Polyimide-Based Membrane Materials for CO2 Separation: A Comparison of Segmented and Aromatic (Co)polyimides
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
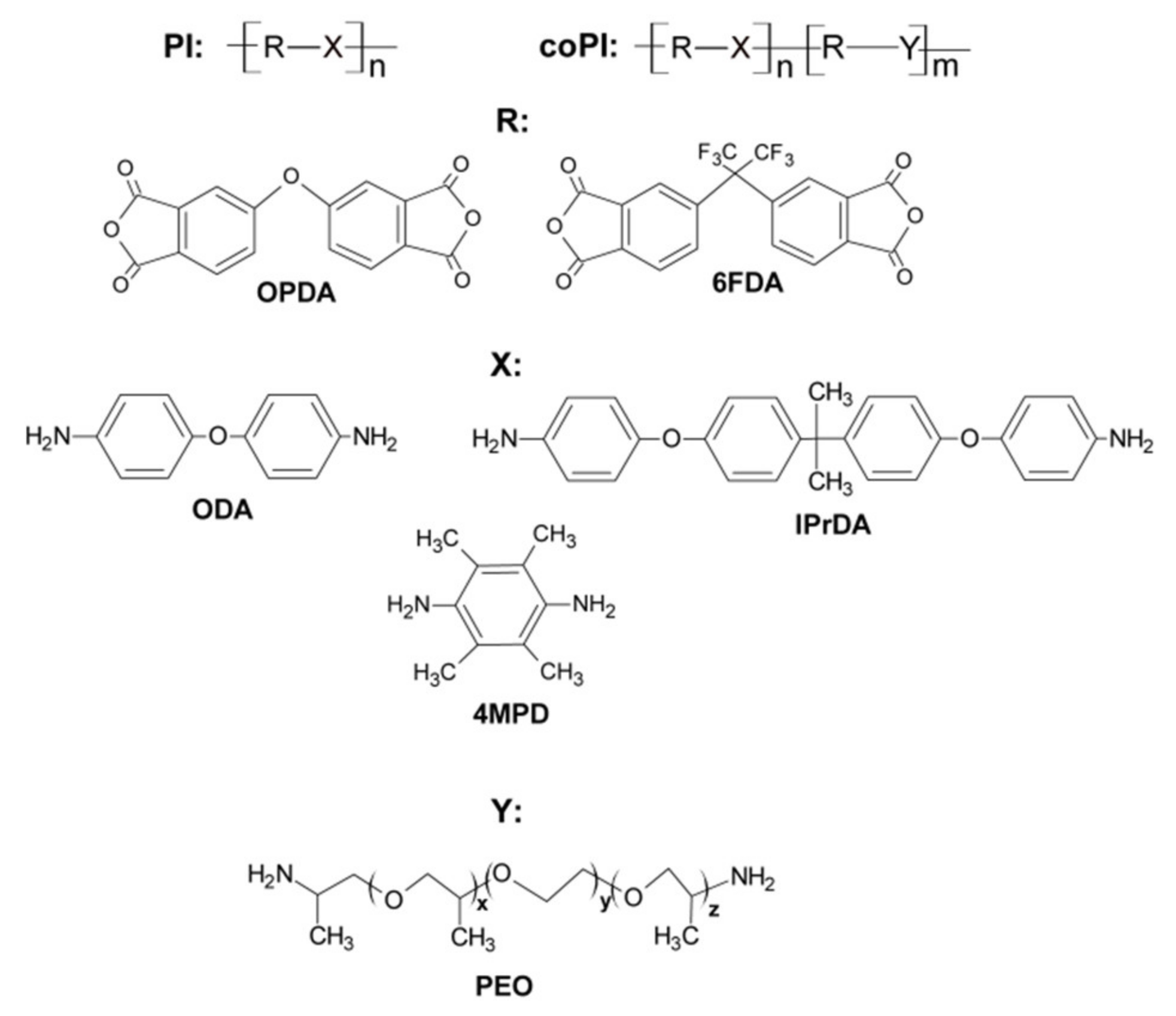
2.2. Synthesis of Aromatic and Segmented (Co)polyimides
2.3. Membrane Formation
2.4. Measurements
3. Results and Discussion
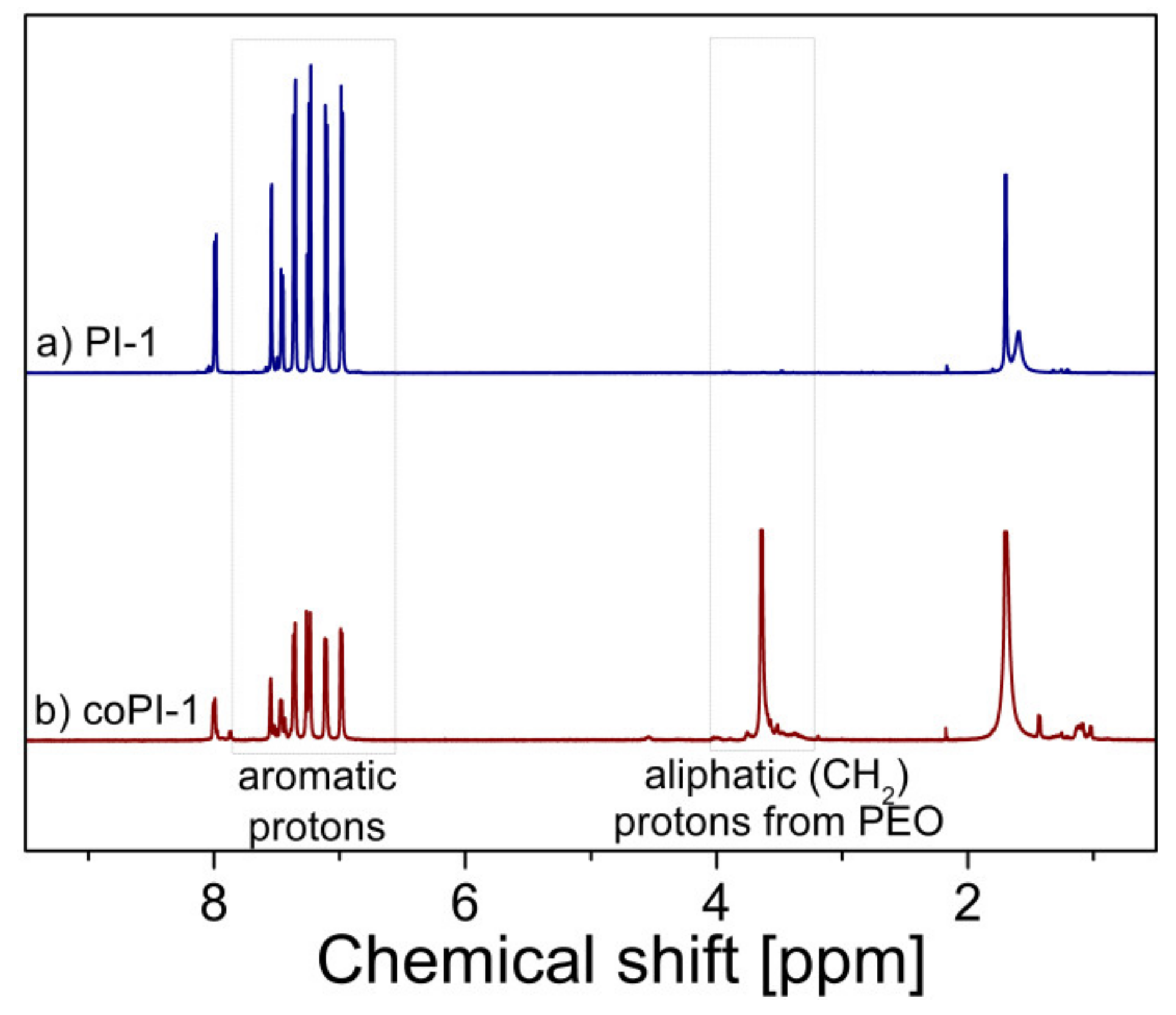
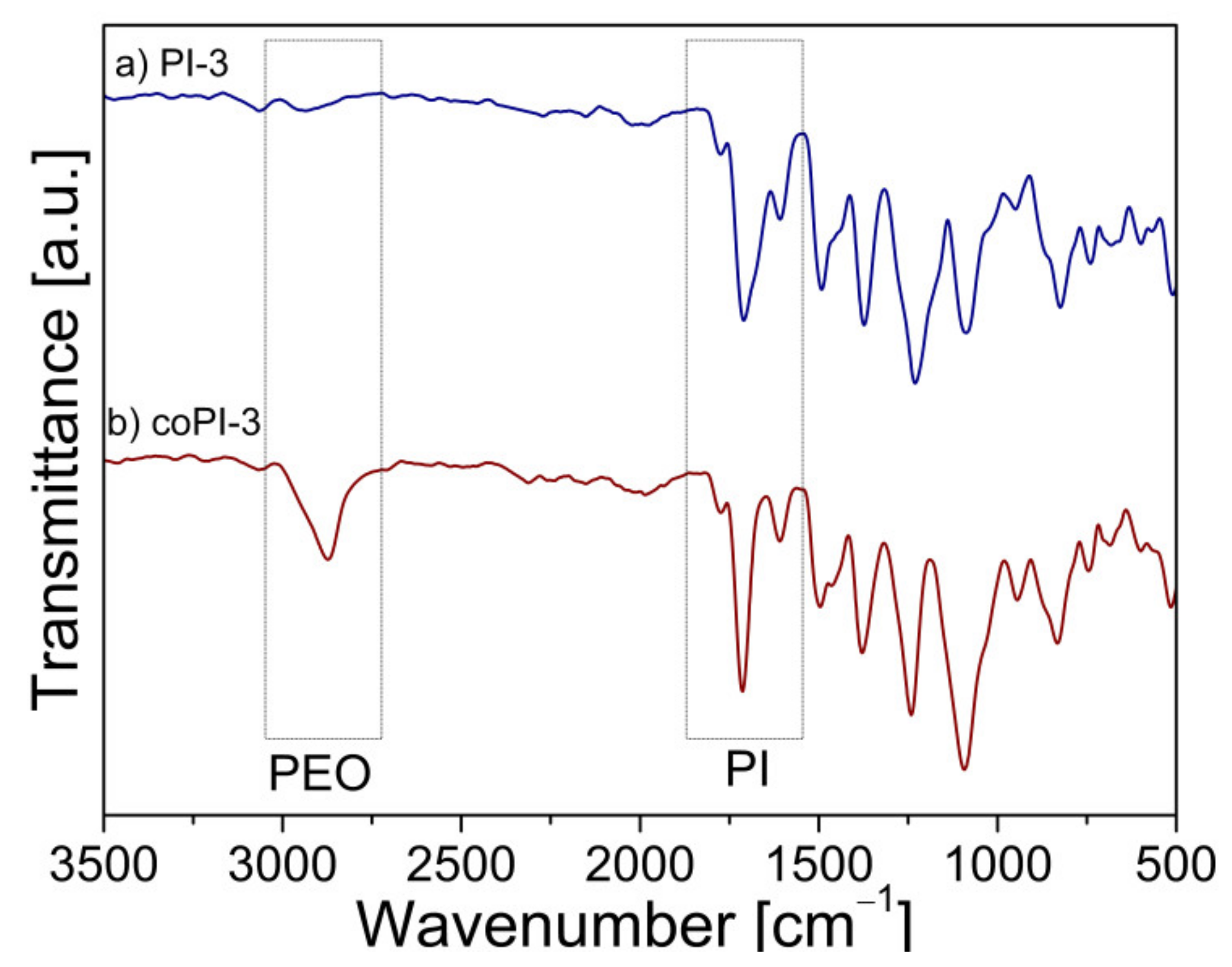
3.1. Characterization of (Co)polyimides
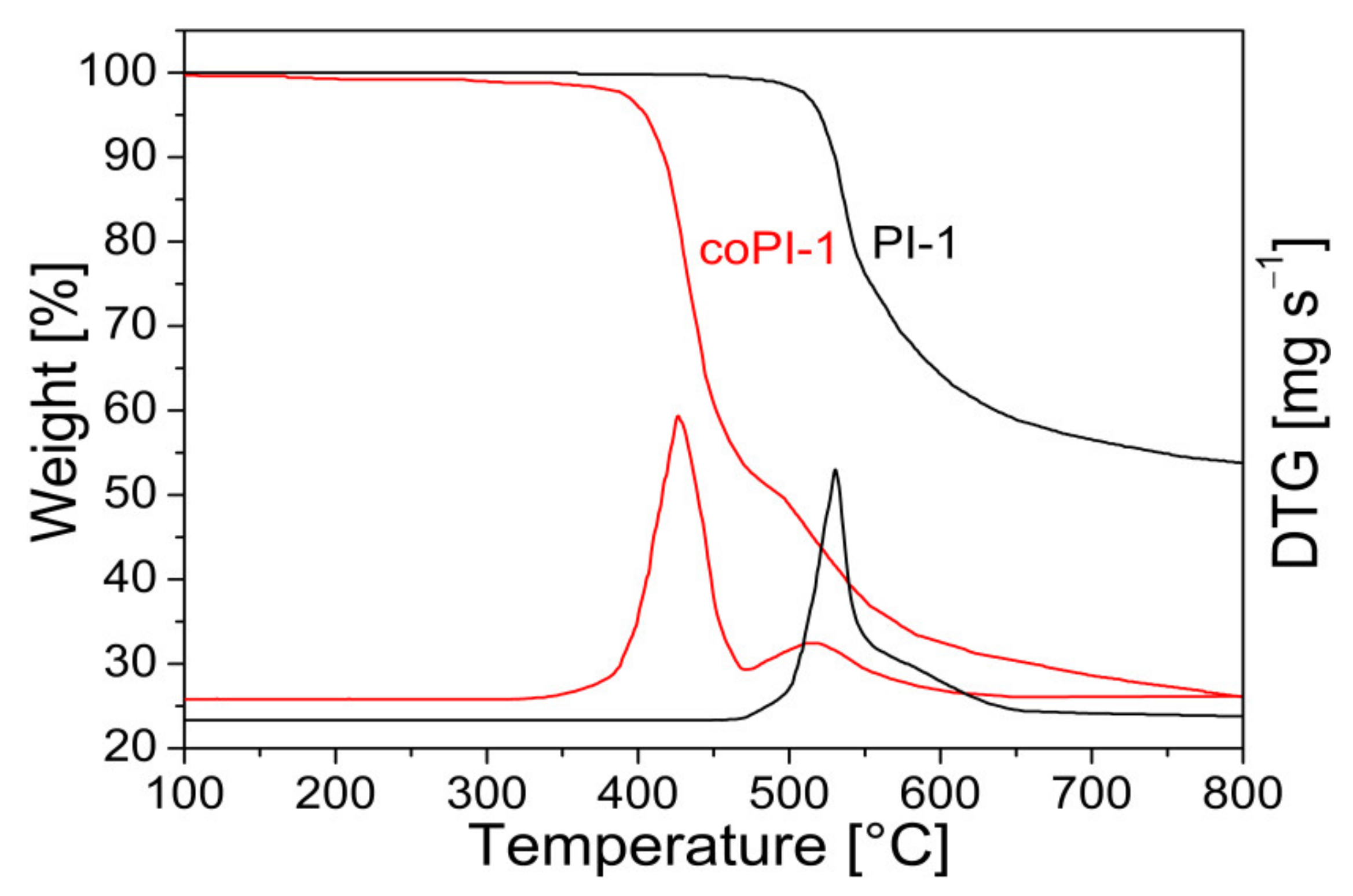
3.2. Thermal Properties
3.3. Wide-Angle-X-ray Diffrection Patterns
3.4. Surface Morphology Characterization by AFM
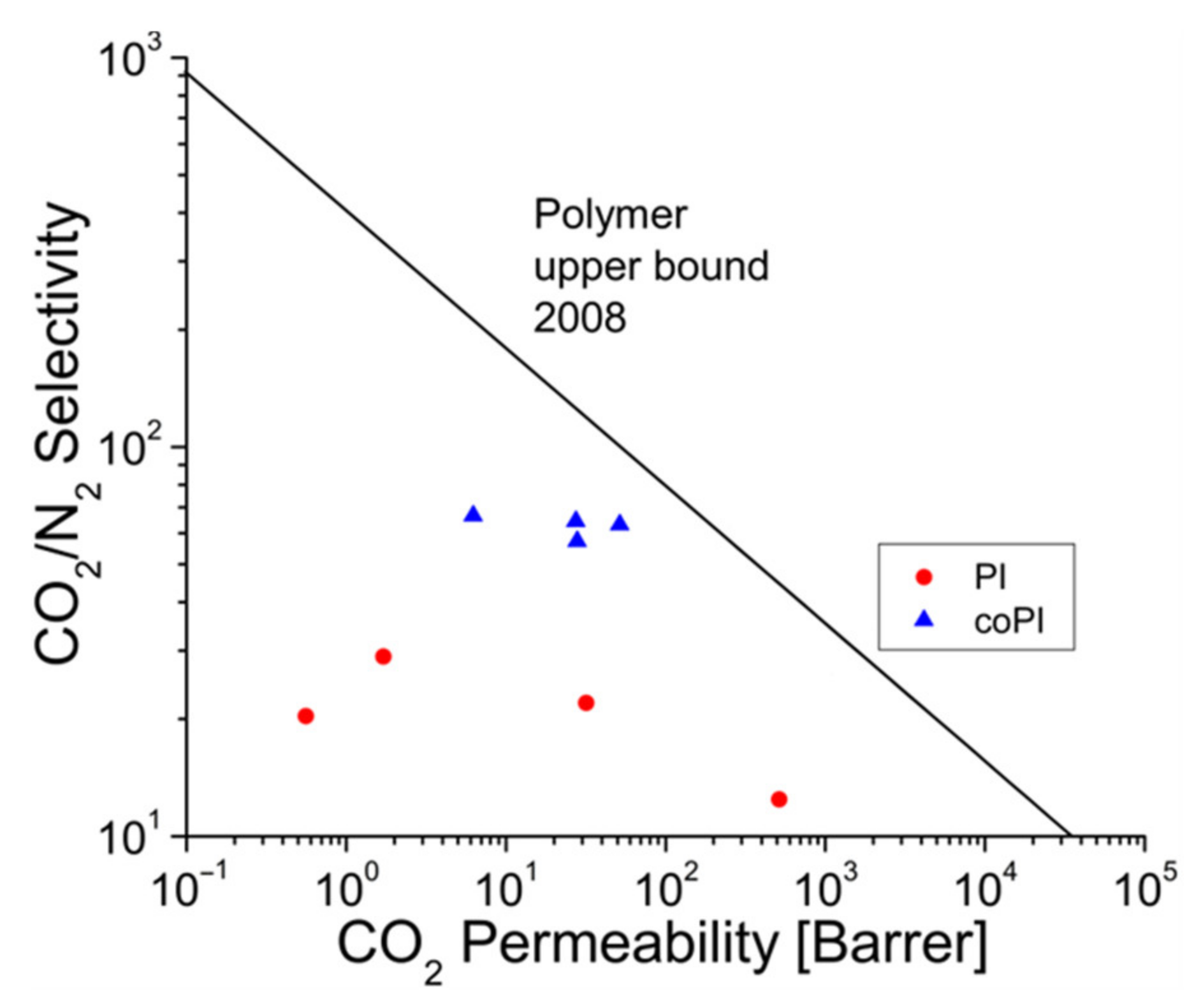
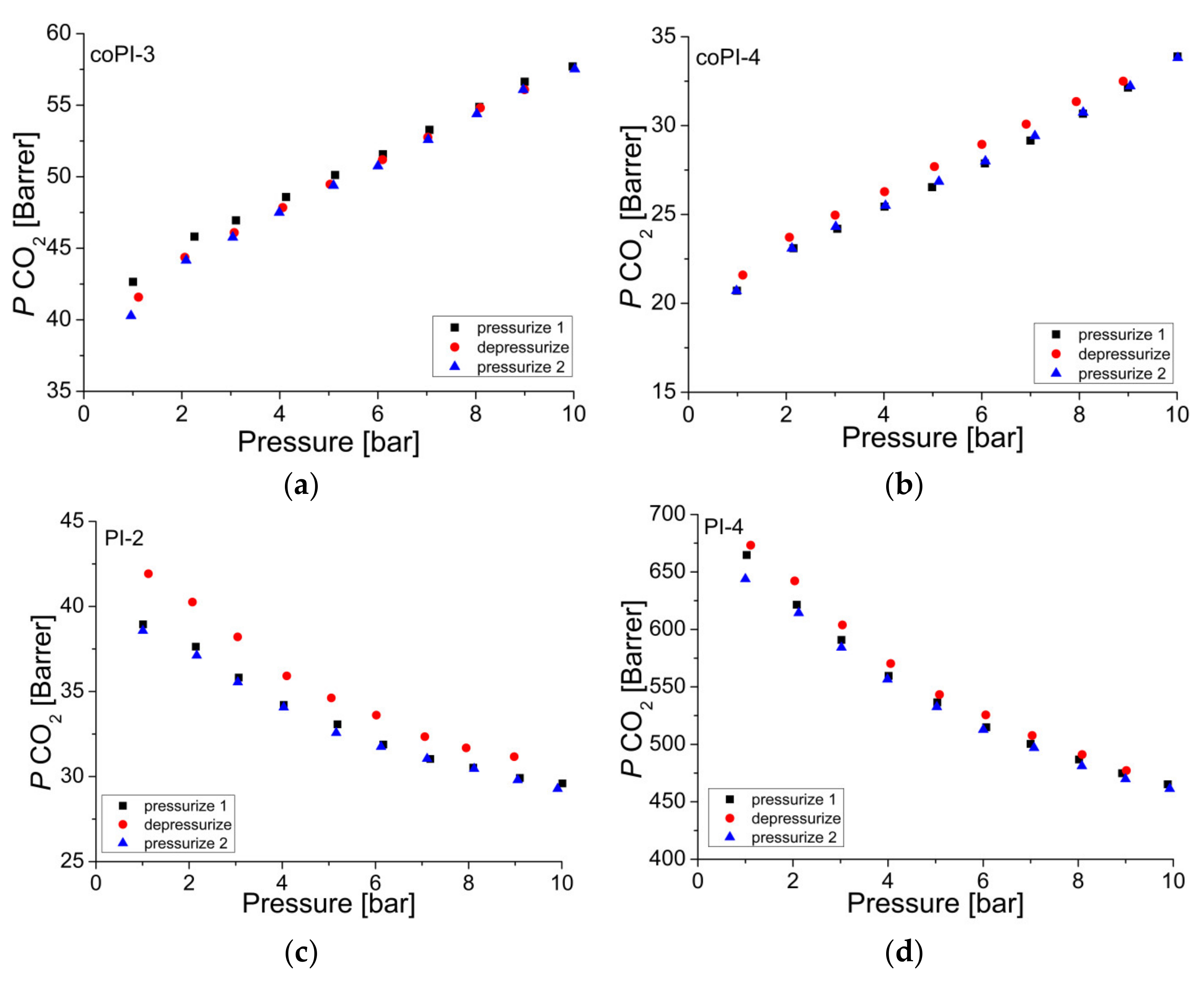
3.5. Gas Transport Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Figueroa, J.D.; Fout, T.; Plasynski, S.; McIlvried, H.; Srivastava, R.D. Advances in CO2 capture technology-The U.S. Department of Energy’s Carbon Sequestration Program. Int. J. Greenh. Gas Con. 2008, 2, 9–20. [Google Scholar] [CrossRef]
- Olajire, A.A. CO2 capture and separation technologies for end-of-pipe applications—A review. Energy 2010, 35, 2610–2628. [Google Scholar] [CrossRef]
- Mondal, M.K.; Balsora, H.K.; Varshney, P. Progress and trends in CO2 capture/separation technologies: A review. Energy 2012, 46, 431–441. [Google Scholar] [CrossRef]
- Khalilpour, R.; Mumford, K.; Zhai, H.; Abbas, A.; Stevens, G.; Rubin, E.S. Membrane-based carbon capture from flue gas: A review. J. Clean. Prod. 2015, 103, 286–300. [Google Scholar] [CrossRef]
- He, X. A review of material development in the field of carbon capture and the application of membrane-based processes in power plants and energy-intensive industries. Energy Sustain. Soc. 2018, 8, 34. [Google Scholar] [CrossRef]
- He, X. The Latest Development on Membrane Materials and Processes for Post-combustion CO2 Capture: A Review. SF J. Mater. Chem. Eng. 2018, 1, 1009. [Google Scholar]
- Liu, J.; Hou, X.; Park, H.B.; Lin, H. High-Performance Polymers for Membrane CO2/N2 Separation. Chem. Eur. J. 2016, 22, 15980–15990. [Google Scholar] [CrossRef] [PubMed]
- Bounaceur, R.; Lape, N.; Roizard, D.; Vallieres, C.; Favre, E. Membrane processes for post-combustion carbon dioxide capture: A parametric study. Energy 2006, 31, 2556–2570. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, Z. Nanostructured Membrane Materials for CO2 Capture: A Critical Review. J. Nanosci. Nanotechnol. 2019, 19, 3173–3179. [Google Scholar] [CrossRef] [PubMed]
- Kargari, A.; Rezaeinia, S. State-of-the-art modification of polymeric membranes by PEO and PEG for carbon dioxide separation: A review of the current status and future perspectives. J. Ind. Eng. Chem. 2020, 84, 1–22. [Google Scholar] [CrossRef]
- Robeson, L.M. Correlation of separation factor versus permeability for polymeric membranes. J. Membr. Sci. 1991, 62, 165–185. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Liu, S.L.; Shao, L.; Chua, M.L.; Lau, C.H.; Wang, H.; Quan, S. Recent progress in the design of advanced PEO-containing membranes for CO2 removal. Prog. Polym. Sci. 2013, 38, 1089–1120. [Google Scholar] [CrossRef]
- Lau, C.H.; Li, P.; Li, F.; Chung, T.-S.; Donald, R.; Paul, R.R. Reverse-selective polymeric membranes for gas separations. Prog. Polym. Sci. 2013, 38, 740–766. [Google Scholar] [CrossRef]
- Metz, S.J.; Mulder, M.H.V.; Wessling, M. Gas-Permeation Properties of Poly(ethylene oxide) Poly(butylene terephthalate) Block Copolymers. Macromolecules 2004, 37, 4590–4597. [Google Scholar] [CrossRef]
- Husken, D.; Visser, T.; Wessling, M.; Gaymans, R.J. CO2 permeation properties of poly(ethylene oxide)-based segmented block copolymers. J. Membr. Sci. 2010, 346, 194–201. [Google Scholar] [CrossRef]
- Okamoto, K.; Fujii, M.; Okamyo, S.; Suzuki, H.; Tanaka, K.; Kita, H. Gas Permeation Properties of Poly(ether imide) Segmented Copolymers. Macromolecules 1995, 28, 6950–6956. [Google Scholar] [CrossRef]
- Yoshino, M.; Ito, K.; Kita, H.; Okamoto, K.-I. Effects of Hard-Segment Polymers on CO2/N2 Gas-Separation Properties of Poly(ethylene oxide)-Segmented Copolymers. J. Polym. Sci. Part B: Polym. Phys. 2000, 38, 1707–1715. [Google Scholar] [CrossRef]
- Chen, K.; Xiao, Y.; Chung, T.-S. Synthesis and characterization of poly (ethylene oxide) containing copolyimides, for hydrogen purification. Polymer 2010, 51, 4077–4086. [Google Scholar] [CrossRef]
- Muñoz, D.M.; Maya, E.M.; de Abajo, J.; de la Campa, J.G.; Lozano, A.E. Thermal treatment of poly(ethylene oxide)-segmented copolyimide based membranes: An effective way to improve the gas separation properties. J. Membr. Sci. 2008, 323, 53–59. [Google Scholar] [CrossRef]
- Tena, A.; Marcos-Fernández, A.; Lozano, A.E.; de Abajo, J.; Palacio, L.; Prádanos, P.; Hernández, A. Influence of the PEO length in gas separation properties of segregating aromatic–aliphatic copoly(ether-imide)s. Chem. Eng. Sci. 2013, 104, 574–585. [Google Scholar] [CrossRef]
- Tena, A.; Marcos-Fernández, A.; Lozano, A.E.; de la Campa, J.G.; de Abajo, J.; Palacio, L.; Prádanos, P.; Hernandez, A. Thermally treated copoly(ether-imide)s made from bpda and alifatic plus aromatic diamines. GAS separation properties with different aromatic diamimes. J. Membr. Sci. 2012, 387-388, 54–65. [Google Scholar] [CrossRef]
- Tena, A.; Marcos-Fernández, A.; Palacio, L.; Prádanos, P.; Lozano, A.E.; deAbajo, J.; Hernández, A. On the influence of the proportion of PEO in thermally controlled phase segregation of copoly(ether-imide)s for gas separation. J. Membr. Sci. 2013, 434, 26–34. [Google Scholar] [CrossRef]
- Tena, A.; Shishatskiy, S.; Filiz, V. Poly(ether–amide) vs. poly(ether–imide) copolymers for post-combustion membrane separation processes. RSC Adv. 2015, 5, 22310–22318. [Google Scholar] [CrossRef]
- Tena, A.; Lozano, A.E.; Palacio, L.; Marcos-Fernández, A.; Prádanos, P.; de Abajo, J.; Hernández, A. Gas separation properties of systems with different amounts of long poly(ethylene oxide) segments for mixtures including carbon dioxide. Int. J. Greenh. Gas Con. 2013, 12, 146–154. [Google Scholar] [CrossRef]
- Solimando, X.; Babin, J.; Arnal-Herault, C.; Wang, M.; Barth, D.; Roizard, D.; Doillon-Halmenschlager, J.-R.; Ponçot, M.; Royaud, I.; Alcouffe, P.; et al. Highly selective multi-block poly(ether-urea-imide)s for CO2/N2 separation: Structure-morphology-properties relationships. Polymer 2017, 131, 56–67. [Google Scholar] [CrossRef]
- Luo, S.; Stevens, K.A.; Park, J.S.; Moon, J.D.; Liu, Q.; Freeman, B.D.; Guo, R. Highly CO2-Selective Gas Separation Membranes Based on Segmented Copolymers of Poly(Ethylene oxide) Reinforced with Pentiptycene-Containing Polyimide Hard Segments. ACS Appl. Mater. Interfaces 2016, 8, 2306–2317. [Google Scholar] [CrossRef]
- Krea, M.; Roizard, D.; Favre, E. Copoly(alkyl ether imide) membranes as promising candidates for CO2 capture applications. Sep. Purif. Technol. 2016, 161, 53–60. [Google Scholar] [CrossRef]
- Sanaeepur, H.; Amooghin, A.E.; Bandehali, S.; Moghadassi, A.; Matsuura, T.; Van der Bruggen, B. Polyimides in membrane gas separation: Monomer’s moleculardesign and structural engineering. Prog. Polym. Sci. 2019, 91, 80–125. [Google Scholar] [CrossRef]
- Kubica, P.; Wolinska-Grabczyk, A. Correlation between Cohesive Energy Density, Fractional Free Volume, and Gas Transport Properties of Poly(ethylene-co-vinyl acetate) Materials. Int. J. Polym. Sci. 2015, 2015, 861979. [Google Scholar] [CrossRef]
- Nocoń-Szmajda, K.; Wolińska-Grabczyk, A.; Jankowski, A.; Szeluga, U.; Wojtowicz, M.; Konieczkowska, J.; Hercog, A. Gas transport properties of mixed matrix membranes based on thermally rearranged poly(hydroxyimide)s filled with inorganic porous particles. Sep. Purif. Technol. 2020, 242, 116778. [Google Scholar] [CrossRef]
- Koros, W.J.; Fleming, G.K. Membrane-based gas separation. J. Membr. Sci. 1993, 83, 1–80. [Google Scholar] [CrossRef]
- Cho, Y.J.; Park, H.B. High Performance Polyimide with High Internal Free Volume Elements. Macromol. Rapid Commun. 2011, 32, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Wolińska-Grabczyk, A.; Kubica, P.; Jankowski, A. Effect of the acetate group content on gas permeation through membranes based on poly(ethylene-co-vinyl acetate) and its blends. J. Membr. Sci. 2013, 443, 227–236. [Google Scholar] [CrossRef]
- Lin, H.; Freeman, B.D. Gas solubility, diffusivity and permeability in poly(ethylene oxide). J. Membr. Sci. 2004, 239, 105–117. [Google Scholar] [CrossRef]
- Qiu, W.; Xu, L.; Chen, C.-C.; Paul, D.R.; Koros, W.J. Gas separation performance of 6FDA-based polyimides with different chemical structures. Polymer 2013, 54, 6226–6235. [Google Scholar] [CrossRef]
- Lin, H.; Freeman, B.D.; Kalakkunnath, S.; Kalika, D.S. Effect of copolymer composition, temperature, and carbon dioxide fugacity on pure- and mixed-gas permeability in poly(ethylene glycol)-based materials: Free volume interpretation. J. Membr. Sci. 2007, 291, 131–139. [Google Scholar] [CrossRef]
- Ghadimi, A.; Sadrzadeh, M.; Shahidi, K.; Mohammadi, T. Ternary gas permeation through a synthesized PDMS membrane: Experimental and modeling. J. Membr. Sci. 2009, 344, 225–236. [Google Scholar] [CrossRef]
- Paul, D.R.; Koros, W.J. Effect of Partially Immobilizing Sorption on Permeability and the Diffusion Time Lag. J. Polym. Sci. Polym. Phys. Ed. 1976, 14, 675–685. [Google Scholar] [CrossRef]
- Bondi, A. Physical Properties of Molecular Crystals, Liquids and Glasses; John Wiley & Sons, Inc.: New York, NY, USA, 1968. [Google Scholar]
- Kanehashi, S.; Nakagawa, T.; Nagai, K.; Duthie, X.; Kentish, S.; Stevens, G. Effects of carbon dioxide-induced plasticization on the gas transport properties of glassy polyimide membranes. J. Membr. Sci. 2007, 298, 147–155. [Google Scholar] [CrossRef]
- Nocon-Szmajda, K.; Wolinska-Grabczyk, A.; Jankowski, A.; Dryzek, J.; Dryzek, E.; Janeczek, H.; Grabiec, E.; Musiol, M. Effects of ionic liquid doping on gas transport properties of thermally rearranged poly(hydroxyimide)s. Sep. Purif. Technol. 2021, 254, 117664. [Google Scholar] [CrossRef]
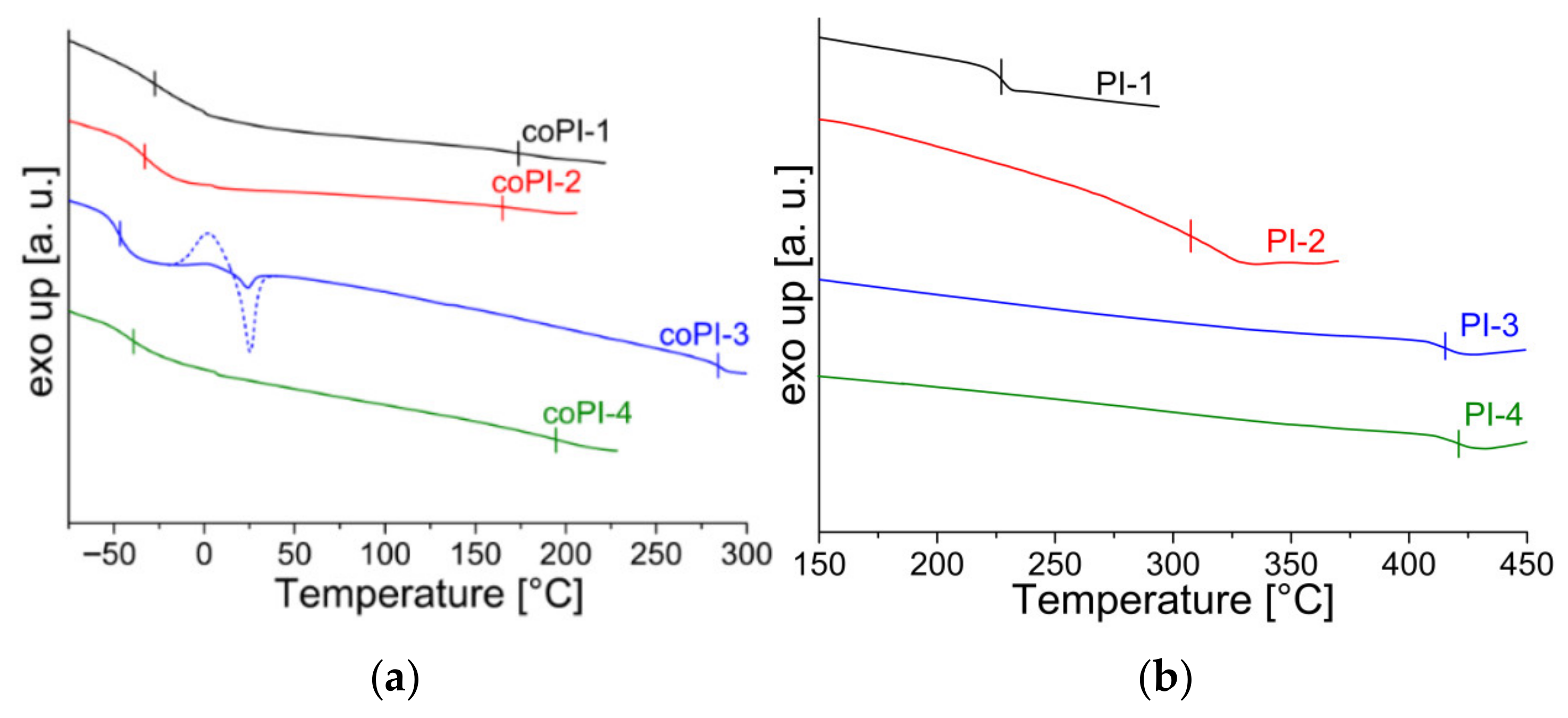
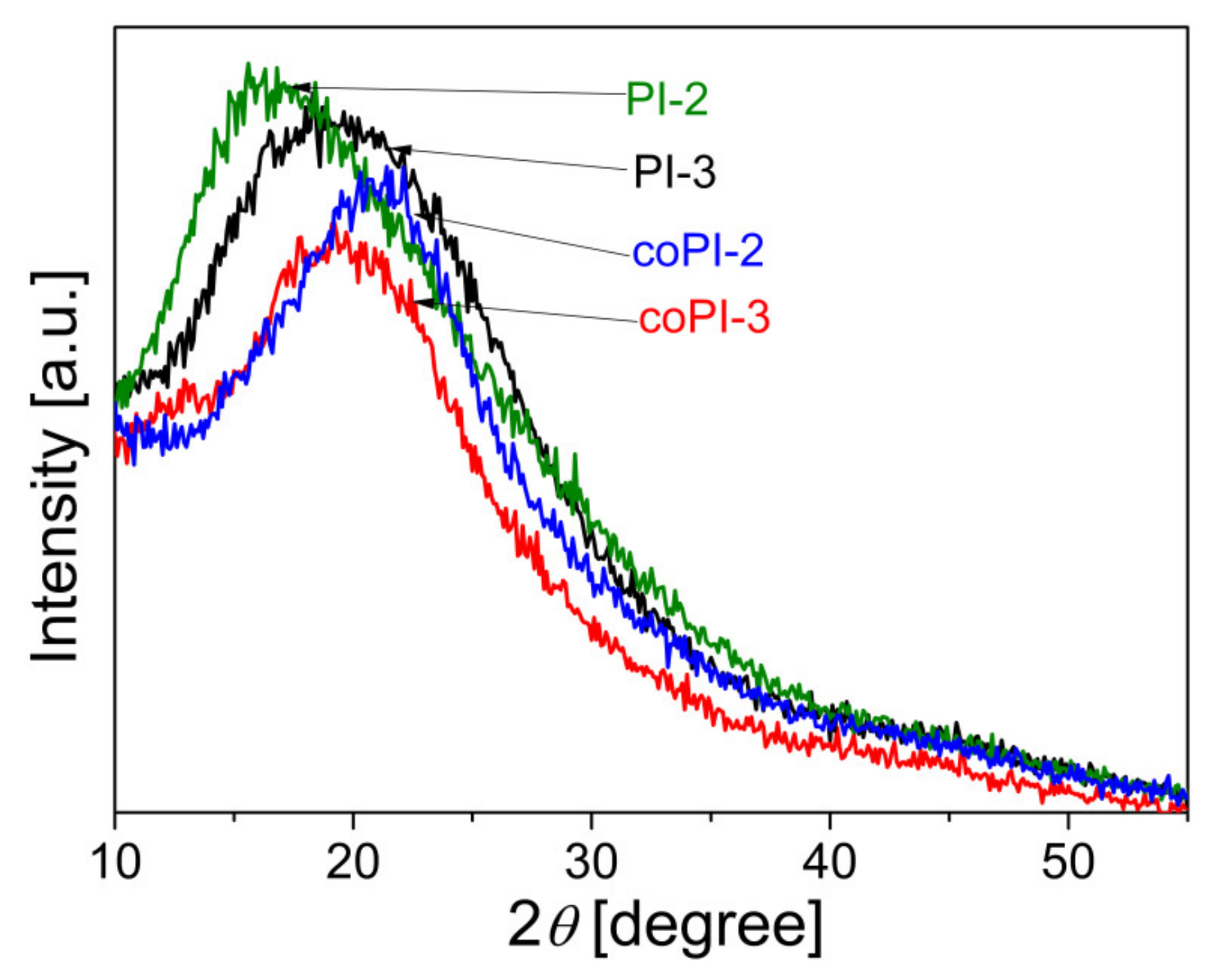
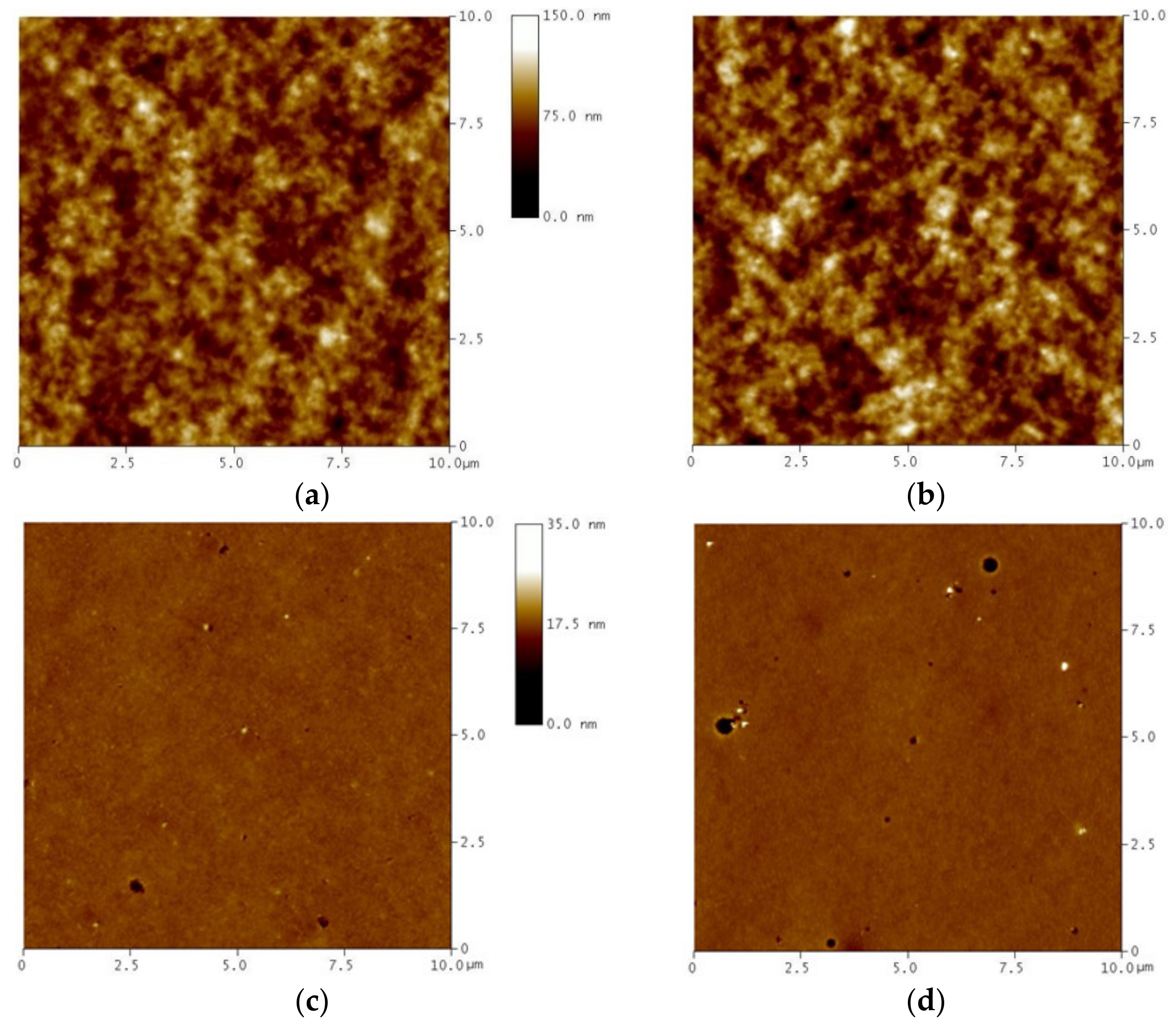
| Code | Structure | TGA | DSC | WAXD | |||||
|---|---|---|---|---|---|---|---|---|---|
| PEO Content[wt.%] | Tmax a [°C] | Char Yield b [%] | Tg [°C] | d-Spacing [Å] | |||||
| calc. | TGA | T1 | T2 | Tg1 | Tg2 | ||||
| PI-1 | ODPA-IPrDA | 550 | 54 | 227 | 5.09 | ||||
| PI-2 | ODPA-4MPD | 520 | 58 | 307 | 5.33 | ||||
| PI-3 | ODPA-ODA | 600 | 59 | 415 | 4.71 | ||||
| PI-4 c | 6FDA-4MPD | 546 | 55 | 421 | 6.1 | ||||
| coPI-1 | ODPA-IPrDA-PEO 3:1 | 44.6 | 49 | 420 | 515 | 27 | −28 | 174 | 4.67 |
| coPI-2 | ODPA-4MPD-PEO 3:1 | 55 | 49 | 400 | 520 | 31 | −33 | 165 | 4.28 |
| coPI-3 | ODPA-ODA-PEO 3:1 | 51.6 | 54 | 400 | 570 | 30 | −47 | 284 | 4.67 |
| coPI-4 | 6FDA-4MPD-PEO 3:1 | 46.3 | 49 | 423 | 520 | −39 | 194 | 5.48 | |
| Sample | Permeability [Barrer] | Selectivity | |||||
|---|---|---|---|---|---|---|---|
| N2 | O2 | He | CO2 | O2/N2 | He/N2 | CO2/N2 | |
| PI-1 | 0.059 | 0.446 | 9.05 | 1.71 | 7.56 | 153.4 | 29.0 |
| PI-2 | 1.45 | 7.79 | 52.4 | 31.9 | 5.37 | 36.1 | 22.0 |
| PI-3 | 0.028 | 0.199 | 2.89 | 0.56 | 7.11 | 103.2 | 20.0 |
| PI-4 | 41.4 | 142.0 | 376 | 515 | 3.43 | 9.1 | 12.4 |
| coPI-1 | 0.426 | 1.37 | 3.75 | 27.4 | 3.22 | 8.8 | 63.3 |
| coPI-2 | 0.094 | 0.368 | 2.40 | 6.25 | 3.91 | 25.5 | 66.5 |
| coPI-3 | 0.816 | 2.32 | 4.61 | 51.6 | 2.84 | 5.6 | 63.2 |
| coPI-4 | 0.487 | 1.63 | 7.05 | 27.9 | 3.35 | 14.5 | 57.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jankowski, A.; Grabiec, E.; Nocoń-Szmajda, K.; Marcinkowski, A.; Janeczek, H.; Wolińska-Grabczyk, A. Polyimide-Based Membrane Materials for CO2 Separation: A Comparison of Segmented and Aromatic (Co)polyimides. Membranes 2021, 11, 274. https://doi.org/10.3390/membranes11040274
Jankowski A, Grabiec E, Nocoń-Szmajda K, Marcinkowski A, Janeczek H, Wolińska-Grabczyk A. Polyimide-Based Membrane Materials for CO2 Separation: A Comparison of Segmented and Aromatic (Co)polyimides. Membranes. 2021; 11(4):274. https://doi.org/10.3390/membranes11040274
Chicago/Turabian StyleJankowski, Andrzej, Eugenia Grabiec, Klaudia Nocoń-Szmajda, Andrzej Marcinkowski, Henryk Janeczek, and Aleksandra Wolińska-Grabczyk. 2021. "Polyimide-Based Membrane Materials for CO2 Separation: A Comparison of Segmented and Aromatic (Co)polyimides" Membranes 11, no. 4: 274. https://doi.org/10.3390/membranes11040274
APA StyleJankowski, A., Grabiec, E., Nocoń-Szmajda, K., Marcinkowski, A., Janeczek, H., & Wolińska-Grabczyk, A. (2021). Polyimide-Based Membrane Materials for CO2 Separation: A Comparison of Segmented and Aromatic (Co)polyimides. Membranes, 11(4), 274. https://doi.org/10.3390/membranes11040274






