Purification of Wet-Process Phosphoric Acid via Donnan Dialysis with a Perfluorinated Sulfonic Acid Cation-Exchange Membrane
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Membranes
2.2. Analysis Methods
2.3. Donnan Dialysis Process
2.4. Absorption Experiments
3. Results and Discussions
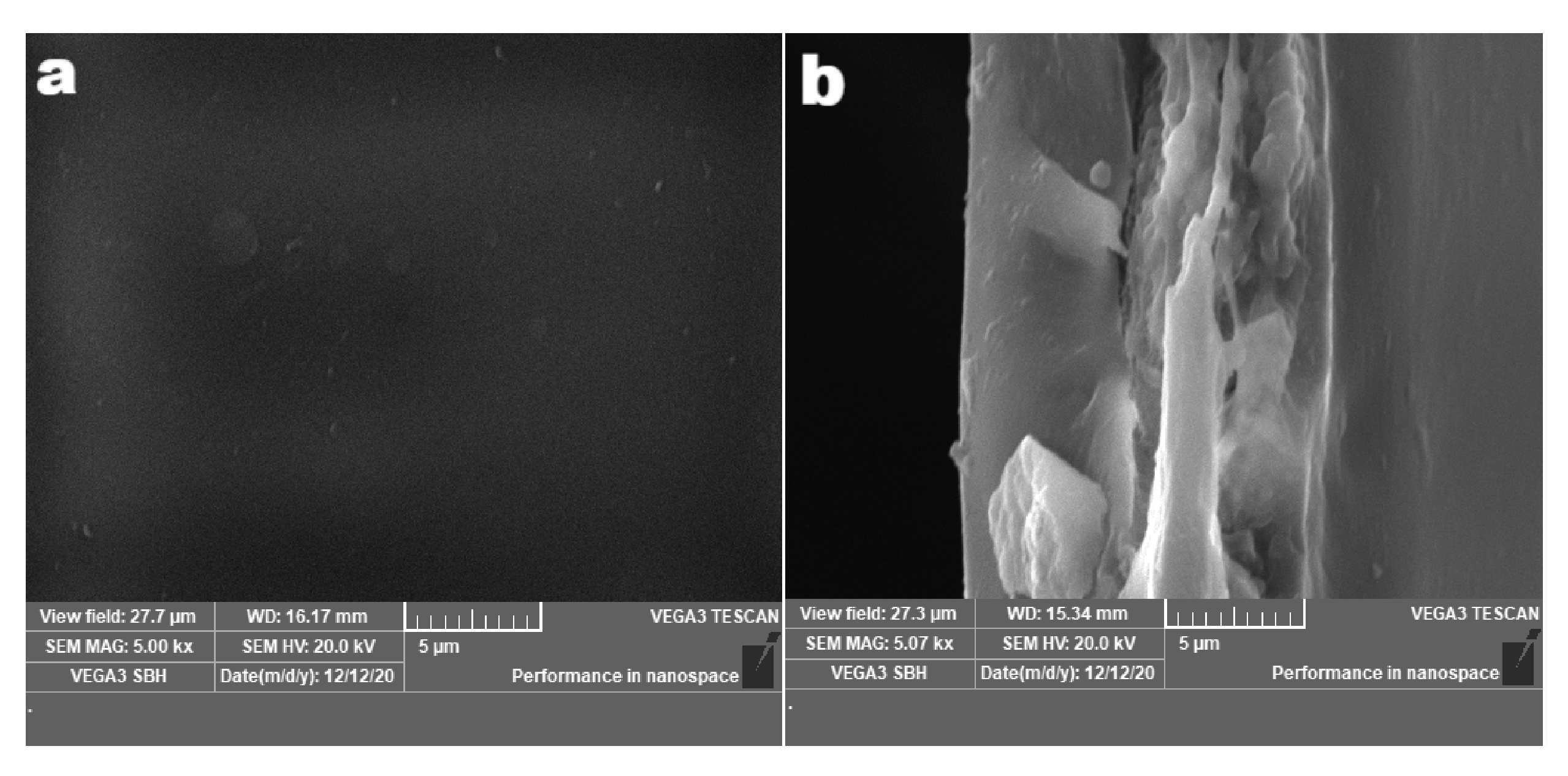
3.1. Membrane Morphology Analysis
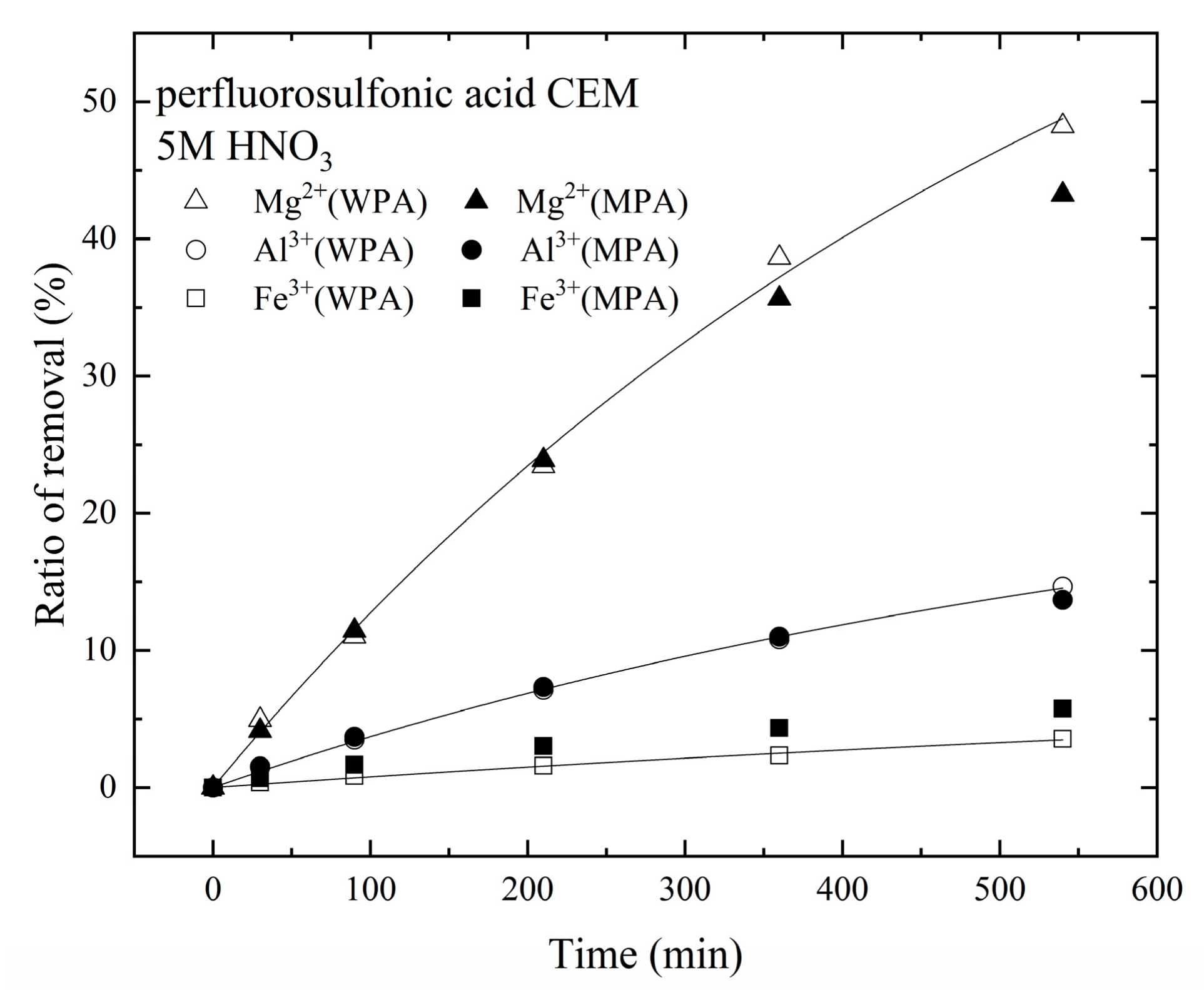
3.2. Removal of Metallic Cations from Industrial Wet-Process Phosphoric Acid
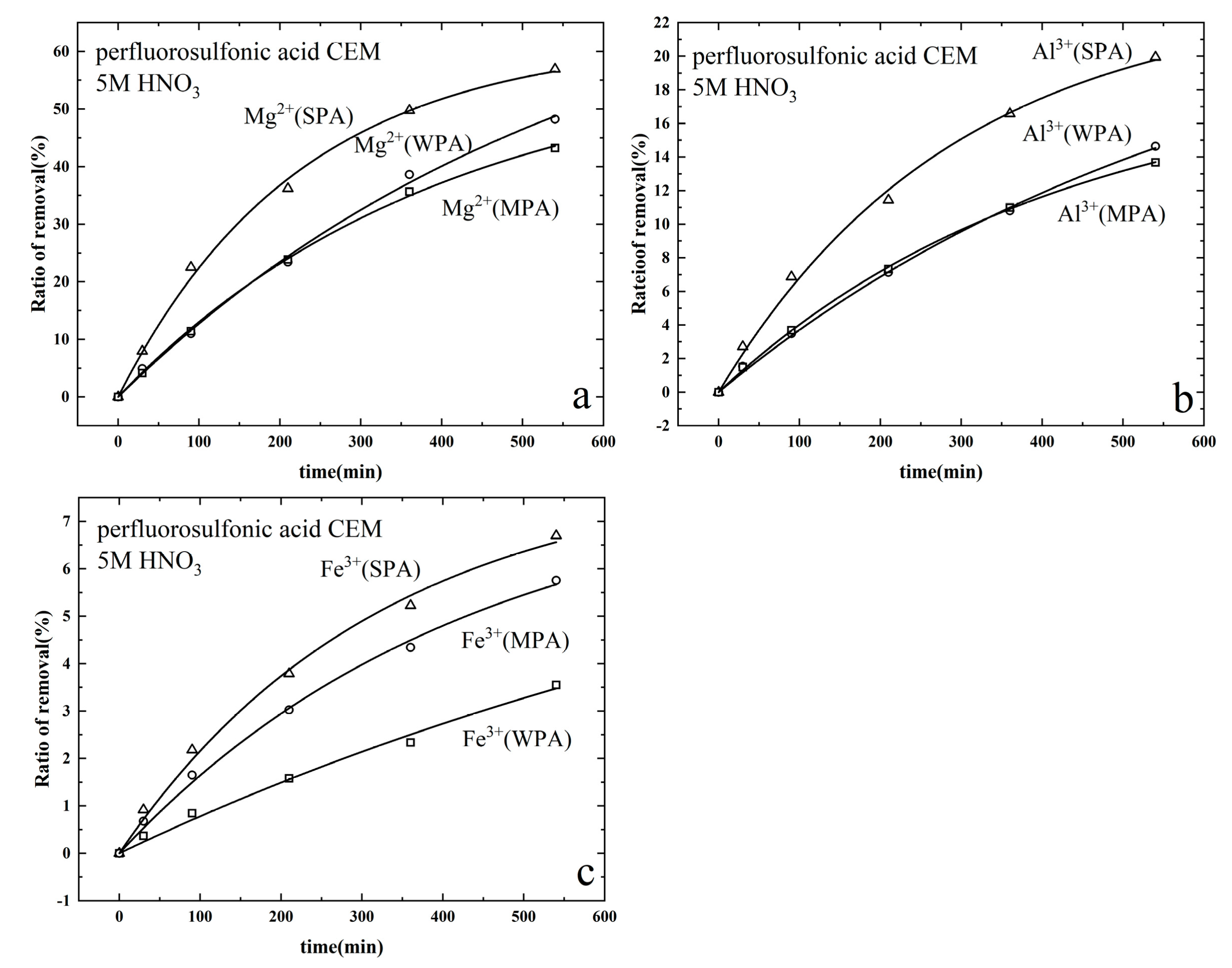
3.3. Removal of Metallic Cations from Synthetic Mixed Metallic Cations Phosphoric Acid
3.4. Removal of Metallic Cations from Synthetic Single Metallic Cations Phosphoric Acid
3.5. Existing form of Metallic Cations in the Membrane
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, T.; Abdu, S.; Wessling, M. Selectivity of ion exchange membranes: A review. J. Membr. Sci. 2018, 555, 429–454. [Google Scholar] [CrossRef]
- Çengeloğlu, Y.; Kir, E.; Ersöz, M. Recovery and concentration of Al(III), Fe(III), Ti(IV), and Na(I) from red mud. J. Colloid Interface Sci. 2001, 244, 342–346. [Google Scholar] [CrossRef]
- Prakhar, P.; David, H.; SenGupta, A.K. Application of homogeneous and heterogeneous cation-exchange membranes in coagulant recovery from water treatment plant residuals using Donnan membrane process. J. Membr. Sci. 2004, 237, 131–144. [Google Scholar]
- Rozanska, A.; Wisniewski, J.; Winnicki, T. Donnan dialysis with anion-exchange membranes in a water desalination system. Desalination 2006, 198, 236–246. [Google Scholar] [CrossRef]
- Lei, Y.; Ma, J. Comparison between neutralization process and extraction process of industrial MAP production from WPA. Phosphate Compd. Fert. 2020, 35, 24–26. [Google Scholar]
- Chen, M.; Li, J.; Jin, Y.; Luo, J.H.; Zhu, X.H.; Yu, D.F. Efficient solvent extraction of phosphoric acid with dibutyl sulfoxide. J. Chem. Technol. Biot. 2018, 93, 467–475. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Luo, J.H.; Jin, Y.; Zou, D. Purification of wet process phosphoric acid by solvent extraction using cyclohexanol. Solvent Extr. Res. Dev. 2017, 24, 23–35. [Google Scholar] [CrossRef]
- Zhu, F.; Zhou, G.Y.; Chen, J.J.; Liu, H.M.; Hu, H.Y. Study on purification of wet-process phosphoric acid by neutralization from sodium hydroxide. Phosphate Compd. Fert. 2017, 32, 11–13. [Google Scholar]
- Yue, H.R.; Ying, J.K. Statistical analysis on removing Al-Mg impurities in wet-process phosphoric acid by complexing precipitation method. Inorg. Chem. Ind. 2009, 41, 51–53. [Google Scholar]
- Tang, C.; Qiu, Y.; Wang, Y.; Wang, X.L.; Zhang, Z.Y.; Yang, L. Kinetic studies on Al3+ removal from phosphoric acid by cation exchange resin. Can. J. Chem. Eng. 2018, 96, 944–954. [Google Scholar] [CrossRef]
- Amin, M.I. Removal of iron from wet process phosphoric acid using ion exchange method by Puromet MTS9570 resin. Int. J. Environ. Anal. Chem. 2020, 50, 1–18. [Google Scholar] [CrossRef]
- Sonoc, A.C.; Jeswiet, J.; Murayama, N.; Shibata, J. A study of the application of Donnan dialysis to the recycling of lithium ion batteries. Hydrometallurgy 2018, 175, 133–143. [Google Scholar] [CrossRef]
- Diallo, H.; Rabiller-Baudry, M.; Khaless, K.; Chaufer, B. On the electrostatic interactions in the transfer mechanisms of iron during nanofiltration in high concentrated phosphoric acid. J. Membr. Sci. 2013, 427, 37–47. [Google Scholar] [CrossRef]
- Moya, A.A. Uphill transport in improved reverse electrodialysis by removal of divalent cations in the dilute solution: A nernst-planck based study. J. Membr. Sci. 2020, 598, 117784. [Google Scholar] [CrossRef]
- Guo, C.M.; Li, X.H.; Li, X.Q. Progress of chemical disposal defluorination of wet -process phosphoric acid. Liaoning Chem. Ind. 2006, 35, 537–539. [Google Scholar]


| Target Components | P2O5(H3PO4) [wt.%] | Fe3+ [wt.%] | Al3+ [wt.%] | Mg2+ [wt.%] |
| 26.57 (36.67) | 0.66 | 0.27 | 0.39 | |
| Other components | Ca2+ [wt.%] | K+ [wt.%] | Na+ [wt.%] | F− [wt.%] |
| 0.08 | 0.03 | 0.01 | 1.47 |
| Product Code | DMR100M | |
|---|---|---|
| Dimensions | Dry film thickness [μm] | 15 ± 1 |
| Wet film thickness [μm] | 19 ± 1 | |
| Parameters | Conductivity [mS cm−1] | 68.6 (H+ form) |
| Areal density [g m−2] | 28 ± 1 | |
| Ion exchange capacity [mmol g−1] | 1.57 | |
| Materials | Perfluorinated sulfonic acid polymer, polypropylene reinforcement | |
| Sample Acid Types | Removed Fe3+ [mg] | Removed Al3+ [mg] | Removed Mg2+ [mg] | Total Charges Removed [mequ.] |
|---|---|---|---|---|
| WPA | 2.79 | 4.76 | 21.91 | 2.48 |
| MPA | 4.86 | 4.27 | 19.07 | 2.30 |
| Fe3+-SPA | 5.60 | / | / | 0.30 |
| Al3+-SPA | / | 6.49 | / | 0.72 |
| Mg2+-SPA | / | / | 24.04 | 1.98 |
| Sample Acid Type | P [mg] | Fe3+ [mg] | Al3+ [mg] | Mg2+ [mg] | Target Metal/P [mol/mol] |
|---|---|---|---|---|---|
| Fe3+-SPA | 0.010 | 0.230 | 0.010 | 0.100 | 13.180 |
| Al3+-SPA | 0.010 | 0.001 | 1.030 | 0 | 118.366 |
| Mg2+-SPA | 0.010 | 0 | 0.015 | 3.091 | 398.581 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, Q.; Luo, T.; Yan, Z.; Yang, L.; Zhang, Z.; Wang, X. Purification of Wet-Process Phosphoric Acid via Donnan Dialysis with a Perfluorinated Sulfonic Acid Cation-Exchange Membrane. Membranes 2021, 11, 298. https://doi.org/10.3390/membranes11040298
Zhong Q, Luo T, Yan Z, Yang L, Zhang Z, Wang X. Purification of Wet-Process Phosphoric Acid via Donnan Dialysis with a Perfluorinated Sulfonic Acid Cation-Exchange Membrane. Membranes. 2021; 11(4):298. https://doi.org/10.3390/membranes11040298
Chicago/Turabian StyleZhong, Qin, Tao Luo, Zhengjuan Yan, Lin Yang, Zhiye Zhang, and Xinlong Wang. 2021. "Purification of Wet-Process Phosphoric Acid via Donnan Dialysis with a Perfluorinated Sulfonic Acid Cation-Exchange Membrane" Membranes 11, no. 4: 298. https://doi.org/10.3390/membranes11040298
APA StyleZhong, Q., Luo, T., Yan, Z., Yang, L., Zhang, Z., & Wang, X. (2021). Purification of Wet-Process Phosphoric Acid via Donnan Dialysis with a Perfluorinated Sulfonic Acid Cation-Exchange Membrane. Membranes, 11(4), 298. https://doi.org/10.3390/membranes11040298






