Characteristics of a Plasticized PVA-Based Polymer Electrolyte Membrane and H+ Conductor for an Electrical Double-Layer Capacitor: Structural, Morphological, and Ion Transport Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrolyte Preparation
2.3. Characterization Techniques
2.3.1. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.2. Field Emission Scanning Electron Microscopy (FESEM)
2.3.3. Electrochemical Impedance Spectroscopy (EIS)
2.4. Electrolyte Characterization
Transference Number Measurement (TNM) and Linear Sweep Voltammetry (LSV)
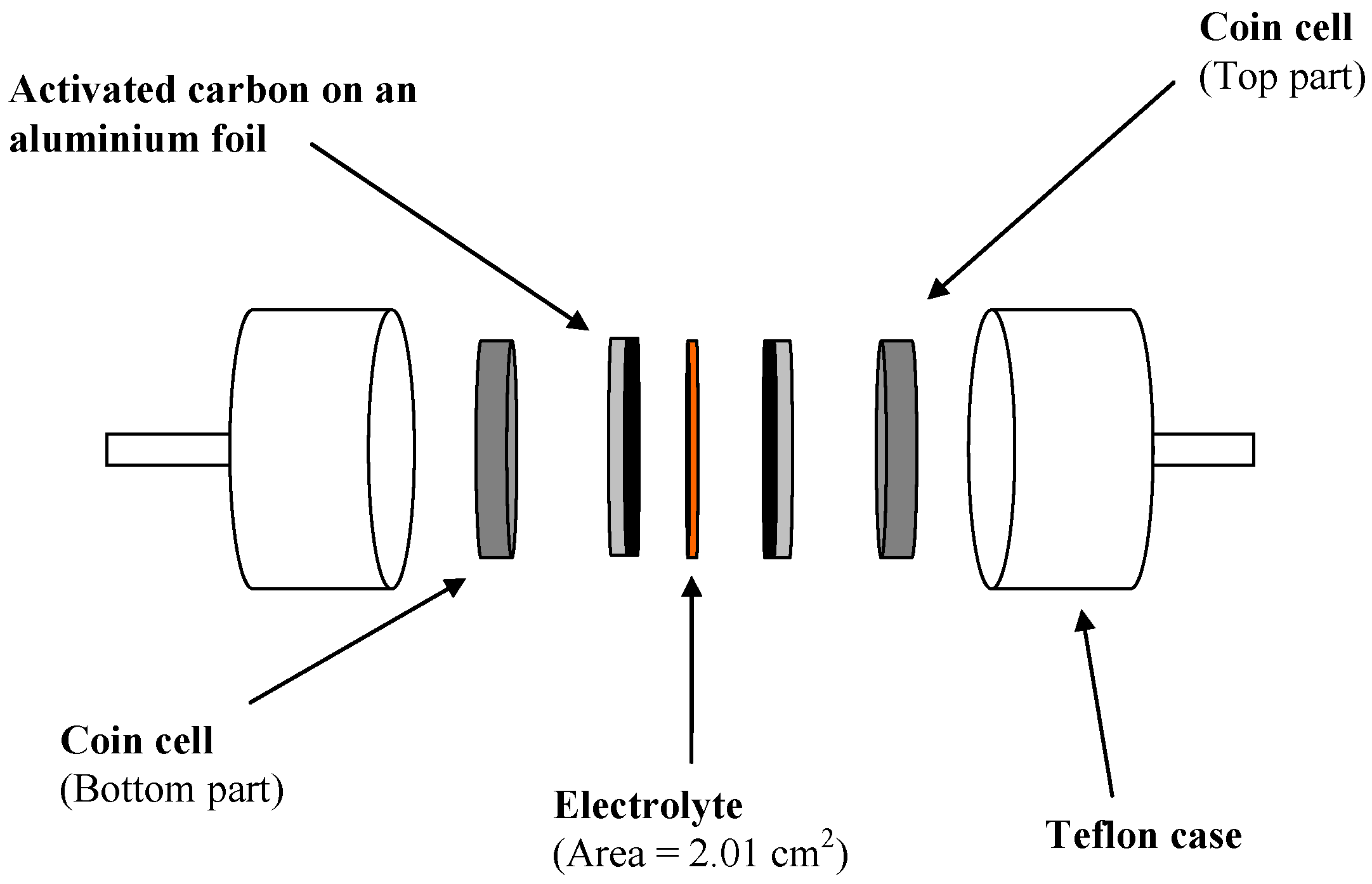
2.5. Electrode Preparation
2.6. EDLC Characterization
3. Results and Discussion
3.1. FTIR Analysis
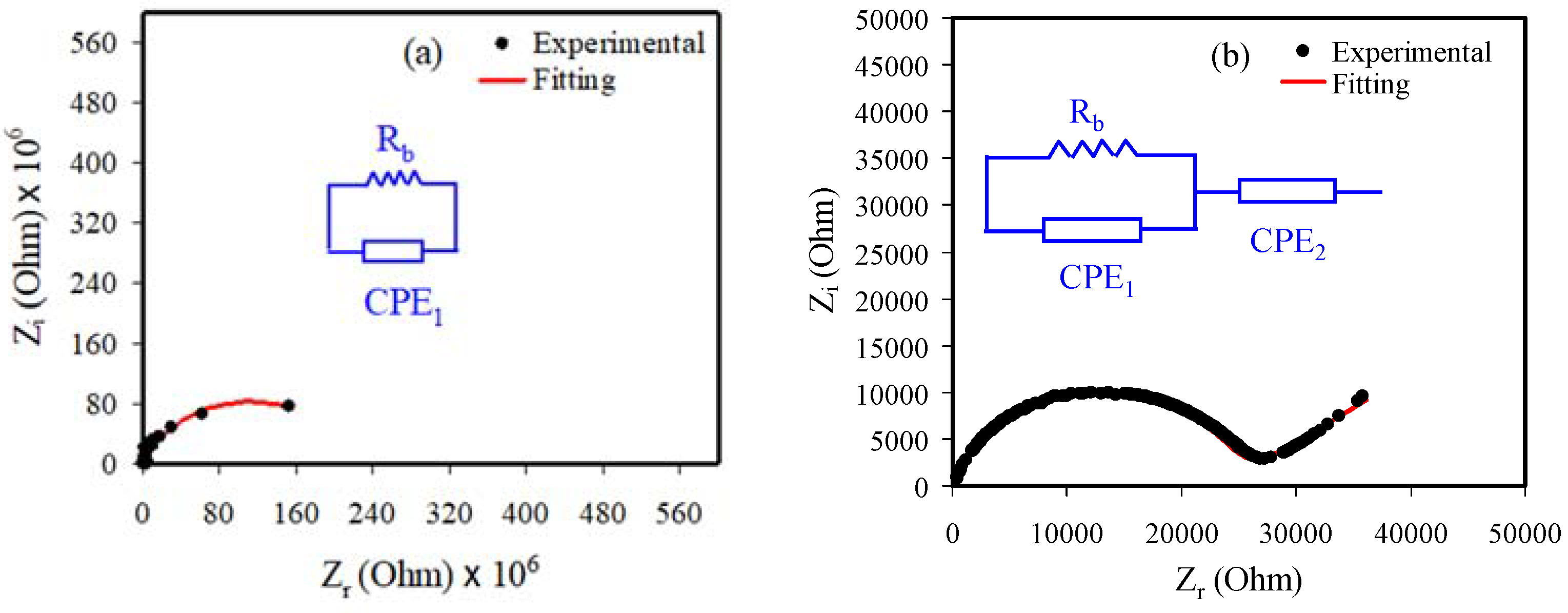
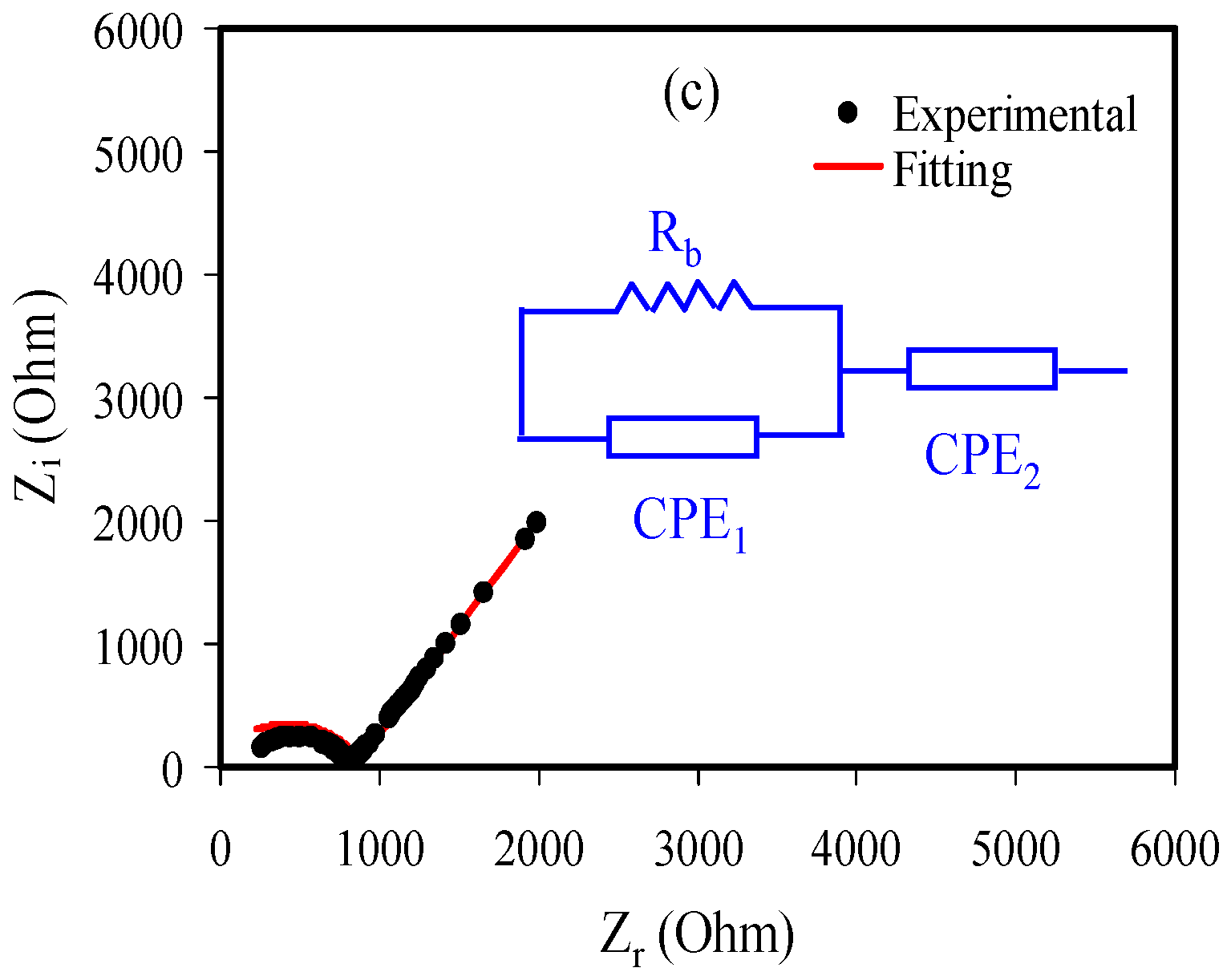
3.2. Impedance Analysis
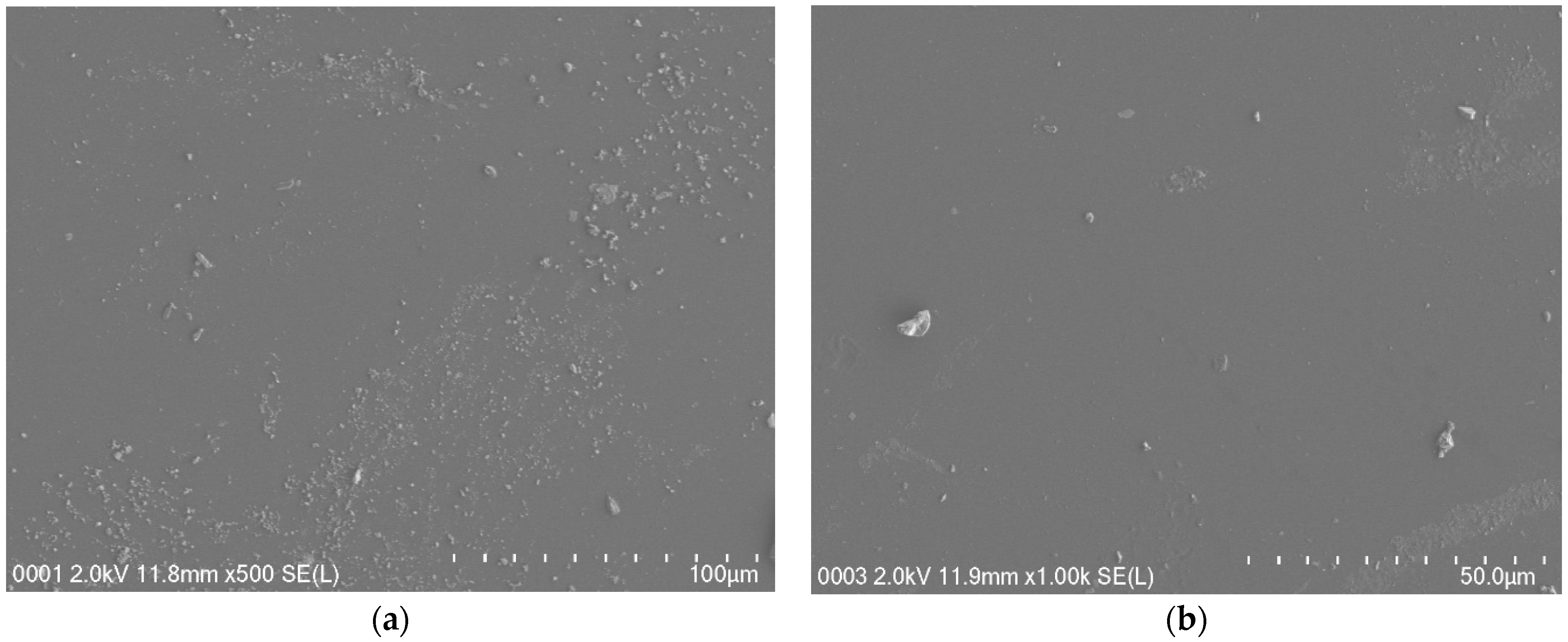
3.3. Field Emission Scanning Electron Microscopy (FESEM)
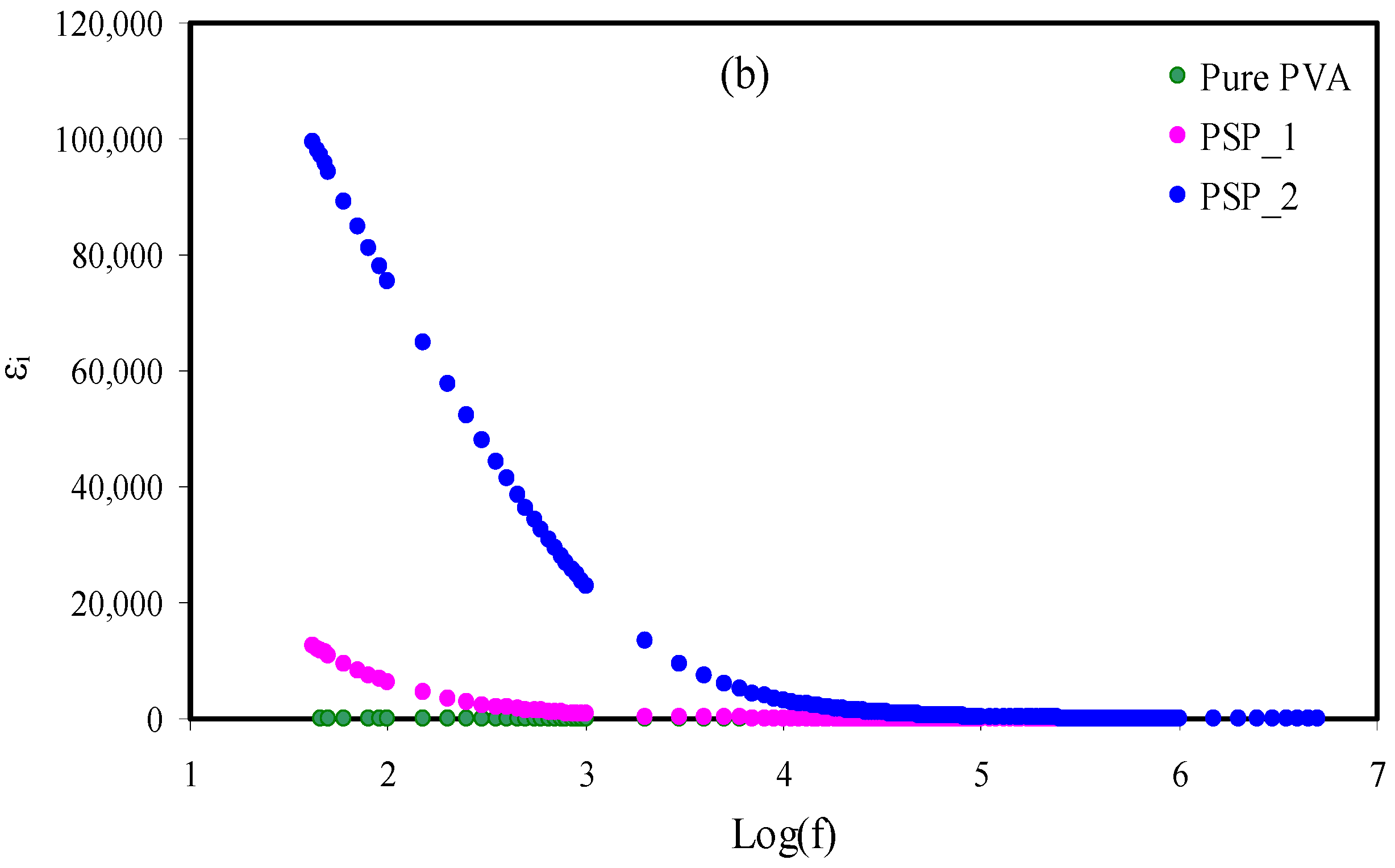
3.4. Dielectric Studies
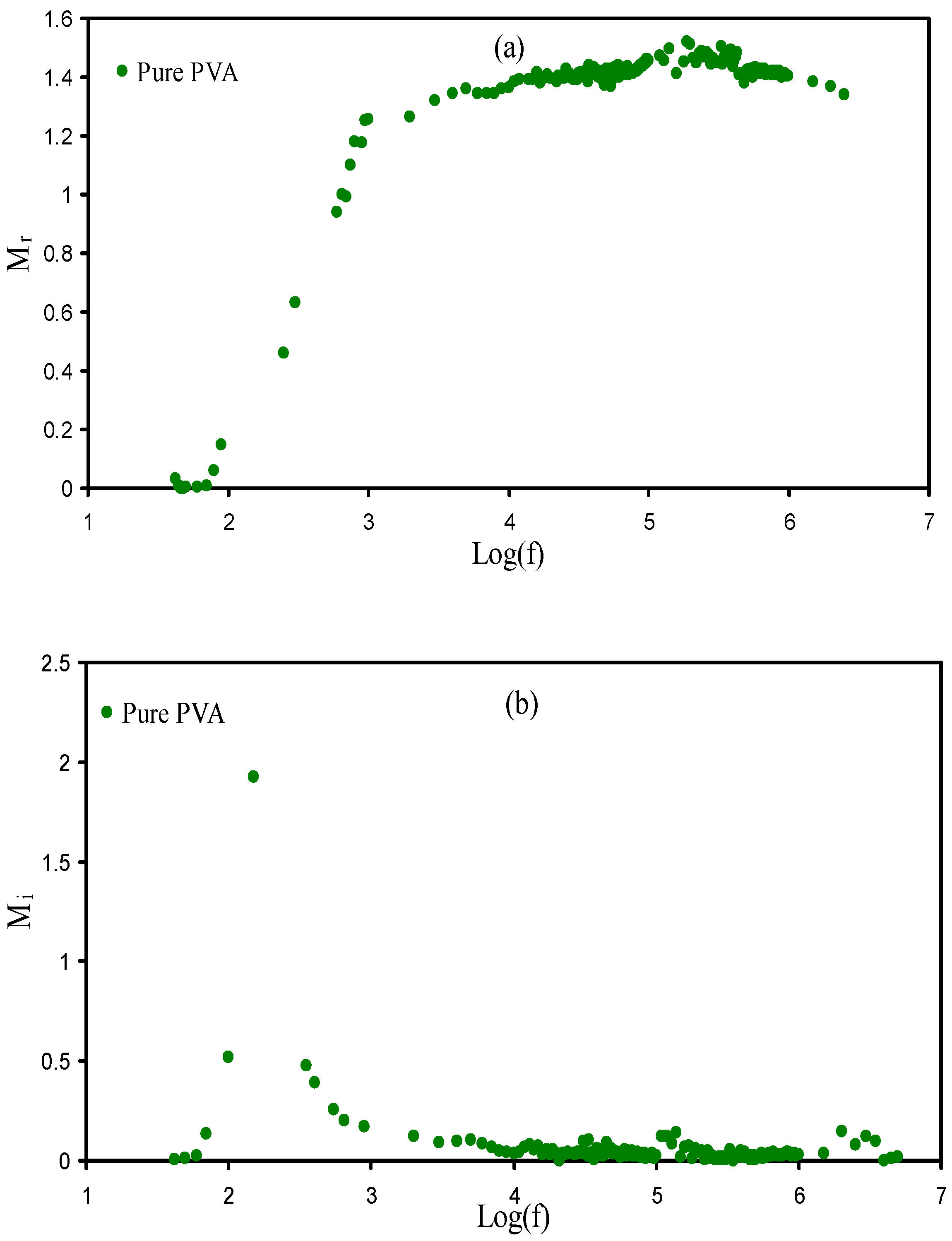
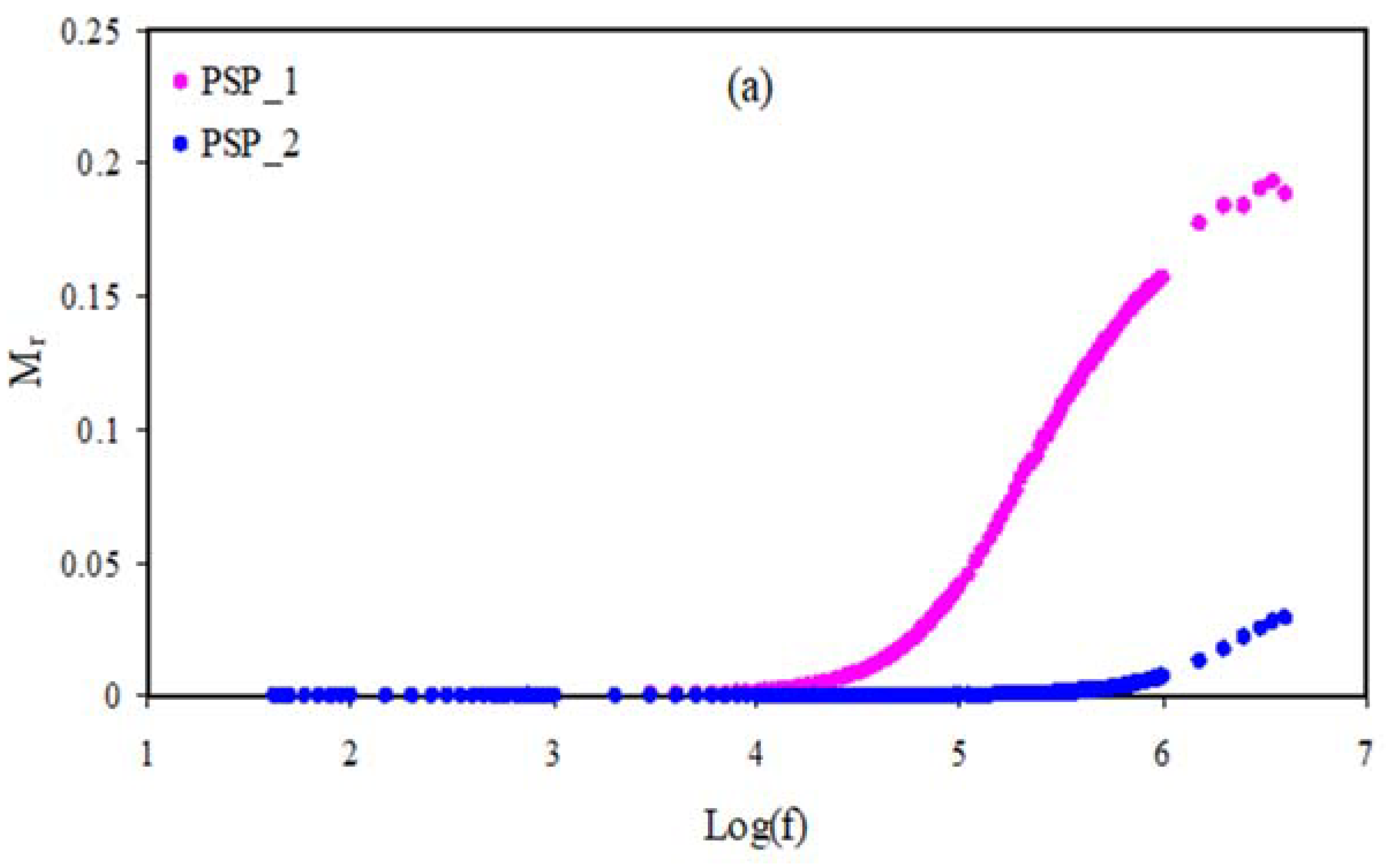
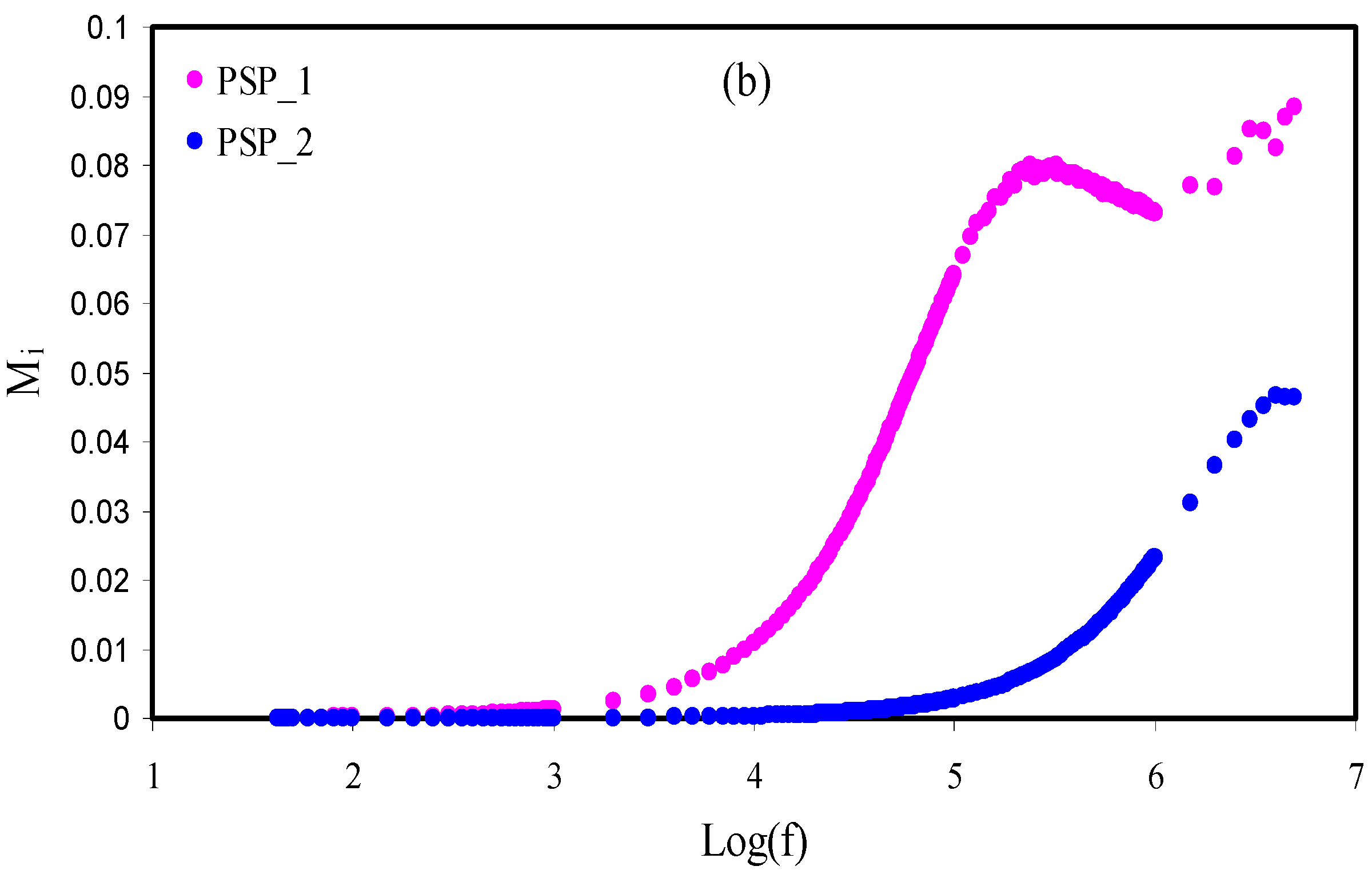
3.5. Electrical Modulus Studies
3.6. EDLC Characteristics
3.6.1. Transference Number Measurement (TNM)
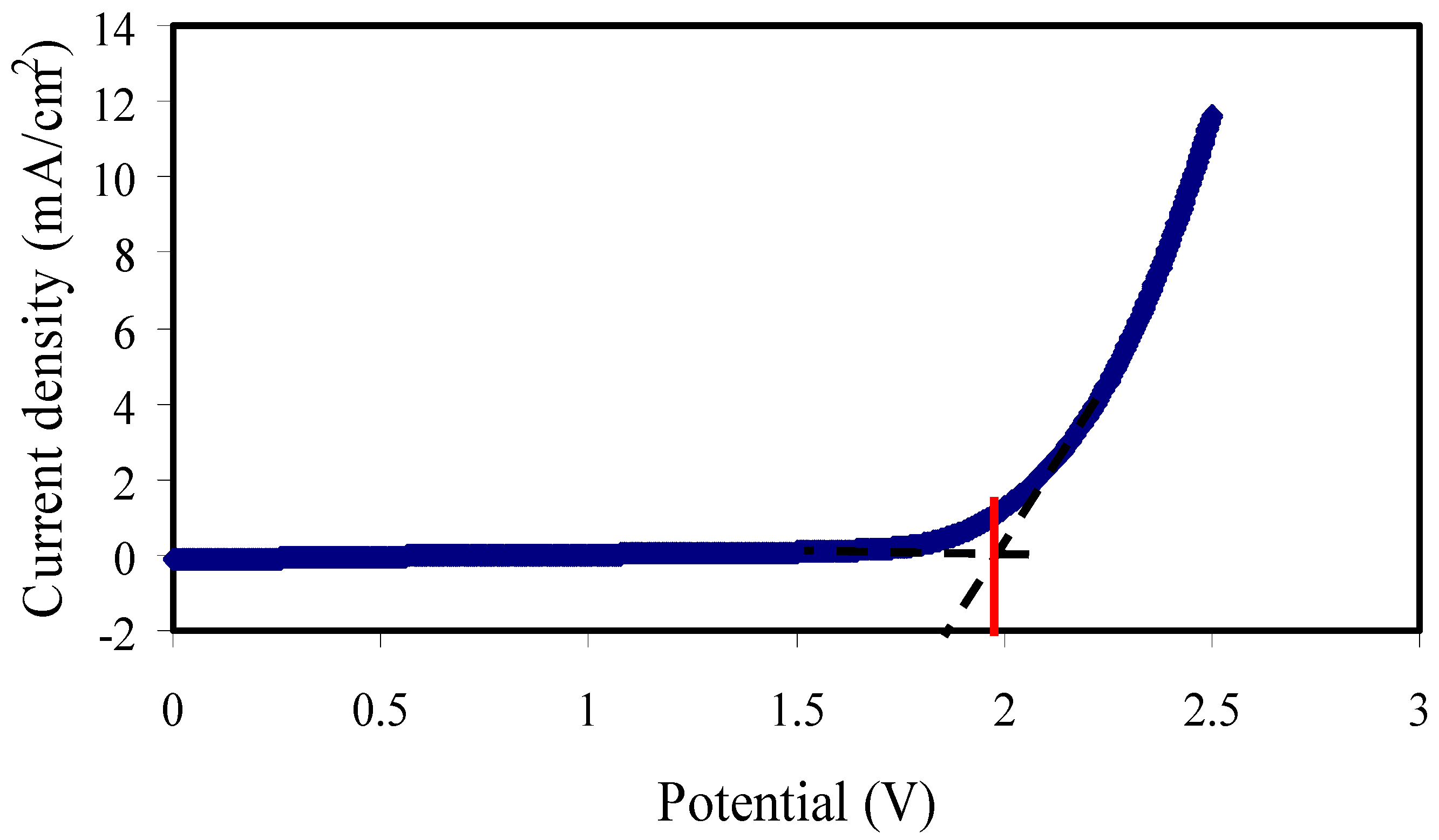
3.6.2. Electrochemical Stability Study
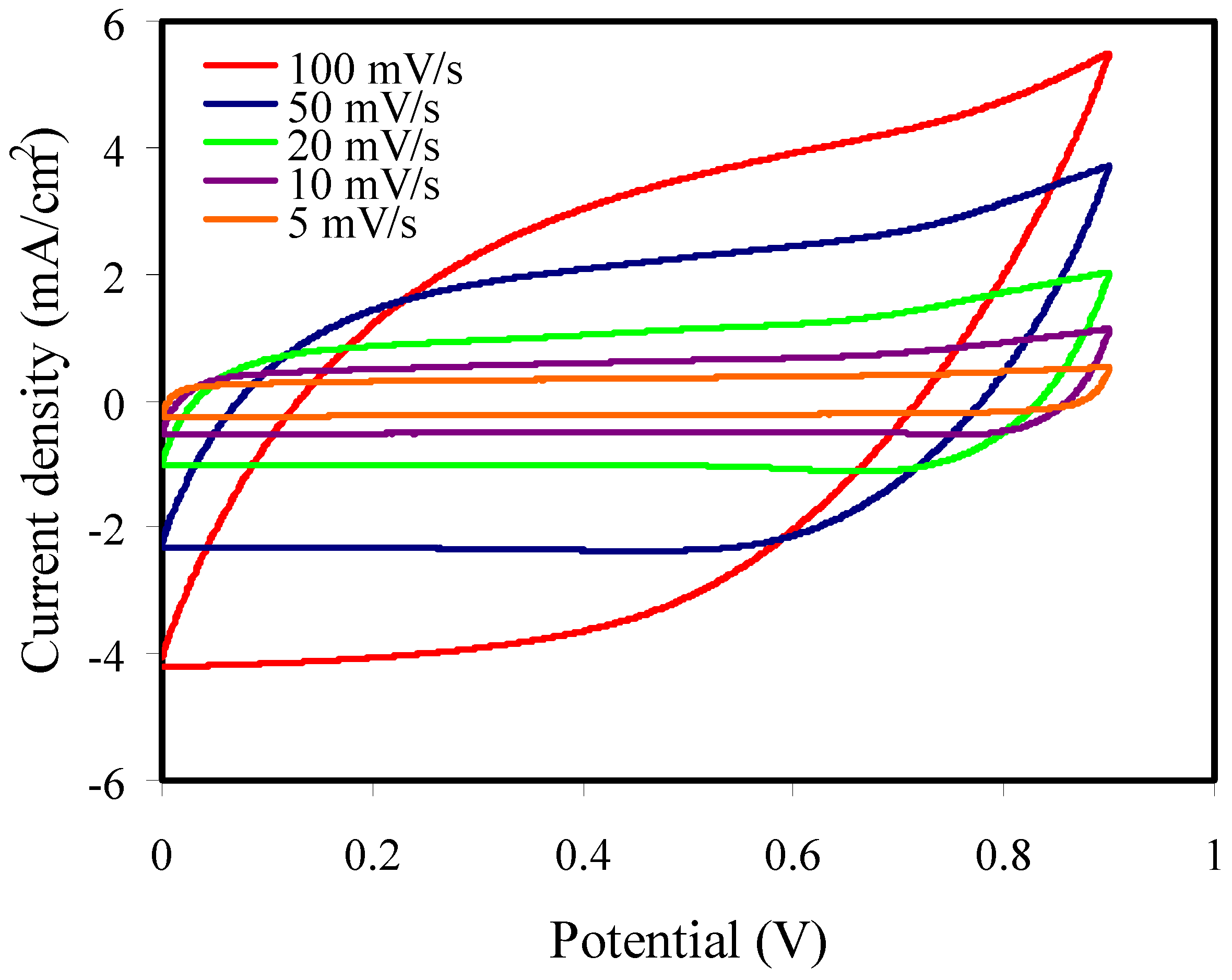
3.6.3. Cyclic Voltammetry Study
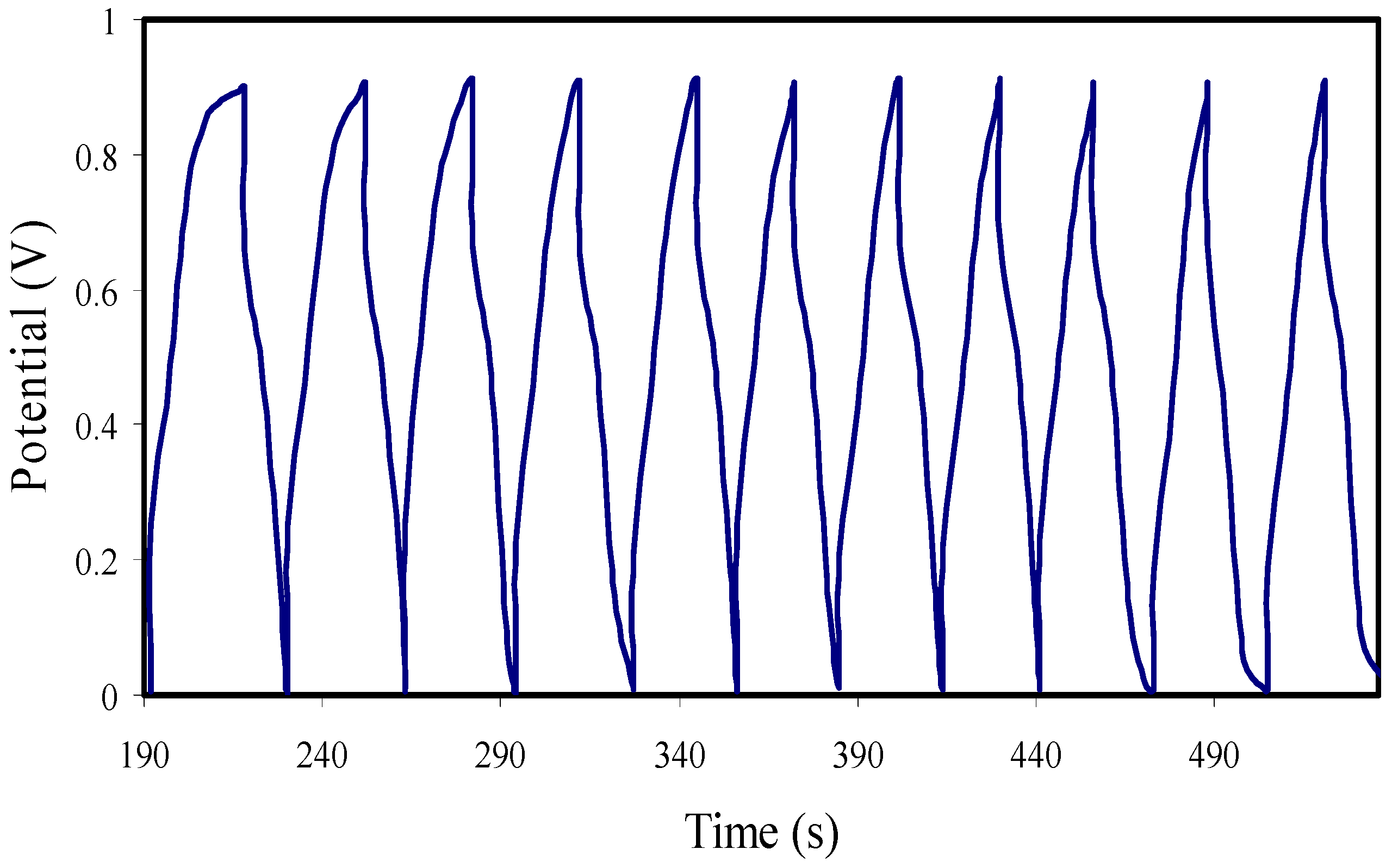
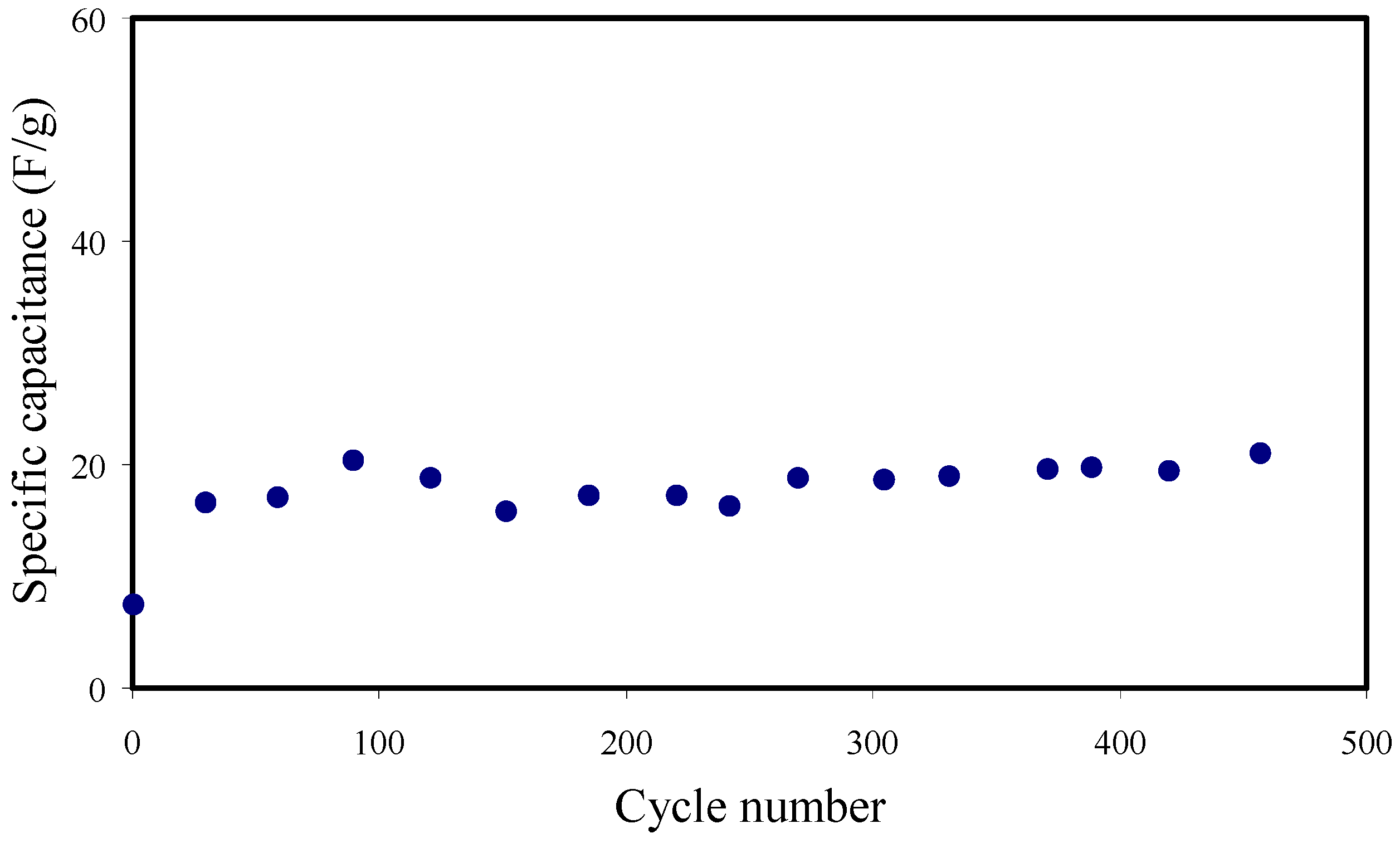
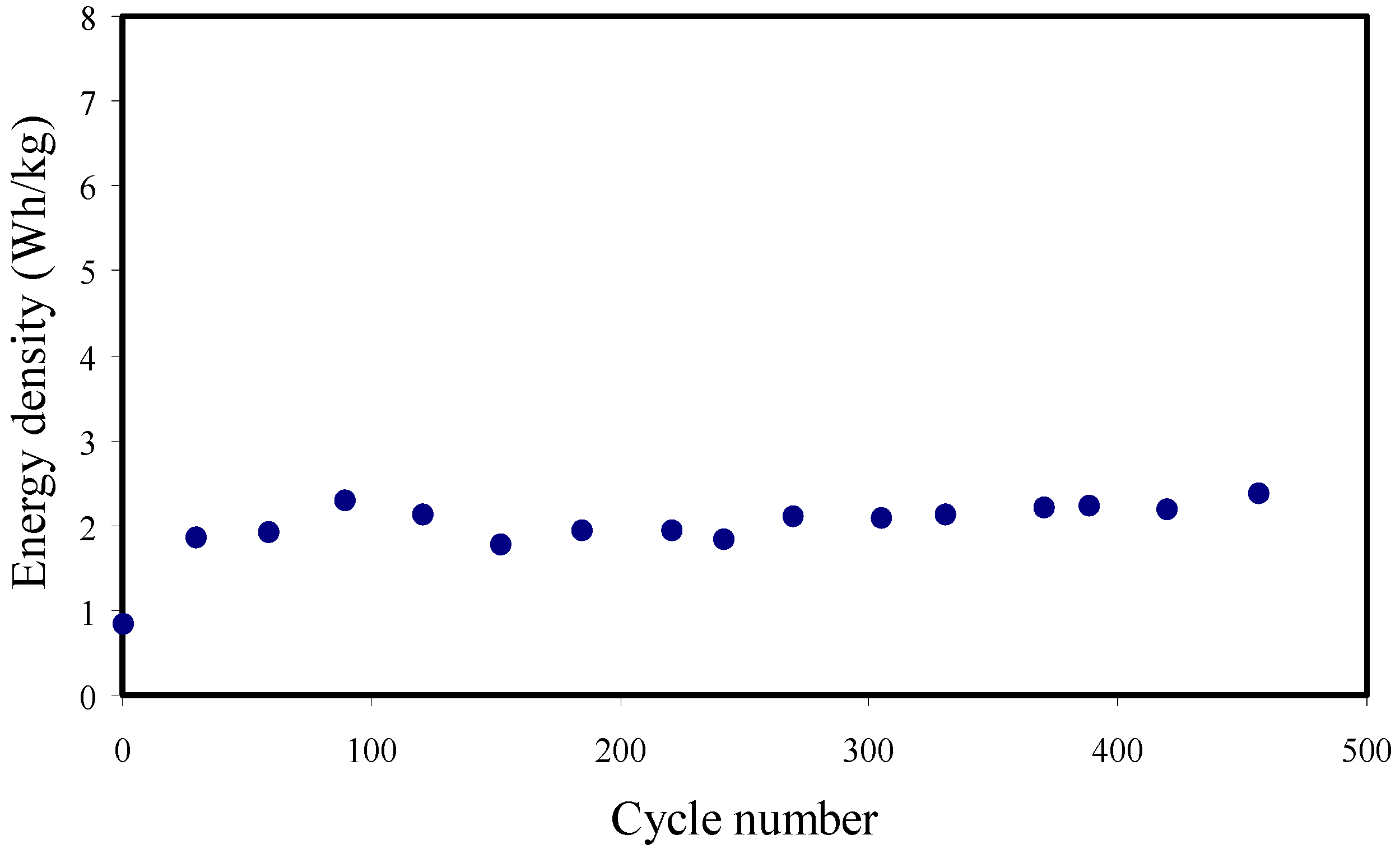
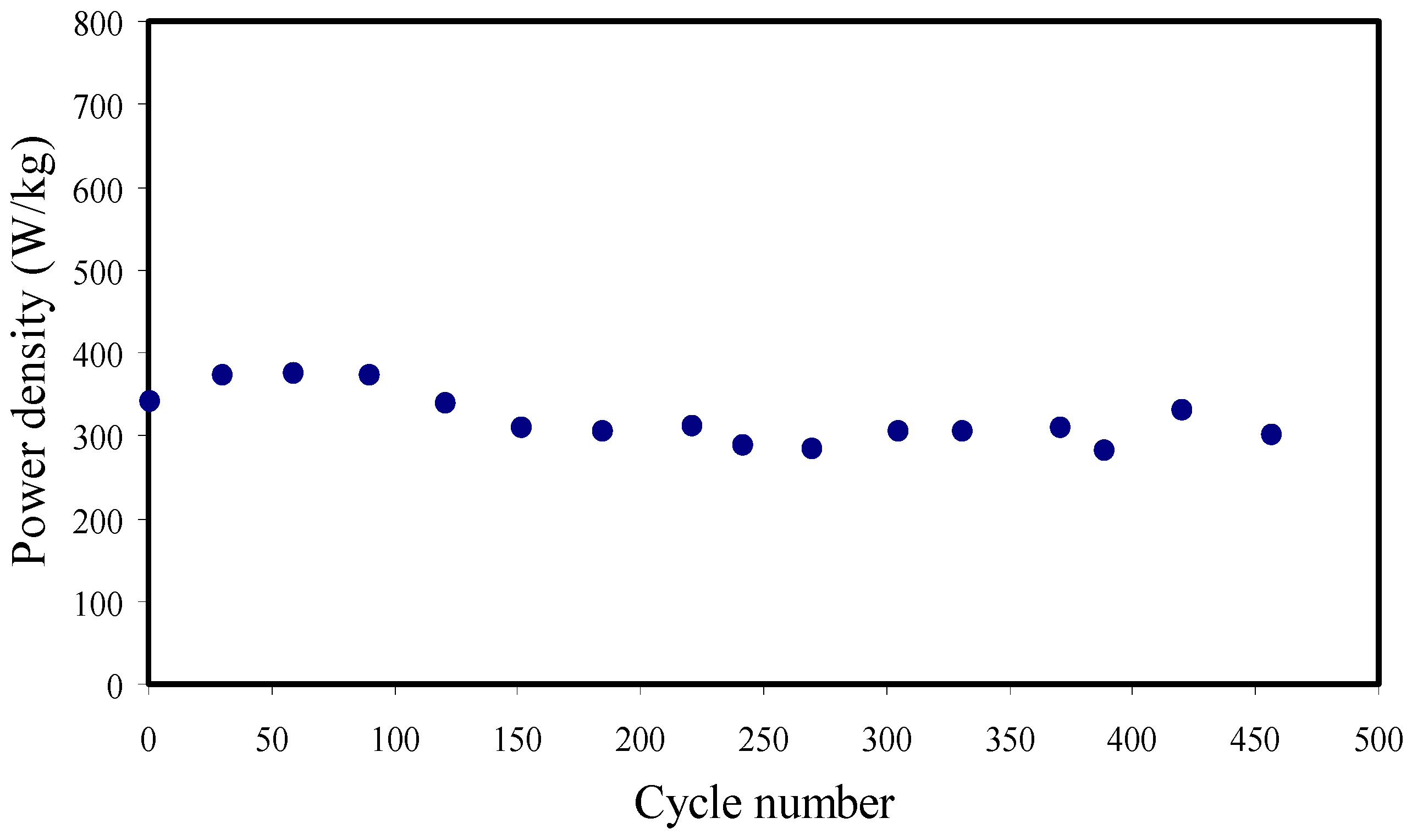
3.7. Galvanostatic Charge-Discharge (GCD) Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burk, A. Ultracapacitors: Why, how, and where is the technology. J. Power Sources 2000, 91, 37–50. [Google Scholar] [CrossRef]
- Kötz, R.; Carlen, M. Principles and applications of electrochemical capacitors. Electrochim. Acta 2000, 45, 2483. [Google Scholar] [CrossRef]
- Brza, M.A.; Aziz, S.B.; Anuar, H.; Ali, F. Structural, Ion Transport Parameter and Electrochemical Properties of Plasticized Polymer Composite Electrolyte Based on PVA: A Novel Approach to Fabricate High Performance EDLC Devices. Polym. Test. 2020, 91, 106813. [Google Scholar] [CrossRef]
- Song, J.Y.; Wang, Y.Y.; Wan, C.C. Review of gel-type polymer electrolytes for lithium-ion batteries. J. Power Sources 1999, 77, 183. [Google Scholar] [CrossRef]
- Groce, F.; Gerace, F.; Dautzemberg, G.; Passerini, S.; Appetecchi, G.B.; Scrosati, B. Synthesis and characterization of highly conducting gel electrolytes. Electrochim. Acta 1994, 39, 2187. [Google Scholar] [CrossRef]
- Pistoia, G.; Antonini, A.; Wang, G. Impedance study on the reactivity of gel polymer electrolytes towards a lithium electrode. J. Power Sources 1996, 58, 139. [Google Scholar] [CrossRef]
- Tsunemi, K.; Ohno, H.; Tsuchida, E. Conduction of lithium ions in polyvinylidene fluoride and its derivatives-I. Electrochim. Acta 1983, 28, 591. [Google Scholar]
- Magistris, A.; Quartarone, E.; Mustarelli, P.; Saito, Y.; Kataoka, H. PVDF-based porous polymer electrolytes for lithium batteries. Solid State Ion. 2002, 152–153, 347. [Google Scholar] [CrossRef]
- Hemalatha, R.; Alagar, M.; Selvasekarapandian, S.; Sundaresan, B.; Moniha, V. Studies of proton conducting polymer electrolyte based on PVA, amino acid proline and NH4SCN. J. Sci. Adv. Mater. Devices 2019, 4, 101–110. [Google Scholar] [CrossRef]
- Hema, M.; Selvasekarapandian, S.; Arunkumar, D.; Sakunthala, A.; Nithya, H. FTIR, XRD and ac impedance spectroscopic study on PVA based polymer electrolyte doped with NH4X (X = Cl, Br, I). J. Non-Cryst. Solids 2009, 355, 84. [Google Scholar] [CrossRef]
- Asnawi, A.S.F.M.; Aziz, S.B.; Brevik, I.; Brza, M.A.; Yusof, Y.M.; Alshehri, S.M.; Ahamad, T.; Kadir, M.F.Z. The study of plasticized sodium ion conducting polymer blend electrolyte membranes based on chitosan/dextran biopolymers: Ion transport, structural, morphological and potential stability. Polymers 2021, 13, 383. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Karan, S.; Dey, A.; De, S.K. Structure, morphology and ionic conductivity of solid polymer electrolyte. Mater. Res. Bull. 2011, 46, 2009–2015. [Google Scholar] [CrossRef]
- Shuhaimi, N.E.A.; Alias, N.A.; Majid, S.R.; Arof, A.K. Electrical double layer capacitor with proton conducting κ-carrageenanchitosan electrolytes. Funct. Mater. Lett. 2008, 1, 195–201. [Google Scholar] [CrossRef]
- Srivastava, N.; Chandra, A.; Chandra, S. Dense branched growth of (SCN)x and ion transport in the poly(ethyleneoxide) NH4SCN polymer electrolyte. Phys. Rev. B 1995, 52, 225–230. [Google Scholar] [CrossRef]
- Pawlicka, A.; Danczuk, M.; Wieczorek, W.; Zygadło-Monikowska, E. Influence of plasticizer type on the properties of polymer electrolytes based on chitosan. J. Phys. Chem. A 2008, 112, 8888. [Google Scholar] [CrossRef]
- Liu, G.; Kim, J.Y.; Wang, M.; Woo, J.Y.; Wang, L.; Zou, D.; Lee, J.K. Soft, Highly Elastic, and Discharge-Current-Controllable Eutectic Gallium–Indium Liquid Metal–Air Battery Operated at Room Temperature. Adv. Energy Mater. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Makled, M.H.; Sheha, E.; Shanap, T.S.; El-Mansy, M.K. Electrical conduction and dielectric relaxation in p-type PVA/CuI polymer composite. J. Adv. Res. 2013, 4, 531. [Google Scholar] [CrossRef]
- Hema, M.; Selvasekerapandian, S.; Sakunthala, A.; Arunkumar, D.; Nithya, H. Structural, vibrational and electrical characterization of PVA-NH4Br polymer electrolyte system. Phys. B Condens. Matter 2008, 403, 2740–2747. [Google Scholar] [CrossRef]
- Noor, N.A.M.; Isa, M.I.N. Structural and Conduction Studies of Solid Biopolymer Electrolytes System Based on Carboxymethyl Cellulose. Am. Eurasian J. Sustain. Agric. 2015, 9, 15. [Google Scholar]
- Liang, S.; Huang, Q.; Liu, L.; Yam, K.L. Microstructure and molecular interaction in glycerol plasticized chitosan/poly(vinyl alcohol) blending films. Macromol. Chem. Phys. 2009, 210, 832. [Google Scholar] [CrossRef]
- Kharazm, A.N.; Faraji, R.M.; Hussin, E.; Saion, W.M.M.; Yunus, M.; Behazad, K. Structural, optical, opto-thermal and thermal properties of ZnS–PVA nanofluids synthesized through a radiolytic approach. Beilstein J. Nanotechnol. 2015, 6, 529. [Google Scholar] [CrossRef]
- Jiang, L.; Yang, T.; Peng, L.; Dan, Y. Acrylamide modified poly(vinyl alcohol): Crystalline and enhanced water solubility. RSC Adv. 2015, 5, 86598. [Google Scholar] [CrossRef]
- Selvasekarapandian, S.; Baskaran, R.; Hema, M. Complex AC impedance, transference number and vibrational spectroscopy studies of proton conducting PVAc–NH4SCN polymer electrolytes. Phys. B 2005, 357, 412. [Google Scholar] [CrossRef]
- Teo, L.P.; Buraidah, M.H.; Nor, A.F.M.; Majid, S.R. Conductivity and dielectric studies of Li2SnO3. Ionics 2012, 18, 655. [Google Scholar] [CrossRef]
- Samsudin, A.S.; Khairul, W.M.; Isa, M.I.N. Characterization on the potential of carboxy methylcellulose for application as proton conducting biopolymer electrolytes. J. Non Cryst. Solids 2012, 358, 1104. [Google Scholar] [CrossRef]
- Malathi, J.; Kumaravadivel, M.; Brahmanandhan, G.M.; Hema, M.; Baskaran, R.; Selvasekarapandian, S. Structural, thermal and electrical properties of PVALiCF3SO3 polymer electrolyte. J. Non-Cryst. Solids 2010, 356, 2277. [Google Scholar] [CrossRef]
- Qian, X.; Gu, N.; Cheng, Z.; Yang, X.; Wang, E.; Dong, S. Impedance study of (PEO)10LiClO4–Al2O3 composite polymer electrolyte with blocking electrodes. Electrochim. Acta 2001, 46, 1829. [Google Scholar] [CrossRef]
- Shuhaimi, N.E.A.; Teo, L.P.; Woo, H.J.; Majid, S.R.; Arof, A.K. Electrical double-layer capacitors with plasticized polymer electrolyte based on methyl cellulose. Polym. Bull. 2012, 69, 807. [Google Scholar] [CrossRef]
- Hamsan, M.H.; Shukur, M.F.; Kadir, M.F.Z. NH4NO3 as charge carrier contributor in glycrolized potato starch-methyl cellulose blend-based polymer electrolyte and the application in electrochemical double-layer capacitor. Ionics 2017, 23, 3429. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abdullah, R.M. Crystalline and amorphous phase identification from the tanδ relaxation peaks and impedance plots in polymer blend electrolytes based on [CS: AgNt ]x: PEO (x-1) (10 ≤ x ≤ 50). Electrochim. Acta 2018, 285, 30. [Google Scholar] [CrossRef]
- Dannoun, E.M.A.; Aziz, S.B.; Brza, M.A.; Nofal, M.M.; Asnawi, A.S.F.M.; Yusof, Y.M.; Al-Zangana, S.; Hamsan, M.H.; Kadir, M.F.Z.; Woo, H.J. The Study of Plasticized Solid Polymer Blend Electrolytes Based on Natural Polymers and Their Application for Energy Storage EDLC Devices. Polymers 2020, 12, 2531. [Google Scholar] [CrossRef]
- Aziz, S.B.; Brza, M.A.; Dannoun, E.M.A.; Hamsan, M.H.; Hadi, J.M.; Kadir, M.F.Z.; Abdulwahid, R.T. The study of electrical and electrochemical properties of magnesium ion conducting CS: PVA based polymer blend electrolytes: Role of lattice energy of magnesium salts on EDLC performance. Molecules 2020, 25, 4503. [Google Scholar] [CrossRef]
- Mustafa, M.S.; Ghareeb, H.O.; Aziz, S.B.; Brza, M.A.; Al-Zangana, S.; Hadi, J.M.; Kadir, M.F.Z. Electrochemical Characteristics of Glycerolized PEO-Based Polymer Electrolytes. Membranes 2020, 10, 116. [Google Scholar] [CrossRef]
- Hamsan, M.H.; Shukur, M.F.; Kadir, M.F.Z. The effect of NH4NO3 towards the conductivity enhancement and electrical behavior in methyl cellulose-starch blend based ionic conductors. Ionics 2016, 23, 1137. [Google Scholar] [CrossRef]
- Gondaliya, N.; Kanchan, D.K.; Sharma, P. Effect of a plasticizer on a solid polymer electrolyte. Soc. Plast. Eng. 2013, 4, 2. [Google Scholar]
- Arof, A.K.; Amirudin, S.; Yusof, S.Z.; Noor, I.M. A method based on impedance spectroscopy to determine transport properties of polymer electrolytes. Phys. Chem. Chem. Phys. 2014, 16, 1856. [Google Scholar] [CrossRef] [PubMed]
- Shukur, M.F.; Ithnin, R.; Kadir, M.F.Z. Electrical properties of proton conducting solid biopolymer electrolytes based on starch–chitosan blend. Ionics 2014, 20, 977. [Google Scholar] [CrossRef]
- YYusof, M.; Shukur, M.F.; Hamsan, M.H.; Jumbri, K.; Kadir, M.F.Z. Plasticized solid polymer electrolyte based on natural polymer blend incorporated with lithium perchlorate for electrical double-layer capacitor fabrication. Ionics 2019, 25, 5473. [Google Scholar] [CrossRef]
- Mobarak, N.N.; Ahmad, A.; Abdullah, M.P.; Ramli, N.; Rahman, M.Y.A. Conductivity enhancement via chemical modification of chitosan based green polymer electrolyte. Electrochim. Acta 2013, 92, 161. [Google Scholar] [CrossRef]
- Hamsan, H.M.; Aziz, S.B.; Kadir, M.F.Z.; Brza, M.A.; Karim, W.O. The study of EDLC device fabricated from plasticized magnesium ion conducting chitosan based polymer electrolyte. Polym. Test. 2020, 90, 106714. [Google Scholar] [CrossRef]
- Woo, H.J.; Majid, S.R.; Arof, A.K. Dielectric properties and morphology of polymer electrolyte based on poly(ɛ-caprolactone) and ammonium thiocyanate. Mater. Chem. Phys. 2012, 134, 755. [Google Scholar] [CrossRef]
- Hamsan, M.H.; Shukur, M.F.; Aziz, S.B.; Kadir, M.F.Z. Dextran from Leuconostoc mesenteroides-doped ammonium salt-based green polymer electrolyte. Bull. Mater. Sci. 2019, 42. [Google Scholar] [CrossRef]
- Khiar, A.S.; Arof, A.K. Conductivity studies of starch-based polymer electrolytes. Ionics 2010, 16, 123–129. [Google Scholar] [CrossRef]
- Aziz, S.B. Study of electrical percolation phenomenon from the dielectric and electric modulus analysis. Bull. Mater. Sci. 2015, 38, 1597–1602. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abidin, Z.H.Z.; Arof, A.K. Influence of silver ion reduction on electrical modulus parameters of solid polymer electrolyte based on chitosan-silver triflate electrolyte membrane. Express Polym. Lett. 2010, 4, 300. [Google Scholar] [CrossRef]
- Aziz, S.B.; Kadir, M.F.Z.; Abidin, Z.H.Z. Structural, morphological and electrochemical impedance study of CS: LiTf based solid polymer electrolyte: Reformulated arrhenius equation for ion transport study. Int. J. Electrochem. Sci. 2016, 11, 9228–9244. [Google Scholar] [CrossRef]
- Aziz, N.A.; Majid, S.R.; Arof, A.K. Synthesis and characterizations of phthaloyl chitosan-based polymer electrolytes. J. Non. Cryst. Solids 2012, 358, 1581–1590. [Google Scholar] [CrossRef]
- Aziz, S.B.; Woo, T.J.; Kadir, M.F.Z.; Ahmed, H.M. A conceptual review on polymer electrolytes and ion transport models. J. Sci. Adv. Mater. Devices 2018, 3, 1–17. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abidin, Z.H.Z. Ion-transport study in nanocomposite solid polymer electrolytes based on chitosan: Electrical and dielectric analysis. J. Appl. Polym. Sci. 2015, 132, 1–10. [Google Scholar] [CrossRef]
- Aziz, S.B. Li+ ion conduction mechanism in poly (ε-caprolactone)-based polymer electrolyte. Iran. Polym. J. 2013, 22, 877. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abidin, Z.H.Z. Electrical and morphological analysis of chitosan:AgTf solid electrolyte. Mater. Chem. Phys. 2014, 144, 280–286. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abdullah, R.M.; Rasheed, M.A.; Ahmed, H.M. Role of ion dissociation on DC conductivity and silver nanoparticle formation in PVA:AgNt based polymer electrolytes: Deep insights to ion transport mechanism. Polymers 2017, 9, 338. [Google Scholar] [CrossRef] [PubMed]
- Shukur, M.F.; Ibrahim, F.M.; Majid, N.A.; Ithnin, R.; Kadir, M.F.Z. Electrical analysis of amorphous corn starch-based polymer electrolyte membranes doped with LiI. Phys. Scr. 2013, 88, 025601. [Google Scholar] [CrossRef]
- Selvasekarapandian, S.; Chithra, D.R. Dielectric studies on a solid electrolyte AgI-PbBr2-Ag2O-B2O3. Mater. Chem. Phys. 1999, 58, 90. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Rafiuddin, S.R. Structural, electrical conductivity and dielectric behavior of Na2SO4–LDT composite solid electrolyte. J. Adv. Res. 2016, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Aziz, S.B.; Marf, A.S.; Dannoun, E.M.A.; Brza, M.A.; Abdullah, R.M. The Study of the Degree of Crystallinity, Electrical Equivalent Circuit, and Dielectric Properties of Polyvinyl Alcohol (PVA)-Based Biopolymer Electrolytes. Polymers 2020, 12, 2184. [Google Scholar] [CrossRef] [PubMed]
- Hadi, J.M.; Aziz, S.B.; Mustafa, M.S.; Brza, M.A.; Hamsan, M.H.; Kadir, M.F.Z.; Ghareeb, H.O.; Hussein, S.A. Electrochemical impedance study of proton conducting polymer electrolytes based on PVC doped with thiocyanate and plasticized with glycerol. Int. J. Electrochem. Sci. 2020, 15, 4671–4683. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abdullah, R.M.; Kadir, M.F.Z.; Ahmed, H.M. Non suitability of silver ion conducting polymer electrolytes based on chitosan mediated by barium titanate (BaTiO3) for electrochemical device applications. Electrochim. Acta 2019, 296, 494–507. [Google Scholar] [CrossRef]
- Aziz, S.B. Occurrence of electrical percolation threshold and observation of phase transition in chitosan (1 − x): AgI x (0.05 ≤ x ≤ 0.2)-based ion-conducting solid polymer composites. Appl. Phys. A Mater. Sci. Process. 2016, 122, 706. [Google Scholar] [CrossRef]
- Aziz, S.B. The mixed contribution of ionic and electronic carriers to conductivity in chitosan based solid electrolytes mediated by CuNt salt. J. Inorg. Organomet. Polym. Mater. 2018, 28, 1942. [Google Scholar] [CrossRef]
- Aziz, S.B.; Brza, M.A.; Saed, S.R.; Hamsan, M.H.; Kadir, M.F.Z. Ion association as a main shortcoming in polymer blend electrolytes based on CS: PS incorporated with various amounts of ammonium tetrafluoroborate. J. Mater. Res. Technol. 2020, 9, 5410. [Google Scholar] [CrossRef]
- Gurusiddappa, J.; Madhuri, W.; Suvarna, R.P.; Dasan, K.P. Conductivity and dielectric behavior of polyethylene oxidelithium perchlorate solid polymer electrolyte films. Indian J. Adv. Chem. Sci. 2016, 4, 14. [Google Scholar]
- Khiar, A.S.A.; Puteh, R.; Arof, A.K. Conductivity studies of a chitosan-based polymer electrolyte. Phys. B 2006, 373, 23. [Google Scholar] [CrossRef]
- Aziz, S.B.; Karim, W.O.; Ghareeb, H.O. The deficiency of chitosan: AgNO3 polymer electrolyte incorporated with titanium dioxide filler for device fabrication and membrane separation technology. J. Mater. Res. Technol. 2020, 9, 4692. [Google Scholar] [CrossRef]
- Hadi, J.M.; Aziz, S.B.; Mustafa, M.S.; Hamsan, M.H.; Abdulwahid, R.T.; Kadir, M.F.Z.; Ghareeb, H.O. Role of nano-capacitor on dielectric constant enhancement in PEO:NH4SCN:xCeO2 polymer nano-composites: Electrical and electrochemical properties. J. Mater. Res. Technol. 2020, 9, 9283–9294. [Google Scholar] [CrossRef]
- Hadi, J.M.; Aziz, S.B.; Saeed, S.R.; Brza, M.A.; Abdulwahid, R.T.; Hamsan, M.H.; Abdullah, R.M.; Kadir, M.F.Z.; Muzakir, S.K. Investigation of ion transport parameters and electrochemical performance of plasticized biocompatible chitosan-based proton conducting polymer composite electrolytes. Membranes 2020, 10, 363. [Google Scholar] [CrossRef]
- Aziz, S.B.; Hamsan, M.H.; Abdullah, R.M.; Kadir, M.F.Z. A promising polymer blend electrolyte based on chitosan: Methyl cellulose for EDLC application with high specific capacitance and energy density. Molecules 2019, 24, 2503. [Google Scholar] [CrossRef]
- Hadi, J.M.; Aziz, S.B.; Nofal, M.M.; Hussen, S.A.; Hamsan, M.H.; Brza, M.A.; Abdulwahid, R.T.; Kadir, M.F.Z.; Woo, H.J. Electrical, dielectric property and electrochemical performances of plasticized silver ion-conducting chitosan-based polymer nanocomposites. Membranes 2020, 10, 151. [Google Scholar] [CrossRef]
- Hamsan, M.H.; Aziz, S.B.; Nofal, M.M.; Brza, M.A.; Abdulwahid, R.T.; Hadi, J.M.; Karim, W.O.; Kadir, M.F.Z. Characteristics of EDLC device fabricated from plasticized chitosan: MgCl2 based polymer electrolyte. J. Mater. Res. Technol. 2020, 9, 10635–10646. [Google Scholar] [CrossRef]
- Kobayashi, T.; Noguchi, Y.; Miyayama, M. Enhanced spontaneous polarization in superlattice structure Bi4Ti3O12-BaBi4Ti4O15 single crystal. Appl. Phys. Lett. 2005, 86, 012907. [Google Scholar] [CrossRef]
- Rani, M.S.A.; Ahmad, A.; Mohamed, N.S. Influence of nano-sized fumed silica on physicochemical and electrochemical properties of cellulose derivatives-ionic liquid biopolymer electrolytes. Ionics 2017, 24, 807. [Google Scholar] [CrossRef]
- Shukur, M.F.; Kadir, M.F.Z. Hydrogen ion conducting starch-chitosan blend based electrolyte for application in electrochemical devices. Electrochim. Acta 2015, 158, 152. [Google Scholar] [CrossRef]
- Aziz, S.B.; Hadi, J.M.; Elham, E.M.; Abdulwahid, R.T.; Saeed, S.R.; Marf, A.S.; Karim, W.O.; Kadir, M.F.Z. The study of plasticized amorphous biopolymer blend electrolytes based on polyvinyl alcohol (PVA): Chitosan with high ion conductivity for energy storage electrical double-layer capacitors (EDLC) device application. Polymers 2020, 12, 1938. [Google Scholar] [CrossRef] [PubMed]
- Azli, A.A.; Manan, N.S.A.; Aziz, S.B.; Kadir, M.F.Z. Structural, impedance and electrochemical double-layer capacitor characteristics of improved number density of charge carrier electrolytes employing potato starch blend polymers. Ionics 2020, 26, 5773. [Google Scholar] [CrossRef]
- Asnawi, A.S.F.M.; Aziz, S.B.; Saeed, S.R.; Yusof, Y.M.; Abdulwahid, R.T.; Al-Zangana, S.; Karim, W.O.; Kadir, M.F.Z. Solid-State EDLC Device Based on Magnesium Ion-Conducting Biopolymer Composite Membrane Electrolytes: Impedance, Circuit Modeling, Dielectric Properties and Electrochemical Characteristics. Membranes 2020, 10, 389. [Google Scholar] [CrossRef]
- Moniha, V.; Alagar, M.; Selvasekarapandian, S.; Sundaresan, B.; Hemalatha, R.; Boopathi, G. Synthesis and characterization of bio-polymer electrolyte based on iota-carrageenan with ammonium thiocyanate and its applications. J. Solid State Electrochem. 2018, 22, 3209–3223. [Google Scholar] [CrossRef]
- Hashmi, S.A.; Latham, R.J.; Linford, R.G.; Schilndwein, S.W. Polymer electrolyte based solid state redox supercapacitors withpoly(3-methyl thiophene) and polypyrrole conducting polymer electrodes. Ionics 1997, 3, 177. [Google Scholar] [CrossRef]
- Kadir, M.F.Z.; Arof, A.K. Application of PVA–chitosan blend polymer electrolyte membrane in electrical double layer capacitor. Materials Research Innovations. Mater. Res. Innov. 2011, 15, s217. [Google Scholar] [CrossRef]
- Teoh, K.H.; Liew, C.W.; Ramesh, S. Electric double layer capacitor based on activated carbon electrode and biodegradable composite polymer electrolyte. Ionics 2014, 20, 251. [Google Scholar]
- Asmara, S.N.; Kufian, M.Z.; Majid, S.R.; Arof, A.K. Preparation and characterization of magnesium ion gel polymer electrolytes for application in electrical double layer capacitors. Electrochim. Acta 2011, 57, 91. [Google Scholar] [CrossRef]
- Wei, Z.; Ren, Y.; Wang, M.; He, J.; Huo, W.; Tang, H. Improving the Conductivity of Solid Polymer Electrolyte by Grain Reforming. Nanoscale Res. Lett. 2020, 15, 122. [Google Scholar] [CrossRef]
- Yu, H.; Liu, G.; Wang, M.; Ren, R.; Shim, G.; Kim, J.Y.; Tran, M.X.; Byun, D.; Lee, J.K. Plasma-Assisted Surface Modification on the Electrode Interface for Flexible Fiber-Shaped Zn-Polyaniline Batteries. ACS Appl. Mater. Interfaces 2020, 12, 5820–5830. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.Z.; Fang, B.; Iwasa, S.; Kumagai, M. A novel electrode material for electric double-layer capacitors. J. Power Source 2005, 141, 386. [Google Scholar] [CrossRef]
- Liew, C.W.; Ramesh, S.; Arof, A.K. Enhanced capacitance of EDLCs (electrical double layer capacitors) based on ionic liquid added polymer electrolytes. Energy 2016, 109, 546. [Google Scholar] [CrossRef]
- Shim, G.; Tran, X.M.; Liu, G.; Byun, D.; Lee, K.J. Flexible, fiber-shaped, quasi-solid-state Zn-polyaniline batteries with methanesulfonic acid-doped aqueous gel electrolyte. Energy Storage Mater. 2021, 35, 739–749. [Google Scholar]
- Aziz, S.B.; Hamsan, M.H.; Brza, M.A.; Kadir, M.F.Z.; Muzakir, S.K.; Abdulwahid, R.T. Effect of glycerol on EDLC characteristics of chitosan: Methylcellulose polymer blend electrolytes. J. Mater. Res. Technol. 2020, 9, 8355. [Google Scholar] [CrossRef]
- Marf, A.S.; Aziz, S.B.; Abdullah, R.M. Plasticized H+ ion-conducting PVA:CS-based polymer blend electrolytes for energy storage EDLC application. J. Mater. Sci. Mater. Electron. 2020, 31, 18554. [Google Scholar] [CrossRef]
- Aziz, S.B.; Hamsan, M.H.; Karim, W.O.; Marif, A.S.; Abdulwahid, R.T.; Kadir, M.F.Z.; Brza, M.A. Study of impedance and solid-state double-layer capacitor behavior of proton (H+)-conducting polymer blend electrolyte-based CS:PS polymers. Ionics 2020, 26, 4635. [Google Scholar] [CrossRef]
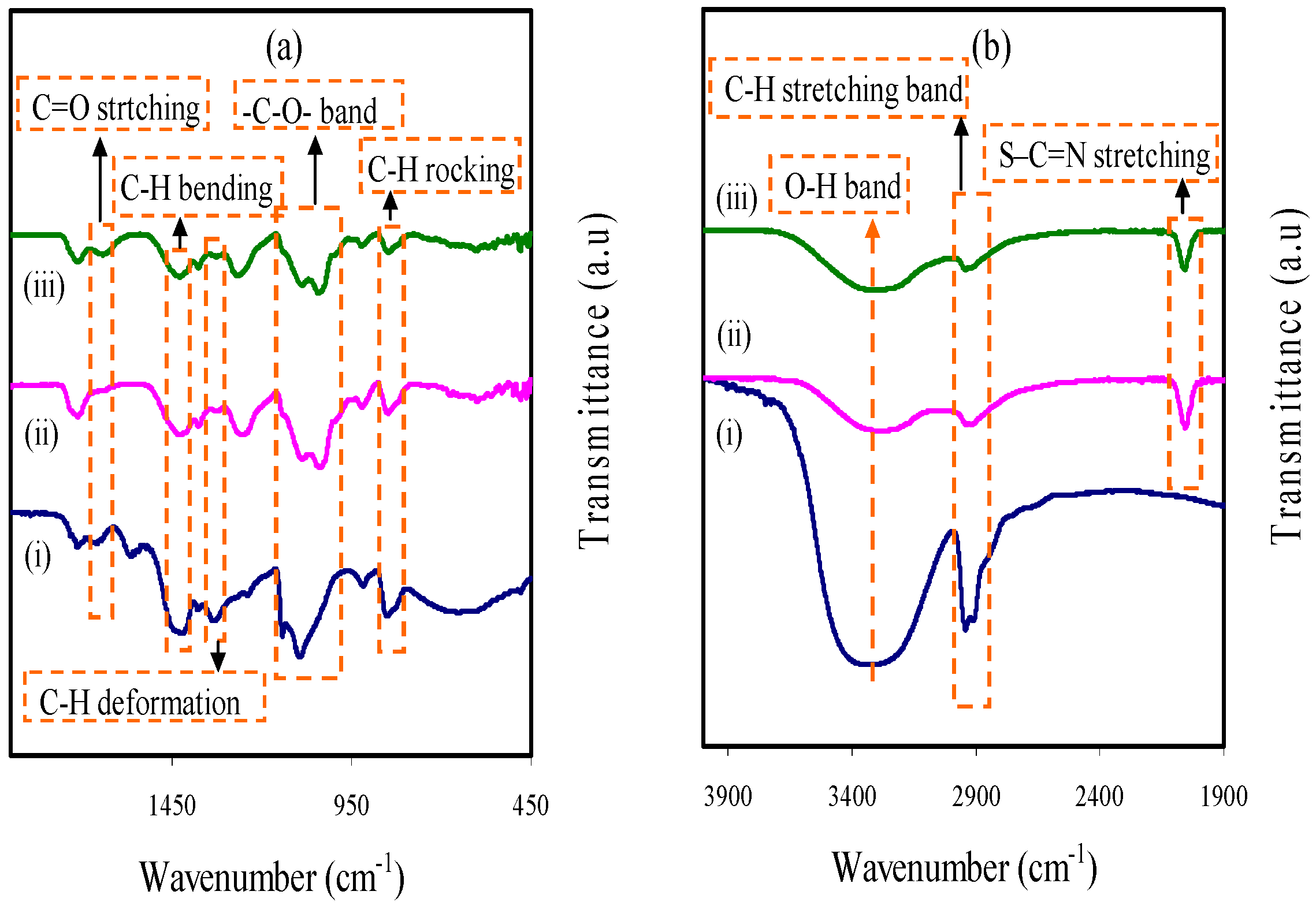



| Assignments | Wavenumber (cm−1) | ||
|---|---|---|---|
| PVA | PSP_1 | PSP_2 | |
| O–H stretching | 3303 | 3305 | 3309 |
| C–H stretching | 2902 | 2914 | 2909 |
| Aromatic S–C = n stretching | - | 2043 | 2042 |
| C=O stretching | 1638 | 1609 | 1605 |
| C–H bending of CH2 | 1412 | 1407 | 1413 |
| C–H deformation | 1318 | 1310 | 1309 |
| –C–O– stretching | 1080 | 1029 | 1038 |
| C–H rocking | 833 | 831 | 822 |
| Sample | P1 (rad) | P2 (rad) | K1 (F−1) | K2 (F−1) | C1 (F) | C2 (F) |
|---|---|---|---|---|---|---|
| PVA | 0.79 | - | 3.6 × 1010 | - | 2.78 × 10−11 | - |
| PSP_1 | 0.86 | 0.40 | 2.2 × 109 | 1.6 × 105 | 4.55 × 10−10 | 6.25 × 10−6 |
| PSP_2 | 0.87 | 0.69 | 3.8 × 108 | 1.09 × 105 | 2.63 × 10−9 | 9.17 × 10−6 |
| Symbol | PVA (wt. %)/NH4SCN (wt. %)/Glycerol (wt. %) | σdc (S cm−1) |
|---|---|---|
| Pure PVA | 50:00:00 | 2.87 × 10−11 |
| PSP_1 | 50:50:30 | 5.45 × 10−7 |
| PSP_2 | 50:50:40 | 1.82 × 10−5 |
| Sample | D (cm2 s−1) | µ (cm2 V−1s) | n (cm−3) | D+ (cm2 s−1) | D− (cm2 s−1) | µ+ (cm2 V−1s) | µ− (cm2 V−1s) |
|---|---|---|---|---|---|---|---|
| PSP_1 | 7.27 × 10−9 | 2.83 × 10−7 | 1.20 × 1019 | 6.55 × 10−9 | 7.12 × 10−10 | 2.55 × 10−7 | 2.77 × 10−8 |
| PSP_2 | 2.59 × 10−8 | 1.01 × 10−6 | 1.12 × 1020 | 2.34 × 10−8 | 2.54 × 10−9 | 9.11 × 10−7 | 9.9 × 10−8 |
| Scan Rate (mVs−1) | CCV (F g−1) |
|---|---|
| 100 | 19.43 |
| 50 | 29.26 |
| 20 | 40.00 |
| 10 | 44.58 |
| 5 | 45.72 |
| Electrolyte Systems | Specific Capacitance (F/g) | Energy Density (Wh/kg) | Power Density (W/kg) | ESR (Ω) | Cycle Number | Ref. |
|---|---|---|---|---|---|---|
| Chitosan/methyl cellulose (MC)/NH4I/glycerol | 9.70 | 1.1 | 578.55 | 136–155 | 100 | [86] |
| Chitosan-κ-carrageenan/NH4NO3 | 18.5 | - | - | - | 20 | [13] |
| MC/NH4NO3/poly(ethylene glycol) | 25 | 2.5 | 130 | 109 | - | [28] |
| PVA/chitosan/NH4I/glycerol | 19.4 | 5.48 | 380 | 75–330 | 250 | [87] |
| MC/potato starch (PS)/NH4NO3/glycerol | 31 | 3.1 | 910–385 | 29–64 | 1000 | [29] |
| Chitosan/PS/NH4F | 4.1 | 0.57 | 155 | 550 | 100 | [88] |
| PVA/NH4SCN/glycerol | 18.3 | 2.06 | 318.73 | 245–278 | 450 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brza, M.A.; Aziz, S.B.; Anuar, H.; Alshehri, S.M.; Ali, F.; Ahamad, T.; Hadi, J.M. Characteristics of a Plasticized PVA-Based Polymer Electrolyte Membrane and H+ Conductor for an Electrical Double-Layer Capacitor: Structural, Morphological, and Ion Transport Properties. Membranes 2021, 11, 296. https://doi.org/10.3390/membranes11040296
Brza MA, Aziz SB, Anuar H, Alshehri SM, Ali F, Ahamad T, Hadi JM. Characteristics of a Plasticized PVA-Based Polymer Electrolyte Membrane and H+ Conductor for an Electrical Double-Layer Capacitor: Structural, Morphological, and Ion Transport Properties. Membranes. 2021; 11(4):296. https://doi.org/10.3390/membranes11040296
Chicago/Turabian StyleBrza, Mohamad A., Shujahadeen B. Aziz, Hazleen Anuar, Saad M. Alshehri, Fathilah Ali, Tansir Ahamad, and Jihad M. Hadi. 2021. "Characteristics of a Plasticized PVA-Based Polymer Electrolyte Membrane and H+ Conductor for an Electrical Double-Layer Capacitor: Structural, Morphological, and Ion Transport Properties" Membranes 11, no. 4: 296. https://doi.org/10.3390/membranes11040296
APA StyleBrza, M. A., Aziz, S. B., Anuar, H., Alshehri, S. M., Ali, F., Ahamad, T., & Hadi, J. M. (2021). Characteristics of a Plasticized PVA-Based Polymer Electrolyte Membrane and H+ Conductor for an Electrical Double-Layer Capacitor: Structural, Morphological, and Ion Transport Properties. Membranes, 11(4), 296. https://doi.org/10.3390/membranes11040296









