Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome: Propensity Score Matching
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Definitions
2.3. Statistical Analysis
3. Results
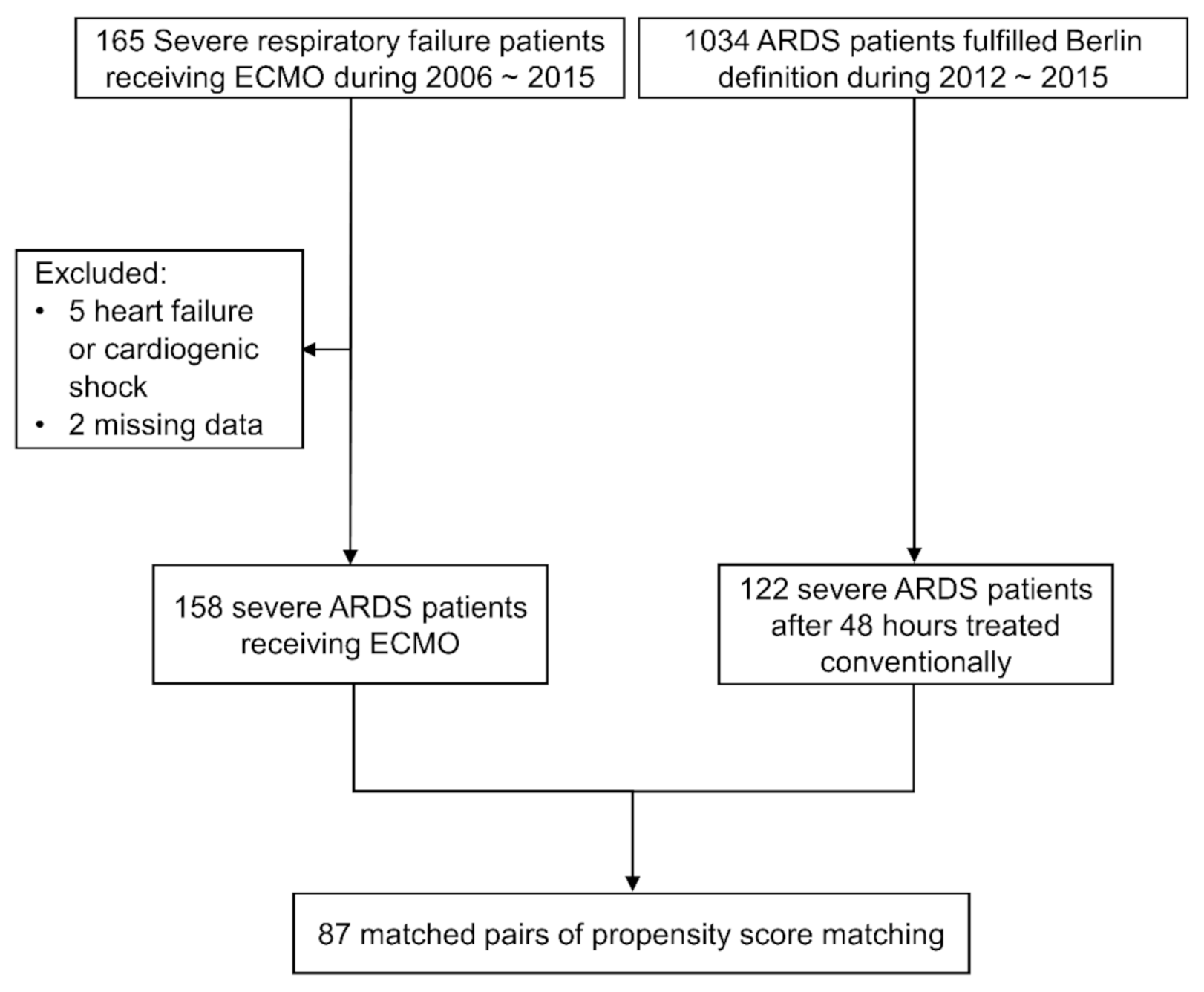
3.1. Patients
3.2. Comparisons of ECMO Group and non-ECMO Group before and after Matching
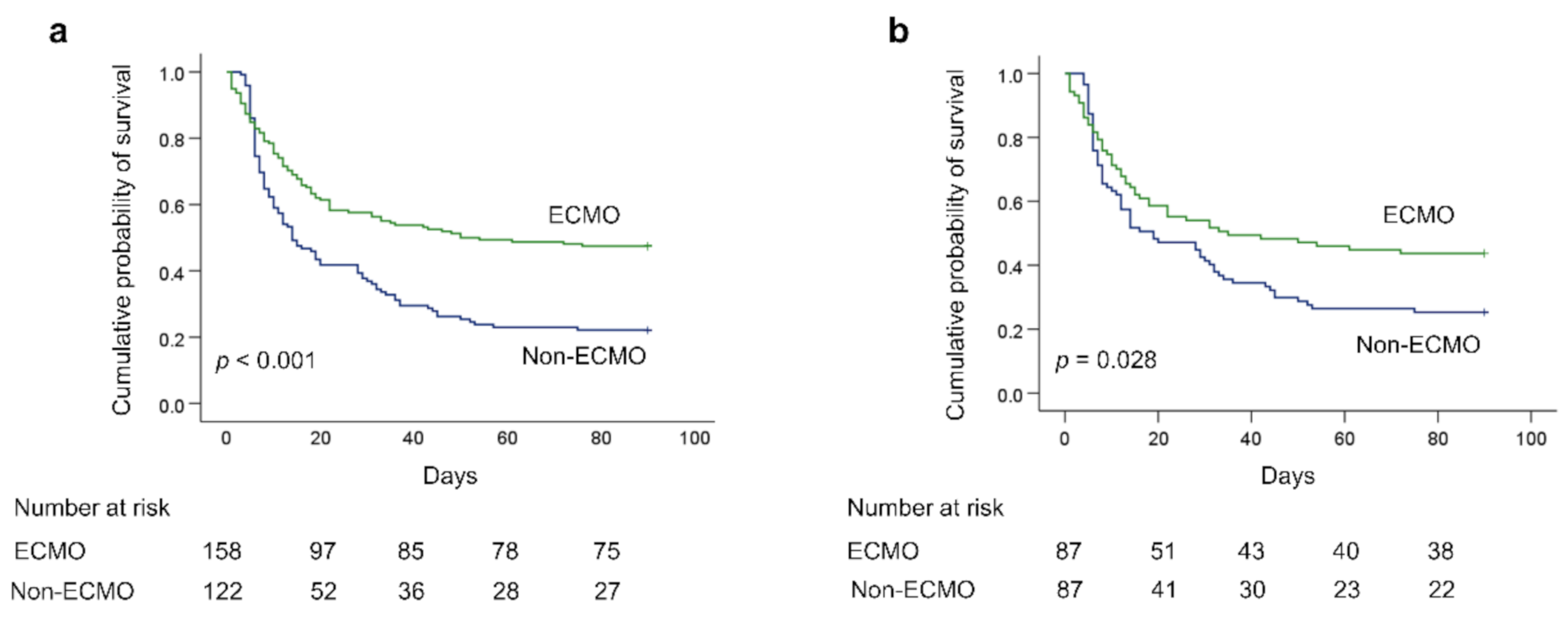
3.3. Outcomes
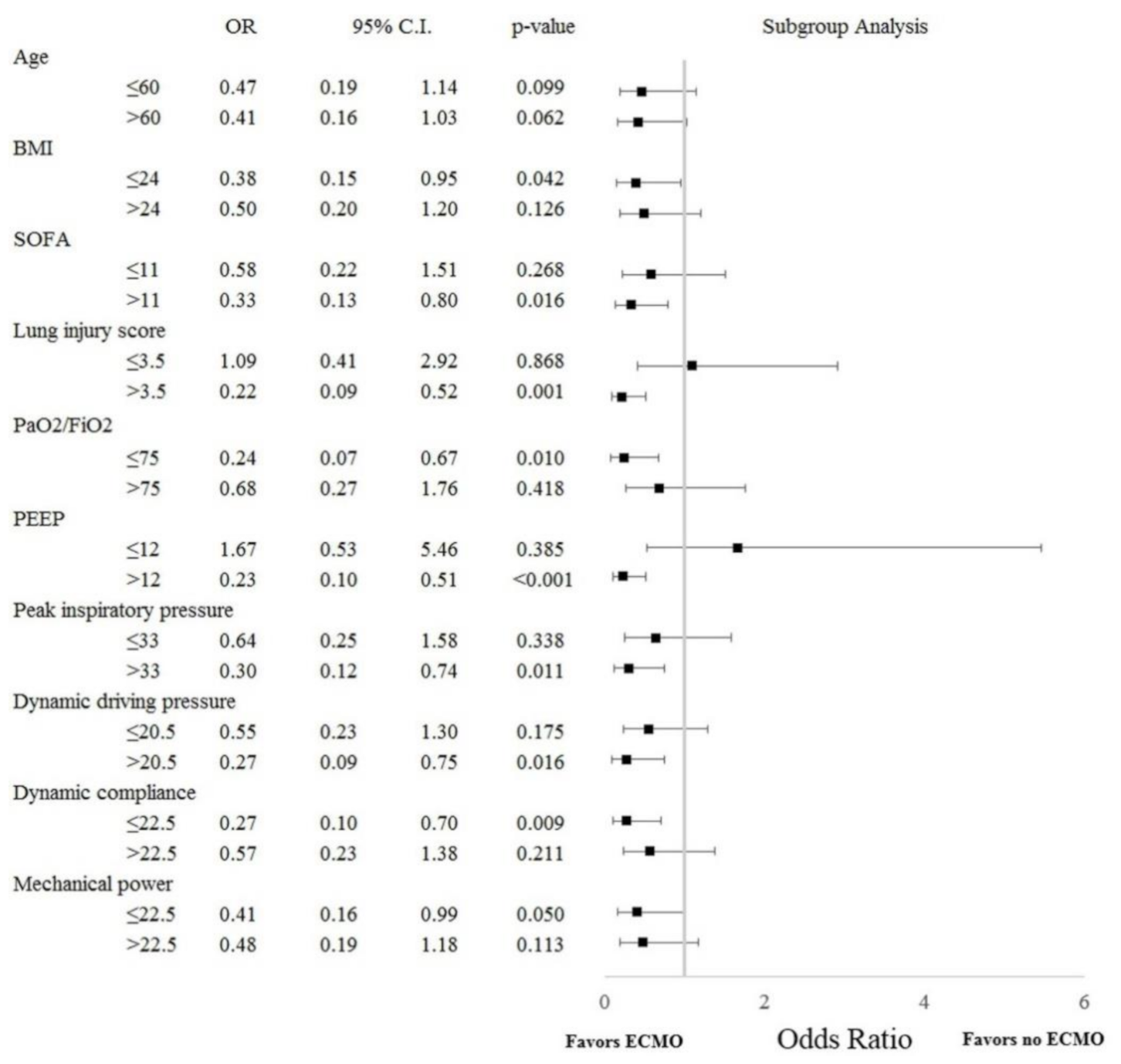
3.4. Subgroup Analysis
3.5. Multivariate Adjustment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar]
- Madotto, F.; Pham, T.; Bellani, G.; Bos, L.D.; Simonis, F.D.; Fan, E.; Artigas, A.; Brochard, L.; Schultz, M.J.; Laffey, J.G. LUNG SAFE Investigators and the ESICM Trials Group. Resolved versus confirmed ARDS after 24 h: Insights from the LUNG SAFE study. Intensive Care Med. 2018, 44, 564–577. [Google Scholar] [CrossRef]
- Fan, E.; Del Sorbo, L.; Goligher, E.C.; Hodgson, C.L.; Munshi, L.; Walkey, A.J.; Adhikari, N.K.J.; Amato, M.B.P.; Branson, R.; Brower, R.G. American Thoracic Society, European Society of Intensive Care Medicine, and Society of Critical Care Medicine. An Official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine Clinical Practice Guideline: Mechanical Ventilation in Adult Patients with Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2017, 195, 1253–1263. [Google Scholar]
- Guérin, C.; Reignier, J.; Richard, J.C.; Beuret, P.; Gacouin, A.; Boulain, T.; Mercier, E.; Badet, M.; Mercat, A.; Baudin, O.; et al. PROSEVA Study Group. Prone positioning in severe acute respiratory distress syndrome. N. Engl. J. Med. 2013, 368, 2159–2168. [Google Scholar] [CrossRef]
- Bullen, E.C.; Teijeiro-Paradis, R.; Fan, E. How I Select Which Patients with ARDS Should Be Treated with Venovenous Extracorporeal Membrane Oxygenation. Chest 2020, 158, 1036–1045. [Google Scholar] [CrossRef]
- Brodie, D.; Slutsky, A.S.; Combes, A. Extracorporeal Life Support for Adults with Respiratory Failure and Related Indications: A Review. JAMA 2019, 322, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Combes, A.; Schmidt, M.; Hodgson, C.L.; Fan, E.; Ferguson, N.D.; Fraser, J.F.; Jaber, S.; Pesenti, A.; Ranieri, M.; Rowan, K.; et al. Extracorporeal life support for adults with acute respiratory distress syndrome. Intensive Care Med. 2020, 46, 2464–2476. [Google Scholar] [CrossRef] [PubMed]
- Barbaro, R.P.; Odetola, F.O.; Kidwell, K.M.; Paden, M.L.; Bartlett, R.H.; Davis, M.M.; Annich, G.M. Association of hospital-level volume of extracorporeal membrane oxygenation cases and mortality. Analysis of the extracorporeal life support organization registry. Am. J. Respir. Crit. Care Med. 2015, 191, 894–901. [Google Scholar] [CrossRef]
- Combes, A.; Hajage, D.; Capellier, G.; Demoule, A.; Lavoué, S.; Guervilly, C.; Da Silva, D.; Zafrani, L.; Tirot, P.; Veber, B.; et al. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2018, 378, 1965–1975. [Google Scholar] [CrossRef] [PubMed]
- Peek, G.J.; Mugford, M.; Tiruvoipati, R.; Wilson, A.; Allen, E.; Thalanany, M.M.; Hibbert, C.L.; Truesdale, A.; Clemens, F.; Cooper, N.; et al. CESAR trial collaboration. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): A multicentre randomised controlled trial. Lancet 2009, 374, 1351–1363. [Google Scholar] [CrossRef]
- Haukoos, J.S.; Lewis, R.J. The Propensity Score. JAMA 2015, 314, 1637–1638. [Google Scholar] [CrossRef]
- Chiu, L.C.; Hu, H.C.; Hung, C.Y.; Chang, C.H.; Tsai, F.C.; Yang, C.T.; Huang, C.C.; Wu, H.P.; Kao, K.C. Dynamic driving pressure associated mortality in acute respiratory distress syndrome with extracorporeal membrane oxygenation. Ann. Intensive Care 2017, 7, 12. [Google Scholar] [CrossRef]
- Chiu, L.C.; Lin, S.W.; Liu, P.H.; Chuang, L.P.; Chang, C.H.; Hung, C.Y.; Li, S.H.; Lee, C.S.; Wu, H.P.; Huang, C.C.; et al. Reclassifying severity after 48 h could better predict mortality in acute respiratory distress syndrome. Ther. Adv. Respir. Dis. 2020, 14, 1–12. [Google Scholar] [CrossRef]
- Pham, T.; Combes, A.; Rozé, H.; Chevret, S.; Mercat, A.; Roch, A.; Mourvillier, B.; Ara-Somohano, C.; Bastien, O.; Zogheib, E.; et al. REVA Research Network. Extracorporeal membrane oxygenation for pandemic influenza A(H1N1)-induced acute respiratory distress syndrome: A cohort study and propensity-matched analysis. Am. J. Respir. Crit. Care Med. 2013, 187, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Giani, M.; Martucci, G.; Madotto, F.; Belliato, M.; Fanelli, V.; Garofalo, E.; Forlini, C.; Lucchini, A.; Panarello, G.; Bottino, N.; et al. Prone Positioning during Venovenous Extracorporeal Membrane Oxygenation in Acute Respiratory Distress Syndrome. A Multicenter Cohort Study and Propensity-matched Analysis. Ann. Am. Thorac. Soc. 2021, 18, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Shaefi, S.; Brenner, S.K.; Gupta, S.; O’Gara, B.P.; Krajewski, M.L.; Charytan, D.M.; Chaudhry, S.; Mirza, S.H.; Peev, V.; Anderson, M.; et al. STOP-COVID Investigators. Extracorporeal membrane oxygenation in patients with severe respiratory failure from COVID-19. Intensive Care Med. 2021, 47, 208–221. [Google Scholar] [CrossRef]
- Urner, M.; Jüni, P.; Hansen, B.; Wettstein, M.S.; Ferguson, N.D.; Fan, E. Time-varying intensity of mechanical ventilation and mortality in patients with acute respiratory failure: A registry-based, prospective cohort study. Lancet Respir. Med. 2020, 8, 905–913. [Google Scholar] [CrossRef]
- Chiu, L.C.; Lin, S.W.; Chuang, L.P.; Li, H.H.; Liu, P.H.; Tsai, F.C.; Chang, C.H.; Hung, C.Y.; Lee, C.S.; Leu, S.W.; et al. Mechanical power during extracorporeal membrane oxygenation and hospital mortality in patients with acute respiratory distress syndrome. Crit Care 2021, 25, 13. [Google Scholar] [CrossRef]
- Schmidt, M.; Pham, T.; Arcadipane, A.; Agerstrand, C.; Ohshimo, S.; Pellegrino, V.; Vuylsteke, A.; Guervilly, C.; McGuinness, S.; Pierard, S.; et al. Mechanical Ventilation Management during Extracorporeal Membrane Oxygenation for Acute Respiratory Distress Syndrome. An International Multicenter Prospective Cohort. Am. J. Respir. Crit. Care Med. 2019, 200, 1002–1012. [Google Scholar] [CrossRef] [PubMed]
- Munshi, L.; Walkey, A.; Goligher, E.; Pham, T.; Uleryk, E.M.; Fan, E. Venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: A systematic review and meta-analysis. Lancet Respir. Med. 2019, 7, 163–172. [Google Scholar] [CrossRef]
- Combes, A.; Peek, G.J.; Hajage, D.; Hardy, P.; Abrams, D.; Schmidt, M.; Dechartres, A.; Elbourne, D. ECMO for severe ARDS Systematic review and individual patient data meta-analysis. Intensive Care Med. 2020, 46, 2048–2057. [Google Scholar] [CrossRef]
- Schmidt, M.; Bailey, M.; Sheldrake, J.; Hodgson, C.; Aubron, C.; Rycus, P.T.; Scheinkestel, C.; Cooper, D.J.; Brodie, D.; Pellegrino, V.; et al. Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score. Am. J. Respir. Crit. Care Med. 2014, 189, 1374–1382. [Google Scholar] [CrossRef]
- Schmidt, M.; Zogheib, E.; Rozé, H.; Repesse, X.; Lebreton, G.; Luyt, C.E.; Trouillet, J.L.; Bréchot, N.; Nieszkowska, A.; Dupont, H.; et al. The PRESERVE mortality risk score and analysis of long-term outcomes after extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Intensive Care Med. 2013, 39, 1704–1713. [Google Scholar] [CrossRef]
- Schmidt, M.; Stewart, C.; Bailey, M.; Nieszkowska, A.; Kelly, J.; Murphy, L.; Pilcher, D.; Cooper, D.J.; Scheinkestel, C.; Pellegrino, V.; et al. Mechanical ventilation management during extracorporeal membrane oxygenation for acute respiratory distress syndrome: A retrospective international multicenter study. Crit Care Med. 2015, 43, 654–664. [Google Scholar] [CrossRef]
- Neto, A.S.; Schmidt, M.; Azevedo, L.C.; Bein, T.; Brochard, L.; Beutel, G.; Combes, A.; Costa, E.L.; Hodgson, C.; Lindskov, C.; et al. ReVA Research Network and the PROVE Network Investigators. Associations between ventilator settings during extracorporeal membrane oxygenation for refractory hypoxemia and outcome in patients with acute respiratory distress syndrome: A pooled individual patient data analysis: Mechanical ventilation during ECMO. Intensive Care Med. 2016, 42, 1672–1684. [Google Scholar]
- Brodie, D.; Bacchetta, M. Extracorporeal membrane oxygenation for ARDS in adults. N. Engl. J. Med. 2011, 365, 1905–1914. [Google Scholar] [CrossRef] [PubMed]
- Amato, M.B.; Meade, M.O.; Slutsky, A.S.; Brochard, L.; Costa, E.L.; Schoenfeld, D.A.; Stewart, T.E.; Briel, M.; Talmor, D.; Mercat, A.; et al. Driving pressure and survival in the acute respiratory distress syndrome. N. Engl. J. Med. 2015, 372, 747–755. [Google Scholar] [CrossRef]
- Laffey, J.G.; Bellani, G.; Pham, T.; Fan, E.; Madotto, F.; Bajwa, E.K.; Brochard, L.; Clarkson, K.; Esteban, A.; Gattinoni, L.; et al. LUNG SAFE Investigators and the ESICM Trials Group. Potentially modifiable factors contributing to outcome from acute respiratory distress syndrome The LUNG SAFE study. Intensive Care Med. 2016, 42, 1865–1876. [Google Scholar] [CrossRef] [PubMed]
- Abrams, D.; Schmidt, M.; Pham, T.; Beitler, J.R.; Fan, E.; Goligher, E.C.; McNamee, J.J.; Patroniti, N.; Wilcox, M.E.; Combes, A.; et al. Mechanical Ventilation for Acute Respiratory Distress Syndrome during Extracorporeal Life Support. Research and Practice. Am. J. Respir. Crit. Care Med. 2020, 201, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Thompson, B.T.; Chambers, R.C.; Liu, K.D. Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2017, 377, 562–572. [Google Scholar] [CrossRef]
- Bellani, G.; Laffey, J.G.; Pham, T.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; van Haren, F.; Larsson, A.; McAuley, D.F.; et al. LUNG SAFE Investigators; ESICM Trials Group. Epidemiology, Patterns of Care, and Mortality for Patients with Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016, 315, 788–800. [Google Scholar] [CrossRef] [PubMed]
| Variables | Before Matching | After Matching | ||||
|---|---|---|---|---|---|---|
| ECMO | Non-ECMO | ECMO | Non-ECMO | |||
| (n = 158) | (n = 122) | p | (n = 87) | (n = 87) | p | |
| Age (years) | 50.3 ± 16.3 | 65.1 ± 14.1 | <0.001 | 58.3 ± 13.2 | 61.9 ± 14.3 | 0.082 |
| Gender (male) | 108 (68.4%) | 79 (64.8%) | 0.526 | 58 (66.7%) | 60 (69%) | 0.746 |
| Body mass index (kg/m2) | 25.8 ± 5.2 | 23.7 ± 4.4 | 0.001 | 24.8 ± 4.0 | 24.2 ± 4.6 | 0.323 |
| ARDS etiologies | ||||||
| Pulmonary cause | 121 (76.6%) | 95 (77.9%) | 0.799 | 66 (75.9%) | 69 (79.3%) | 0.585 |
| Extrapulmonary cause | 37 (23.4%) | 27 (22.1%) | 0.799 | 21 (24.1%) | 18 (20.7%) | 0.585 |
| Comorbidities | ||||||
| Diabetes mellitus | 40 (25.3%) | 33 (27%) | 0.743 | 29 (33.3%) | 19 (21.8%) | 0.09 |
| Cerebrovascular accident | 10 (6.3%) | 12 (9.8%) | 0.280 | 9 (10.3%) | 4 (4.6%) | 0.248 |
| Congestive heart failure | 13 (8.2%) | 5 (4.1%) | 0.162 | 9 (10.3%) | 3 (3.4%) | 0.132 |
| Coronary artery disease | 6 (3.8%) | 6 (4.9%) | 0.646 | 6 (6.9%) | 4 (4.6%) | 0.747 |
| Chronic lung disease | 16 (10.1%) | 16 (13.1%) | 0.436 | 7 (8%) | 14 (16.1%) | 0.103 |
| Liver cirrhosis or chronic hepatitis | 22 (13.9%) | 21 (17.2%) | 0.449 | 16 (18.4%) | 17 (19.5%) | 0.847 |
| Chronic kidney disease | 18 (11.4%) | 14 (11.5%) | 0.983 | 14 (16.1%) | 9 (10.3%) | 0.263 |
| Malignancies | 24 (15.2%) | 20 (16.4%) | 0.784 | 16 (18.4%) | 15 (17.2%) | 0.843 |
| SOFA score | 10.9 ± 3.2 | 12.2 ± 3.5 | 0.001 | 11.4 ± 3.2 | 11.8 ± 3.5 | 0.417 |
| Lung injury score | 3.4 ± 0.4 | 3.4 ± 0.4 | 0.225 | 3.4 ± 0.4 | 3.4 ± 0.4 | 0.482 |
| ARDS duration before ECMO (h) | 28 (7–129) | 40 (8–153) | ||||
| PaO2/FiO2 (mm Hg) | 81.1 ± 50.4 | 78.7 ± 17.8 | 0.576 | 80.4 ± 45.7 | 79.2 ± 18.8 | 0.809 |
| PaCO2 (mm Hg) | 52.2 ± 18.8 | 52.8 ± 19.0 | 0.803 | 50.6 ± 17.9 | 53.9 ± 20.4 | 0.258 |
| Ventilator settings | ||||||
| Tidal volume (ml/kg PBW) | 7.7 ± 2.4 | 8.2 ± 2.4 | 0.098 | 8.2 ± 2.6 | 8.1 ± 2.4 | 0.795 |
| PEEP (cm H2O) | 12.0 ± 2.8 | 12.5 ± 2.6 | 0.123 | 12 ± 2.6 | 12. 3 ± 2.6 | 0.428 |
| Peak inspiratory pressure (cm H2O) | 33.9 ± 6.5 | 32.8 ± 5.9 | 0.130 | 33.1 ± 5.8 | 33.3 ± 5.4 | 0.861 |
| Dynamic driving pressure (cm H2O) | 21.9 ± 6.2 | 20.3 ± 5.5 | 0.022 | 21.0 ± 6.1 | 20.9 ± 5.0 | 0.935 |
| Dynamic compliance (ml/cm H2O) | 22.5 ± 11.2 | 25.6 ± 16.6 | 0.072 | 24.1 ± 12.7 | 23.8 ± 9.8 | 0.828 |
| Total respiratory rate (breaths/min) | 24.2 ± 7.1 | 25.2 ± 4.7 | 0.142 | 24.2 ± 6.6 | 25.3 ± 4.8 | 0.207 |
| Minute ventilation (L/min) | 10.6 ± 3.8 | 10.7 ± 3.2 | 0.858 | 10.8 ± 4.0 | 10.8 ± 3.0 | 1.000 |
| Mechanical power (J/min) | 23.7 ± 9.6 | 23.6 ± 7.7 | 0.910 | 23.8 ± 10.0 | 23.9 ± 7.0 | 0.946 |
| MP/PBW (×10−3 J/min/kg) | 415 ± 172 | 437 ± 170 | 0.297 | 430 ± 188 | 433 ± 152 | 0.889 |
| MP/Compliance (J/min/mL/cm H2O) | 1.27 ± 0.76 | 1.12 ± 0.54 | 0.065 | 1.18 ± 0.66 | 1.15 ± 0.51 | 0.751 |
| Duration of mechanical ventilator (days) | 20 (11.5–38) | 14 (8–28.3) | 0.001 | 20 (12–35.9) | 16 (8–30) | 0.019 |
| Length of ICU stay (days) | 23 (13–43) | 16 (8–30) | 0.003 | 23 (15–43) | 17 (10–31) | 0.031 |
| Length of hospital stay (days) | 38.5 (20.8–64) | 29.5 (15–51.3) | 0.017 | 37 (20–68) | 30 (14–52) | 0.035 |
| Hospital mortality, n (%) | 87 (55.1%) | 97 (79.5%) | <0.001 | 52 (59.8%) | 67 (77%) | 0.014 |
| Outcomes | ECMO | Non-ECMO | p |
|---|---|---|---|
| (n = 87) | (n = 87) | ||
| Mortality | |||
| 28 day hospital mortality, n (%) | 40 (46%) | 46 (52.9%) | 0.371 |
| 60 day hospital mortality, n (%) | 47 (54%) | 64 (73.6%) | 0.025 |
| 90 day hospital mortality, n (%) | 49 (56.3%) | 65 (74.7%) | 0.028 |
| Other outcomes | |||
| Ventilator-free days on day 28 | 2.8 ± 6.1 | 2.8 ± 6.0 | 0.974 |
| Ventilator-free days on day 60 | 12.8 ± 19.1 | 9.4 ± 17.5 | 0.218 |
| Ventilator-free days on day 90 | 23.9 ± 32.6 | 16.4 ± 29.8 | 0.114 |
| ICU-free days on day 90 | 21.8 ± 29.4 | 13.3 ± 26.4 | 0.046 |
| Hospital-free days on day 90 | 12.9 ± 21.7 | 9.3 ± 20.1 | 0.266 |
| Factors | Odds Ratio (95% CI) | p |
|---|---|---|
| ECMO | 0.40 (0.19–0.81) | 0.013 |
| SOFA score | 1.25 (1.11–1.43) | <0.001 |
| Dynamic driving pressure | 1.18 (1.02–1.39) | 0.038 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, L.-C.; Chuang, L.-P.; Leu, S.-W.; Lin, Y.-J.; Chang, C.-J.; Li, H.-H.; Tsai, F.-C.; Chang, C.-H.; Hung, C.-Y.; Lin, S.-W.; et al. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome: Propensity Score Matching. Membranes 2021, 11, 393. https://doi.org/10.3390/membranes11060393
Chiu L-C, Chuang L-P, Leu S-W, Lin Y-J, Chang C-J, Li H-H, Tsai F-C, Chang C-H, Hung C-Y, Lin S-W, et al. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome: Propensity Score Matching. Membranes. 2021; 11(6):393. https://doi.org/10.3390/membranes11060393
Chicago/Turabian StyleChiu, Li-Chung, Li-Pang Chuang, Shaw-Woei Leu, Yu-Jr Lin, Chee-Jen Chang, Hsin-Hsien Li, Feng-Chun Tsai, Chih-Hao Chang, Chen-Yiu Hung, Shih-Wei Lin, and et al. 2021. "Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome: Propensity Score Matching" Membranes 11, no. 6: 393. https://doi.org/10.3390/membranes11060393
APA StyleChiu, L.-C., Chuang, L.-P., Leu, S.-W., Lin, Y.-J., Chang, C.-J., Li, H.-H., Tsai, F.-C., Chang, C.-H., Hung, C.-Y., Lin, S.-W., Hu, H.-C., Huang, C.-C., Wu, H.-P., & Kao, K.-C. (2021). Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome: Propensity Score Matching. Membranes, 11(6), 393. https://doi.org/10.3390/membranes11060393






