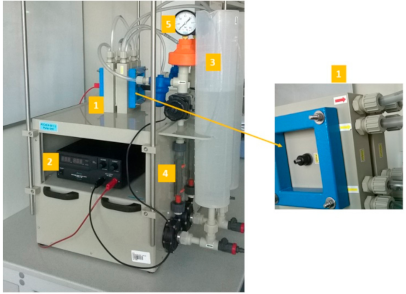
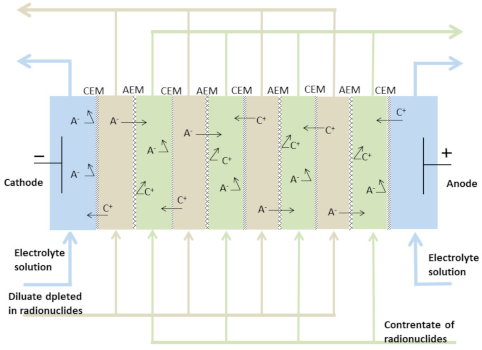
3.1. Experiments Using Model Solutions
The first experiments were conducted using non-radioactive model solutions that were prepared based on the chemical composition of radioactive liquid waste collected at the RWMP. The types of organic compounds present in this waste were not specified; only their content was known (measured as the TOC). The TOC in different waste samples collected from the RWMP ranged from 15 to 75 mg/dm
3. Therefore, when preparing the model solutions, it was assumed that both ionic and non-ionic organic compounds were present. In this work, citric acid and octylphenyl ethoxylate (Triton X-102) were added as the model organic substances, which may be present in waste containing decontamination agents from cleaning procedures. Detergents of Triton X-type (e.g., Triton X-100) are also used as a scintillation cocktail in beta scintillation counters, so they may be present in liquid waste originating from radioisotope applications. Four series of solutions, differing in salinity and type of organic substance admixture, were prepared according to
Table 2 and employed as model liquid waste in ED experiments.
The preliminary study focused on evaluating the effects of the initial salt concentration of the feed solution and the applied current on the process’s performance. Two current density values were used for each solution: specifically, 18.8 and 35.9 A/m
2, which were 50% and 96% of the limiting current (for 70% desalination), respectively. The results of these tests are presented in
Table 3 and
Table 4.
Electrodialysis conducted at a high current was more efficient than the process at a lower amperage (
Table 3). Moreover, the results indicated that the total salinity of the solution affected the efficiency of the electrolysis. At lower salt concentrations (solution No. II), a higher desalination of the model solutions was achieved compared with the process applied to solutions with a higher salt concentration. In general, the higher the amperage, the more efficient the removal of citric acid, which is expressed as a decreasing TOC. When a current density of 35.9 A/m
2 was applied, the TOC decreased by almost 90% in the case of solutions No. I and II. In contrast, in the presence of a lower current density (18.8 A/m
2), the TOC diminished by 71% and 80% for solutions No. I and II, respectively. The model organic compound (citric acid) added to solutions No. I and II also passed through the membranes, so during the process it was removed from the diluate reservoir and collected in the concentrate container. However, the citrate anions were probably transported from the diluate to the concentrate stream more slowly compared to the other ions, as indicated by lower degree of TOC removal (approximately 85–90%), while the other ions were removed with an efficiency of close to 100%. The transport of ionic organic compounds through the membranes during ED can be influenced by various parameters, such as current density, size, and type of ion charge, as well as the functional groups on these ions [
18]. It has been reported that mono-selective anion-exchange membranes can be used to improve the separation of anionic organic substances from salts [
19]. However, the separation of ionic organic substances from inorganic compounds present in the model solutions was not observed with the membranes and process conditions applied in this work.
A different situation emerged when Triton X-102 was used as the model non-ionic substance added to the solution (solutions No. III and IV). In this case, the organic substance was retained by the membranes and only a small amount of Triton X-102 passed to the concentrate stream (
Table 4).
This experiment demonstrated the suitability of ED for the separation of ionic substances from non-ionic components in solution. These results demonstrated the possibility of selectively separating non-ionic organic contaminants of radioactive liquid waste (which interfere with numerous processes) to treat such waste in the subsequent stages of predisposal. This valuable feature of ED has been used in many fields [
20,
21,
22].
3.2. Treatment of Radioactive Solutions Using Electrodialysis
One of the main objectives of this work was to assess the possibility of (i) using ED to remove radionuclides from liquid waste, (ii) concentrating them in a small volume, and (iii) simultaneously obtaining a stream of purified water that can be safely discharged into the environment. For this purpose, two model solutions containing inorganic salts and one of two radionuclides commonly present in real waste collected from Polish laboratories (
137Cs and
60Co) were prepared and subjected to the ED process. The compositions of the model radioactive solutions are presented in
Table 5.
The electrodialysis was performed using the process parameters selected during the preliminary tests, which provided the best salt removal effects: the voltage (U) was 20 V, the mean velocities (u) of the diluate and concentrate were the same at 0.16 cm/s, and the current density (i) was 31.3 A/m
2. The results of these experiments are presented in
Figure 4 and
Figure 5.
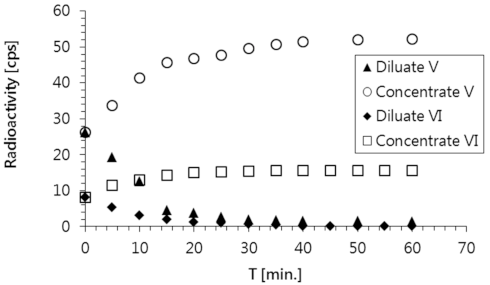
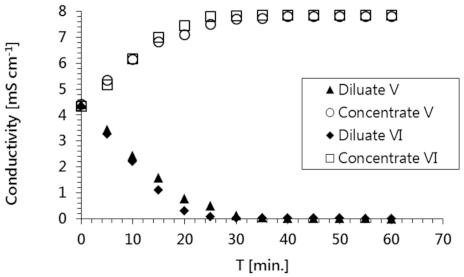
These experimental results confirmed that the tested solutions containing radioactive substances were purified with very high efficiency using the applied ED process. Both radionuclides (i.e.,
137Cs in solution No. V, and
60Co in solution No. VI) were completely removed in 40 min (
Figure 4). In the same amount of time, a corresponding concentration of these species was detected in the concentrate stream. The efficiency of a radioactive waste purification process is typically described by the decontamination factor (DF), which is calculated using Equation (2),
where
A0 and
Ai represent the radioactivity of the solution at the end of the experiment and at a given time, respectively (in counts per minute, cpm).
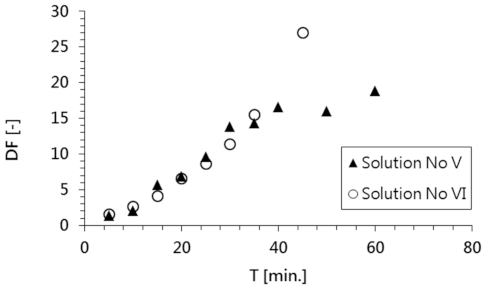
A comparison of the changes in the decontamination factor of both model solutions is shown in
Figure 6. High DF values (19 and 27 for solutions No. V and VI, respectively) were achieved as a result of the electrodialysis process used to decontaminate both model solutions.
The remaining components in the model solutions (i.e., inorganic salt ions) accumulated in the concentrate, leaving the diluate free of impurities (
Figure 5). The decline in conductivity observed for the diluate stream was slightly faster than the decline in this stream’s radioactivity; the diluate attained a conductivity close to that of pure water in just 30 min. Similar behavior was observed for both model radioactive solutions.
Finally, a sample of real waste received from the RWMP was subjected to the electrodialysis treatment process. Its characteristics are summarized in
Table 6.
The sample contained
137Cs,
60Co,
85Sr, and
241Am radionuclides. The parameters of the applied electrodialysis process were the same as those applied for the treatment of model radioactive solutions. The results of these experiments are presented in
Figure 7a,b.
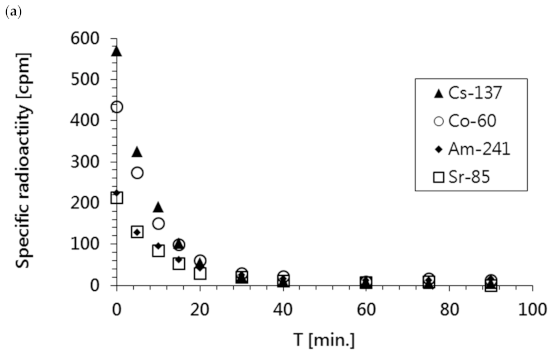
As shown in
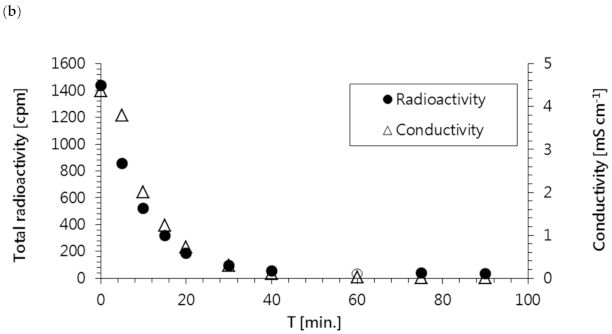
Figure 7a, the specific radioactivity of all radionuclides in the waste sample rapidly decreased over time; this was confirmed by the reduction in total activity shown in
Figure 7b. Additionally, it was observed that the radioactivities of all radioisotopes and the concentrations of ions present in the solution (represented as conductivity) dropped to the levels of pure water within the first 60 min of the ED process.
In summary, treating the real waste sample using the developed ED process led to efficient salt and radionuclide removal (nearly 100%) after 1 h of treatment, as shown in
Table 7.
The content of organic compounds in the final solution (diluate) also diminished. However, at the same time it was observed that organic matter did not accumulate in the concentrate stream—the concentration of organic substances in the concentrate stream slightly decreased as well. This can be explained by membrane fouling caused by organic substances such as ionic surfactants. There are many reports in the literature on the occurrence of membrane fouling during the electrodilysis process of solutions containing surfactants, peptides, or humates [
23,
24]. They can accumulate on the membrane surface and damage the membrane. This phenomenon occurs mainly when solutions of high concentration are treated or at the end of experiments when a high concentration of electrolyte appears near the membrane surface. It also seems possible to pass the components of the diluate and concentrate into the electrode rinsing solution. However, further research is required to explain the behavior of the organic compounds present in the sample of radioactive wastewater under examination.
The fouling of the ion exchange membranes is one of the main limiting factors for wide ED implementation, as for other membrane processes used for industrial wastewater treatment. Before implementing the process for the treatment of liquid radioactive waste on an industrial scale, the risk of membrane fouling will certainly require consideration, and the phenomenon itself will necessitate further experiments.
However, we already know many ways to deal with such problems—e.g., by adjusting the hydrodynamic conditions in the apparatus, adding appropriate substances to minimize membrane fouling [
25,
26], or periodic membrane cleaning. In the described case, fouling can be prevented by adding a small amount of acid (e.g., nitric acid) to the concentrate or by subjecting the wastewater to a preliminary treatment that consists of removing non-ionizable substances (silica, bacteria, soluble organic compounds, etc.).
Overall, the results obtained in this study regarding the treatment of model radioactive solutions and real radioactive waste indicated that the developed ED process (
Table 8) could be applied to effectively remove radionuclides as well as inorganic salts present in solutions. Very high levels of salt removal (>99.5%) and high DF values were obtained as a result of this process.
During the experiments, no significant decrease in the efficiency of the process was observed. However, membrane fouling and scaling can occur during the treatment of real radioactive wastewater by the ED, especially in the case of a long-term process. The membrane supplier predicts the lifetime of the membranes to be 1–3 years. If fouling occurs, cleaning with 5–10% HCl or 4% NaOH can be applied for membrane regeneration. Moreover, the mechanical cleaning of the membrane surfaces should also be carried out periodically.
Certainly, in the further stages of the development of the ED process for the treatment of specific real radioactive wastewater, it will be necessary to carry out longer test cycles to draw final conclusions about the feasibility of the process and to design an industrial system with an electrodialysis chamber.