Oxytocin Downregulates the CaV1.2 L-Type Ca2+ Channel via Gi/cAMP/PKA/CREB Signaling Pathway in Cardiomyocytes
Abstract
1. Introduction
2. Results
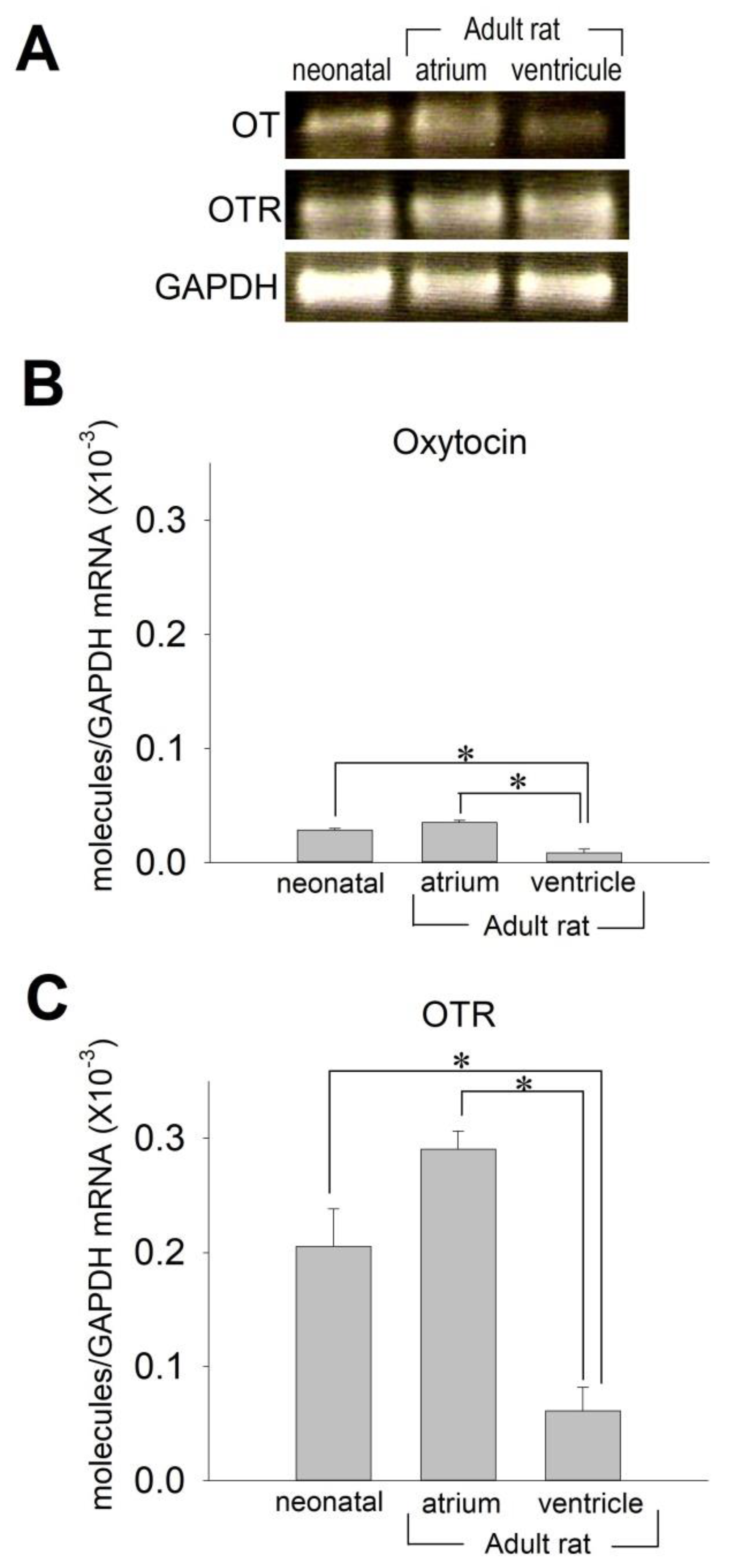
2.1. OT and OTR in Cardiomyocytes
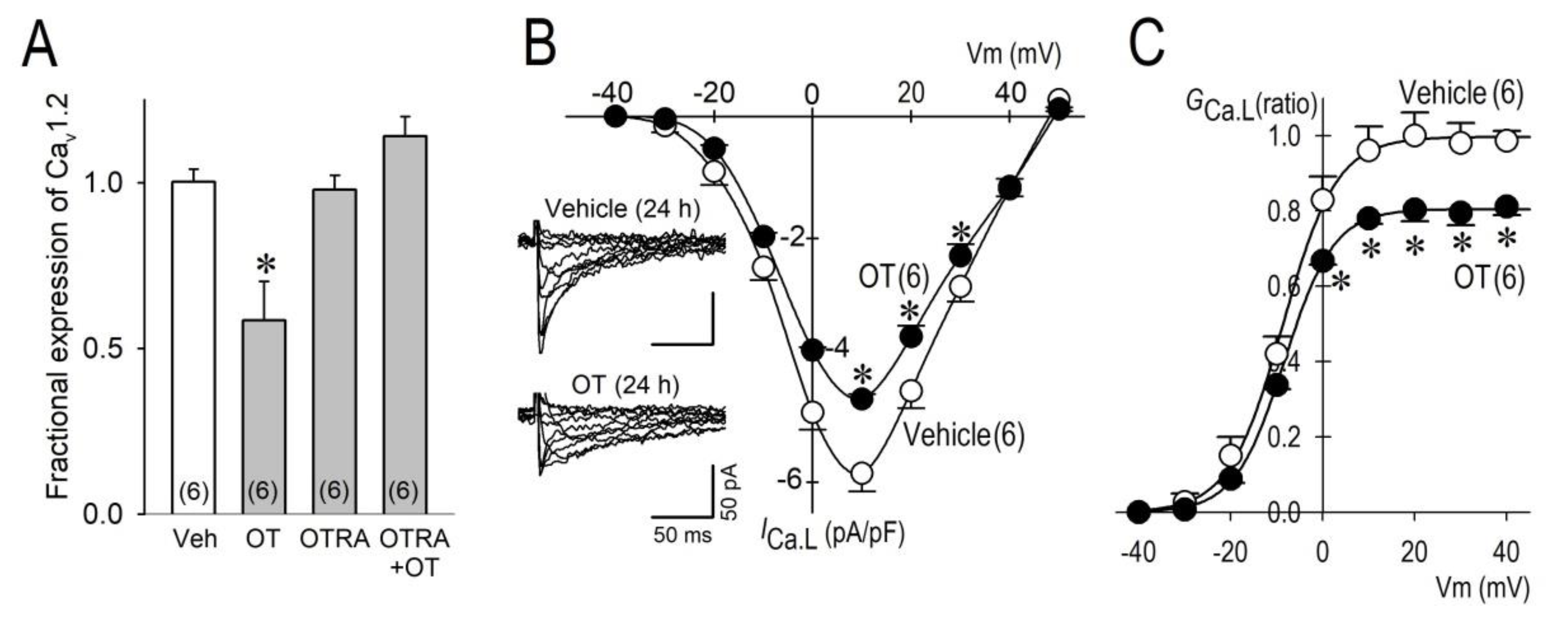
2.2. Long-Term Effects of OT on ICa.L
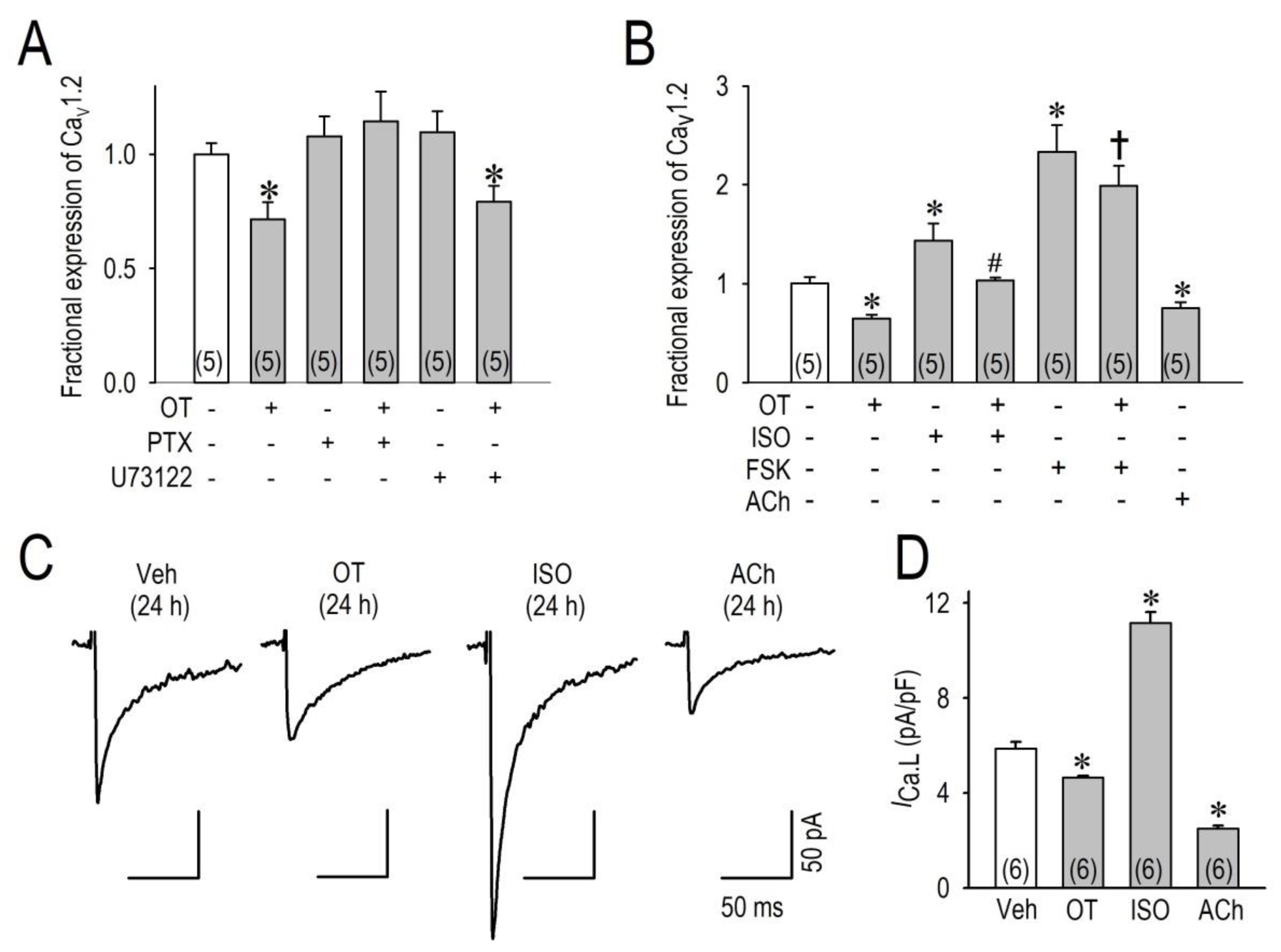
2.3. OT Downregulates Cav1.2 mRNA and ICa.L via the Gi Protein
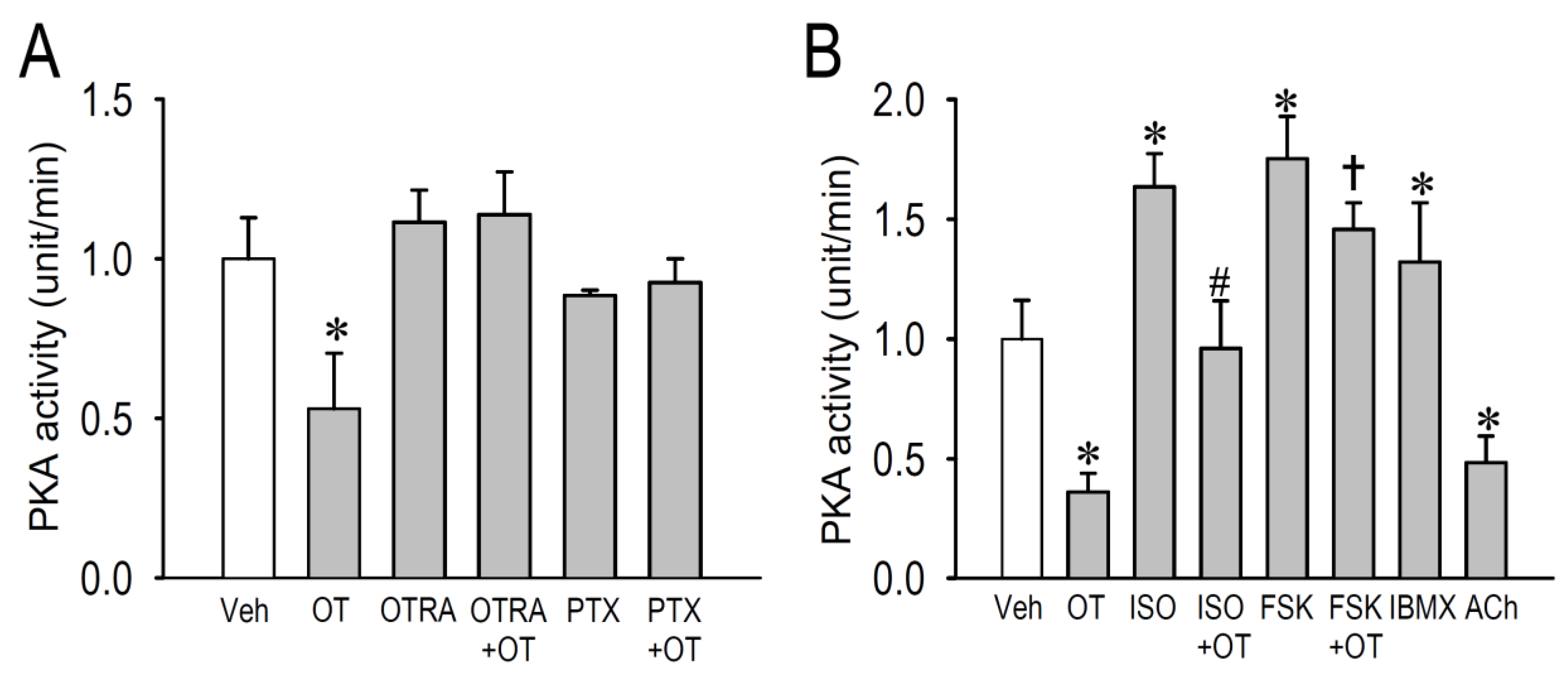
2.4. Effects of OT on Protein Kinase Activity
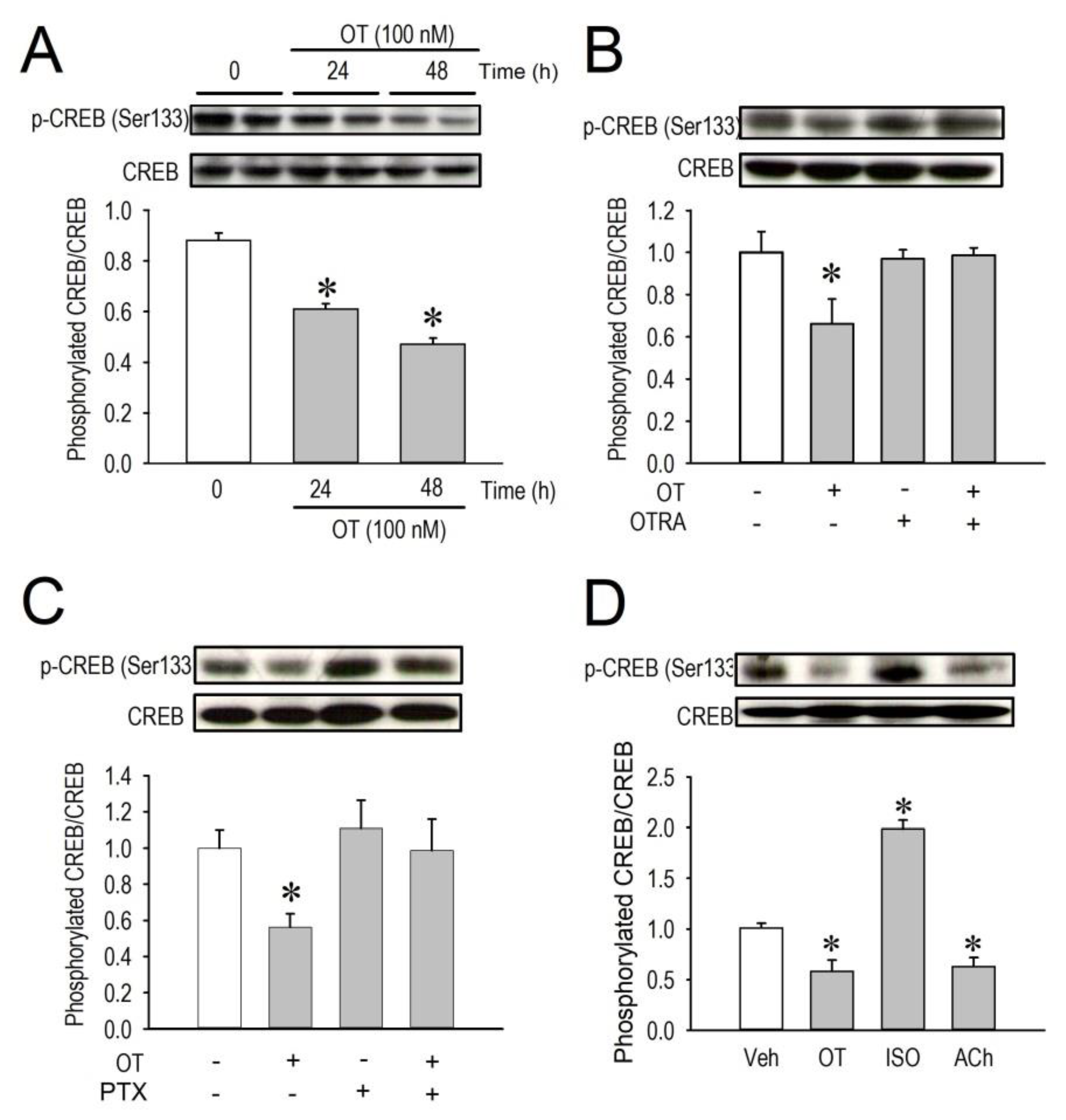
2.5. Effects of OT on CREB Phosphorylation
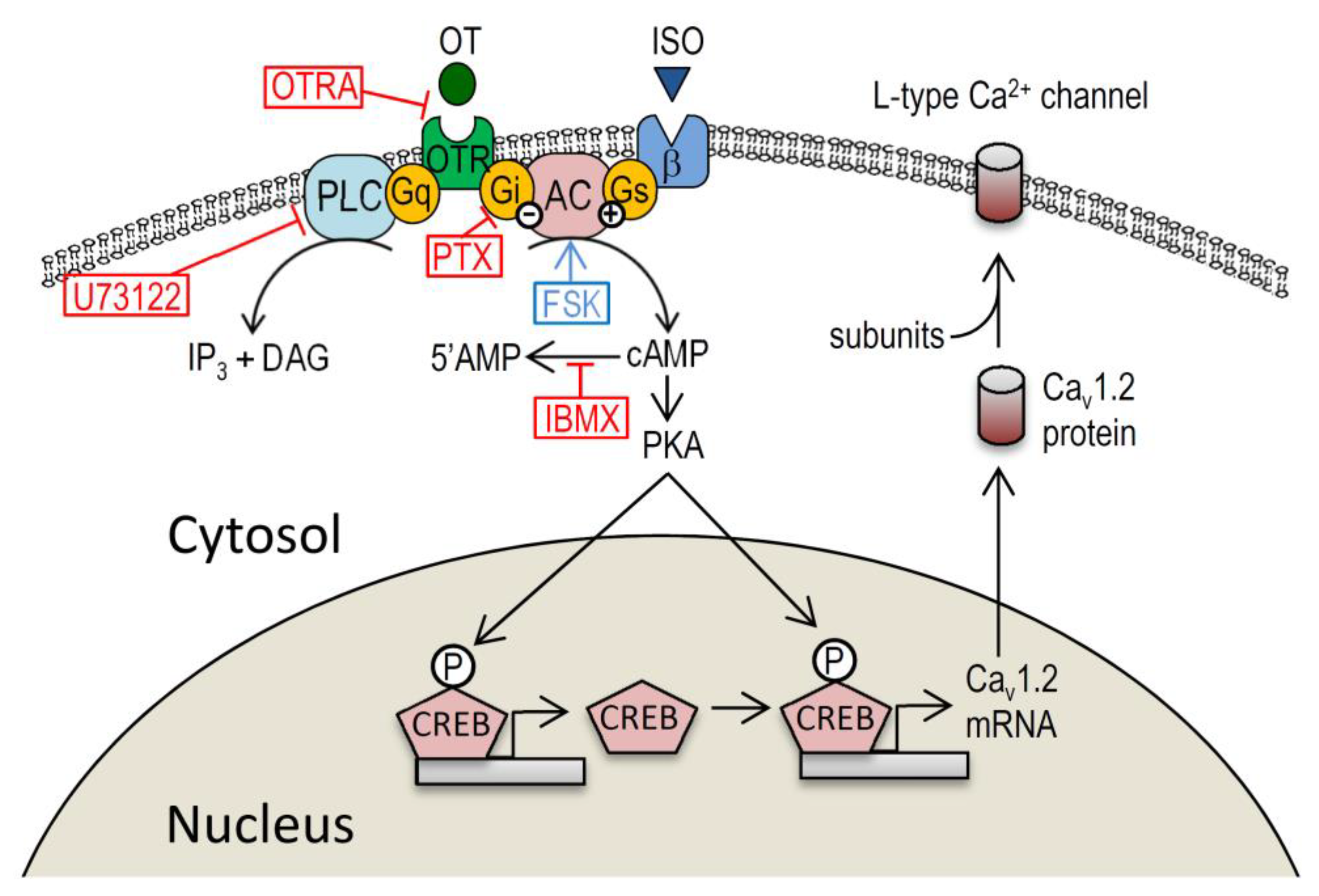
3. Discussion
3.1. Myocardial Ca2+ Homeostasis Modulated by OT
3.2. Action of OT on the Cardiovascular System though Gi-Dependent Pathway
3.3. Study Limitation
3.4. Conclusions
4. Materials and Methods
4.1. Animals and Surgery
4.2. Chemicals
4.3. Inhibitor Experiments
4.4. Electrophysiological Measurements
4.5. Quantitative Real-Time PCR
4.6. Western Blot Analysis
4.7. Protein Kinase A (PKA) Assay
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OT | oxytocin |
| OTR | oxytocin receptor |
| OTRA | oxytocin receptor antagonist |
| cAMP | adenosine-3′,5′-cyclic monophosphate |
| CREB | cAMP response element binding protein |
| PKA | protein kinase A |
| PKC | protein kinase C |
| Gi | inhibitory GTP-binding regulators of adenylate cyclase |
| PLC | phospholipase C |
| DAG | diacylglycerol |
| IP3 | inositol 1,4,5-trisphosphate |
| ICa.L | L-type Ca2+ channel current |
| NO | nitric oxide |
| ANP | atrial natriuretic peptide |
References
- Gutkowska, J.; Jankowski, M.; Lambert, C.; Mukaddam-Daher, S.; Zingg, H.H.; McCann, S.M. Oxytocin releases atrial natriuretic peptide by combining with oxytocin receptors in the heart. Proc. Natl. Acad. Sci. USA 1997, 94, 11704–11709. [Google Scholar] [CrossRef]
- Jankowski, M.; Hajjar, F.; Kawas, S.A.; Mukaddam-Daher, S.; Hoffman, G.; McCann, S.M.; Gutkowska, J. Rat heart: A site of oxytocin production and action. Proc. Natl. Acad. Sci. USA 1998, 95, 14558–14563. [Google Scholar] [CrossRef]
- Petersson, M. Cardiovascular effects of oxytocin. Prog. Brain Res. 2002, 139, 281–288. [Google Scholar] [PubMed]
- Jankowski, M.; Bissonauth, V.; Gao, L. Anti-inflammatory effect of oxytocin in rat myocardial infraction. Basic. Res. Cardiol. 2010, 105, 205–218. [Google Scholar] [CrossRef]
- Gukowska, J.; Jankowski, M. Oxytocin revisited: Its role in cardiovascular regulation. J. Neuroendocrinol. 2012, 24, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Mukaddam-Daher, M.; Yin, Y.L.; Roy, J.; Gutkowska, J.; Cardinal, R. Negative inotropic and chronotropic effects of oxytocin. Hypertension 2001, 38, 292–296. [Google Scholar] [CrossRef]
- Gassanov, N.; Devost, D.; Danalache, B.; Noiseux, N.; Jankowski, M.; Zingg, H.H.; Gutkowska, J. Functional activity of the carboxyl-terminally extended oxytocin precursor peptide during cardiac differentiation of embryonic stem cells. Stem. Cells 2008, 26, 45–54. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.; Pfeffer, M.A. Heart failure. Lancet 2005, 365, 1877–1889. [Google Scholar] [CrossRef]
- Lohse, M.J.; Engelhardt, S.; Eschenhagen, T. What is the role of beta-adrenergic signaling in heart failure? Circ. Res. 2003, 93, 896–906. [Google Scholar] [CrossRef]
- Reuter, H. Localization of beta adrenergic receptors, and effects of noradrenaline and cyclic nucleotides on action potentials, ionic currents and tension in mammalian cardiac muscle. J. Physiol. 1974, 242, 429–451. [Google Scholar] [CrossRef]
- Morishima, M.; Tahara, S.; Wang, Y.; Kaku, T.; Ono, K. Nonapeptide hormones oxytocin and vasopression distinctly regulate Cav1.2-L-type calcium channel expression in cardiomyocytes. J. Arrhyth. 2010, 26, 111–118. [Google Scholar] [CrossRef]
- Zhou, X.B.; Lutz, S.; Steffens, F.; Korth, M.; Wieland, T. Oxytocin receptors differentially signal via Gq and Gi proteins in pregnant and nonpregnant rat uterine myocytes: Implications for myometrial contractility. Mol. Endocrinol. 2007, 21, 740–752. [Google Scholar] [CrossRef]
- Jankowski, M.; Wang, D.; Hajjar, F.; Mukaddam-Daher, S.; McCann, S.M.; Gutkowska, J. Oxytocin and its receptors are synthesized in the rat vasculature. Proc. Natl. Acad. Sci. USA 2000, 97, 6207–6211. [Google Scholar] [CrossRef]
- Stoop, R. Neuromodulation by oxytocin and vasopressin. Neuron 2012, 76, 142–159. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, M.; Broderick, T.L.; Gutkowska, J. The Role of oxytocin in cardiovascular protection. Front. Psychol. 2020, 11, 2139. [Google Scholar] [CrossRef] [PubMed]
- Jurek, B.; Slattery, D.A.; Hiraoka, Y.; Liu, Y.; Nishimori, K.; Aguilera, G.; Neumann, I.D.; van den Burg, E.H. Oxytocin regulates stress-induced Crf gene transcription through CREB-regulated transcription coactivator 3. J. Neurosci. 2015, 35, 12248–12260. [Google Scholar] [CrossRef]
- Schulte, J.S.; Seidl, M.D.; Nunes, F.; Freese, C.; Schneider, M.; Schmitz, W.; Müller, F.U. CREB critically regulates action potential shape and duration in the adult mouse ventricle. Am. J. Physiol. Heart. Circ. Physiol. 2012, 302, H1998–H2007. [Google Scholar] [CrossRef]
- Mayr, B.; Montminy, M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat. Rev. Mol. Cell. Biol. 2001, 8, 599–609. [Google Scholar] [CrossRef]
- Tsai, C.T.; Wang, D.L.; Chen, W.P.; Hwang, J.J.; Hsieh, C.S.; Hsu, K.L.; Tseng, C.D.; Lai, L.P.; Tseng, Y.Z.; Chiang, F.T.; et al. Angiotensin II increases expression of α1c subunit of L-type calcium channel through a reactive oxygen species and cAMP response element-binding protein-dependent pathway in HL-1 myocytes. Circ. Res. 2007, 100, 1476–1485. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Mao, X.; Xu, G.; Xing, S.; Chattopadhyay, A.; Jin, S.; Salama, G. Estradiol up-regulates L-type Ca(2+) channels via membrane-bound estrogen receptor/phosphoinositide-3-kinase/Akt/cAMP response element-binding protein signaling pathway. Heart Rhythm. 2018, 15, 741–749. [Google Scholar] [CrossRef]
- Kumaresan, P.; Anandarangam, P.B.; Dianzon, W.; Vasicka, A. Plasma oxytocin levels during human pregnancy and labor as determined by radioimmunoassay. Am., J. Obstet. Gynecol. 1974, 119, 215–223. [Google Scholar] [CrossRef]
- Vargas-Martínez, F.; Schanler, R.J.; Abrams, S.A.; Hawthorne, K.M.; Landers, S.; Guzman-Bárcenas, J.; Muñoz, O.; Henriksen, T.; Petersson, M.; Uvnäs-Moberg, K.; et al. Oxytocin, a main breastfeeding hormone, prevents hypertension acquired in utero: A therapeutics preview. Biochim. Biophys. Acta. Gen. Subj. 2017, 1861, 3071–3084. [Google Scholar] [CrossRef]
- Morishima, M.; Iwata, E.; Nakada, C.; Tsukamoto, Y.; Takanari, H.; Miyamoto, S.; Moriyama, M.; Ono, K. Atrial Fibrillation-Mediated Upregulation of miR-30d Regulates Myocardial Electrical Remodeling of the G-Protein-Gated K+ Channel, IK.Ach. Circ. J. 2016, 80, 1346–1355. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Yang, M.; Sanborn, B.M. Extracellular signal-regulated kinase 1/2 activation by myometrial oxytocin receptor involves Gαq/Gβγ and epidermal growth factor receptor tyrosine kinase activation. Endocrinology 2003, 144, 2947–2956. [Google Scholar] [CrossRef]
- Wang, Y.; Morishima, M.; Zheng, M.Q.; Uchino, T.; Mannen, K.; Takahashi, A.; Nakaya, Y.; Komuro, I.; Ono, K. Transcription factors Csx/Nkx2.5 and GATA4 distinctly regulate expression of Ca2+ channels in neonatal rat heart. J. Mol. Cell. Cardiol. 2007, 42, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morishima, M.; Tahara, S.; Wang, Y.; Ono, K. Oxytocin Downregulates the CaV1.2 L-Type Ca2+ Channel via Gi/cAMP/PKA/CREB Signaling Pathway in Cardiomyocytes. Membranes 2021, 11, 234. https://doi.org/10.3390/membranes11040234
Morishima M, Tahara S, Wang Y, Ono K. Oxytocin Downregulates the CaV1.2 L-Type Ca2+ Channel via Gi/cAMP/PKA/CREB Signaling Pathway in Cardiomyocytes. Membranes. 2021; 11(4):234. https://doi.org/10.3390/membranes11040234
Chicago/Turabian StyleMorishima, Masaki, Shintaro Tahara, Yan Wang, and Katsushige Ono. 2021. "Oxytocin Downregulates the CaV1.2 L-Type Ca2+ Channel via Gi/cAMP/PKA/CREB Signaling Pathway in Cardiomyocytes" Membranes 11, no. 4: 234. https://doi.org/10.3390/membranes11040234
APA StyleMorishima, M., Tahara, S., Wang, Y., & Ono, K. (2021). Oxytocin Downregulates the CaV1.2 L-Type Ca2+ Channel via Gi/cAMP/PKA/CREB Signaling Pathway in Cardiomyocytes. Membranes, 11(4), 234. https://doi.org/10.3390/membranes11040234



