The Antimicrobial Peptide Gramicidin S Enhances Membrane Adsorption and Ion Pore Formation Potency of Chemotherapy Drugs in Lipid Bilayers
Abstract
1. Introduction
2. Materials and Methods
2.1. Electrophysiology Experiments
2.2. Direct Detection Method
3. Results
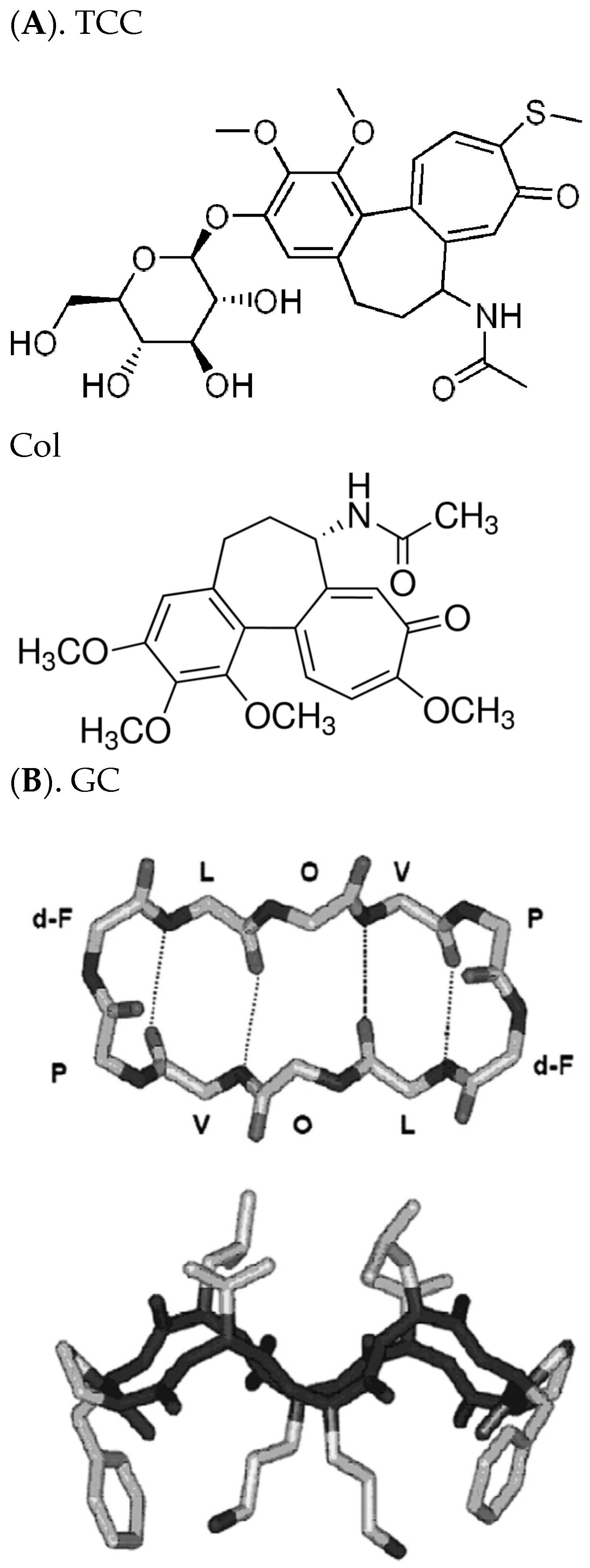
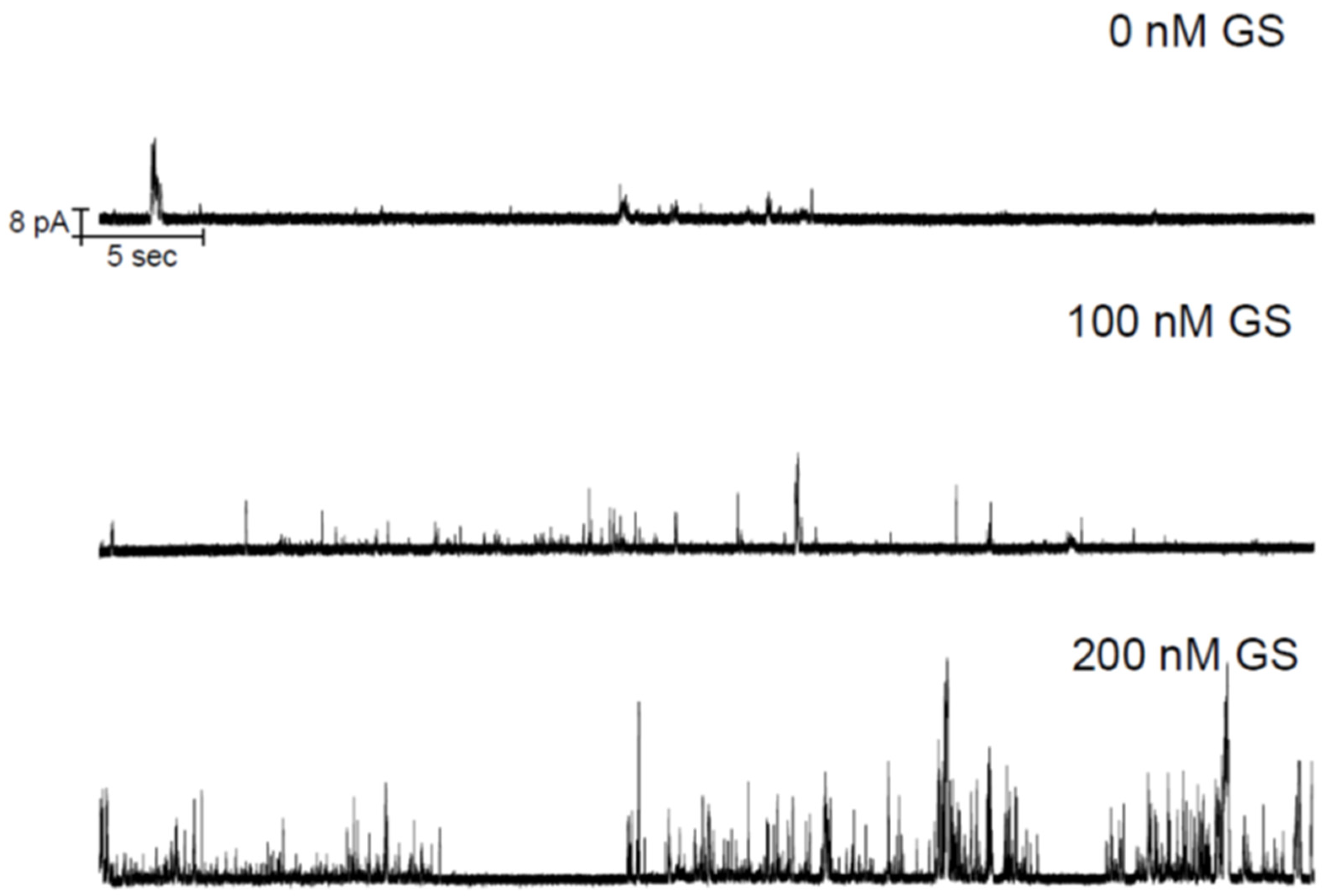
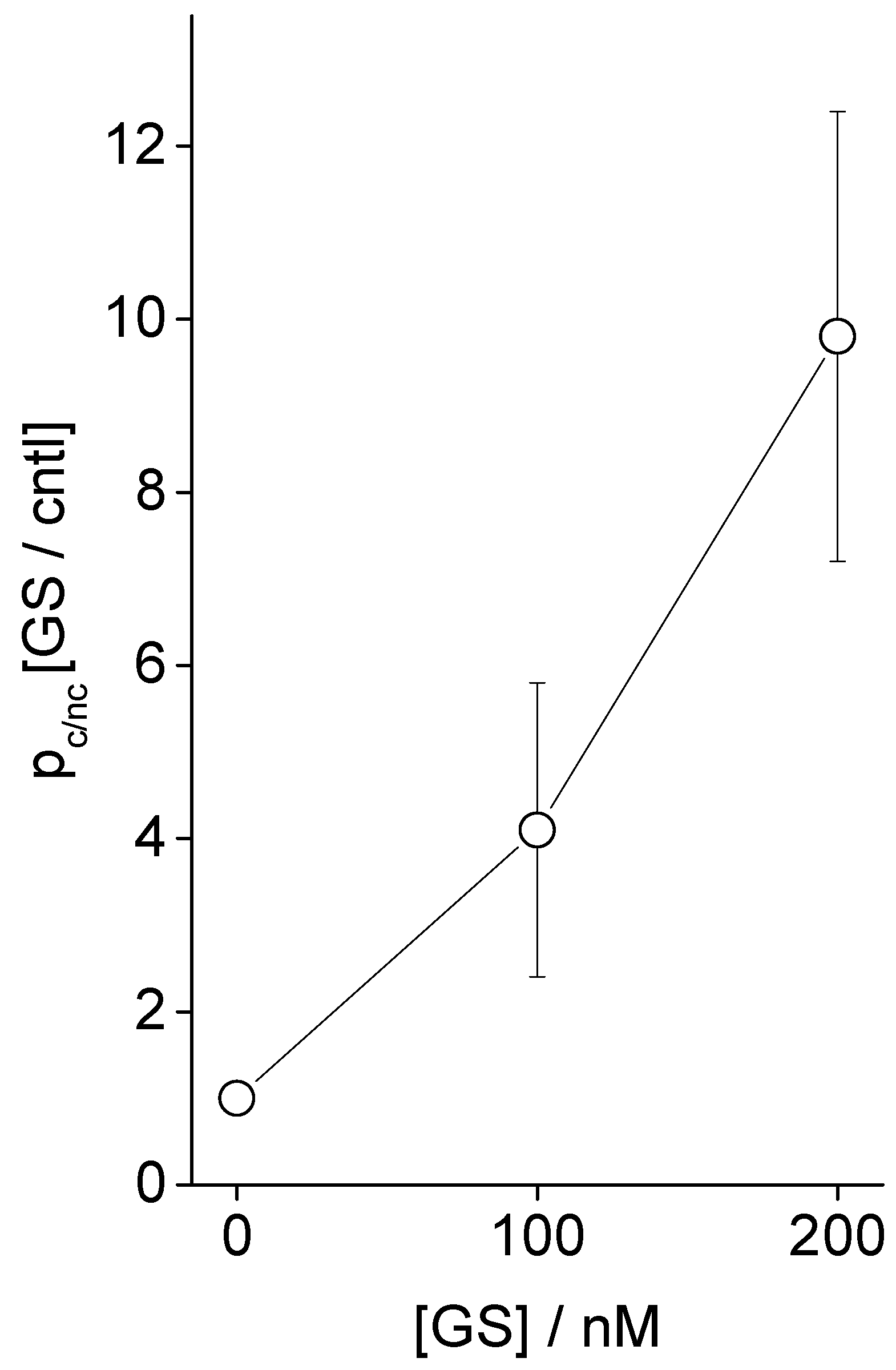
3.1. Electrophysiology Experiments Demonstrating GS Effects on TCC-Induced Lipid Bilayer Currents
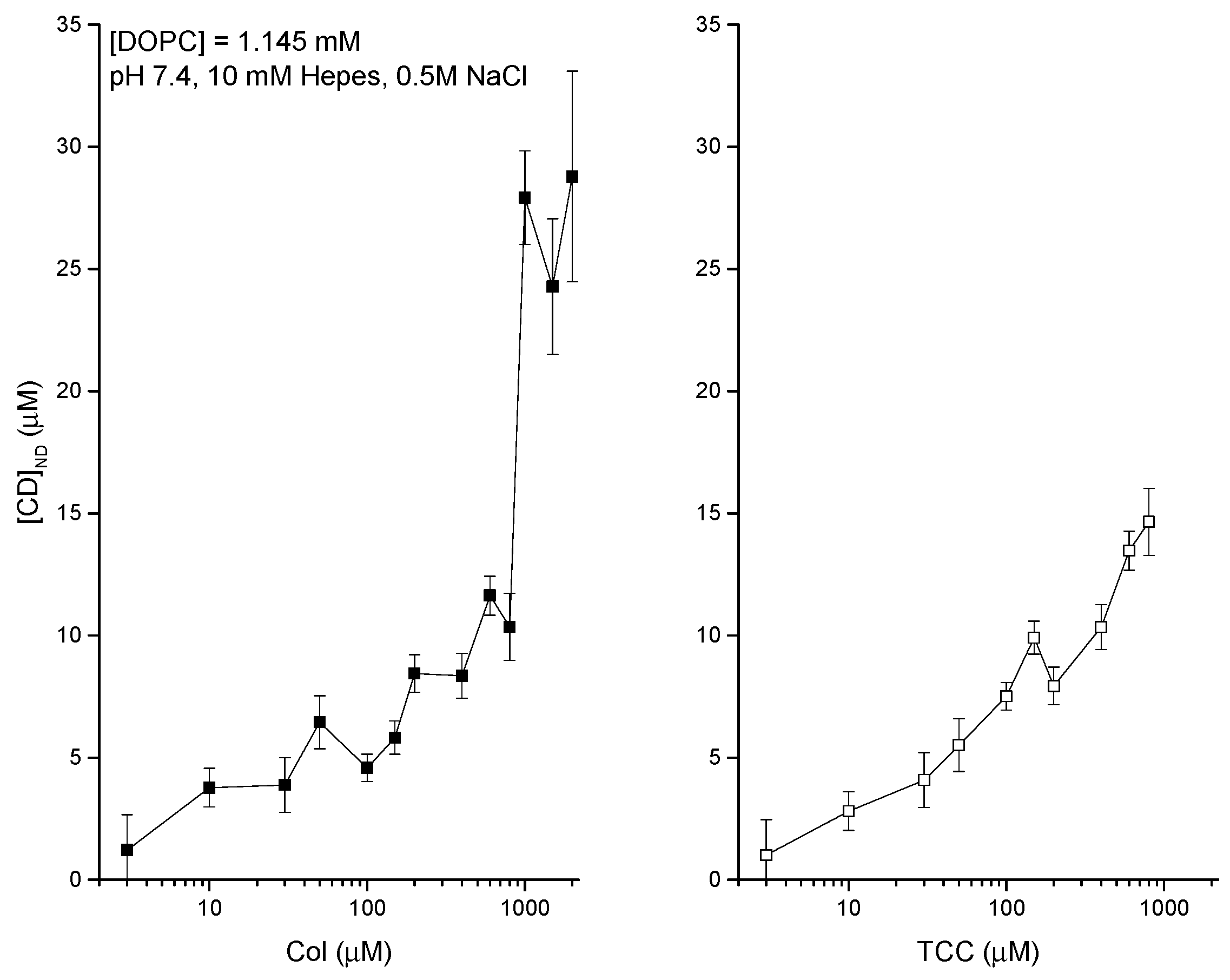
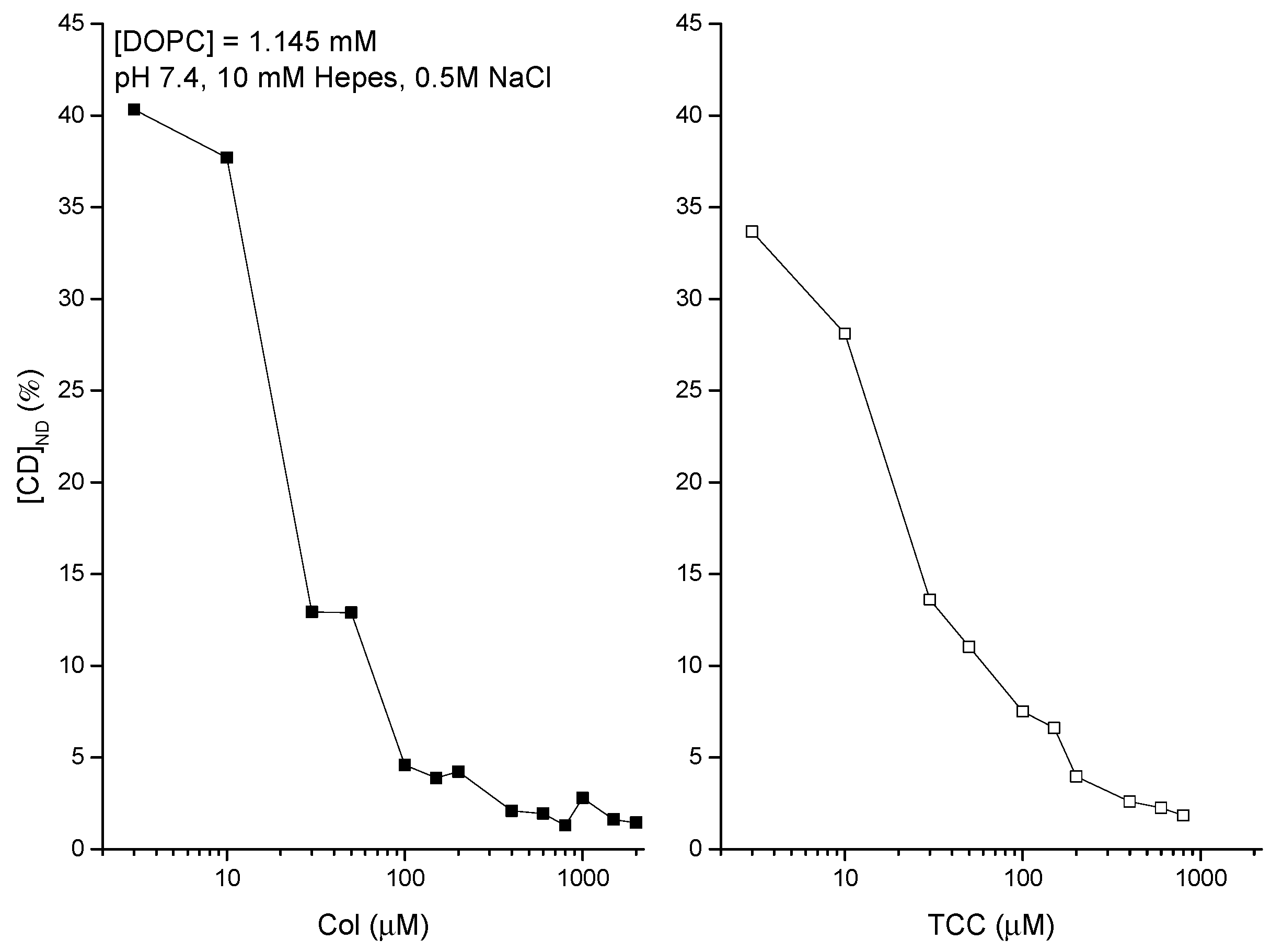
3.2. Liposome Binding of CDs Dissolved in Aqueous Phase-DDM to Detect Agents Directly at the Liposome
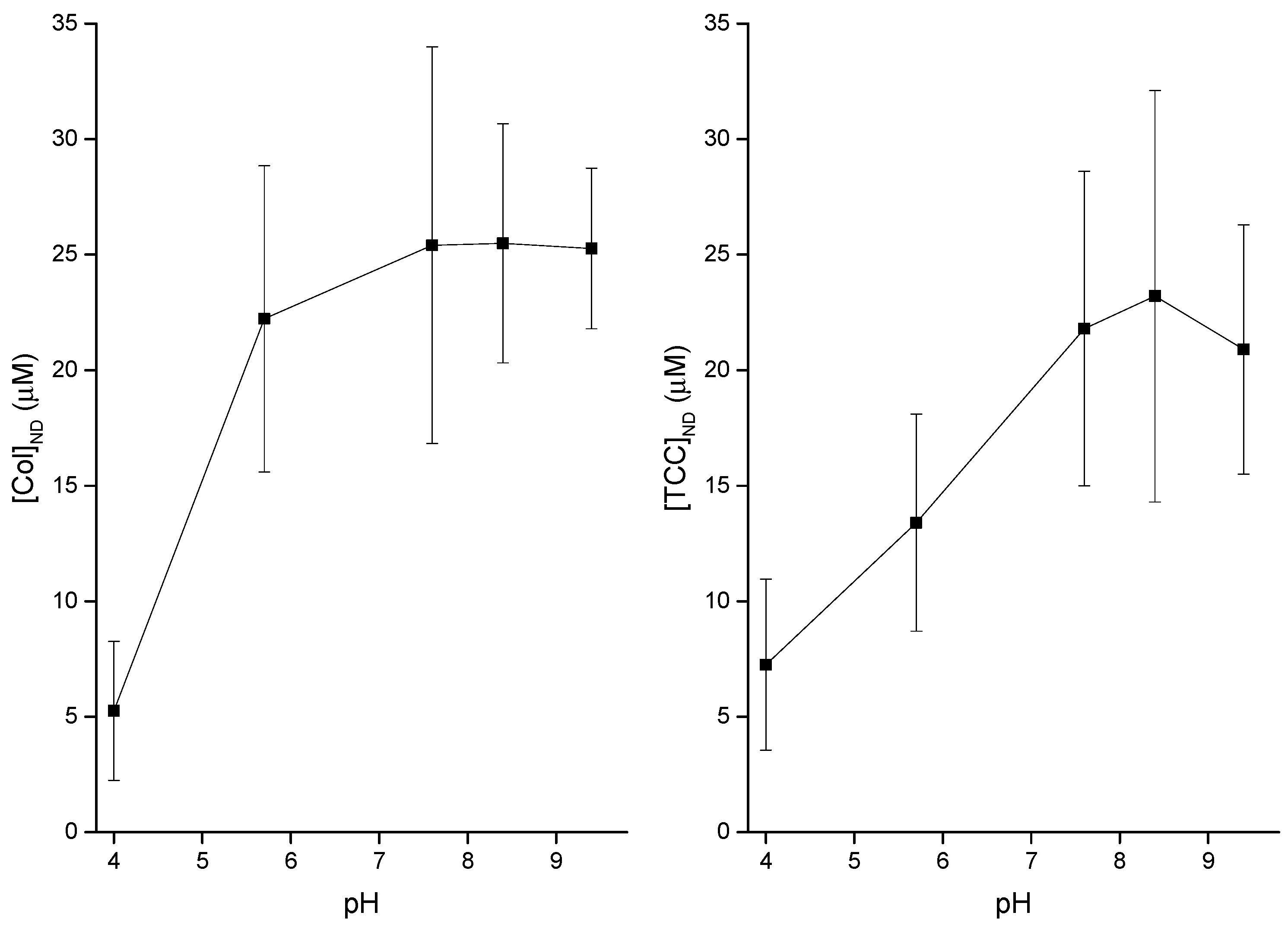
3.3. pH Effects on Liposome Binding of CDs
4. Discussion
4.1. Modest Effects of pH on Both Membrane Adsorption of CDs and Induction of CD Pores
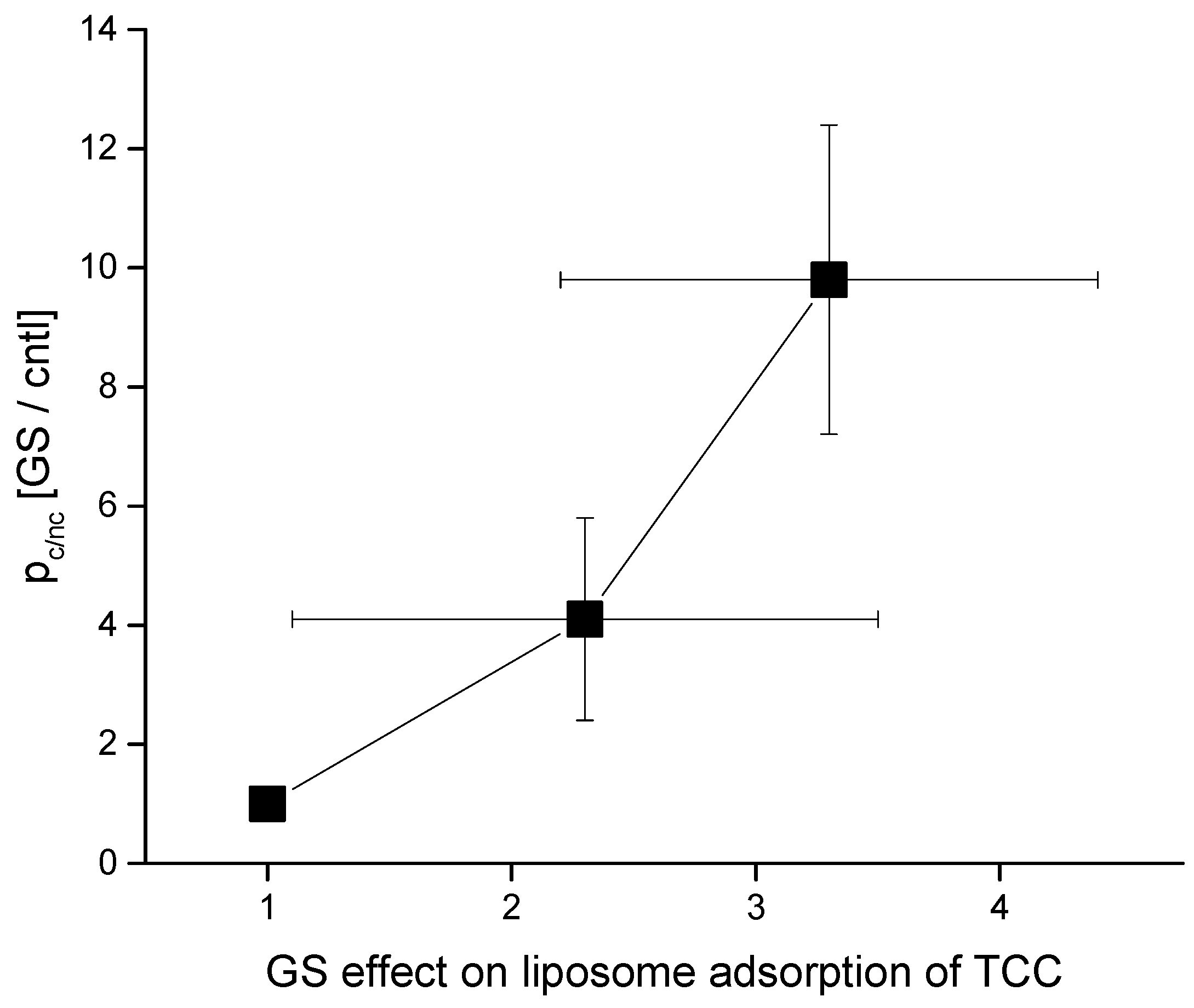
4.2. GS Effects on CD pore Formation and Liposome Adsorption Are Mutually Correlated
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ashrafuzzaman, M.; Duszyk, M.; Tuszynski, J.A. Chemotherapy Drugs Thiocolchicoside and Taxol Permeabilize Lipid Bilayer Membranes by Forming Ion Pores. J. Physics Conf. Ser. 2011, 329, 012029. [Google Scholar] [CrossRef]
- Ashrafuzzaman, M.; Tseng, C.-Y.; Duszyk, M.; Tuszynski, J.A. Chemotherapy Drugs Form Ion Pores in Membranes Due to Physical Interactions with Lipids. Chem. Biol. Drug Des. 2012, 80, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- Ludtke, S.J.; He, K.; Heller, W.T.; Harroun, T.A.; Yang, A.L.; Huang, H.W. Membrane Pores Induced by Magainin†. Biochemistry 1996, 35, 13723–13728. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Murase, O.; Fujii, N.; Miyajima, K. An Antimicrobial Peptide, Magainin 2, Induced Rapid Flip-Flop of Phospholipids Coupled with Pore Formation and Peptide Translocation†. Biochemistry 1996, 35, 11361–11368. [Google Scholar] [CrossRef]
- Boheim, G. Statistical analysis of alamethicin channels in black lipid membranes. J. Membr. Biol. 1974, 19, 277–303. [Google Scholar] [CrossRef] [PubMed]
- Andersen, O. Ion movement through gramicidin A channels. Studies on the diffusion-controlled association step. Biophys. J. 1983, 41, 147–165. [Google Scholar] [CrossRef]
- Andersen, O.S. Gramicidin Channels. Annu. Rev. Physiol. 1984, 46, 531–548. [Google Scholar] [CrossRef]
- He, K.; Ludtke, S.; Worcester, D.; Huang, H. Neutron Scattering in the Plane of Membranes: Structure of Alamethicin Pores. Biophys. J. 1996, 70, 2659–2666. [Google Scholar] [CrossRef]
- Ashrafuzzaman, M.; Andersen, O.S.; McElhaney, R.N. The antimicrobial peptide gramicidin S permeabilizes phospholipid bilayer membranes without forming discrete ion channels. Biochim. et Biophys. Acta (BBA) Biomembr. 2008, 1778, 2814–2822. [Google Scholar] [CrossRef] [PubMed]
- Seideman, P.; Fjellner, B.; Johannesson, A. Psoriatic arthritis treated with oral colchicine. J. Rheumatol. 1987, 14, 777–779. [Google Scholar]
- Callen, J.P. Colchicine is effective in controlling chronic cutaneous leukocytoclastic vasculitis. J. Am. Acad. Dermatol. 1985, 13, 193–200. [Google Scholar] [CrossRef]
- Rosenman, S.J.; Ganji, A.A.; Gallatin, W.M. Contact dependent redistribution of cell surface adhesion and activation molecules reorganization. FASEB J. 1991, 5, 1603. [Google Scholar]
- Rosenman, S.J.; Ganji, A.A.; Tedder, T.F.; Gallatin, W.M. Syn- capping of human T lymphocyte adhesion/activation molecules and their redistribution during interaction with endothelial cells. J. Leukoc. Biol. 1993, 53, 1–10. [Google Scholar] [CrossRef]
- Mekori, Y.A.; Baram, D.; Goldberg, A.; Klajman, A. Inhibition of delayed hypersensitivity reactions in mice by colchicine. Cell. Immunol. 1989, 120, 330–340. [Google Scholar] [CrossRef]
- Borisy, G.G.; Taylor, E.W. The mechanism of action of colchicine. J. Cell Biol. 1967, 34, 525–533. [Google Scholar] [CrossRef]
- Matsumoto, G.; Sakai, H. Microtubules inside the plasma membrane of squid giant axons and their possible physiological function. J. Membr. Biol. 1979, 50, 1–14. [Google Scholar] [CrossRef]
- Haga, T.; Kurokawa, M. Microtubule formation from two components separated by gel filtration of a tubulin preparation. Biochim. et Biophys. Acta (BBA) Gen. Subj. 1975, 392, 335–345. [Google Scholar] [CrossRef]
- Agutter, P.S.; Suckling, K.E. Effect of colchicine on mammalian liver nuclear envelope and on nucleo-cytoplasmic RNA transport. Biochim. et Biophys. Acta (BBA) Gene Struct. Expr. 1982, 698, 223–229. [Google Scholar] [CrossRef]
- Izumiya, N.; Kato, T.; Aoyagi, H.; Waki, M.; Kondo, M. Synthetic Aspects of Biologically Active Cyclic Peptides—Gramicidin S and Tyrocidines; Waring, M.J., Ed.; John Wiley (Halsted): New York, NY, USA; Kodansha: Tokyo, Japan, 1980; Volume 21, pp. 591–592. [Google Scholar] [CrossRef]
- Waki, M.; Izumiya, N. Recent advances in the biotechnology of β-lactams and microbial bioactive peptides. In Biochemistry of Peptide Antibiotics; Walter de Gruyter: Berlin, Germany, 1990; pp. 205–240. [Google Scholar]
- Jelokhani-Niaraki, M.; Hodges, R.S.; Meissner, J.E.; Hassenstein, U.E.; Wheaton, L. Interaction of Gramicidin S and its Aromatic Amino-Acid Analog with Phospholipid Membranes. Biophys. J. 2008, 95, 3306–3321. [Google Scholar] [CrossRef] [PubMed]
- Hall, R.; Collis, C. Origins and evolution of antibiotic and multiple antibiotic resistance in bacteria. In Development of Novel Antimicrobial Agents: Emerging Strategies; Horizon Scientific Press: Wymondham, UK, 2001. [Google Scholar]
- Grant, T.; Lehrer, R. Antimicrobial peptides in innate immunity. In Development of Novel Antimicrobial Agents: Emerging Strategies; Horizon Scientific Press: Wymondham, UK, 2001. [Google Scholar]
- Prenner, E.J.; Lewis, R.N.; McElhaney, R.N. The interaction of the antimicrobial peptide gramicidin S with lipid bilayer model and biological membranes. Biochim. et Biophys. Acta (BBA) Biomembr. 1999, 1462, 201–221. [Google Scholar] [CrossRef]
- Prenner, E.J.; Lewis, R.N.; Kondejewski, L.H.; Hodges, R.S.; McElhaney, R.N. Differential scanning calorimetric study of the effect of the antimicrobial peptide gramicidin S on the thermotropic phase behavior of phosphatidylcholine, phosphatidylethanolamine and phosphatidylglycerol lipid bilayer membranes. Biochim. et Biophys. Acta (BBA) Biomembr. 1999, 1417, 211–223. [Google Scholar] [CrossRef]
- Prenner, E.; Lewis, R.; Mcelhaney, R. Biophysical studies of the interaction of the antimicrobial peptide gramicidin S with lipid bilayer model and biological membranes. Phys. Can. 2004, 60, 121–129. [Google Scholar]
- Krivanek, R.; Rybar, P.; Prenner, E.J.; McElhaney, R.N.; Hianik, T. Interaction of the antimicrobial peptide gramicidin S with dimyristoyl–phosphatidylcholine bilayer membranes: A densitometry and sound velocimetry study. Biochim. et Biophys. Acta (BBA) Biomembr. 2001, 1510, 452–463. [Google Scholar] [CrossRef]
- Prenner, E.J.; Lewis, R.N.A.H.; Neuman, K.C.; Gruner, S.M.; Kondejewski, L.H.; Hodges, R.S.; McElhaney, R.N. Nonlamellar Phases Induced by the Interaction of Gramicidin S with Lipid Bilayers. A Possible Relationship to Membrane-Disrupting Activity†. Biochemistry 1997, 36, 7906–7916. [Google Scholar] [CrossRef] [PubMed]
- Staudegger, E.; Prenner, E.J.; Kriechbaum, M.; Degovics, G.; Lewis, R.N.; McElhaney, R.N.; Lohner, K. X-ray studies on the interaction of the antimicrobial peptide gramicidin S with microbial lipid extracts: Evidence for cubic phase formation. Biochim. et Biophys. Acta (BBA) Biomembr. 2000, 1468, 213–230. [Google Scholar] [CrossRef]
- Lewis, R.N.A.H.; Prenner, E.J.; Kondejewski, L.H.; Flach, C.R.; Mendelsohn, R.; Hodges, R.S.; McElhaney, R.N. Fourier transform infrared spectroscopic studies of the interaction of the antimicrobial peptide gramicidin S with lipid micelles and with lipid monolayer and bilayer membranes. Biochemistry 1999, 38, 15193–15203. [Google Scholar] [CrossRef]
- Wu, M.; Maier, E.; Benz, A.R.; Hancock, R.E.W. Mechanism of Interaction of Different Classes of Cationic Antimicrobial Peptides with Planar Bilayers and with the Cytoplasmic Membrane of Escherichia coli†. Biochemistry 1999, 38, 7235–7242. [Google Scholar] [CrossRef]
- Ashrafuzzaman, M.; McElhamney, R.; Andersen, O. One antimicrobial peptide (gramicidin S) can affect the function of another (gramicidin A or alamethicin) via effects on the phospholipid bilayer. Biophys. J. 2008, 94, 21. [Google Scholar] [CrossRef]
- Lundbæk, J.A.; Birn, P.; Tape, S.E.; Toombes, G.E.S.; Søgaard, R.; Koeppe, R.E.; Gruner, S.M.; Hansen, A.J.; Andersen, O.S. Capsaicin Regulates Voltage-Dependent Sodium Channels by Altering Lipid Bilayer Elasticity. Mol. Pharmacol. 2005, 68, 680–689. [Google Scholar] [CrossRef]
- Lundbaek, J.A.; Koeppe, R.E.; Andersen, O.S. Amphiphile regulation of ion channel function by changes in the bilayer spring constant. Proc. Natl. Acad. Sci. USA 2010, 107, 15427–15430. [Google Scholar] [CrossRef]
- Ashrafuzzaman, A.; Lampson, M.; Greathouse, D.V.; Koeppe, R.E.; Andersen, O.S. Manipulating lipid bilayer material properties using biologically active amphipathic molecules. J. Physics Condens. Matter 2006, 18, S1235–S1255. [Google Scholar] [CrossRef]
- Greisen, P.; Lum, K.; Ashrafuzzaman, M.; Greathouse, D.V.; Andersen, O.S.; Lundbaek, J.A.; Lundbæk, J.A. Linear rate-equilibrium relations arising from ion channel-bilayer energetic coupling. Proc. Natl. Acad. Sci. USA 2011, 108, 12717–12722. [Google Scholar] [CrossRef]
- Sun, D.; Peyear, T.A.; Bennett, W.F.D.; Holcomb, M.; He, S.; Zhu, F.; Lightstone, F.C.; Andersen, O.S.; Ingólfsson, H.I. Assessing the Perturbing Effects of Drugs on Lipid Bilayers Using Gramicidin Channel-Based In Silico and In Vitro Assays. J. Med. Chem. 2020, 63, 11809–11818. [Google Scholar] [CrossRef]
- Ashrafuzzaman, M.; Koeppe, R., II; Andersen, O. Lipid bilayer elasticity and intrinsic curvature as regulators of channel function: A single molecule study. Biophys. J. BPS Annu. Meet. Abstr. 2007, 421A. [Google Scholar]
- Ashrafuzzaman, M.; Tseng, C. US9529006B1—Method for Direct Detection of Lipid Binding Agents in Membrane. 2016. Available online: https://patents.google.com/patent/US9529006B1/en (accessed on 27 December 2016).
- Ashrafuzzaman, M.; Tseng, C.-Y.; Tuszynski, J. Charge-based interactions of antimicrobial peptides and general drugs with lipid bilayers. J. Mol. Graph. Model. 2020, 95, 107502. [Google Scholar] [CrossRef] [PubMed]
- Muvaffak, A.; Gürhan, I.; Hasirci, N. Prolonged cytotoxic effect of colchicine released from biodegradable microspheres. J. Biomed. Mater. Res. 2004, 71, 295–304. [Google Scholar] [CrossRef]
- Lin, Z.-Y.; Kuo, C.-H.; Wu, D.-C.; Chuang, W.-L. Anticancer effects of clinically acceptable colchicine concentrations on human gastric cancer cell lines. Kaohsiung J. Med Sci. 2016, 32, 68–73. [Google Scholar] [CrossRef]
- Persi, E.; Duran-Frigola, M.; Damaghi, M.; Roush, W.R.; Aloy, P.; Cleveland, J.L.; Gillies, R.J.; Ruppin, E. Systems analysis of intracellular pH vulnerabilities for cancer therapy. Nat. Commun. 2018, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Webb, B.A.; Chimenti, M.S.; Jacobson, M.P.; Barber, D.L. Dysregulated pH: A perfect storm for cancer progression. Nat. Rev. Cancer 2011, 11, 671–677. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashrafuzzaman, M. The Antimicrobial Peptide Gramicidin S Enhances Membrane Adsorption and Ion Pore Formation Potency of Chemotherapy Drugs in Lipid Bilayers. Membranes 2021, 11, 247. https://doi.org/10.3390/membranes11040247
Ashrafuzzaman M. The Antimicrobial Peptide Gramicidin S Enhances Membrane Adsorption and Ion Pore Formation Potency of Chemotherapy Drugs in Lipid Bilayers. Membranes. 2021; 11(4):247. https://doi.org/10.3390/membranes11040247
Chicago/Turabian StyleAshrafuzzaman, Md. 2021. "The Antimicrobial Peptide Gramicidin S Enhances Membrane Adsorption and Ion Pore Formation Potency of Chemotherapy Drugs in Lipid Bilayers" Membranes 11, no. 4: 247. https://doi.org/10.3390/membranes11040247
APA StyleAshrafuzzaman, M. (2021). The Antimicrobial Peptide Gramicidin S Enhances Membrane Adsorption and Ion Pore Formation Potency of Chemotherapy Drugs in Lipid Bilayers. Membranes, 11(4), 247. https://doi.org/10.3390/membranes11040247





