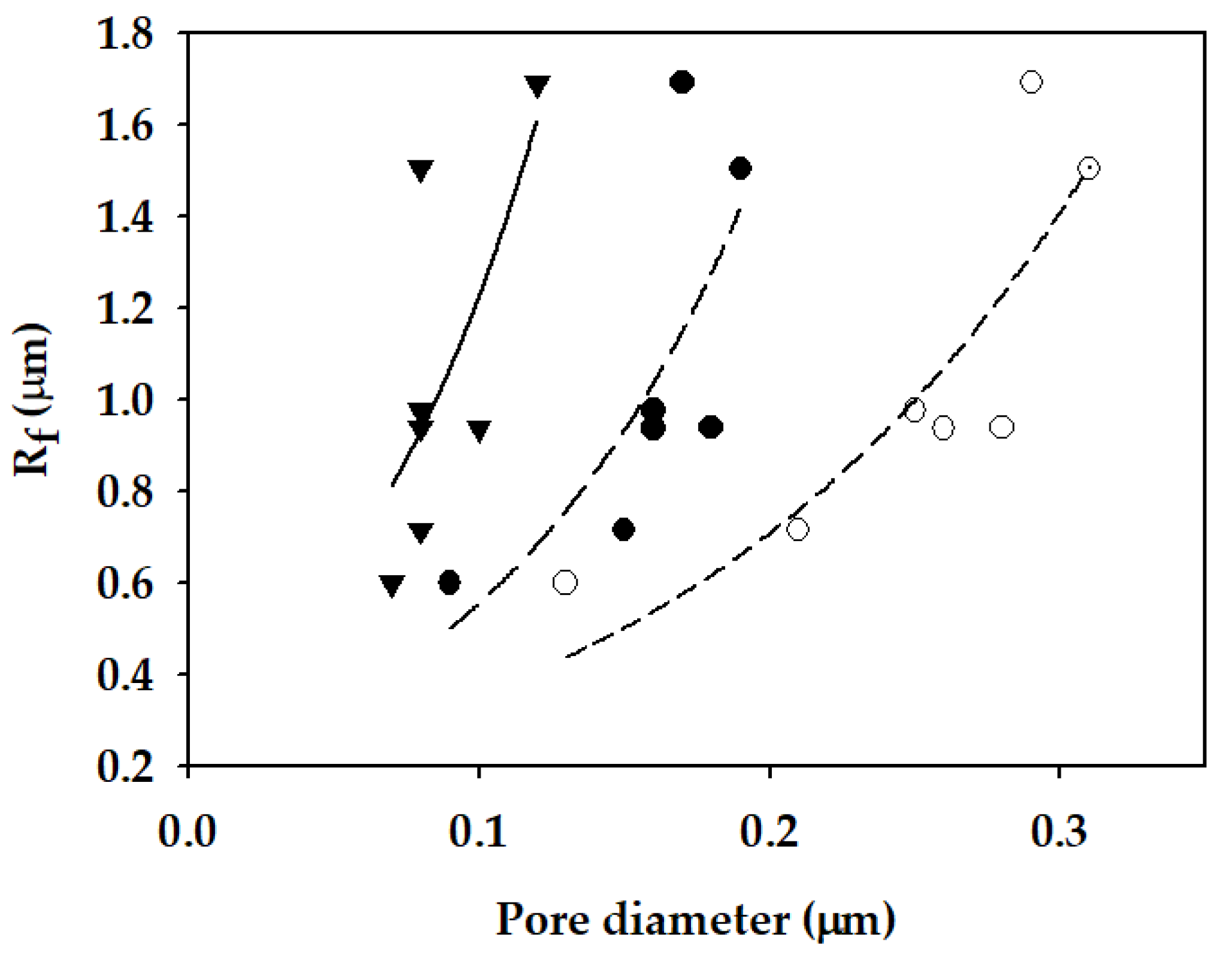
- Rf was calculated in the range from 0.598 μm to 1.690 μm. Since the Rf was relatively small, the prepared membranes can have high LEP (more than 1.13 bar) even at low CA (less than 90.8°). Rf was found to be correlated with the surface roughness measured by AFM.
- An efficient MD membrane should have a high flux, rejection, and LEP with low fouling propensity. The results in this study suggest that the pore size should be high to ensure high Bw but Rf should be small to lower Lw. However, care should be taken in this approach. Since there is a trade-off between pore size and Rf, it may not be possible to simultaneously increase both properties. In addition, an increase in the pore size above a critical value is not allowed due to high risk of the wetting.
- If Rf is sufficiently small, it is plausible to fabricate membranes using moderately hydrophobic materials, which is beneficial to retard fouling due to hydrophobic foulants. Nevertheless, further work should be done to examine this hypothesis.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Am | membrane area (m2) |
| aw | activity of water (-) |
| Bw | water vapor permeability (s/m) |
| cf | salt concentration in the feed (kg/m3) |
| cf,0 | initial feed concentration (kg/m3) |
| cp | permeate concentration (kg/m3) |
| cp,distill | salt concentration in the distilled water (kg/m3) |
| cp,leak | salt concentration in the water leakage (kg/m3) |
| cp,net | salt concentration of the water transferred through the membrane (kg/m3) |
| De | specific gravity of the ethanol (g/cm3) |
| Dp | specific gravity of the PVDF material (g/cm3) |
| Jv | permeate (water) flux (kg/m2-h) |
| Lw | leakage ratio (-) |
| m1 | mass of the saturated membrane (g) |
| m2 | mass of the dry membrane (g) |
| pw | vapor pressure (bar) |
| Rapp | apparent salt rejection (-) |
| Rint | intrinsic rejection (-) |
| subscript f | feed |
| subscript p | permeate |
| superscript 0 | pure water |
| xNaCl | mole fraction of NaCl (-) |
| xw | mole fraction of water (-) |
| Δmg | increased mass of the permeate (g) |
| Δpw | vapor pressure difference (bar) |
| Δt | time interval (s) |
| γ | activity coefficient (-) |
| γ w,f | activity coefficient of water in the feed (-) |
| ρ | density of water (kg/m3) |
References
- Lu, S.; Bai, X.; Li, W.; Wang, N. Impacts of climate change on water resources and grain production. Technol. Forecast. Soc. Chang. 2019, 143, 76–84. [Google Scholar] [CrossRef]
- Zhuang, X.W.; Li, Y.P.; Nie, S.; Fan, Y.R.; Huang, G.H. Analyzing climate change impacts on water resources under uncertainty using an integrated simulation-optimization approach. J. Hydrol. 2018, 556, 523–538. [Google Scholar] [CrossRef]
- Pistocchi, A.; Bleninger, T.; Breyer, C.; Caldera, U.; Dorati, C.; Ganora, D.; Millán, M.M.; Paton, C.; Poullis, D.; Herrero, F.S.; et al. Can seawater desalination be a win-win fix to our water cycle? Water Res. 2020, 182, 115906. [Google Scholar] [CrossRef]
- Aziz, N.I.H.A.; Hanafiah, M.M. Application of life cycle assessment for desalination: Progress, challenges and future directions. Environ. Pollut. 2021, 268, 115948. [Google Scholar] [CrossRef] [PubMed]
- Takabatake, H.; Taniguchi, M.; Kurihara, M. Advanced Technologies for Stabilization and High Performance of Seawater RO Membrane Desalination Plants. Membranes 2021, 11, 138. [Google Scholar] [CrossRef]
- Suwaileh, W.; Johnson, D.; Hilal, N. Membrane desalination and water re-use for agriculture: State of the art and future outlook. Desalination 2020, 491, 114559. [Google Scholar] [CrossRef]
- Ibrahim, Y.; Ismail, R.A.; Ogungbenro, A.; Pankratz, T.; Banat, F.; Arafat, H.A. The sociopolitical factors impacting the adoption and proliferation of desalination: A critical review. Desalination 2021, 498, 114798. [Google Scholar] [CrossRef]
- Lee, S.; Choi, J.; Park, Y.-G.; Shon, H.; Ahn, C.H.; Kim, S.-H. Hybrid desalination processes for beneficial use of reverse osmosis brine: Current status and future prospects. Desalination 2019, 454, 104–111. [Google Scholar] [CrossRef]
- Panagopoulos, A.; Haralambous, K.-J. Environmental impacts of desalination and brine treatment—Challenges and mitigation measures. Mar. Pollut. Bull. 2020, 161, 111773. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.N.; Guen, F.Z.; Ahmed, S.A.; Saleem, H.; Khalil, M.J.; Zaidi, S.J. Energy consumption and environmental impact assessment of desalination plants and brine disposal strategies. Process Saf. Environ. Prot. 2021, 147, 589–608. [Google Scholar] [CrossRef]
- Cho, H.; Choi, J.; Choi, Y.; Lee, S. Ultrasonic-assisted removal of inorganic scales in high-salinity wastewater treatment using membrane distillation. Desalination Water Treat. 2019, 157, 383–392. [Google Scholar] [CrossRef]
- Panagopoulos, A.; Haralambous, K.-J.; Loizidou, M. Desalination brine disposal methods and treatment technologies—A review. Sci. Total Environ. 2019, 693, 133545. [Google Scholar] [CrossRef]
- Choi, Y.; Naidu, G.; Lee, S.; Vigneswaran, S. Recovery of sodium sulfate from seawater brine using fractional submerged membrane distillation crystallizer. Chemosphere 2020, 238, 124641. [Google Scholar] [CrossRef]
- Elcik, H.; Fortunato, L.; Alpatova, A.; Soukane, S.; Orfi, J.; Ali, E.; AlAnsary, H.; Leiknes, T.; Ghaffour, N. Multi-effect distillation brine treatment by membrane distillation: Effect of antiscalant and antifoaming agents on membrane performance and scaling control. Desalination 2020, 493, 114653. [Google Scholar] [CrossRef]
- Drioli, E.; Ali, A.; Macedonio, F. Membrane distillation: Recent developments and perspectives. Desalination 2015, 356, 56–84. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Darwish, N.; Hilal, N. Membrane distillation: A comprehensive review. Desalination 2012, 287, 2–18. [Google Scholar] [CrossRef]
- Tijing, L.D.; Woo, Y.C.; Choi, J.-S.; Lee, S.; Kim, S.-H.; Shon, H.K. Fouling and its control in membrane distillation—A review. J. Membr. Sci. 2015, 475, 215–244. [Google Scholar] [CrossRef]
- Ashoor, B.; Mansour, S.; Giwa, A.; Dufour, V.; Hasan, S. Principles and applications of direct contact membrane distillation (DCMD): A comprehensive review. Desalination 2016, 398, 222–246. [Google Scholar] [CrossRef]
- Park, Y.; Lee, S. Analysis of thermal energy efficiency for hollow fiber membranes in direct contact membrane distillation. Environ. Eng. Res. 2019, 24, 347–353. [Google Scholar] [CrossRef]
- Warsinger, D.M.; Swaminathan, J.; Guillen-Burrieza, E.; Arafat, H.A.; Lienhard, V.J.H. Scaling and fouling in membrane distillation for desalination applications: A review. Desalination 2015, 356, 294–313. [Google Scholar] [CrossRef]
- Ahmed, F.E.; Lalia, B.S.; Hashaikeh, R.; Hilal, N. Alternative heating techniques in membrane distillation: A review. Desalination 2020, 496, 114713. [Google Scholar] [CrossRef]
- Schwantes, R.; Chavan, K.; Winter, D.; Felsmann, C.; Pfafferott, J. Techno-economic comparison of membrane distillation and MVC in a zero liquid discharge application. Desalination 2018, 428, 50–68. [Google Scholar] [CrossRef]
- Alsebaeai, M.K.; Ahmad, A.L. Membrane distillation: Progress in the improvement of dedicated membranes for enhanced hydrophobicity and desalination performance. J. Ind. Eng. Chem. 2020, 86, 13–34. [Google Scholar] [CrossRef]
- Chew, N.G.P.; Zhao, S.; Wang, R. Recent advances in membrane development for treating surfactant-and oil-containing feed streams via membrane distillation. Adv. Colloid Interface Sci. 2019, 273, 102022. [Google Scholar] [CrossRef]
- Guan, G.; Yang, X.; Wang, R.; Fane, A.G. Modular matrix design for large-scale membrane distillation system via Aspen simulations. Desalination 2018, 428, 207–217. [Google Scholar] [CrossRef]
- Eykens, L.; De Sitter, K.; Dotremont, C.; Pinoy, L.; Van der Bruggen, B. Membrane synthesis for membrane distillation: A review. Sep. Purif. Technol. 2017, 182, 36–51. [Google Scholar] [CrossRef]
- Tibi, F.; Charfi, A.; Cho, J.; Kim, J. Fabrication of polymeric membranes for membrane distillation process and application for wastewater treatment: Critical review. Process Saf. Environ. Prot. 2020, 141, 190–201. [Google Scholar] [CrossRef]
- Lu, K.J.; Chen, Y.; Chung, T.-S. Design of omniphobic interfaces for membrane distillation–A review. Water Res. 2019, 162, 64–77. [Google Scholar] [CrossRef]
- Afsari, M.; Shon, H.K.; Tijing, L.D. Janus membranes for membrane distillation: Recent advances and challenges. Adv. Colloid Interface Sci. 2021, 102362. [Google Scholar] [CrossRef]
- Fadhil, S.; Marino, T.; Makki, H.F.; Alsalhy, Q.F.; Blefari, S.; Macedonio, F.; Di Nicolò, E.; Giorno, L.; Drioli, E.; Figoli, A. Novel PVDF-HFP flat sheet membranes prepared by triethyl phosphate (TEP) solvent for direct contact membrane distillation. Chem. Eng. Process. Process Intensif. 2016, 102, 16–26. [Google Scholar] [CrossRef]
- Woo, Y.C.; Kim, Y.; Shim, W.-G.; Tijing, L.D.; Yao, M.; Nghiem, L.D.; Choi, J.-S.; Kim, S.-H.; Shon, H.K. Graphene/PVDF flat-sheet membrane for the treatment of RO brine from coal seam gas produced water by air gap membrane distillation. J. Membr. Sci. 2016, 513, 74–84. [Google Scholar] [CrossRef]
- Zheng, L.; Wu, Z.; Wei, Y.; Zhang, Y.; Yuan, Y.; Wang, J. Preparation of PVDF-CTFE hydrophobic membranes for MD application: Effect of LiCl-based mixed additives. J. Membr. Sci. 2016, 506, 71–85. [Google Scholar] [CrossRef]
- Munirasu, S.; Banat, F.; Durrani, A.A.; Haija, M.A. Intrinsically superhydrophobic PVDF membrane by phase inversion for membrane distillation. Desalination 2017, 417, 77–86. [Google Scholar] [CrossRef]
- Hou, D.; Fan, H.; Jiang, Q.; Wang, J.; Zhang, X. Preparation and characterization of PVDF/nonwoven fabric flat-sheet composite membranes for desalination through direct contact membrane distillation. Sep. Purif. Technol. 2014, 135, 211–222. [Google Scholar] [CrossRef]
- Thomas, R.; Guillen-Burrieza, E.; Arafat, H.A. Pore structure control of PVDF membranes using a 2-stage coagulation bath phase inversion process for application in membrane distillation (MD). J. Membr. Sci. 2014, 452, 470–480. [Google Scholar] [CrossRef]
- Baghbanzadeh, M.; Rana, D.; Matsuura, T.; Lan, C.Q. Effects of hydrophilic CuO nanoparticles on properties and performance of PVDF VMD membranes. Desalination 2015, 369, 75–84. [Google Scholar] [CrossRef]
- Ahmad, A.; Ramli, W. Hydrophobic PVDF membrane via two-stage soft coagulation bath system for Membrane Gas Absorption of CO2. Sep. Purif. Technol. 2013, 103, 230–240. [Google Scholar] [CrossRef]
- Chang, H.-H.; Chang, L.-K.; Yang, C.-D.; Lin, D.-J.; Cheng, L.-P. Effect of polar rotation on the formation of porous poly (vinylidene fluoride) membranes by immersion precipitation in an alcohol bath. J. Membr. Sci. 2016, 513, 186–196. [Google Scholar] [CrossRef]
- Kuo, C.-Y.; Lin, H.-N.; Tsai, H.-A.; Wang, D.-M.; Lai, J.-Y. Fabrication of a high hydrophobic PVDF membrane via nonsolvent induced phase separation. Desalination 2008, 233, 40–47. [Google Scholar] [CrossRef]
- Park, Y.; Ju, J.; Woo, Y.; Choi, J.-S.; Lee, S. Fabrication and characterization of moderately hydrophobic membrane with enhanced permeability using a phase-inversion method in membrane distillation. Desalination Water Treat. 2020, 183, 173–181. [Google Scholar] [CrossRef]
- Kim, Y.; Choi, Y.; Choi, J.; Lee, S. Powdered activated carbon (PAC)—vacuum-assisted air gap membrane distillation (V-AGMD) hybrid system to treat wastewater containing surfactants: Effect of operating conditions. Environ. Eng. Res. 2021, 26, 200377. [Google Scholar] [CrossRef]
- Tijing, L.D.; Woo, Y.C.; Johir, M.A.H.; Choi, J.-S.; Shon, H.K. A novel dual-layer bicomponent electrospun nanofibrous membrane for desalination by direct contact membrane distillation. Chem. Eng. J. 2014, 256, 155–159. [Google Scholar] [CrossRef]
- Choudhury, M.R.; Anwar, N.; Jassby, D.; Rahaman, M.S. Fouling and wetting in the membrane distillation driven wastewater reclamation process–A review. Adv. Colloid Interface Sci. 2019, 269, 370–399. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Choi, J.; Shin, Y.; Cho, H.; Sohn, J.; Lee, S. A feasibility study on sewage discharge water treatment for water reuse by direct contact membrane distillation. Membr. J. 2016, 26, 70–75. [Google Scholar] [CrossRef]
- Khayet, M.; Matsuura, T. Chapter 10—Direct Contact Membrane Distillation. In Membrane Distillation; Khayet, M., Matsuura, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 249–293. [Google Scholar]
- Rahimpour, M.R.; Esmaeilbeig, M.A. Chapter 6—Membrane Wetting in Membrane Distillation. In Current Trends and Future Developments on (Bio-) Membranes; Basile, A., Curcio, E., Inamuddin, Eds.; Elsevier: Melbourne, Australia, 2019; pp. 143–174. [Google Scholar]
- Servi, A.T.; Kharraz, J.; Klee, D.; Notarangelo, K.; Eyob, B.; Guillen-Burrieza, E.; Liu, A.; Arafat, H.A.; Gleason, K.K. A systematic study of the impact of hydrophobicity on the wetting of MD membranes. J. Membr. Sci. 2016, 520, 850–859. [Google Scholar] [CrossRef]
- Kim, B.-S.; Harriott, P. Critical entry pressure for liquids in hydrophobic membranes. J. Colloid Interface Sci. 1987, 115, 1–8. [Google Scholar] [CrossRef]
- Pagliero, M.; Bottino, A.; Comite, A.; Costa, C. Novel hydrophobic PVDF membranes prepared by nonsolvent induced phase separation for membrane distillation. J. Membr. Sci. 2020, 596, 117575. [Google Scholar] [CrossRef]
- Guillen, G.R.; Pan, Y.; Li, M.; Hoek, E.M. Preparation and characterization of membranes formed by nonsolvent induced phase separation: A review. Ind. Eng. Chem. Res. 2011, 50, 3798–3817. [Google Scholar] [CrossRef]




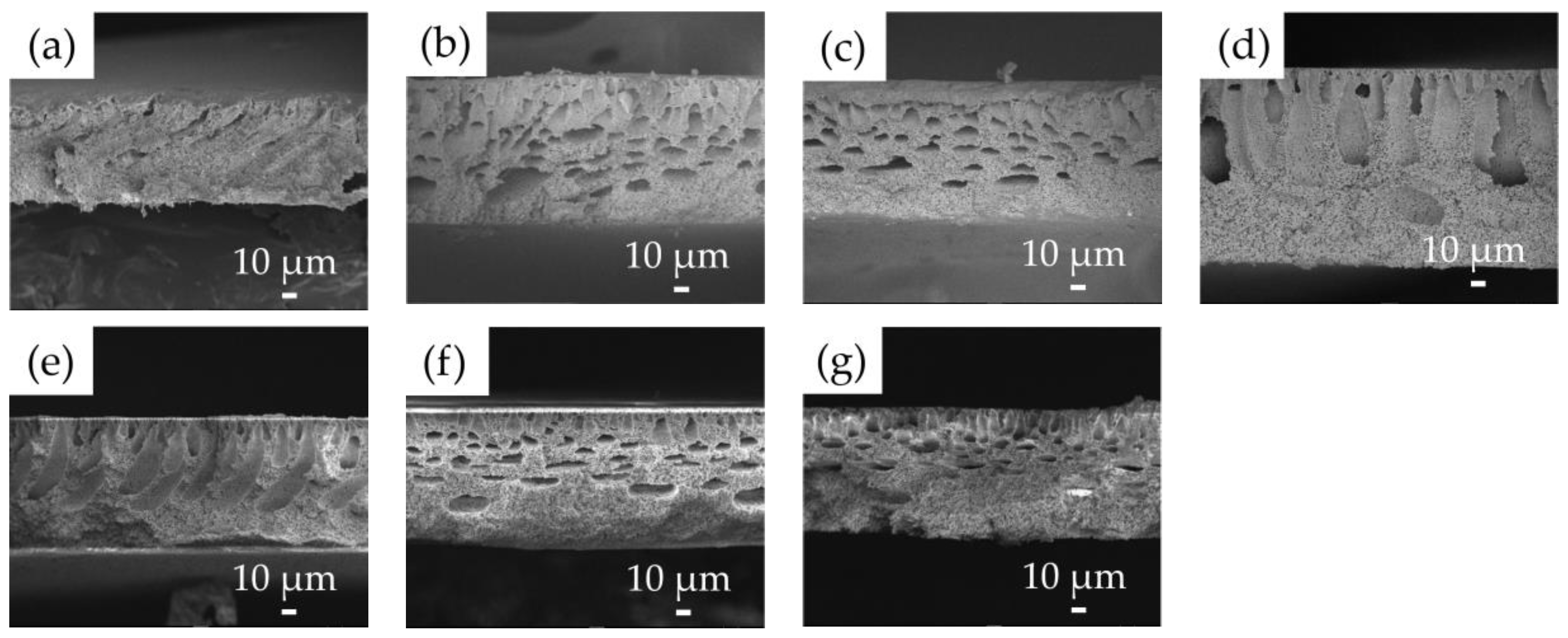



| Membrane Sample | PVDF Concentration [w/w] | LiCl Concentration [w/w] | Solvent | Non-Solvent [v/v] |
|---|---|---|---|---|
| S1 | 14.0% | 0.0% | DMF | Water (100%) |
| S2 | 14.0% | 3.0% | DMF | Water (100%) |
| S3 | 16.0% | 3.0% | DMF | Water (100%) |
| S4 | 18.0% | 3.0% | DMF | Water (100%) |
| S5 | 16.0% | 3.0% | DMF | Water (90%) + EtOH (10%) |
| S6 | 16.0% | 3.0% | DMF | Water (80%) + EtOH (20%) |
| S7 | 16.0% | 3.0% | DMF | Water (70%) + EtOH (30%) |
| Membrane Sample | Contact Angle (°) | LEP (bar) | Membrane Thickness (μm) | Porosity (%) | dmean (μm) | dmax (μm) | dmin (μm) |
|---|---|---|---|---|---|---|---|
| S1 | 76.9 ± 3.3 | 3.19 ± 0.57 | 68.0 ± 3.4 | 85.4 ± 2.5 | 0.09 | 0.13 | 0.07 |
| S2 | 75.1 ± 1.1 | 1.13 ± 0.09 | 80.3 ± 3.3 | 88.3 ± 2.3 | 0.17 | 0.29 | 0.12 |
| S3 | 75.7 ± 1.7 | 1.77 ± 0.23 | 85.2 ± 6.4 | 85.2 ± 2.0 | 0.18 | 0.28 | 0.08 |
| S4 | 76.9 ± 1.9 | 1.87 ± 0.14 | 98.8 ± 3.0 | 84.9 ± 1.3 | 0.16 | 0.26 | 0.10 |
| S5 | 77.4 ± 2.8 | 2.45 ± 0.18 | 78.3 ± 2.4 | 86.1 ± 4.2 | 0.15 | 0.21 | 0.08 |
| S6 | 83.6 ± 2.1 | 2.29 ± 0.06 | 79.7 ± 7.5 | 81.9 ± 1.5 | 0.16 | 0.25 | 0.08 |
| S7 | 90.3 ± 3.6 | 2.08 ± 0.07 | 76.8 ± 0.8 | 83.6 ± 1.1 | 0.19 | 0.31 | 0.08 |
| Membrane Sample | Flux (kg/m2-h) | Apparent Rejection (%) |
|---|---|---|
| S1 | 5.45 ± 0.50 | 99.95 ± 0.005 |
| S2 | 14.19 ± 1.14 | 99.75 ± 0.146 |
| S3 | 13.32 ± 0.33 | 99.87 ± 0.061 |
| S4 | 12.21 ± 0.78 | 99.91 ± 0.0043 |
| S5 | 13.11 ± 0.19 | 99.95 ± 0.019 |
| S6 | 13.73 ± 0.46 | 99.92 ± 0.0190 |
| S7 | 13.87 ± 0.21 | 99.93 ± 0.041 |
| Membrane Sample | Water Vapor Permeability, Bw (×10−8 s/m) | Salt Leakage Ratio, Lw (-) | Fiber Radius, Rf (μm) |
|---|---|---|---|
| S1 | 7.76 ± 0.71 | 0.0020 ± 0.0019 | 0.598 |
| S2 | 20.19 ± 1.62 | 0.0151 ± 0.0043 | 1.690 |
| S3 | 18.96 ± 0.47 | 0.0073 ± 0.0049 | 0.938 |
| S4 | 17.38 ± 1.11 | 0.0054 ± 0.0033 | 0.936 |
| S5 | 18.66 ± 0.27 | 0.0024 ± 0.0006 | 0.714 |
| S6 | 19.54 ± 0.65 | 0.0026 ± 0.0010 | 0.975 |
| S7 | 19.74 ± 0.30 | 0.0045 ± 0.0012 | 1.503 |
| Membrane Sample | Ra (nm) a | Rq (nm) b | Rmax (nm) c |
|---|---|---|---|
| S3 | 20.01 | 25.47 | 89.87 |
| S5 | 18.51 | 23.97 | 83.49 |
| S6 | 27.03 | 34.09 | 103.22 |
| S7 | 40.80 | 51.13 | 129.44 |
| Membrane Sample | Contact Angle (°) | LEP (bar) | dmean (μm) | Flux (kg/m2h) | Apparent Rejection (%) | Water Vapor Permeability, Bw, (×10−8 s/m) | Salt Leakage Ratio, Lw (-) | Fiber Radius, Rf (μm) |
|---|---|---|---|---|---|---|---|---|
| S6 | 83.6 ± 2.1 | 2.29 ± 0.06 | 0.16 | 13.73 ± 0.46 | 99.92 ± 0.0190 | 19.54 ± 0.65 | 0.0026 ± 0.0010 | 0.975 |
| S7 | 90.3 ± 3.6 | 2.08 ± 0.07 | 0.19 | 13.87 ± 0.21 | 99.93 ± 0.0410 | 19.74 ± 0.30 | 0.0045 ± 0.0012 | 1.503 |
| M1 [40] | 75.7 ± 1.4 | 2.93 ± 0.06 | 0.11 | 20.20 ± 0.10 | 99.93 ± 0.0182 | 28.73 ± 0.27 | 0.0015 ± 0.0003 | 0.618 |
| M2 [40] | 73.2 ± 2.7 | 4.16 ± 0.25 | 0.07 | 8.6 ± 0.11 | 99.93 ± 0.0145 | 12.26 ± 0.12 | 0.0026 ± 0.0007 | 0.350 |
| Commercial PVDF [40] | 126.8 ± 1.1 | 1.81 ± 0.16 | 0.22 | 15.10 ± 0.61 | 99.93 ± 0.0087 | 21.69 ± 0.65 | 0.0023 ± 0.0009 | 0.429 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, M.; Cho, H.; Choi, Y.; Lee, S.; Lee, S. Analysis of Polyvinylidene Fluoride Membranes Fabricated for Membrane Distillation. Membranes 2021, 11, 437. https://doi.org/10.3390/membranes11060437
Ahn M, Cho H, Choi Y, Lee S, Lee S. Analysis of Polyvinylidene Fluoride Membranes Fabricated for Membrane Distillation. Membranes. 2021; 11(6):437. https://doi.org/10.3390/membranes11060437
Chicago/Turabian StyleAhn, Minchul, Hyeongrak Cho, Yongjun Choi, Seockheon Lee, and Sangho Lee. 2021. "Analysis of Polyvinylidene Fluoride Membranes Fabricated for Membrane Distillation" Membranes 11, no. 6: 437. https://doi.org/10.3390/membranes11060437
APA StyleAhn, M., Cho, H., Choi, Y., Lee, S., & Lee, S. (2021). Analysis of Polyvinylidene Fluoride Membranes Fabricated for Membrane Distillation. Membranes, 11(6), 437. https://doi.org/10.3390/membranes11060437






