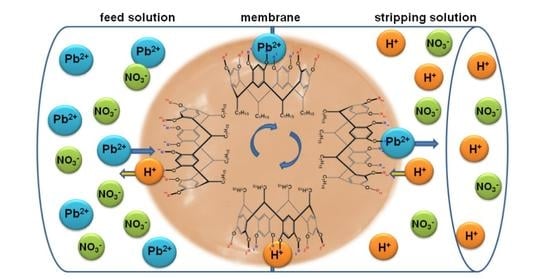
Calixresorcin[4]arene-Mediated Transport of Pb(II) Ions through Polymer Inclusion Membrane
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Apparatus
2.2. Membranes Preparation
2.3. Transport Studies
2.4. Membrane Characterization
2.5. Calculations
3. Results and discussion
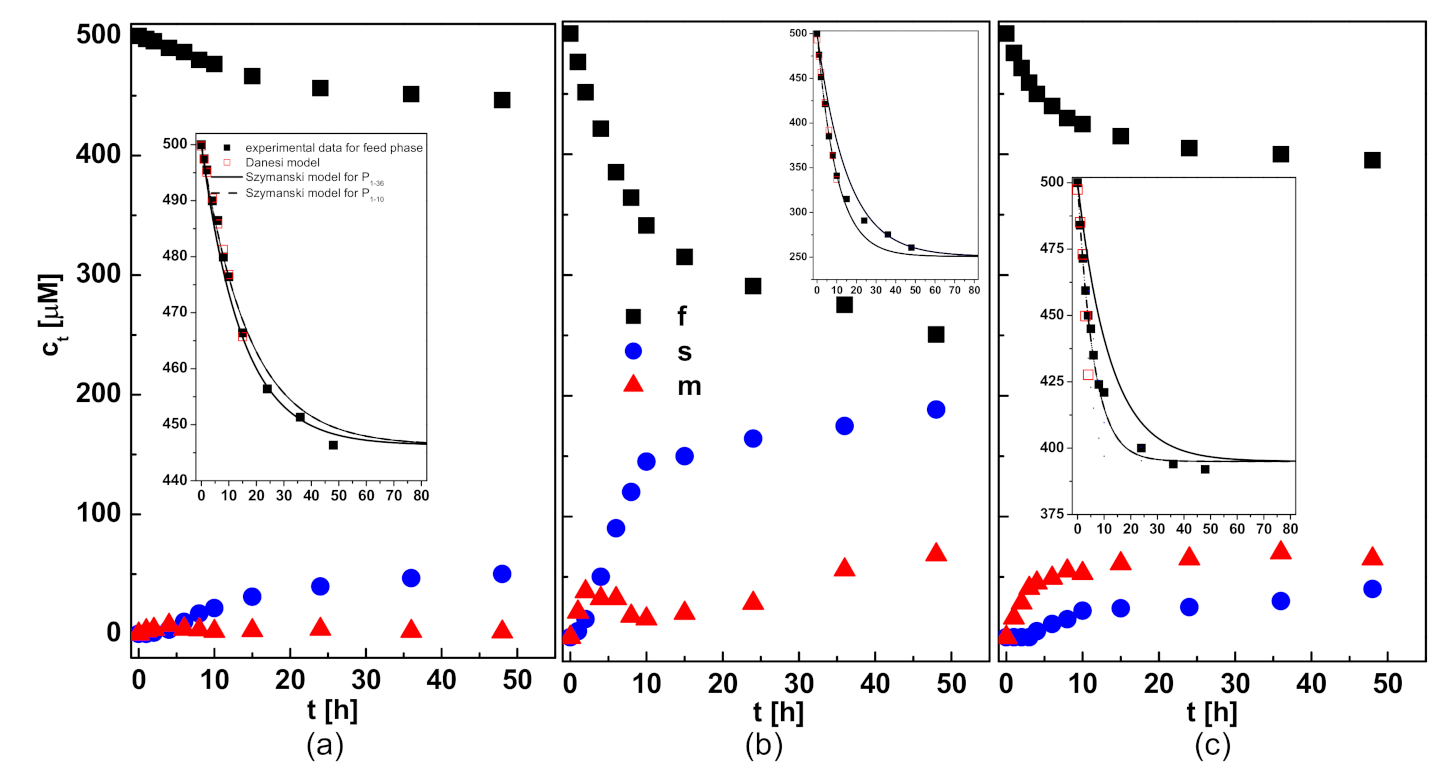
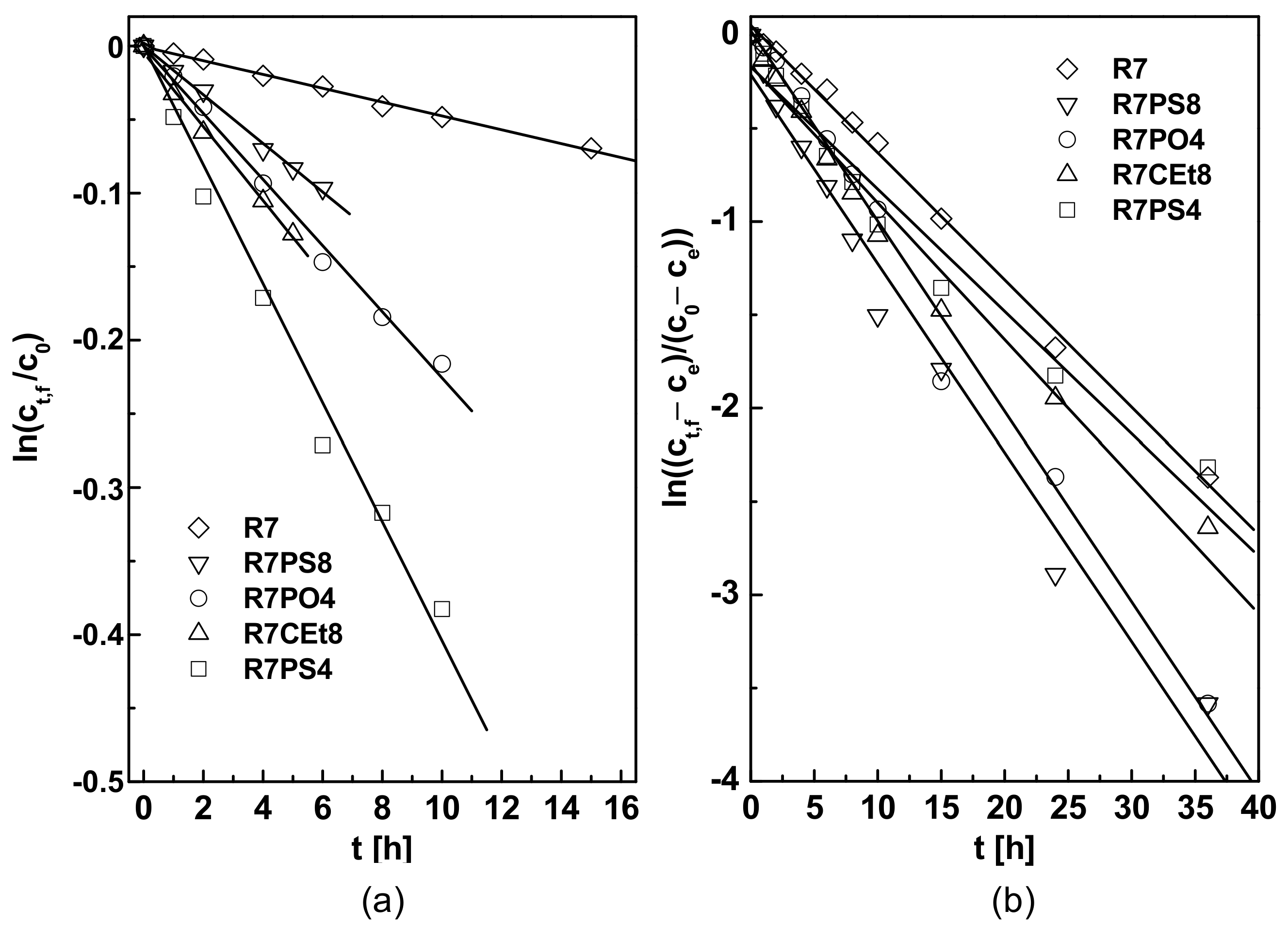
3.1. Kinetics of Pb(II) Transport
3.2. Influence of the Stripping Phase Composition
3.3. Influence of the Membrane Phase Composition
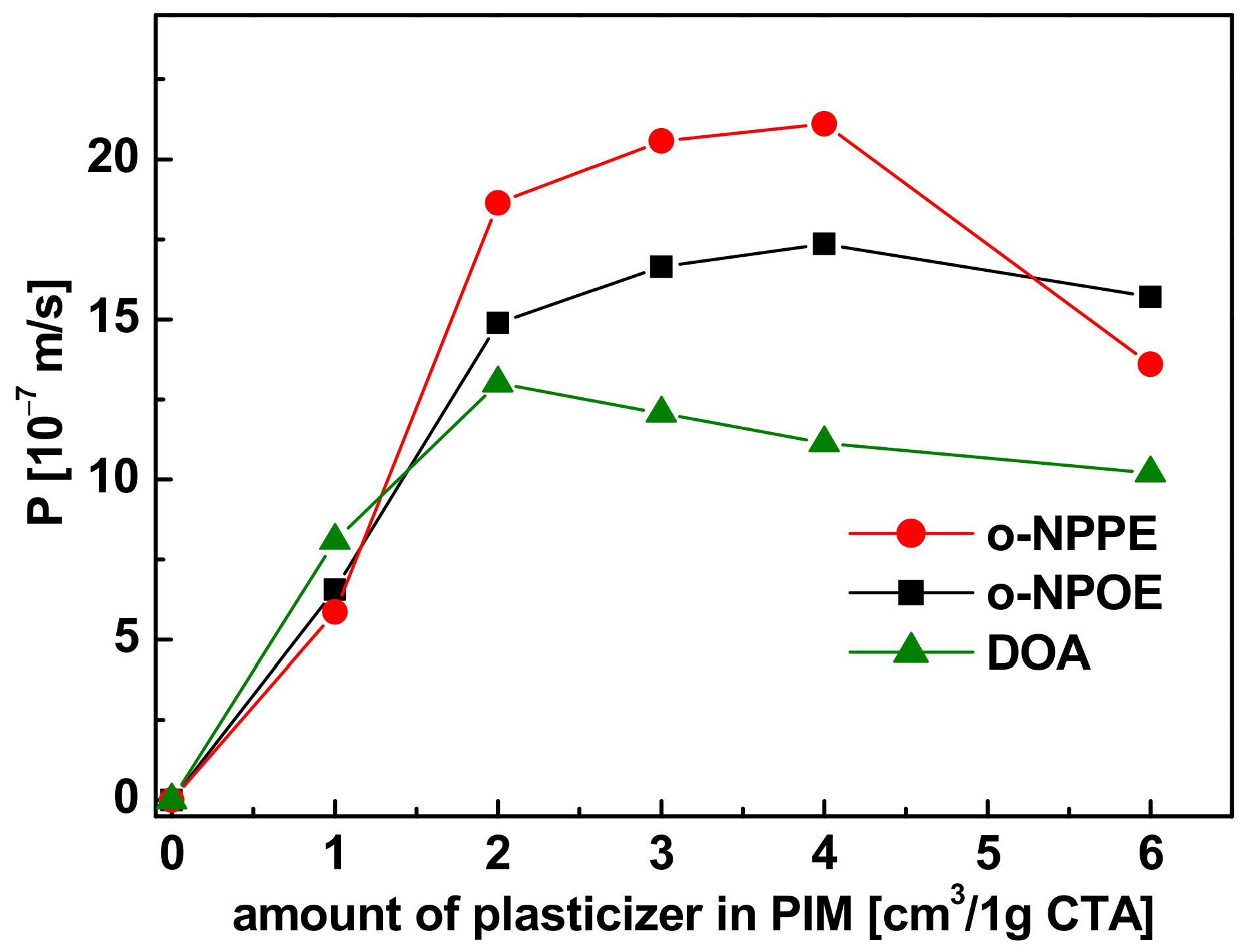
3.3.1. Plasticizer
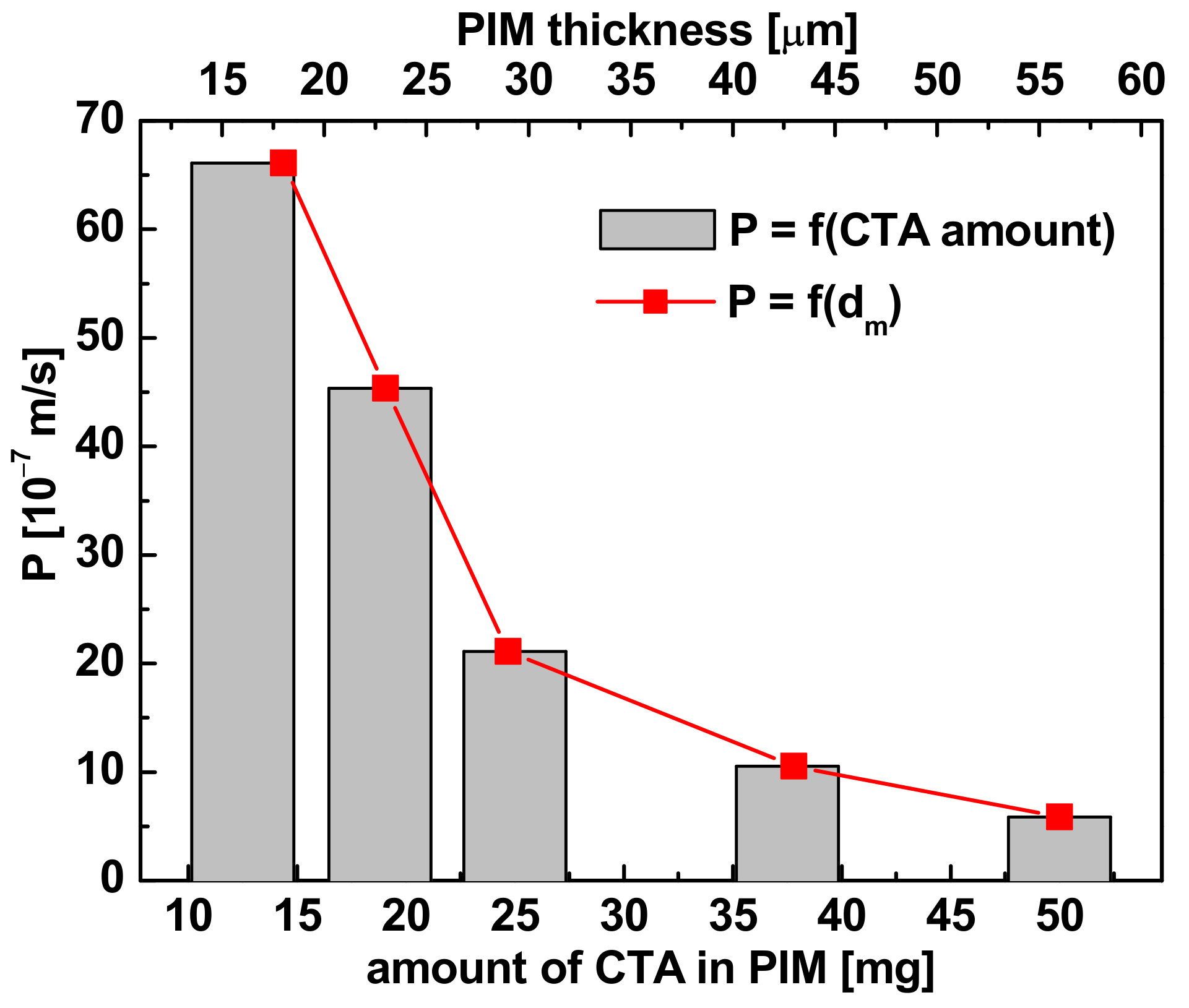
3.3.2. Polymer Support
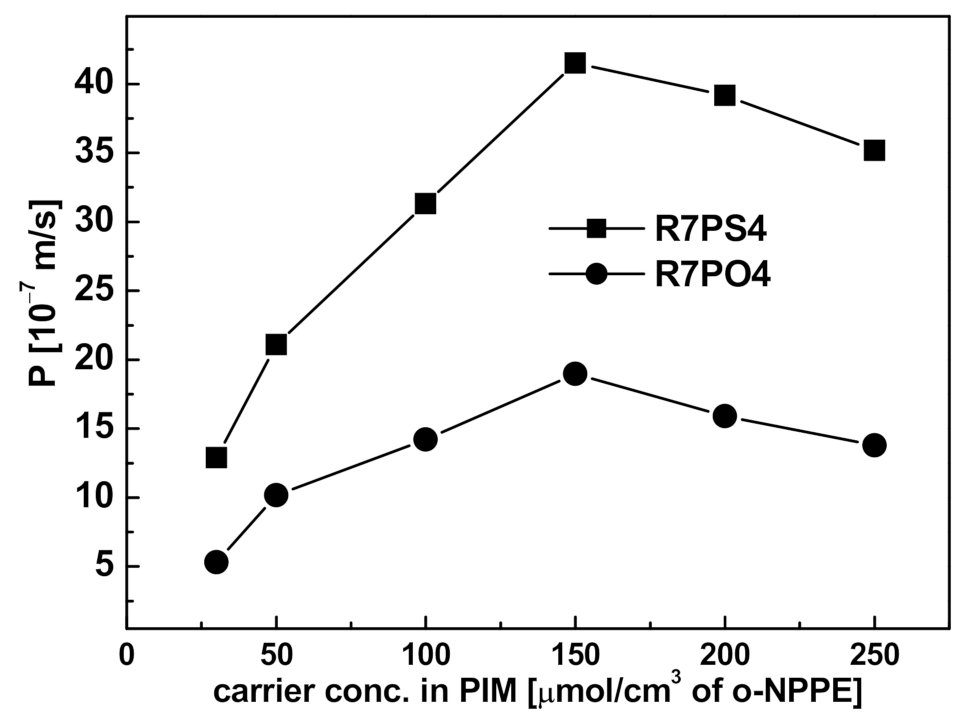
3.3.3. Carrier
3.4. Influence of the Metal Concentration in the Feed Phase
3.5. Influence of the Temperature
3.6. Separation of Pb(II), Zn(II), Cd(II) and Cr(III) Ions
3.7. Membrane Stability
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ordinance of the Polish Minister of Maritime Economy and Inland Navigation of 13 September 2019. Available online: https://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20190001747/O/D20191747.pdf (accessed on 16 March 2021). (In Polish)
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead toxicity: A review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef]
- Kolev, S.D. Membrane Techniques. Liquid Membranes, 3rd ed.; Elsevier Inc.: Aalborg, Denmark, 2019; ISBN 9780081019832. [Google Scholar]
- Moulahcene, L.; Skiba, M.; Bounoure, F.; Benamor, M.; Milon, N.; Hallouard, F.; Lahiani-Skiba, M. New Polymer Inclusion Membrane Containing β-Cyclodextrin Polymer: Application for Pharmaceutical Pollutant Removal from Waste Water. Int. J. Environ. Res. Public Health 2019, 16, 414. [Google Scholar] [CrossRef]
- Vera, R.; Insa, S.; Fontàs, C.; Anticó, E. A new extraction phase based on a polymer inclusion membrane for the detection of chlorpyrifos, diazinon and cyprodinil in natural water samples. Talanta 2018, 185, 291–298. [Google Scholar] [CrossRef]
- Nghiem, L.; Mornane, P.; Potter, I.; Perera, J.; Cattrall, R.; Kolev, S. Extraction and transport of metal ions and small organic compounds using polymer inclusion membranes (PIMs). J. Membr. Sci. 2006, 281, 7–41. [Google Scholar] [CrossRef]
- Zulkefeli, N.S.W.; Weng, S.K.; Halim, N.S.A. Removal of Heavy Metals by Polymer Inclusion Membranes. Curr. Pollut. Rep. 2018, 4, 84–92. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E.; Pyszka, I.; Ulewicz, M. Separation of Zn(II), Cr(III), and Ni(II) Ions Using the Polymer Inclusion Membranes Containing Acetylacetone Derivative as the Carrier. Membranes 2020, 10, 88. [Google Scholar] [CrossRef]
- Konczyk, J.; Kozlowski, C.; Walkowiak, W. Removal of chromium(III) from acidic aqueous solution by polymer inclusion membranes with D2EHPA and Aliquat 336. Desalination 2010, 263, 211–216. [Google Scholar] [CrossRef]
- Croft, C.F.; Almeida, M.I.G.; Cattrall, R.W.; Kolev, S.D. Separation of lanthanum(III), gadolinium(III) and ytterbium(III) from sulfuric acid solutions by using a polymer inclusion membrane. J. Membr. Sci. 2018, 545, 259–265. [Google Scholar] [CrossRef]
- Pyszka, I.; Radzyminska-Lenarcik, E. New Polymer Inclusion Membrane in the Separation of Nonferrous Metal Ion from Aqueous Solutions. Membranes 2020, 10, 385. [Google Scholar] [CrossRef] [PubMed]
- Sedkaoui, Y.; Abdellaoui, N.; Arous, O.; Lounici, H.; Nasrallah, N.; Szymczyk, A. Elaboration and characterization of multilayer polymeric membranes: Effect of the chemical nature of polymers. J. Polym. Eng. 2021, 41, 127–136. [Google Scholar] [CrossRef]
- Albaraka, Z. Carrier-mediated liquid membrane systems for lead (II) ion separations. Chem. Pap. 2019, 74, 77–88. [Google Scholar] [CrossRef]
- Engin, M.S.; Sayin, S.; Cay, S. Utilization of transport efficacy of novel calix[4]arene-embedded polymer inclusion membrane towards trace metals. J. Incl. Phenom. Macrocycl. Chem. 2018, 92, 173–179. [Google Scholar] [CrossRef]
- Kozlowski, C.A.; Kudelska, W.; Konczyk, J. ChemInform Abstract: New Trends in Modifications and Applications of Resorcinarenes. ChemInform 2011, 42. [Google Scholar] [CrossRef]
- Konczyk, J.; Miroshnychenko, S.; Kozlowski, C. Extraction properties of tetraheptylresorcin[4]arenes in relation to Cr(III) ions. Physicochem. Probl. Miner. Process. 2016, 52, 835–844. [Google Scholar] [CrossRef]
- Kończyk, J. Selective solvent extraction of some heavy metal ions from aqueous solutions by octafunctionalized resorcin[4]arenes. Physicochem. Probl. Miner. Process. 2020, 56, 271–285. [Google Scholar] [CrossRef]
- Ugur, A.; Şener, I.; Alpoguz, H.K. The Removal of Zn(II) Through Calix[4]Recorcinarene Derivative Based Polymer Inclusion Membrane From Aqueous Solution. J. Macromol. Sci. Part A 2015, 52, 801–808. [Google Scholar] [CrossRef]
- Utomo, S.B.; Jumina, J.; Siswanta, D.; Mustofa, M. Kinetics and equilibrium model of Pb(II) AND Cd(II) adsorption onto tetrakis-thiomethyl-C-4-methoxyphenylcalix[4]resorcinarene. Indones. J. Chem. 2012, 12, 49–56. [Google Scholar] [CrossRef][Green Version]
- Alshahateet, S.F.; Jiries, A.G.; Al-Trawneh, S.A.; ElDouhaibi, A.S.; Al-Mahadeen, M.M. Kinetic, equilibrium and selectivity studies of heavy metal ions (Pb(II), Co(II), Cu(II), Mn(II), and Zn(II)) removal from water using synthesizedC-4-methoxyphenylcalix[4]resorcinarene adsorbent. Desalin. Water Treat. 2014, 1–11. [Google Scholar] [CrossRef]
- Anwar, C.; Jumina; Siswanta, D.; Santosa, S.J.; Fiani, A.; Mardjan, M.I.D. Adsorption Study of Pb(II), Cd(II), Cu(II) and Cr(III) ions in Aqueous Medium using C-4-Hydroxy-3-methoxyphenylcalix[4]resorcinarene Dodecaacetate. Orient. J. Chem. 2017, 33, 979–984. [Google Scholar] [CrossRef][Green Version]
- Konczyk, J.; Nowik-Zajac, A.; Kozlowski, C.A. Calixarene-based extractants for heavy metal ions removal from aqueous solutions. Sep. Sci. Technol. 2016, 51, 2394–2410. [Google Scholar] [CrossRef]
- Benosmane, N.; Hamdi, S.M.; Hamdi, M.; Boutemeur, B. Selective transport of metal ions across polymer inclusion membranes (PIMs) containing calix[4]resorcinarenes. Sep. Purif. Technol. 2009, 65, 211–219. [Google Scholar] [CrossRef]
- Ugur, A.; Şener, I.; Hol, A.; Alpoguz, H.K.; Elci, L. Facilitated Transport of Zn(II) and Cd(II) Ions Through Polymer Inclusion Membranes Immobilized With a Calix[4]resorcinarene Derivative. J. Macromol. Sci. Part A 2014, 51, 611–618. [Google Scholar] [CrossRef]
- Zawierucha, I.; Nowik-Zajac, A.; Kozlowski, C.A. Removal of Pb(II) Ions Using Polymer Inclusion Membranes Containing Calix[4]resorcinarene Derivative as Ion Carrier. Polymers 2019, 11, 2111. [Google Scholar] [CrossRef]
- Benosmane, N.; Guedioura, B.; Hamdi, S.M.; Hamdi, M.; Boutemeur, B. Preparation, characterization and thermal studies of polymer inclusion cellulose acetate membrane with calix[4]resorcinarenes as carriers. Mater. Sci. Eng. C 2010, 30, 860–867. [Google Scholar] [CrossRef]
- Danesi, P.R. Separation of Metal Species by Supported Liquid Membranes. Sep. Sci. Technol. 1984, 19, 857–894. [Google Scholar] [CrossRef]
- Szczepański, P. Some Critical Remarks about Mathematical Model Used for the Description of Transport Kinetics in Polymer Inclusion Membrane Systems. Membranes 2020, 10, 411. [Google Scholar] [CrossRef] [PubMed]
- Gadelmawla, E.; Koura, M.; Maksoud, T.; Elewa, I.; Soliman, H. Roughness parameters. J. Mater. Process. Technol. 2002, 123, 133–145. [Google Scholar] [CrossRef]
- Ulewicz, M.; Bocheńska, M.; Lesińska, U.; Walkowiak, W. Studies on removal of Zn(II), Cd(II) and Pb(II) ions in polymer inclusion membrane transport with calix[4]-crown-6 derivatives. Physicochem. Probl. Miner. Process. 2005, 39, 107–116. [Google Scholar]
- Ulewicz, M.; Lesinska, U.; Bochenska, M.; Walkowiak, W. Facilitated transport of Zn(II), Cd(II) and Pb(II) ions through polymer inclusion membranes with calix[4]-crown-6 derivatives. Sep. Purif. Technol. 2007, 54, 299–305. [Google Scholar] [CrossRef]
- Kozlowski, C.A.; Walkowiak, W. Applicability of liquid membranes in chromium(VI) transport with amines as ion carriers. J. Membr. Sci. 2005, 266, 143–150. [Google Scholar] [CrossRef]
- Almeida, M.I.G.; Cattrall, R.W.; Kolev, S.D. Recent trends in extraction and transport of metal ions using polymer inclusion membranes (PIMs). J. Membr. Sci. 2012, 415-416, 9–23. [Google Scholar] [CrossRef]
- Lamb, J.D. Lead(II) ion sorption and transport using polymer inclusion membranes containing tri-octylphosphine oxide. J. Membr. Sci. 1997, 134, 255–259. [Google Scholar] [CrossRef]
- Kebiche-Senhadji, O.; Mansouri, L.; Tingry, S.; Seta, P.; Benamor, M. Facilitated Cd(II) transport across CTA polymer inclusion membrane using anion (Aliquat 336) and cation (D2EHPA) metal carriers. J. Membr. Sci. 2008, 310, 438–445. [Google Scholar] [CrossRef]
- Arous, O.; Amara, M.; Kerdjoudj, H. Selective transport of metal ions using polymer inclusion membranes containing crown ethers and cryptands. Arab. J. Sci. Eng. 2010, 35, 79. [Google Scholar]
- Smail, F.; Arous, O.; Amara, M.; Kerdjoudj, H. A competitive transport across polymeric membranes. Study of complexation and separation of ions. Comptes Rendus Chim. 2013, 16, 605–612. [Google Scholar] [CrossRef]
- Salazaralvarez, G.; Bautistaflores, A.; Desanmiguel, E.; Muhammed, M.; DeGyves, J. Transport characterisation of a PIM system used for the extraction of Pb(II) using 2 as carrier. J. Membr. Sci. 2005, 250, 247–257. [Google Scholar] [CrossRef]
- Aguilar, J.C. Cd(II) and Pb(II) extraction and transport modeling in SLM and PIM systems using Kelex 100 as carrier. J. Membr. Sci. 2001, 190, 107–118. [Google Scholar] [CrossRef]
- Ulewicz, M.; Lesińska, U.; Bocheńska, M. Transport of lead across polymer inclusion membrane with p-tert -butylcalix[4]arene derivative. Physicochem. Probl. Miner. Process. 2010, 44, 245–256. [Google Scholar]
- Lazarova, Z.; Boyadzhiev, L. Kinetic aspects of copper (II) transport across liquid membrane containing LIX-860 as a carrier. J. Membr. Sci. 1993, 78, 239–245. [Google Scholar] [CrossRef]
- Reichwein-Buitenhuis, E.G.; Visser, H.C.; de Jong, F.; Reinhoudt, D. N Kinetic versus diffusion control in carrier-mediated cotransport of alkali cations through supported liquid membranes. J. Am. Chem. Soc. 1995, 117, 3913–3921. [Google Scholar] [CrossRef][Green Version]
- Radzyminska-Lenarcik, E.; Ulewicz, M. THE use of 1-alkylimidzoles for selective separation of zinc ions in the transport process across a polymeric inclusion membrane. Physicochem. Probl. Miner. Process. 2014, 50, 131–142. [Google Scholar] [CrossRef]
- Nazarenko, A.Y.; Lamb, J.D. Selective Transport of Lead(II) and Strontium(II) Through a Crown Ether-Based Polymer Inclusion Membrane Containing Dialkylnaphthalenesulfonic Acid. J. Incl. Phenom. Macrocycl. Chem. 1997, 29, 247–258. [Google Scholar] [CrossRef]
- Song, J.; Huang, T.; Qiu, H.; Niu, X.; Li, X.-M.; Xie, Y.; He, T. A critical review on membrane extraction with improved stability: Potential application for recycling metals from city mine. Desalination 2018, 440, 18–38. [Google Scholar] [CrossRef]
- Zhang, B.; Gozzelino, G.; Baldi, G. Membrane liquid loss of supported liquid membrane based on n-decanol. Colloids Surfaces A Physicochem. Eng. Asp. 2001, 193, 61–70. [Google Scholar] [CrossRef]
- Kim, J.S. Selective transport of cesium ion in polymeric CTA membrane containing calixcrown ethers. Talanta 2000, 52, 1143–1148. [Google Scholar] [CrossRef]
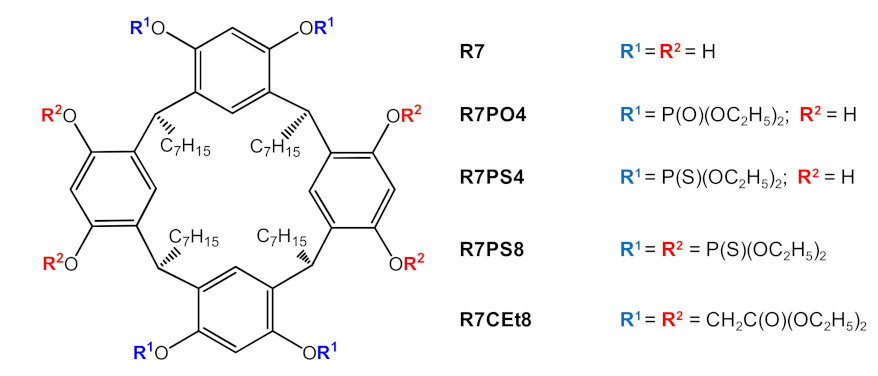
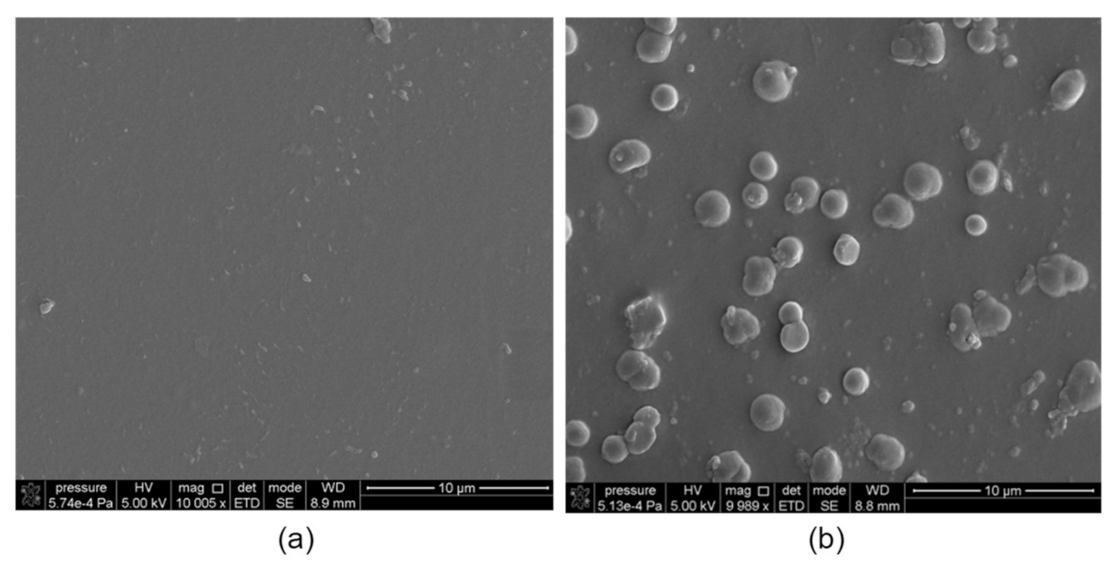

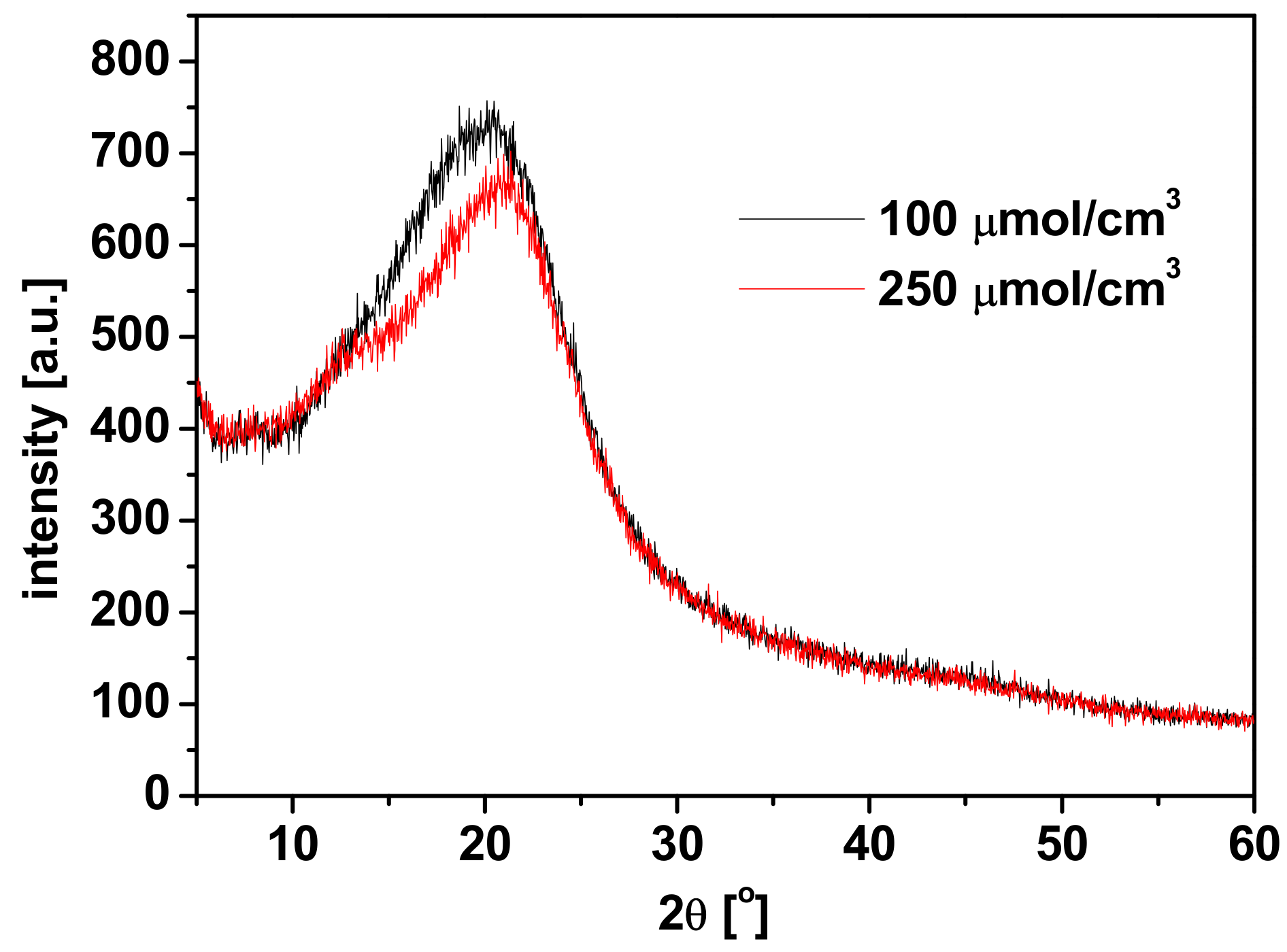

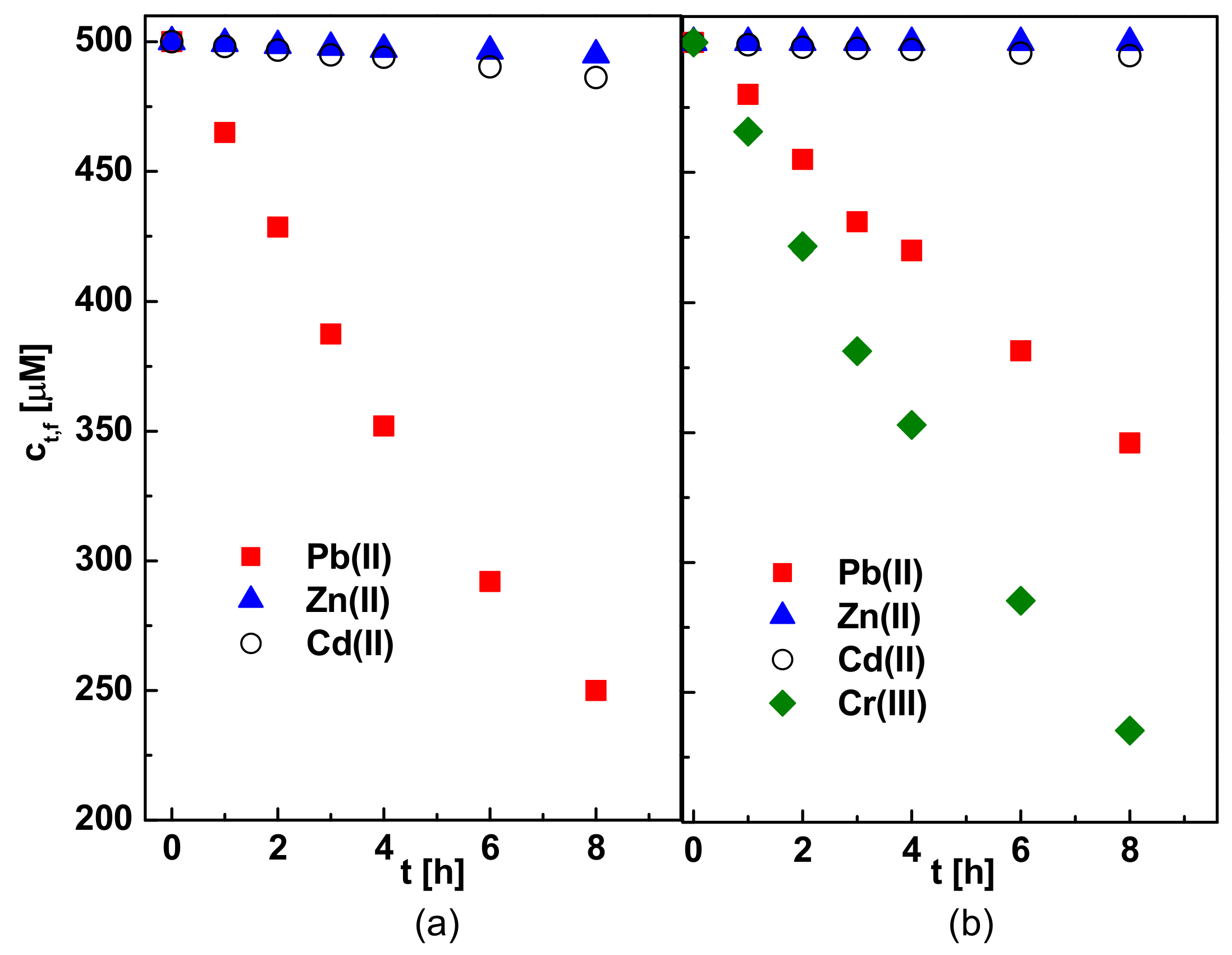
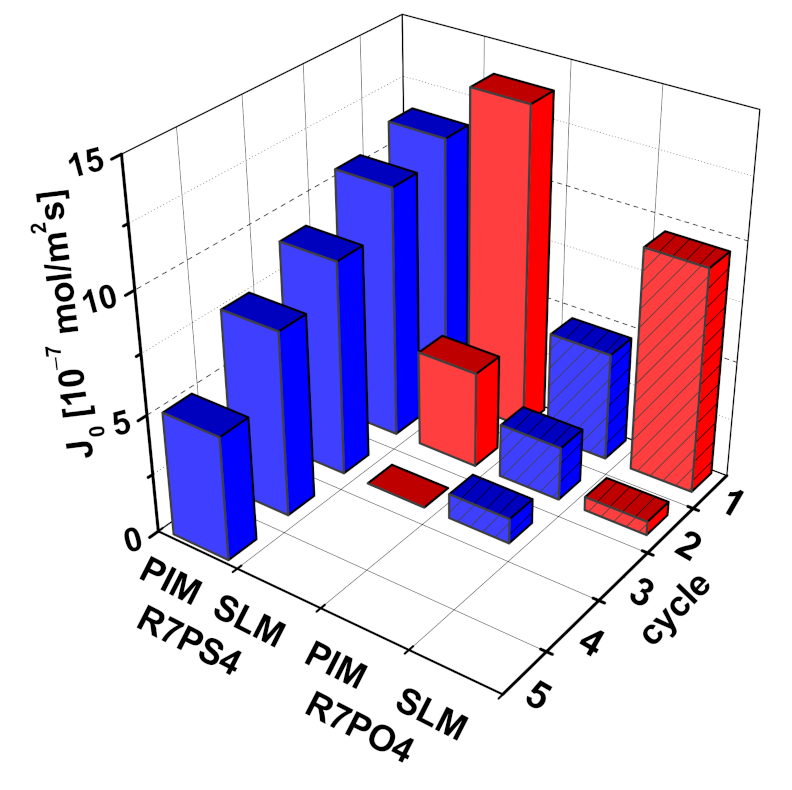
| Parameter\ Carrier | R7 | R7PS4 | R7PO4 | R7PS8 | R7CEt8 |
|---|---|---|---|---|---|
| Em [%] | 5 | 32 | 21 | 14 | 23 |
| REm [%] | 4 | 30 | 20 | 5 | 7 |
| Danesi model | |||||
| t [h] | 15 | 10 | 10 | 6 | 5 |
| P1 [10−7 m/s] | 1.70 | 14.89 | 8.33 | 6.22 | 9.33 |
| J0 [10−7 mol/m2s] | 0.85 | 7.44 | 4.17 | 3.11 | 4.67 |
| R2 [%] | 99.57 | 99.29 | 99.37 | 99.52 | 99.39 |
| Szczepanski model for t = 36 h | |||||
| P2–36 [10−7 m/s] | 2.71 | 12.13 | 9.52 | 3.64 | 6.62 |
| J0 [10−7 mol/m2s] | 1.36 | 6.06 | 4.76 | 1.82 | 3.31 |
| R2 [%] | 99.62 | 96.29 | 97.86 | 95.06 | 96.96 |
| Szczepanski model for t = 10 h | |||||
| P2–10 [10−7 m/s] | 2.32 | 18.55 | 9.36 | 6.38 | 8.50 |
| J0 [10−7 mol/m2s] | 1.16 | 9.28 | 4.68 | 3.19 | 4.25 |
| R2 [%] | 99.02 | 99.84 | 99.64 | 97.29 | 97.90 |
| Carrier | TG | DTG | DSC | |||
|---|---|---|---|---|---|---|
| Temperature Range [°C] | Weight Loss [%] | Decomposition Rate Constant (tgα) | dm/dt [mg/min] | Temperature [°C] | ∆H [J/g] | |
| R7PS4 | 25–150 | 0 | 0 | - | 104.9 | +18.6 |
| 150–290 | 0 | 0 | - | 259.5 290.6 | +25.9 +1.8 | |
| 290–350 | 76.3 | 4.64 | 24.23 | 311.0 | +1.1 | |
| 350–500 | 0 | 0 | - | 356.7 492.2 | −24.7 −78.4 | |
| R7PO4 | 25–150 | 3.80 | 0.11 | 1.10 | 90.6 | +14.8 |
| 150–260 | 0 | 0 | - | 258.3 | +21.7 | |
| 260–350 | 50.4 | 3.84 | 14.57 | 290.6 310.2 | +1.8 +1.6 | |
| 350–500 | 20.9 | 0.71 | 6.04 | 360.3 491.7 | −2.4 −74.5 | |
| System | Pb(II) | Zn(II) | Cd(II) | Cr(III) | Sm | |
|---|---|---|---|---|---|---|
| J0 [10−7 mol/m2s] | ||||||
| Pb(II), Zn(II), Cd(II) | 16.48 | 0.22 | 0.63 | - | Pb(II)/Zn(II) | 74.91 |
| Pb(II)/Cd(II) | 26.15 | |||||
| Pb(II), Zn(II), Cd(II), Cr(III) | 8.46 | 0 | 0.22 | 17.69 | Cr(III)/Pb(II) | 2.09 * |
| Cr(III)/Zn(II) | ∞ * | |||||
| Cr(III)/Cd(II) | 80.41 * | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konczyk, J.; Ciesielski, W. Calixresorcin[4]arene-Mediated Transport of Pb(II) Ions through Polymer Inclusion Membrane. Membranes 2021, 11, 285. https://doi.org/10.3390/membranes11040285
Konczyk J, Ciesielski W. Calixresorcin[4]arene-Mediated Transport of Pb(II) Ions through Polymer Inclusion Membrane. Membranes. 2021; 11(4):285. https://doi.org/10.3390/membranes11040285
Chicago/Turabian StyleKonczyk, Joanna, and Wojciech Ciesielski. 2021. "Calixresorcin[4]arene-Mediated Transport of Pb(II) Ions through Polymer Inclusion Membrane" Membranes 11, no. 4: 285. https://doi.org/10.3390/membranes11040285
APA StyleKonczyk, J., & Ciesielski, W. (2021). Calixresorcin[4]arene-Mediated Transport of Pb(II) Ions through Polymer Inclusion Membrane. Membranes, 11(4), 285. https://doi.org/10.3390/membranes11040285