Synergistic Effect of Chemical Penetration Enhancers on Lidocaine Permeability Revealed by Coarse-Grained Molecular Dynamics Simulations
Abstract
1. Introduction
2. Materials and Methods
2.1. Coarse-Grained Models of Ethanol, Linoleic Acid, Lidocaine and SC Membrane
2.2. Details of Coarse-Grained Simulations and Analysis
3. Results
3.1. Equilibrium MD Simulations of Lidocaine in the Absence and Presence of the CPEs
3.2. PMF Calculations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anselmo, A.C.; Mitragotri, S. An overview of clinical and commercial impact of drug delivery systems. J. Control. Release 2014, 190, 15–28. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A.P. Essential oils and their constituents as skin penetration enhancer for transdermal drug delivery: A review. J. Pharm. Pharmacol. 2015, 67, 473–485. [Google Scholar] [CrossRef]
- Williams, A.C.; Barry, B.W. Penetration enhancers. Adv. Drug Deliv. Rev. 2004, 56, 603–618. [Google Scholar] [CrossRef]
- Kapoor, A.; Mishra, S.K.; Verma, D.K.; Pandey, P. Chemical penetration enhancers for transdermal drug delivery system. J. Drug Deliv. Ther. 2018, 8, 62–66. [Google Scholar] [CrossRef]
- Chen, W.; Liu, C.; Ji, X.; Joseph, J.; Tang, Z.; Ouyang, J.; Xiao, Y.; Kong, N.; Joshi, N.; Farokhzad, O.C.; et al. Stanene-based nanosheets for β-elemene delivery and ultrasound-mediated combination cancer therapy. Angew. Chem. Weinh. Bergstr. Ger. 2021, 133, 7231–7240. [Google Scholar] [CrossRef]
- Prashar, M.; Aggarwal, G.; Harikumar, S.L. Synergistic action of penetration enhancers in transdermal drug delivery. J. Drug Deliv. Ther. 2014, 4, 45–51. [Google Scholar] [CrossRef]
- Chen, W.; Cheng, C.-A.; Lee, B.-Y.; Clemens, D.L.; Huang, W.-Y.; Horwitz, M.A.; Zink, J.I. Facile strategy enabling both high loading and high release amounts of the water-insoluble drug clofazimine using mesoporous silica nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 31870–31881. [Google Scholar] [CrossRef]
- Alkilani, A.Z.; McCrudden, M.T.C.; Donnelly, R.F. Transdermal drug delivery: Innovative pharmaceutical developments based on disruption of the barrier properties of the stratum corneum. Pharmaceutics 2015, 7, 438–470. [Google Scholar] [CrossRef]
- Ghafourian, T.; Nokhodchi, A.; Kaialy, W. Surfactants as Penetration Enhancers for Dermal and Transdermal Drug Delivery. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement; Springer: Berlin/Heidelberg, Germany, 2015; pp. 207–230. [Google Scholar]
- Amjadi, M.; Mostaghaci, B.; Sitti, M. Recent advances in skin penetration enhancers for transdermal gene and drug delivery. Curr. Gene Ther. 2017, 17, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Mitragotri, S. Discovery of transdermal penetration enhancers for drug delivery. Biophys. J. 2010, 98, 436a. [Google Scholar] [CrossRef][Green Version]
- Priyanka, K.; Singh, S. A Review on skin targeted delivery of bioactives as ultradeformable vesicles: Overcoming the penetration problem. Curr. Drug Targets 2014, 15, 184–198. [Google Scholar] [CrossRef]
- Karande, P.; Jain, A.; Mitragotri, S. Insights into synergistic interactions in binary mixtures of chemical permeation enhancers for transdermal drug delivery. J. Control. Release 2006, 115, 85–93. [Google Scholar] [CrossRef]
- Karande, P.; Mitragotri, S. Enhancement of transdermal drug delivery via synergistic action of chemicals. Biochim. Biophys. Acta 2009, 1788, 2362–2373. [Google Scholar] [CrossRef]
- Karande, P.; Jain, A.; Mitragotri, S. Discovery of transdermal penetration enhancers by high-throughput screening. Nat. Biotechnol. 2004, 22, 192–197. [Google Scholar] [CrossRef]
- Das, C.; Noro, M.G.; Olmsted, P.D. Simulation studies of stratum corneum lipid mixtures. Biophys. J. 2009, 97, 1941–1951. [Google Scholar] [CrossRef]
- Guo, S.; Moore, T.C.; Iacovella, C.R.; Strickland, L.A.; McCabe, C. Simulation study of the structure and phase behavior of ceramide bilayers and the role of lipid head group chemistry. J. Chem. Theory Comput. 2013, 9, 5116–5126. [Google Scholar] [CrossRef]
- Das, C.; Olmsted, P.D.; Noro, M.G. Water permeation through stratum corneum lipid bilayers from atomistic simulations. Soft Matter 2009, 5, 4549. [Google Scholar] [CrossRef]
- Paloncýová, M.; Vávrová, K.; Sovová, Ž.; DeVane, R.; Otyepka, M.; Berka, K. Structural changes in ceramide bilayers rationalize increased permeation through stratum corneum models with shorter acyl tails. J. Phys. Chem. B 2015, 119, 9811–9819. [Google Scholar] [CrossRef]
- Paloncýová, M.; DeVane, R.H.; Murch, B.P.; Berka, K.; Otyepka, M. Rationalization of reduced penetration of drugs through ceramide gel phase membrane. Langmuir 2014, 30, 13942–13948. [Google Scholar] [CrossRef]
- Sovová, Ž.; Berka, K.; Otyepka, M.; Jurečka, P. Coarse-grain simulations of skin ceramide ns with newly derived parameters clarify structure of melted phase. J. Phys. Chem. B 2015, 119, 3988–3998. [Google Scholar] [CrossRef]
- Kwak, S.; Lafleur, M. Effect of dimethyl sulfoxide on the phase behavior of model stratum corneum lipid mixtures. Chem. Phys. Lipids 2009, 161, 11–21. [Google Scholar] [CrossRef]
- Moore, T.C.; Hartkamp, R.; Iacovella, C.R.; Bunge, A.L.; McCabe, C. Effect of ceramide tail length on the structure of model stratum corneum lipid bilayers. Biophys. J. 2018, 114, 113–125. [Google Scholar] [CrossRef]
- Wang, E.; Klauda, J.B. Simulations of pure ceramide and ternary lipid mixtures as simple interior stratum corneum models. J. Phys. Chem. B 2018, 122, 2757–2768. [Google Scholar] [CrossRef]
- Gupta, R.; Sridhar, D.B.; Rai, B. Molecular dynamics simulation study of permeation of molecules through skin lipid bilayer. J. Phys. Chem. B 2016, 120, 8987–8996. [Google Scholar] [CrossRef]
- Gupta, R.; Dwadasi, B.S.; Rai, B. Molecular dynamics simulation of skin lipids: Effect of ceramide chain lengths on bilayer properties. J. Phys. Chem. B 2016, 120, 12536–12546. [Google Scholar] [CrossRef]
- Gupta, R.; Rai, B. Effect of size and surface charge of gold nanoparticles on their skin permeability: A molecular dynamics study. Sci. Rep. 2017, 7, 45292. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Kashyap, N.; Rai, B. Transdermal cellular membrane penetration of proteins with gold nanoparticles: A molecular dynamics study. Phys. Chem. Chem. Phys. 2017, 19, 7537–7545. [Google Scholar] [CrossRef]
- Gupta, R.; Rai, B. In-silico design of nanoparticles for transdermal drug delivery application. Nanoscale 2018, 10, 4940–4951. [Google Scholar] [CrossRef] [PubMed]
- Thind, R.; O’Neill, D.W.; Del Regno, A.; Notman, R. Ethanol induces the formation of water-permeable defects in model bilayers of skin lipids. Chem. Commun. 2015, 51, 5406–5409. [Google Scholar] [CrossRef]
- Del Regno, A.; Notman, R. Permeation pathways through lateral domains in model membranes of skin lipids. Phys. Chem. Chem. Phys. 2018, 20, 2162–2174. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Klauda, J.B. Molecular structure of the long periodicity phase in the stratum corneum. Biophys. J. 2019, 116, 571a. [Google Scholar] [CrossRef]
- Hughes, Z.E.; Mancera, R.L. Molecular dynamics simulations of mixed dopc–β-sitosterol bilayers and their interactions with DMSO. Soft Matter 2013, 9, 2920. [Google Scholar] [CrossRef]
- Wang, H.; Meng, F. The permeability enhancing mechanism of menthol on skin lipids: A molecular dynamics simulation study. J. Mol. Modeling 2017, 23, 23. [Google Scholar] [CrossRef]
- Notman, R.; den Otter, W.K.; Noro, M.G.; Briels, W.J.; Anwar, J. The permeability enhancing mechanism of dmso in ceramide bilayers simulated by molecular dynamics. Biophys. J. 2007, 93, 2056–2068. [Google Scholar] [CrossRef] [PubMed]
- Lundborg, M.; Wennberg, C.L.; Narangifard, A.; Lindahl, E.; Norlén, L. Predicting drug permeability through skin using molecular dynamics simulation. J. Control. Release 2018, 283, 269–279. [Google Scholar] [CrossRef]
- Gupta, R.; Badhe, Y.; Rai, B.; Mitragotri, S. Molecular mechanism of the skin permeation enhancing effect of ethanol: A molecular dynamics study. RSC Adv. 2020, 10, 12234–12248. [Google Scholar] [CrossRef]
- Johnson, M.E.; Mitragotri, S.; Patel, A.; Blankschtein, D.; Langer, R. Synergistic effects of chemical enhancers and therapeutic ultrasound on transdermal drug delivery. J. Pharm. Sci. 1996, 85, 670–679. [Google Scholar] [CrossRef]
- Dragicevic, N.; Maibach, H.I. Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement: Modification of the Stratum Corneum; Springer: Berlin/Heilderberg, Germany, 2015; ISBN 9783662470398. [Google Scholar]
- Sieniawska, E.; Sawicki, R.; Swatko-Ossor, M.; Napiorkowska, A.; Przekora, A.; Ginalska, G.; Augustynowicz-Kopec, E. The effect of combining natural terpenes and antituberculous agents against reference and clinical mycobacterium tuberculosis strains. Molecules 2018, 23, 176. [Google Scholar] [CrossRef]
- Zhao, Q.; Dai, C.; Fan, S.; Lv, J.; Nie, L. Synergistic efficacy of salicylic acid with a penetration enhancer on human skin monitored by oct and diffuse reflectance spectroscopy. Sci. Rep. 2016, 6, 34954. [Google Scholar] [CrossRef]
- Mitragotri, S. Synergistic effect of enhancers for transdermal drug delivery. Pharm. Res. 2000, 17, 1354–1359. [Google Scholar] [CrossRef]
- Karande, P.; Jain, A.; Ergun, K.; Kispersky, V.; Mitragotri, S. Design principles of chemical penetration enhancers for transdermal drug delivery. Proc. Natl. Acad. Sci. USA 2005, 102, 4688–4693. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Quan, P.; Liu, X.; Wang, M.; Fang, L. Novel chemical permeation enhancers for transdermal drug delivery. Asian J. Pharm. Sci. 2014, 9, 51–64. [Google Scholar] [CrossRef]
- Lopes, L.B.; Garcia, M.T.J.; Bentley, M.V.L.B. Chemical penetration enhancers. Ther. Deliv. 2015, 6, 1053–1061. [Google Scholar] [CrossRef]
- Lee, P.J.; Ahmad, N.; Langer, R.; Mitragotri, S.; Prasad Shastri, V. Evaluation of chemical enhancers in the transdermal delivery of lidocaine. Int. J. Pharm. 2006, 308, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.W.; Peterson, M.R.; DeBerard, S.C. Regional anesthesia. Nerve blocks of the extremities and face. Postgrad. Med. 1999, 106, 69–73, 77–78. [Google Scholar] [CrossRef] [PubMed]
- Miwa, Y.; Hamamoto, H.; Ishida, T. Lidocaine self-sacrificially improves the skin permeation of the acidic and poorly water-soluble drug etodolac via its transformation into an ionic liquid. Eur. J. Pharm. Biopharm. 2016, 102, 92–100. [Google Scholar] [CrossRef]
- Coderch, L.; Collini, I.; Carrer, V.; Barba, C.; Alonso, C. Assessment of finite and infinite dose in vitro experiments in transdermal drug delivery. Pharmaceutics 2021, 13, 364. [Google Scholar] [CrossRef] [PubMed]
- Kushla, G.P.; Zatz, J.L. Influence of pH on lidocaine penetration through human and hairless mouse skin in vitro. Int. J. Pharm. 1991, 71, 167–173. [Google Scholar] [CrossRef]
- Sarpotdar, P.P.; Zatz, J.L. Evaluation of penetration enhancement of lidocaine by nonionic surfactants through hairless mouse skin in vitro. J. Pharm. Sci. 1986, 75, 176–181. [Google Scholar] [CrossRef]
- Sarheed, O.; Dibi, M.; Ramesh, K.V.R.N.S.; Drechsler, M. Fabrication of alginate-based O/W nanoemulsions for transdermal drug delivery of lidocaine: Influence of the oil phase and surfactant. Molecules 2021, 26, 2556. [Google Scholar] [CrossRef]
- Ben-Shabat, S.; Baruch, N.; Sintov, A.C. Conjugates of unsaturated fatty acids with propylene glycol as potentially less-irritant skin penetration enhancers. Drug Dev. Ind. Pharm. 2007, 33, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Marrink, S.J.; Tieleman, D.P. Perspective on the martini model. Chem. Soc. Rev. 2013, 42, 6801–6822. [Google Scholar] [CrossRef] [PubMed]
- Alessandri, R.; Grünewald, F.; Marrink, S.J. The martini model in materials science. Adv. Mater. 2021, e2008635. [Google Scholar] [CrossRef] [PubMed]
- Alessandri, R.; Souza, P.C.T.; Thallmair, S.; Melo, M.N.; de Vries, A.H.; Marrink, S.J. Pitfalls of the martini model. J. Chem. Theory Comput. 2019, 15, 5448–5460. [Google Scholar] [CrossRef]
- Stark, A.C.; Andrews, C.T.; Elcock, A.H. Toward Optimized potential functions for protein-protein interactions in aqueous solutions: Osmotic second virial coefficient calculations using the MARTINI coarse-grained force field. J. Chem. Theory Comput. 2013, 9, 4176–4185. [Google Scholar] [CrossRef]
- Marrink, S.J.; Corradi, V.; Souza, P.C.T.; Ingólfsson, H.I.; Tieleman, D.P.; Sansom, M.S.P. Computational modeling of realistic cell membranes. Chem. Rev. 2019, 119, 6184–6226. [Google Scholar] [CrossRef] [PubMed]
- Orekhov, P.S.; Kholina, E.G.; Bozdaganyan, M.E.; Nesterenko, A.M.; Kovalenko, I.B.; Strakhovskaya, M.G. Molecular mechanism of uptake of cationic photoantimicrobial phthalocyanine across bacterial membranes revealed by molecular dynamics simulations. J. Phys. Chem. B 2018, 122, 3711–3722. [Google Scholar] [CrossRef]
- Bereau, T.; Kremer, K. Automated Parametrization of the coarse-grained martini force field for small organic molecules. J. Chem. Theory Comput. 2015, 11, 2783–2791. [Google Scholar] [CrossRef]
- Qi, Y.; Ingólfsson, H.I.; Cheng, X.; Lee, J.; Marrink, S.J.; Im, W. CHARMM-GUI martini maker for coarse-grained simulations with the martini force field. J. Chem. Theory Comput. 2015, 11, 4486–4494. [Google Scholar] [CrossRef]
- Wassenaar, T.A.; Ingólfsson, H.I.; Böckmann, R.A.; Tieleman, D.P.; Marrink, S.J. Computational lipidomics with insane: A versatile tool for generating custom membranes for molecular simulations. J. Chem. Theory Comput. 2015, 11, 2144–2155. [Google Scholar] [CrossRef]
- de Jong, D.H.; Baoukina, S.; Ingólfsson, H.I.; Marrink, S.J. Martini straight: Boosting performance using a shorter cutoff and GPUs. Comput. Phys. Commun. 2016, 199, 1–7. [Google Scholar] [CrossRef]
- Yesylevskyy, S.O.; Schäfer, L.V.; Sengupta, D.; Marrink, S.J. Polarizable water model for the coarse-grained MARTINI force field. PLoS Comput. Biol. 2010, 6, e1000810. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Bozdaganyan, M.E.; Lokhmatikov, A.V.; Voskoboynikova, N.; Cherepanov, D.A.; Steinhoff, H.-J.; Shaitan, K.V.; Mulkidjanian, A.Y. Proton leakage across lipid bilayers: Oxygen atoms of phospholipid ester linkers align water molecules into transmembrane water wires. Biochim. Biophys. Acta Bioenerg. 2019, 1860, 439–451. [Google Scholar] [CrossRef]
- Kholina, E.G.; Kovalenko, I.B.; Bozdaganyan, M.E.; Strakhovskaya, M.G.; Orekhov, P.S. Cationic antiseptics facilitate pore formation in model bacterial membranes. J. Phys. Chem. B 2020, 124, 8593–8600. [Google Scholar] [CrossRef]
- Michaud-Agrawal, N.; Denning, E.J.; Woolf, T.B.; Beckstein, O. MDAnalysis: A Toolkit for the Analysis of Molecular Dynamics Simulations. J. Comput. Chem. 2011, 32, 2319–2327. [Google Scholar] [CrossRef]
- Gupta, R.; Dwadasi, B.S.; Rai, B.; Mitragotri, S. Effect of chemical permeation enhancers on skin permeability: In silico screening using molecular dynamics simulations. Sci. Rep. 2019, 9, 1456. [Google Scholar] [CrossRef]
- Lundborg, M.; Narangifard, A.; Wennberg, C.L.; Lindahl, E.; Daneholt, B.; Norlén, L. Human skin barrier structure and function analyzed by cryo-em and molecular dynamics simulation. J. Struct. Biol. 2018, 203, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Vasyuchenko, E.P.; Orekhov, P.S.; Armeev, G.A.; Bozdaganyan, M.E. CPE-DB: An open database of chemical penetration enhancers. Pharmaceutics 2021, 13, 66. [Google Scholar] [CrossRef]
- Panchagnula, R.; Salve, P.S.; Thomas, N.S.; Jain, A.K.; Ramarao, P. Transdermal delivery of naloxone: Effect of water, propylene glycol, ethanol and their binary combinations on permeation through rat skin. Int. J. Pharm. 2001, 219, 95–105. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Li, S.K. Efficiency of fatty acids as chemical penetration enhancers: Mechanisms and structure enhancement relationship. Pharm. Res. 2010, 27, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Čižinauskas, V.; Elie, N.; Brunelle, A.; Briedis, V. Skin penetration enhancement by natural oils for dihydroquercetin delivery. Molecules 2017, 22, 1536. [Google Scholar] [CrossRef] [PubMed]
- Golden, G.M.; McKie, J.E.; Potts, R.O. Role of stratum corneum lipid fluidity in transdermal drug flux. J. Pharm. Sci. 1987, 76, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Gurtovenko, A.A.; Anwar, J. Interaction of ethanol with biological membranes: The formation of non-bilayer structures within the membrane interior and their significance. J. Phys. Chem. B 2009, 113, 1983–1992. [Google Scholar] [CrossRef] [PubMed]
- Van der Merwe, D.; Riviere, J.E. Comparative studies on the effects of water, ethanol and water/ethanol mixtures on chemical partitioning into porcine stratum corneum and silastic membrane. Toxicol. In Vitro 2005, 19, 69–77. [Google Scholar] [CrossRef]


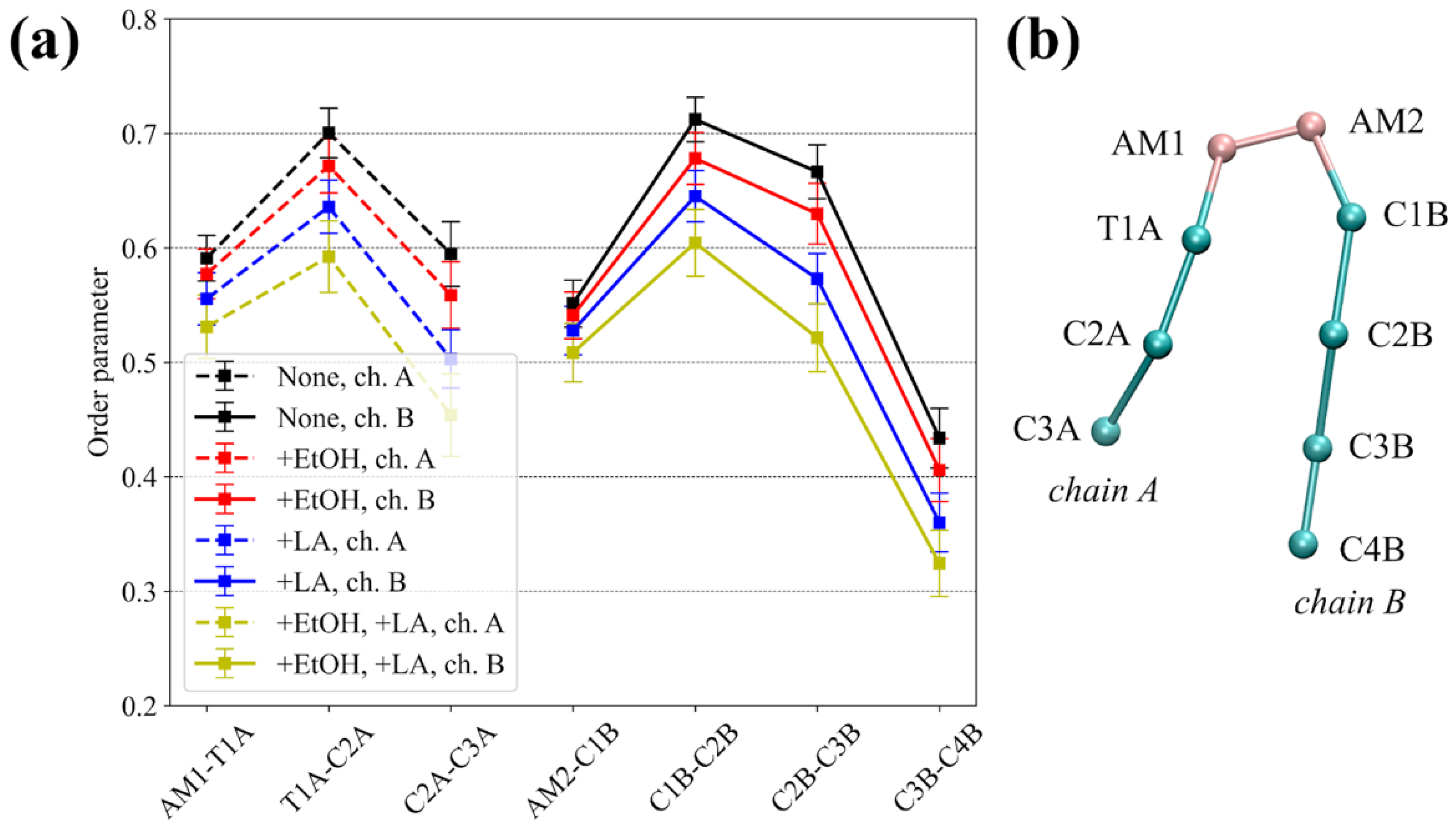

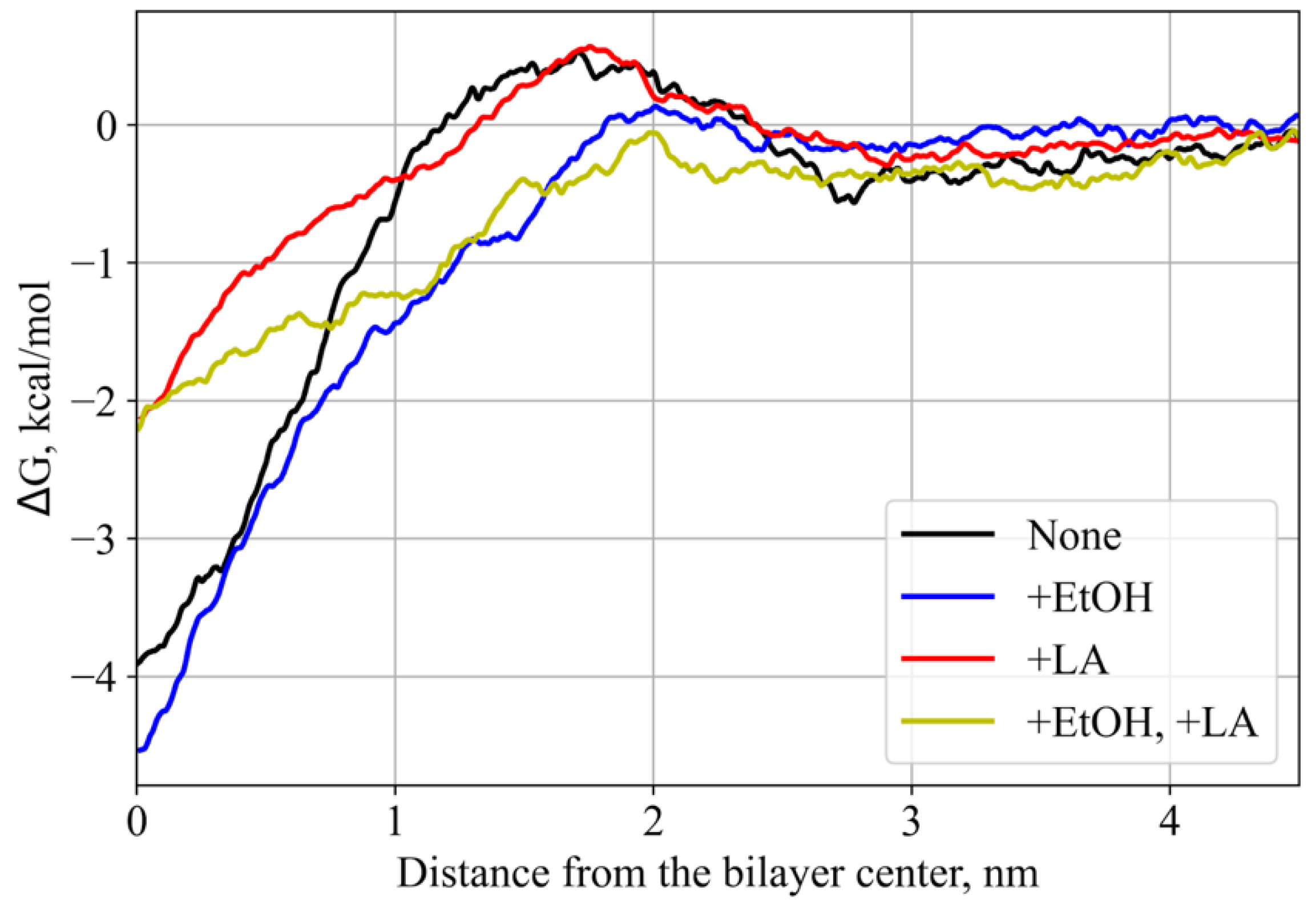
| # | System Composition: Name/Molecules | Simulation Type | Total Simulation Time, μs |
|---|---|---|---|
| 1 | SC membrane (208 CHOL + 209 DPCE + 208 BCN) 1:1:1 + 21740 PW + 397 Na+ + 81 Cl− | Equilibrium | 1 |
| 2 | SC membrane (208 CHOL + 209 DPCE + 208 BCN + 57 LA) 18:18:18:5 + 21729 PW + 419 Na+ + 59 Cl− | Equilibrium | 1 |
| 3 | SC membrane (208 CHOL + 209 DPCE + 208 BCN) 1:1:1 + 10870 PW + 10870 EtOH + 397 Na+ + 81 Cl− | Equilibrium | 1 |
| 4 | SC membrane (208 CHOL + 209 DPCE + 208 BCN + 57 LA) 18:18:18:5 + 10870 PW + 10870 EtOH + 419Na+ + 59 Cl− | Equilibrium | 1 |
| 5 | SC membrane (208 CHOL + 209 DPCE + 208 BCN) 1:1:1 + 1 LID + 21740 PW + 397 Na+ + 81 Cl− | PMF | 0.46 |
| 6 | SC membrane (208 CHOL + 209 DPCE + 208 FFA + 57 LA) 18:18:18:5 + 1 LID + 21729 PW + 419 Na+ + 59 Cl− | PMF | 0.47 |
| 7 | SC membrane (208 CHOL + 209 DPCE + 208 FFA) 1:1:1 + 1 LID + 10870 PW + 10870 EtOH + 397 Na+ + 81 Cl− | PMF | 0.46 |
| 8 | SC membrane (208 CHOL + 209 DPCE + 208 BCN + 57 LA) 18:18:18:5 + 1 LID + 10870 PW + 10870 EtOH + 419 Na+ + 59 Cl− | PMF | 0.47 |
| System | Interleaflet Distance, Å | ||
|---|---|---|---|
| DPCE | Behenic Acid | Cholesterol | |
| None | 24.78 | 38.55 | 30.54 |
| +EtOH | 22.53 | 37.55 | 28.54 |
| +LA | 23.28 | 37.80 | 29.54 |
| +EtOH, +LA | 22.03 | 36.30 | 27.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bozdaganyan, M.E.; Orekhov, P.S. Synergistic Effect of Chemical Penetration Enhancers on Lidocaine Permeability Revealed by Coarse-Grained Molecular Dynamics Simulations. Membranes 2021, 11, 410. https://doi.org/10.3390/membranes11060410
Bozdaganyan ME, Orekhov PS. Synergistic Effect of Chemical Penetration Enhancers on Lidocaine Permeability Revealed by Coarse-Grained Molecular Dynamics Simulations. Membranes. 2021; 11(6):410. https://doi.org/10.3390/membranes11060410
Chicago/Turabian StyleBozdaganyan, Marine E., and Philipp S. Orekhov. 2021. "Synergistic Effect of Chemical Penetration Enhancers on Lidocaine Permeability Revealed by Coarse-Grained Molecular Dynamics Simulations" Membranes 11, no. 6: 410. https://doi.org/10.3390/membranes11060410
APA StyleBozdaganyan, M. E., & Orekhov, P. S. (2021). Synergistic Effect of Chemical Penetration Enhancers on Lidocaine Permeability Revealed by Coarse-Grained Molecular Dynamics Simulations. Membranes, 11(6), 410. https://doi.org/10.3390/membranes11060410








