Evaluation of Performance of Existing RO Drinking Water Stations in the North Central Province, Sri Lanka
Abstract
1. Introduction
2. Materials and Methods
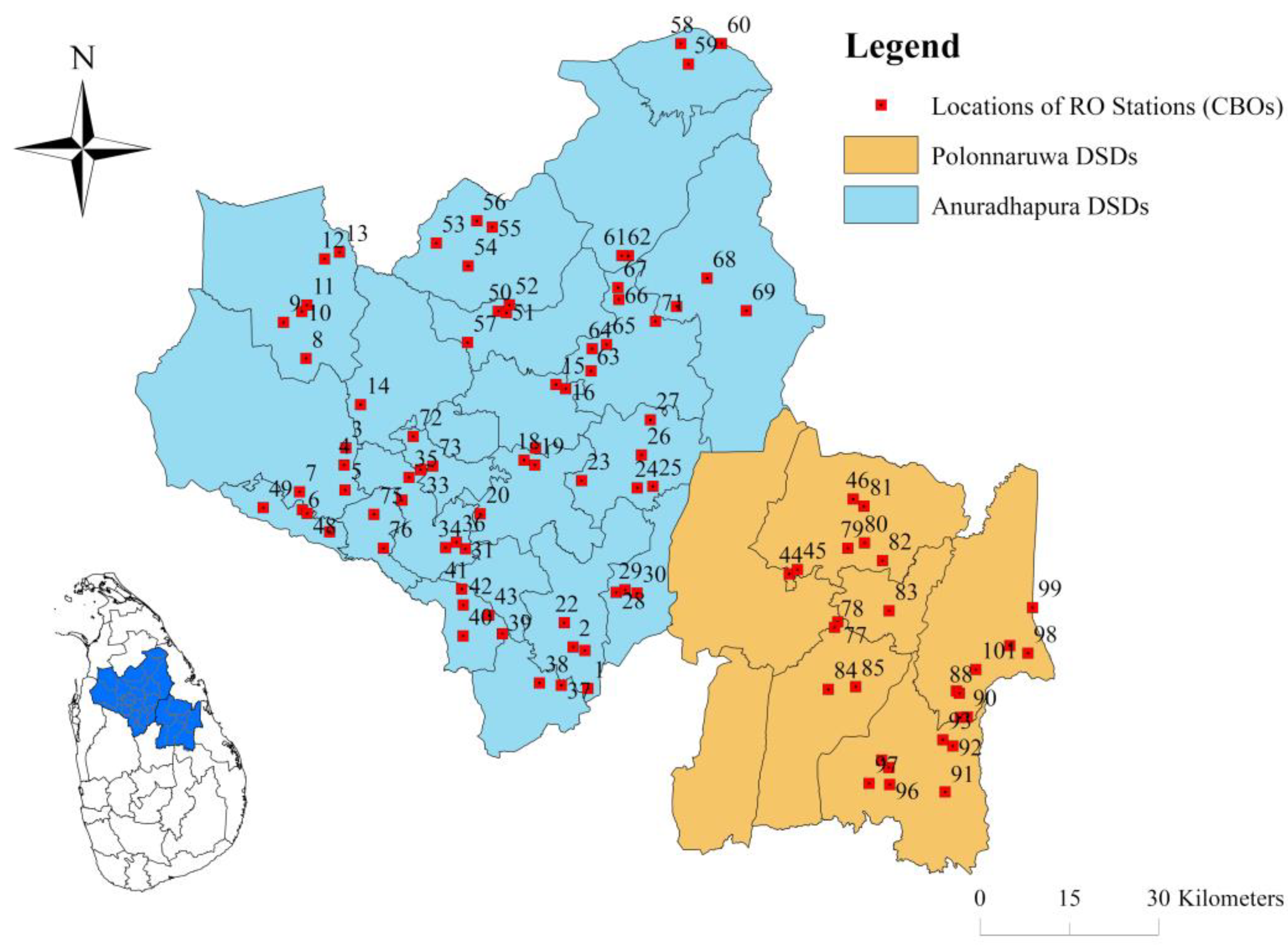
2.1. Study Area
2.2. Sampling and Data Collection
2.3. Water Quality Analysis
2.4. Data Analysis
3. Results and Discussion
3.1. Comparison of RO Treatment Process
3.1.1. Pre-Treatment Process
3.1.2. RO Filtration
3.1.3. Post-Treatment Process
3.1.4. Reject Water Handling
3.2. Operation and Maintenance
3.3. Performance Analysis of RO Systems
3.3.1. Water Quality
3.3.2. Comparison of RO Performances
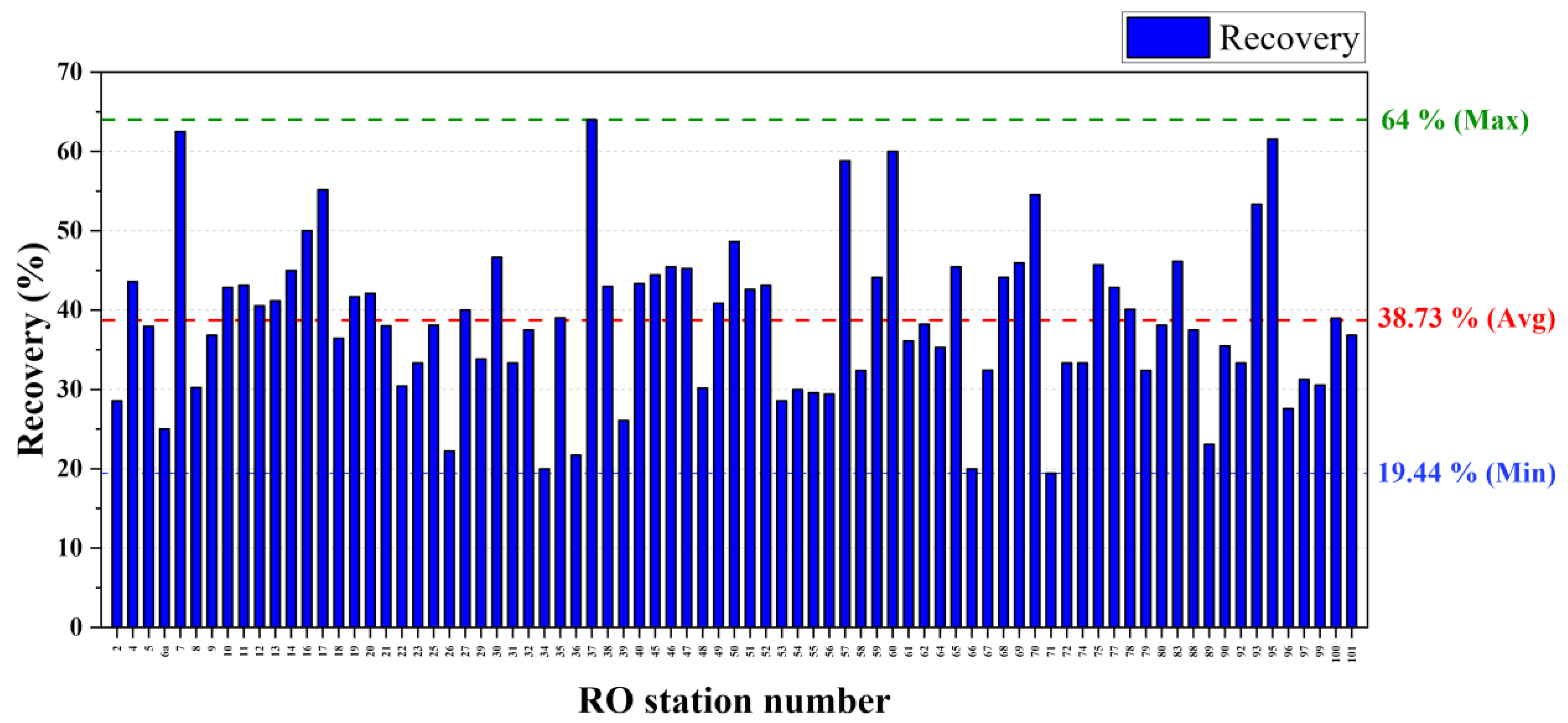
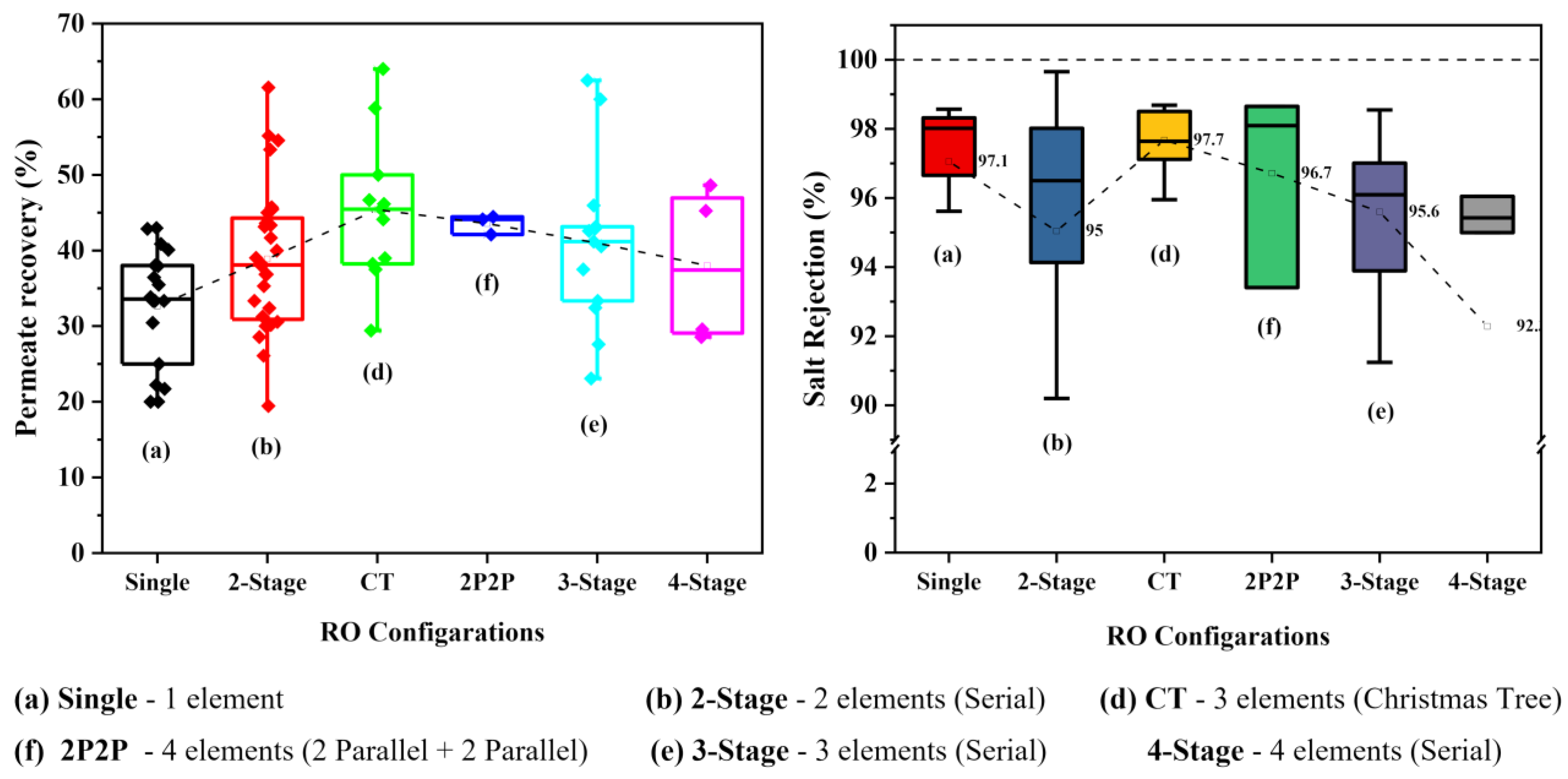
Permeate Recovery
Hardness and Alkalinity Rejection
Salt Rejection
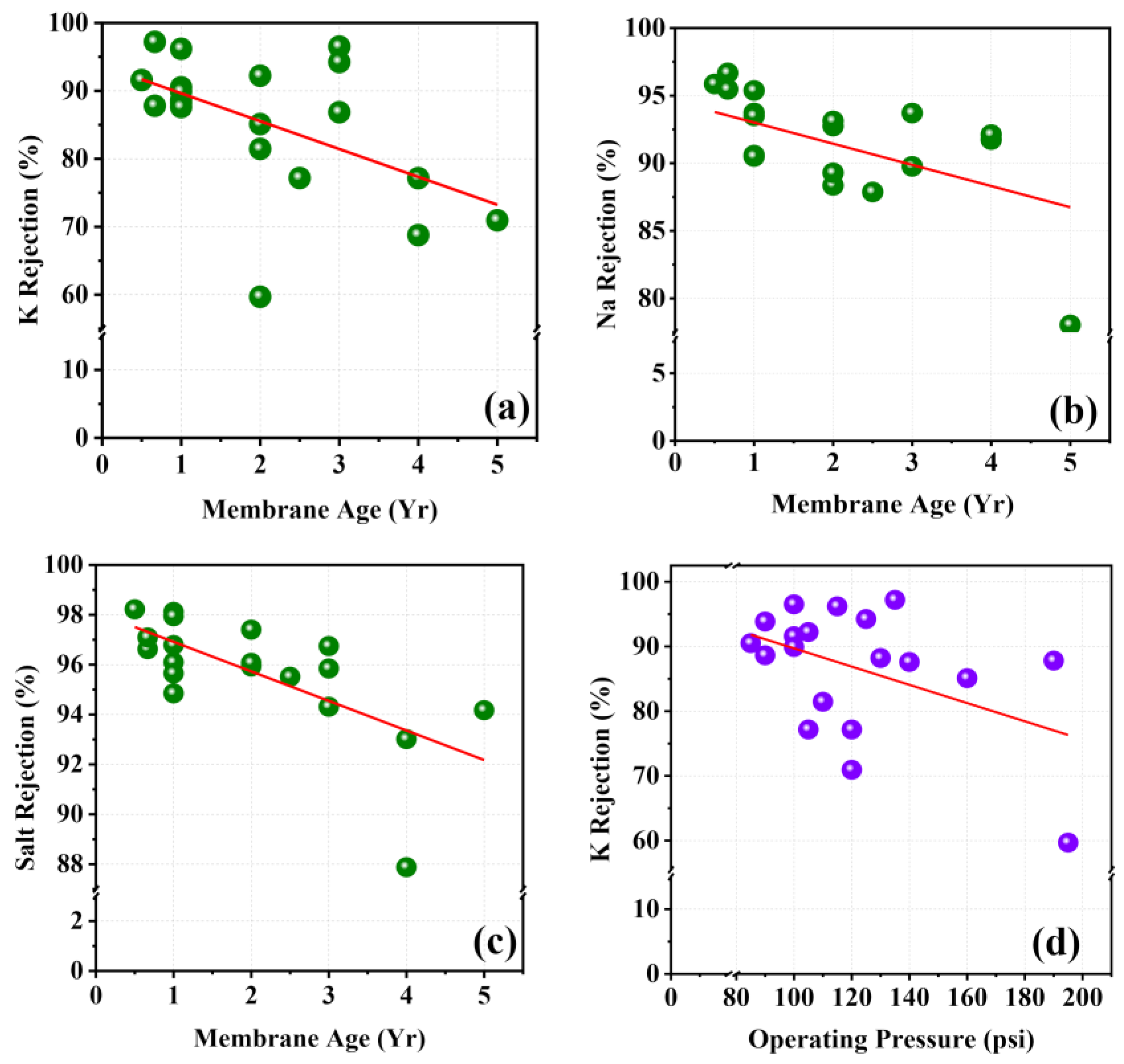
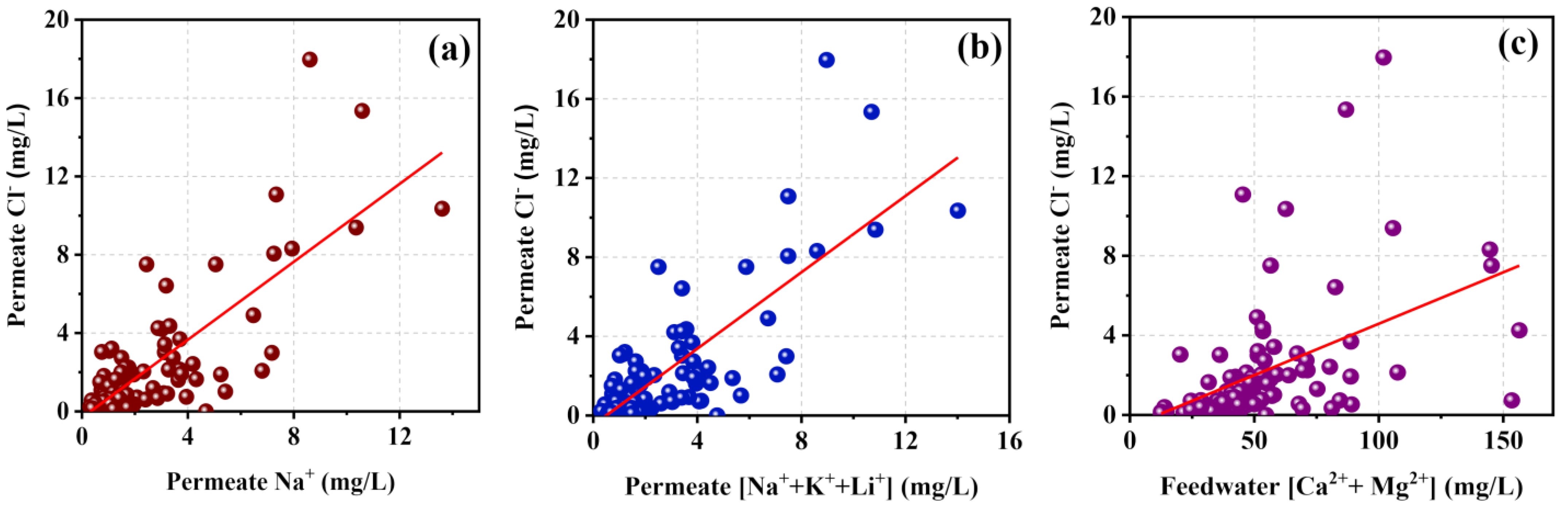
Factors of Ion Rejection
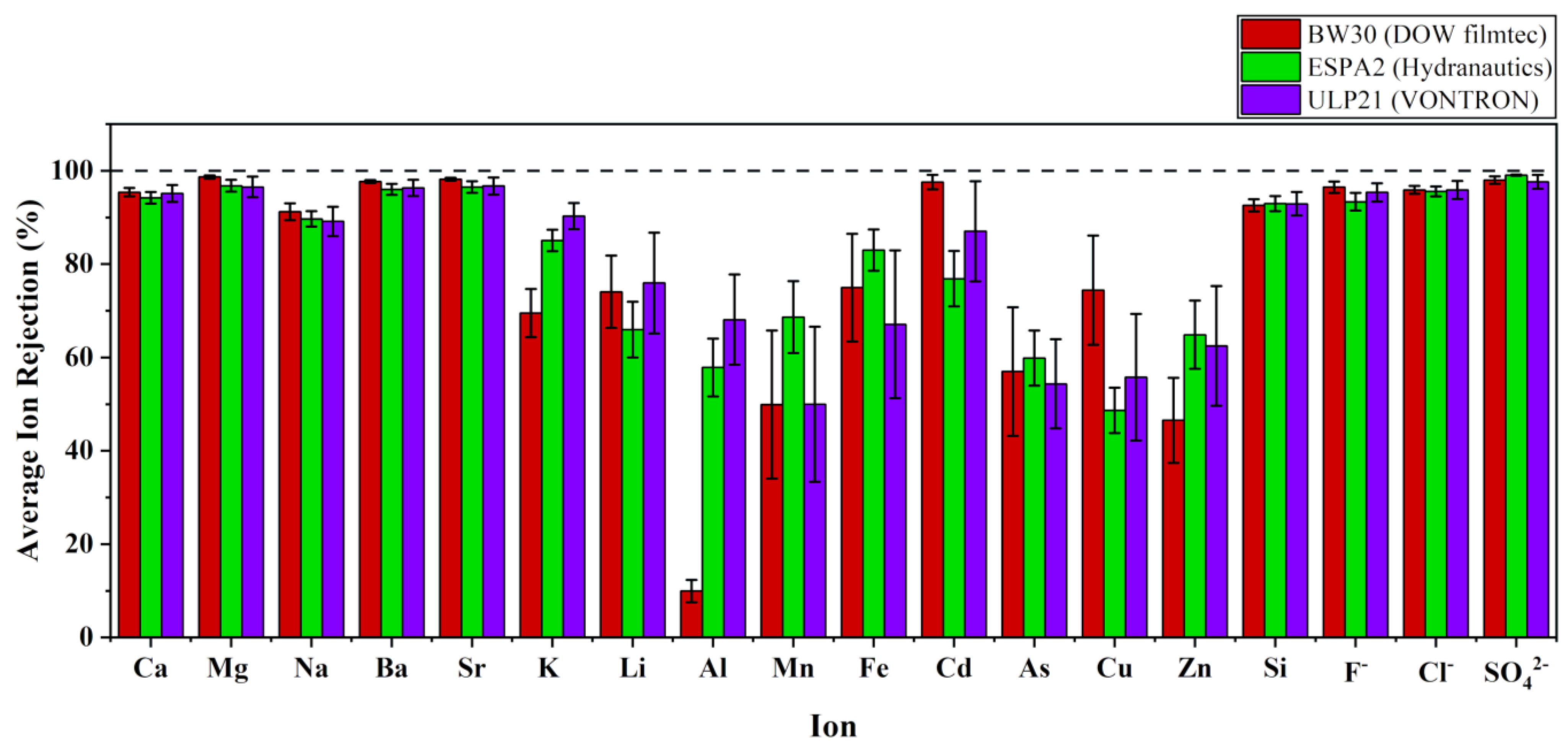
Variation of Performances with Different RO Configurations
Ion Selectivity and Permeability of Polyamide TFC RO Membranes
- Selectivity and permeability of cations
- II
- Permeability of anions
3.4. Cost Analysis
4. Challenges and Recommendations
4.1. Water Quality Issues
4.2. CBO Organizational Issues
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dharma-Wardana, M.W.C.; Amarasiri, S.L.; Dharmawardene, N.; Panabokke, C.R. Chronic kidney disease of unknown aetiology and ground-water ionicity: Study based on Sri Lanka. Environ. Geochem. Health 2015, 37, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.A.; Wimalawansa, S.J. Impact of changing agricultural practices on human health: Chronic kidney disease of multi-factorial origin in Sri Lanka. Wudpecker J. Agric. Res. 2014, 3, 110–124. [Google Scholar]
- Wimalawansa, S.J. The role of ions, heavy metals, fluoride, and agrochemicals: Critical evaluation of potential aetiological factors of chronic kidney disease of multifactorial origin (CKDmfo/CKDu) and recommendations for its eradication. Environ. Geochem. Health 2016, 38, 639–678. [Google Scholar] [CrossRef] [PubMed]
- Perera, W.P.R.T.; Dayananda, M.D.N.R.; Liyanage, J.A. Exploring the root cause for chronic kidney disease of unknown etiology (CKDU) via analysis of metal ion and counterion contaminants in drinking water: A study in Sri Lanka. J. Chem. 2020, 2020. [Google Scholar] [CrossRef]
- Pinto, U.; Thoradeniya, B.; Maheshwari, B. Water quality and chronic kidney disease of unknown aetiology (CKDu) in the dry zone region of Sri Lanka: Impacts on well-being of village communities and the way forward. Environ. Sci. Pollut. Res. 2020, 27, 3892–3907. [Google Scholar] [CrossRef] [PubMed]
- Balasooriya, S.; Munasinghe, H.; Herath, A.T.; Diyabalanage, S.; Ileperuma, O.A.; Manthrithilake, H.; Daniel, C.; Amann, K.; Zwiener, C.; Barth, J.A.C.; et al. Possible links between groundwater geochemistry and chronic kidney disease of unknown etiology (CKDu): An investigation from the Ginnoruwa region in Sri Lanka. Expo. Heal. 2019. [Google Scholar] [CrossRef]
- Wanasinghe, W.C.S.; Gunarathna, M.H.J.P.; Herath, H.M.P.I.K.; Jayasinghe, G.Y. Drinking Water Quality on Chronic Kidney Disease of Unknown Aetiology (CKDu) in Ulagalla Cascade, Sri Lanka. Sabaragamuwa Univ. J. 2018, 16, 17–27. [Google Scholar] [CrossRef]
- Cooray, T.; Wei, Y.; Zhong, H.; Zheng, L.; Weragoda, S.; Weerasooriya, R. Assessment of Groundwater Quality in CKDu Affected Areas of Sri Lanka: Implications for Drinking Water Treatment. Int. J. Environ. Res. Public Health 2019, 16, 1698. [Google Scholar] [CrossRef]
- Jayasumana, C.; Ranasinghe, O.; Ranasinghe, S.; Siriwardhana, I.; Gunatilake, S.; Siribaddana, S. Reverse osmosis plant maintenance and efficacy in chronic kidney disease endemic region in Sri Lanka. Environ. Health Prev. Med. 2016, 21, 591–596. [Google Scholar] [CrossRef]
- Imbulana, S.; Oguma, K.; Takizawa, S. Evaluation of groundwater quality and reverse osmosis water treatment plants in the endemic areas of Chronic Kidney Disease of Unknown Etiology (CKDu) in Sri Lanka. Sci. Total Environ. 2020, 745, 140716. [Google Scholar] [CrossRef]
- Sri Lanka Navy Navy Built RO Plants in Ampara Vested with the Public. Available online: https://news.navy.lk/eventnews/2021/02/06/202102061300/ (accessed on 22 April 2021).
- Baker, R.W. Membrane Technology and Applications, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2012; ISBN 1118359690. [Google Scholar]
- Verma, K.C.; Kushwaha, A.S. Demineralization of drinking water: Is it prudent? Med. J. Armed Forces India 2014, 70, 377–379. [Google Scholar] [CrossRef]
- Kozisek, F. Health Risk from Drinking Demineralized Water. Roll. Revis. WHO Guidel. Drink. Water Qual. 2004, 1, 148–163. [Google Scholar]
- Cooray, T.; Wei, Y.; Zhang, J.; Zheng, L.; Zhong, H.; Weragoda, S.K.; Weerasooriya, R. Drinking-Water supply for CKDu affected areas of Sri Lanka, using nanofiltration membrane technology: From laboratory to practice. Water 2019, 11, 2512. [Google Scholar] [CrossRef]
- Balasubramanya, S.; Stifel, D.; Horbulyk, T.; Kafle, K. Chronic kidney disease and household behaviors in Sri Lanka: Historical choices of drinking water and agrochemical use. Econ. Hum. Biol. 2020, 37, 100862. [Google Scholar] [CrossRef]
- Horbulyk, T.; Kafle, K.; Balasubramanya, S. Community response to the provision of alternative water supplies: A focus on chronic kidney disease of unknown aetiology (CKDu) in rural Sri Lanka. Water Int. 2021, 46, 37–58. [Google Scholar] [CrossRef]
- Makehelwala, M.; Wei, Y.; Weragoda, S.K.; Weerasooriya, R. Ca2+ and SO42− interactions with dissolved organic matter: Implications of groundwater quality for CKDu incidence in Sri Lanka. J. Environ. Sci. 2020, 88, 326–337. [Google Scholar] [CrossRef]
- Makehelwala, M.; Wei, Y.; Weragoda, S.K.; Weerasooriya, R.; Zheng, L. Characterization of dissolved organic carbon in shallow groundwater of chronic kidney disease affected regions in Sri Lanka. Sci. Total Environ. 2019, 660, 865–875. [Google Scholar] [CrossRef]
- Hansima, M.A.C.K.; Makehelwala, M.; Jinadasa, K.B.S.N.; Wei, Y.; Nanayakkara, K.G.N.; Herath, A.C.; Weerasooriya, R. Fouling of ion exchange membranes used in the electrodialysis reversal advanced water treatment: A review. Chemosphere 2021, 263, 127951. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 19th ed.; Water Environment Federation: Alexandria, VA, USA; American Public Health Association: New York, NY, USA, 1995. [Google Scholar]
- Jamaly, S.; Darwish, N.N.; Ahmed, I.; Hasan, S.W. A short review on reverse osmosis pretreatment technologies. Desalination 2014, 354, 30–38. [Google Scholar] [CrossRef]
- Cooray, P.G. An Introduction to the Geology of Sri Lanka (Ceylon); National Museums of Sri Lanka Publication: Colombo, Sri Lanka, 1984; Volume 38. [Google Scholar]
- Thivya, C.; Chidambaram, S.; Singaraja, C.; Thilagavathi, R.; Prasanna, M.V.; Anandhan, P.; Jainab, I. A study on the significance of lithology in groundwater quality of Madurai district, Tamil Nadu (India). Environ. Dev. Sustain. 2013, 15, 1365–1387. [Google Scholar] [CrossRef]
- Zheng, L.; Cooray, T.; Zhong, H.; Weragoda, S.; Weerasooriyae, R.; Makehelwala, M.; Wei, Y. Critical challenges and solutions on ground drinking water in chronic kidney disease of unknown etiology (CKDu) affected regions in Sri Lanka. Chin. J. Environ. Eng. 2020, 14, 2100–2111. [Google Scholar] [CrossRef]
- Tennakoon, T. Dental fluorosis in anuradhapura district, Sri Lanka. In Proceedings of the 4th International Workshop on Fluorosis Prevention and Defluoridation of Water, Colombo, Sri Lanka, 2–6 March 2004; pp. 19–22. [Google Scholar]
- Ranasinghe, N.; Kruger, E.; Chandrajith, R.; Tennant, M. Groundwater fluoride in Sri Lanka: Opportunities to mitigate the risk at maximum contaminant level. Ceylon Med. J. 2018, 63, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Dharmaratne, R.W. Fluoride in drinking water and diet: The causative factor of chronic kidney diseases in the North Central Province of Sri Lanka. Environ. Health Prev. Med. 2015, 20, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Naidu, L.; Saravanan, S.; Manickam, C.; Goel, M.; Das, A.; Babu, J. Nanofiltration in transforming surface water into healthy water: Comparison with reverse osmosis. J. Chem. 2015, 2015, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Al-Amshawee, S.; Yunus, M.Y.B.M.; Azoddein, A.A.M.; Hassell, D.G.; Dakhil, I.H.; Hasan, H.A. Electrodialysis desalination for water and wastewater: A review. Chem. Eng. J. 2020, 380, 122231. [Google Scholar] [CrossRef]
- Vingerhoeds, M.H.; Nijenhuis-de Vries, M.A.; Ruepert, N.; van der Laan, H.; Bredie, W.L.P.; Kremer, S. Sensory quality of drinking water produced by reverse osmosis membrane filtration followed by remineralisation. Water Res. 2016, 94, 42–51. [Google Scholar] [CrossRef]
- Udeshani, W.A.C.; Dissanayake, H.M.K.P.; Gunatilake, S.K.; Chandrajith, R. Assessment of groundwater quality using water quality index (WQI): A case study of a hard rock terrain in Sri Lanka. Groundw. Sustain. Dev. 2020, 11. [Google Scholar] [CrossRef]
- Hasson, D.; Bendrihem, O. Modeling remineralization of desalinated water by limestone dissolution. Desalination 2006, 190, 189–200. [Google Scholar] [CrossRef]
- Vries, D.; Korevaar, M.; Ghanbari, S.; van Houwelingen, G.; van der Meer, W. Data fusion to monitor remineralisation of desalinated groundwater in calcite contactors. J. Water Process Eng. 2021, 41, 102011. [Google Scholar] [CrossRef]
- Bason, S.; Oren, Y.; Freger, V. Ion transport in the polyamide layer of RO membranes: Composite membranes and free-standing films. J. Memb. Sci. 2011, 367, 119–126. [Google Scholar] [CrossRef]
- Hao, X.; Gao, S.; Tian, J.; Sun, Y.; Cui, F.; Tang, C.Y. Calcium-carboxyl intrabridging during interfacial polymerization: A novel strategy to improve antifouling performance of thin film composite membranes. Environ. Sci. Technol. 2019, 53, 4371–4379. [Google Scholar] [CrossRef]
- Tiraferri, A.; Elimelech, M. Direct quantification of negatively charged functional groups on membrane surfaces. J. Memb. Sci. 2012, 389, 499–508. [Google Scholar] [CrossRef]
- Tzotzi, C.; Pahiadaki, T.; Yiantsios, S.; Karabelas, A.; Andritsos, N. A study of CaCO 3 scale formation and inhibition in RO and NF membrane processes. J. Membr. Sci. 2007, 296, 171–184. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Z.; Epsztein, R.; Zhan, C.; Li, W.; Fortner, J.D.; Pham, T.A.; Kim, J.-H.; Elimelech, M. Intrapore energy barriers govern ion transport and selectivity of desalination membranes. Sci. Adv. 2020, 6, eabd9045. [Google Scholar] [CrossRef]
- Shen, M.; Keten, S.; Lueptow, R. Rejection mechanisms for contaminants in polyamide reverse osmosis membranes. J. Memb. Sci. 2016, 509. [Google Scholar] [CrossRef]
- Chan, E.; Young, A.; Lee, J.-H.; Chung, J.; Stafford, C. Swelling of ultrathin crosslinked polyamide water desalination membranes. J. Polym. Sci. B Polym. Phys. 2013, 51, 385–391. [Google Scholar] [CrossRef]
- Dražević, E.; Kosutic, K.; Freger, V. Permeability and selectivity of reverse osmosis membranes: Correlation to swelling revisited. Water Res. 2013, 49. [Google Scholar] [CrossRef]
- Tansel, B.; Sager, J.; Rector, T.; Garland, J.; Strayer, R.F.; Levine, L.; Roberts, M.; Hummerick, M.; Bauer, J. Significance of hydrated radius and hydration shells on ionic permeability during nanofiltration in dead end and cross flow modes. Sep. Purif. Technol. 2006, 51, 40–47. [Google Scholar] [CrossRef]
- Sigurdardottir, S.B.; DuChanois, R.M.; Epsztein, R.; Pinelo, M.; Elimelech, M. Energy barriers to anion transport in polyelectrolyte multilayer nanofiltration membranes: Role of intra-pore diffusion. J. Memb. Sci. 2020, 603, 117921. [Google Scholar] [CrossRef]
- Kiriukhin, M.Y.; Collins, K.D. Dynamic hydration numbers for biologically important ions. Biophys. Chem. 2002, 99, 155–168. [Google Scholar] [CrossRef]
- Pandian, S.; Katha, A.R.; Moon, J.H.; Kolake, S.M.; Han, S. Exploring the effect of additives on polyamide membrane surface for seawater desalination using density functional tools. Desalination 2015, 367, 28–36. [Google Scholar] [CrossRef]
- Freger, V. Ion partitioning and permeation in charged low-T* membranes. Adv. Colloid Interface Sci. 2020, 277, 102107. [Google Scholar] [CrossRef]
- Park, H.G.; Kwon, Y.N. Investigation on the factors determining permeate pH in reverse osmosis membrane processes. Desalination 2018, 430, 147–158. [Google Scholar] [CrossRef]
- Qin, J.-J.; Oo, M.; Lee, H.; Coniglio, B. Effect of feed pH on permeate pH and ion rejection under acidic conditions in NF process. J. Memb. Sci. 2004, 232, 153–159. [Google Scholar] [CrossRef]
- Stenina, I.; Golubenko, D.; Nikonenko, V.; Yaroslavtsev, A. Selectivity of Transport Processes in Ion-Exchange Membranes: Relationship with the Structure and Methods for Its Improvement. Int. J. Mol. Sci. 2020, 21, 5517. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.H. Generation of resting membrane potential. Adv. Physiol. Educ. 2004, 28, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Haines, T.S.; Lloyd, J.W. Controls on silica in groundwater environments in the United Kingdom. J. Hydrol. 1985, 81, 277–295. [Google Scholar] [CrossRef]



| Parameter | Units | Average | Max | Min | WHO MALs | SLS MALs | Unacceptable Samples (%) |
|---|---|---|---|---|---|---|---|
| Feedwater (groundwater) | |||||||
| General parameters | |||||||
| pH | 7.1 | 8.3 | 6.95 | 6.5–8.5 | 6.5–8.5 | 0 | |
| EC | µS/cm | 770 | 2430 | 112 | 400 | 750 | 98 |
| Hardness | mg/L (CaCO3) | 179.5 | 814 | 2.42 | - | 250 | 17 |
| Alkalinity | mg/L (CaCO3) | 320.4 | 1120 | 90 | - | 200 | 86 |
| Anions | |||||||
| F− | mg/L | 1.16 | 4.9 | 0.03 | 1.5 | 1.0 | 21.8 |
| Cl− | mg/L | 78.9 | 473.6 | 0.29 | - | 250 | 4 |
| Br− | mg/L | 0.44 | 1.6 | 0.08 | 2 | - | 0 |
| NO3− | mg/L | 1.82 | 14.8 | 0.03 | 50 | 50 | 0 |
| PO43− | mg/L | 4.08 | 6.3 | 0.93 | - | - | 0 |
| SO42− | mg/L | 25.3 | 490.9 | 0.10 | 250 | 250 | 1 |
| Cations | |||||||
| Ca2+ | mg/L | 31.9 | 275.8 | 0.37 | - | 100 | 3.2 |
| Mg2+ | mg/L | 23.94 | 64.01 | 0.37 | - | 30 | 33.7 |
| Na+ | mg/L | 44.27 | 196.06 | 3.2 | 200 | 250 | 0 |
| K+ | mg/L | 2.85 | 88.21 | 0.14 | - | - | 0 |
| Li+ | µg/L | 7.47 | 26.65 | 0.354 | - | - | 0 |
| Sr2+ | µg/L | 606.92 | 2763.05 | 2.960 | - | - | 0 |
| Ba2+ | µg/L | 192.34 | 1698.17 | 0.340 | 1300 | - | 1 |
| Mn2+ | µg/L | 0.72 | 25.86 | 0.057 | 100 | 100 | 0 |
| Fe2+ | µg/L | 1.99 | 28.87 | 0.023 | 200 | 300 | 0 |
| Cd2+ | µg/L | 0.2 | 0.59 | 0.010 | 3 | 3 | 0 |
| As3+ | µg/L | 1.89 | 4.43 | 0.410 | 10 | 10 | 0 |
| Cu2+ | µg/L | 2.17 | 14.27 | 0.055 | 2000 | 1000 | 0 |
| Zn2+ | µg/L | 7.19 | 163.04 | 0.071 | 3000 | - | 0 |
| Cr3+ | µg/L | - | ND | ND | 50 | - | 0 |
| Hg2+ | µg/L | - | ND | ND | 6 | - | 0 |
| Si | mg/L | 45.03 | 95.95 | 3.690 | - | - | 0 |
| Parameter | Units | Average | Max | Min | WHO MALs | SLS MALs | Unacceptable Samples (%) |
|---|---|---|---|---|---|---|---|
| RO product (drinking) water | |||||||
| General parameters | |||||||
| pH | 6.95 | 7.45 | 6.57 | 6.5–8.5 | 6.5–8.5 | 0 | |
| EC | µS/cm | 27.6 | 153 | 1.7 | 400 | 750 | 0 |
| Hardness | mg/L (CaCO3) | 7.2 | 141.5 | 0.37 | - | 250 | 0 |
| Alkalinity | mg/L (CaCO3) | 35 | 95 | 0 | - | 200 | 0 |
| Anions | |||||||
| F− | mg/L | 0.07 | 0.9 | 0.02 | 1.5 | 1.0 | 0 |
| Cl− | mg/L | 2.6 | 41.8 | 0.07 | - | 250 | 0 |
| Br− | mg/L | 0.14 | 0.15 | 0.13 | 2 | - | 0 |
| NO3− | mg/L | 0.31 | 1.5 | 0.10 | 50 | 50 | 0 |
| PO43− | mg/L | 0.69 | 2.0 | 0.25 | - | - | - |
| SO42− | mg/L | 0.58 | 14.8 | 0.08 | 250 | 250 | 0 |
| Cations | |||||||
| Ca2+ | mg/L | 1.684 | 20.82 | 0.126 | - | 100 | 0 |
| Mg2+ | mg/L | 0.741 | 24.53 | 0.013 | - | 30 | 0 |
| Na+ | mg/L | 3.164 | 51.65 | 0.310 | 200 | 250 | 0 |
| K+ | mg/L | 0.292 | 3.01 | 0.011 | - | - | - |
| Li+ | µg/L | 2.773 | 14.29 | 0.090 | - | - | - |
| Sr2+ | µg/L | 17.37 | 380.85 | 0.378 | - | - | - |
| Ba2+ | µg/L | 5.989 | 109.47 | 0.306 | 1300 | - | 0 |
| Mn2+ | µg/L | 0.198 | 5.63 | 0.012 | 100 | 100 | 0 |
| Fe2+ | µg/L | 0.938 | 9.32 | 0.010 | 200 | 300 | 0 |
| Cd2+ | µg/L | 0.033 | 0.34 | 0.002 | 3 | 3 | 0 |
| As3+ | µg/L | 0.796 | 2.37 | 0.031 | 10 | 10 | 0 |
| Cu2+ | µg/L | 1.035 | 12.44 | 0.009 | 2000 | 1000 | 0 |
| Zn2+ | µg/L | 4.453 | 107 | 0.011 | 3000 | - | 0 |
| Cr3+ | µg/L | - | ND | ND | 50 | - | 0 |
| Hg2+ | µg/L | - | ND | ND | 6 | - | 0 |
| Si | mg/L | 2.333 | 20.28 | 0.157 | - | - | - |
| Parameter | Membrane Age | Operating Pressure | Feed Water EC |
|---|---|---|---|
| Salt rejection | −0.681 ** | −0.026 | 0.182 |
| Ca rejection | −0.421 | −0.117 | 0.195 |
| Mg rejection | −0.442 * | −0.049 | 0.008 |
| K rejection | −0.550 ** | −0.414 | 0.410 |
| Li rejection | −0.563 * | 0.360 | −0.058 |
| Na rejection | −0.456 * | 0.060 | 0.264 |
| As rejection | 0.283 | −0.229 | −0.158 |
| Si rejection | −0.396 | 0.132 | 0.198 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Indika, S.; Wei, Y.; Hu, D.; Ketharani, J.; Ritigala, T.; Cooray, T.; Hansima, M.A.C.K.; Makehelwala, M.; Jinadasa, K.B.S.N.; Weragoda, S.K.; et al. Evaluation of Performance of Existing RO Drinking Water Stations in the North Central Province, Sri Lanka. Membranes 2021, 11, 383. https://doi.org/10.3390/membranes11060383
Indika S, Wei Y, Hu D, Ketharani J, Ritigala T, Cooray T, Hansima MACK, Makehelwala M, Jinadasa KBSN, Weragoda SK, et al. Evaluation of Performance of Existing RO Drinking Water Stations in the North Central Province, Sri Lanka. Membranes. 2021; 11(6):383. https://doi.org/10.3390/membranes11060383
Chicago/Turabian StyleIndika, Suresh, Yuansong Wei, Dazhou Hu, Jegetheeswaran Ketharani, Tharindu Ritigala, Titus Cooray, M. A. C. K. Hansima, Madhubashini Makehelwala, K. B. S. N. Jinadasa, Sujithra K. Weragoda, and et al. 2021. "Evaluation of Performance of Existing RO Drinking Water Stations in the North Central Province, Sri Lanka" Membranes 11, no. 6: 383. https://doi.org/10.3390/membranes11060383
APA StyleIndika, S., Wei, Y., Hu, D., Ketharani, J., Ritigala, T., Cooray, T., Hansima, M. A. C. K., Makehelwala, M., Jinadasa, K. B. S. N., Weragoda, S. K., & Weerasooriya, R. (2021). Evaluation of Performance of Existing RO Drinking Water Stations in the North Central Province, Sri Lanka. Membranes, 11(6), 383. https://doi.org/10.3390/membranes11060383






