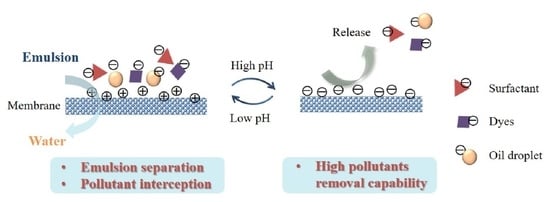
pH-Sensitive Membranes with Smart Cleaning Capability for Efficient Emulsion Separation and Pollutant Removal
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. The Preparation of PP-g-pDMAEMA Membrane
2.3. Oil/Water Separation Experiment
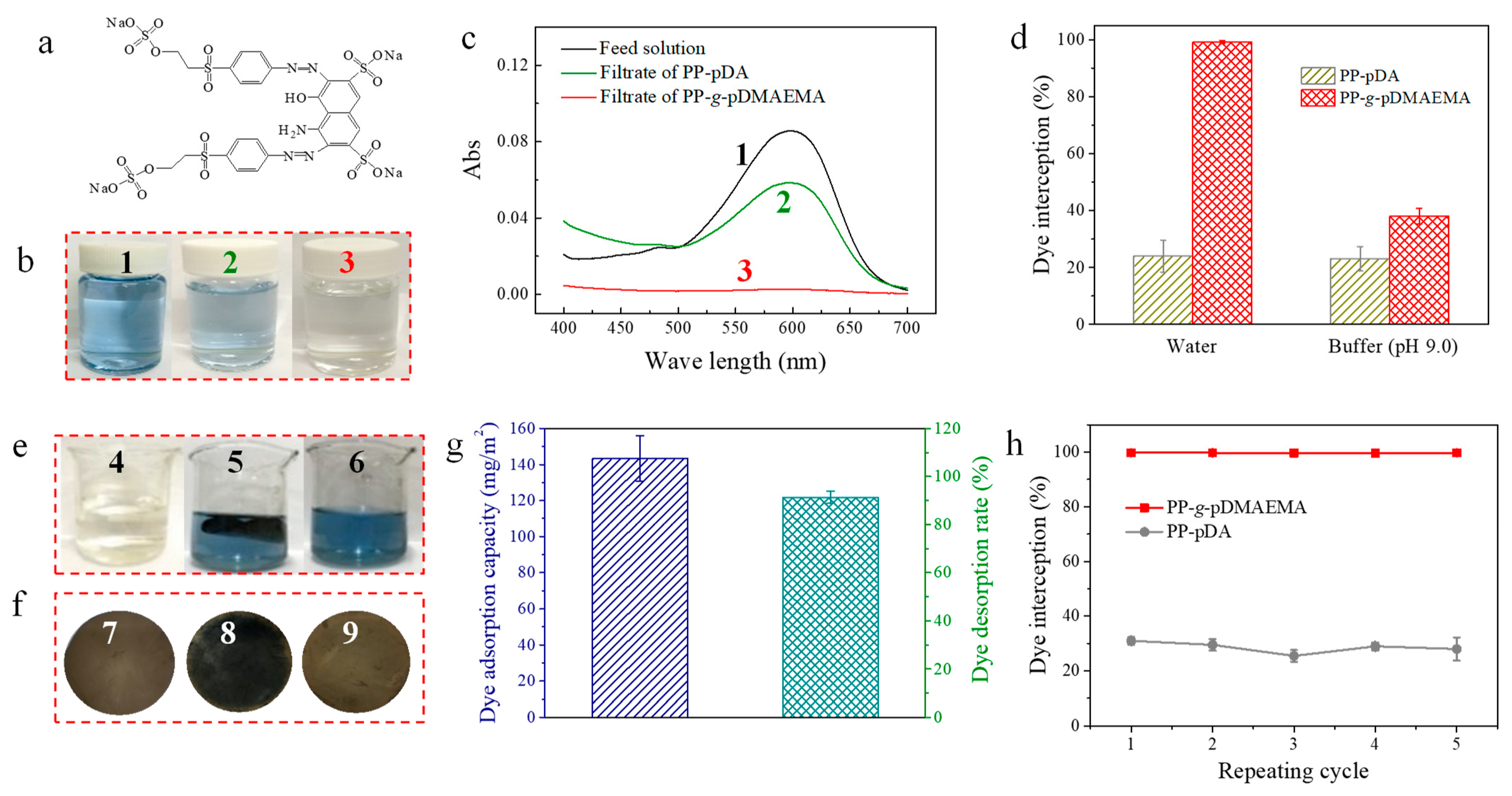
2.4. The Adsorption and Desorption of Dyes and Surfactants
2.5. Instruments and Characterization
3. Results and Discussion
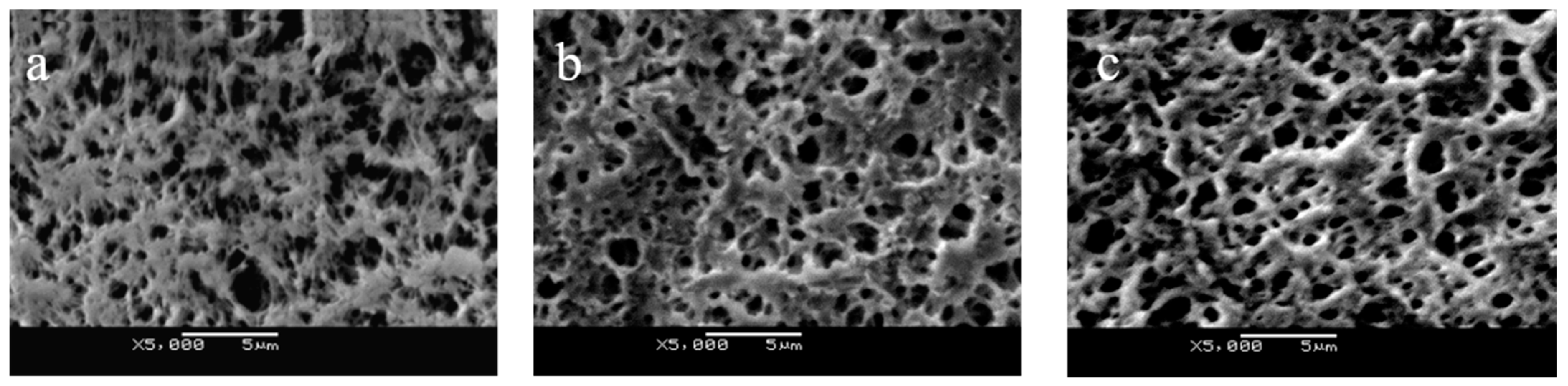
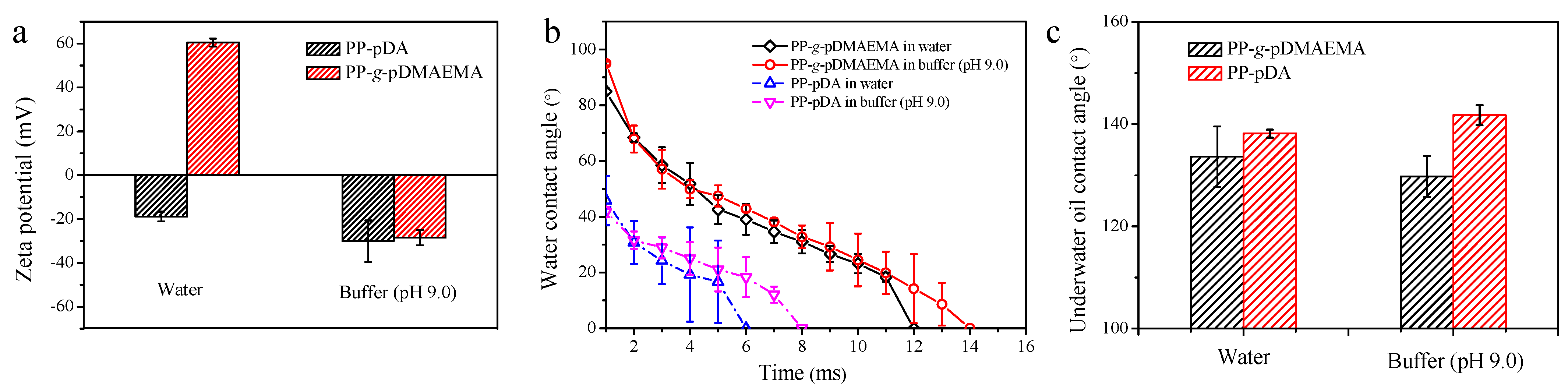
3.1. Surface Characterization of PP-g-pDMAEMA Membrane
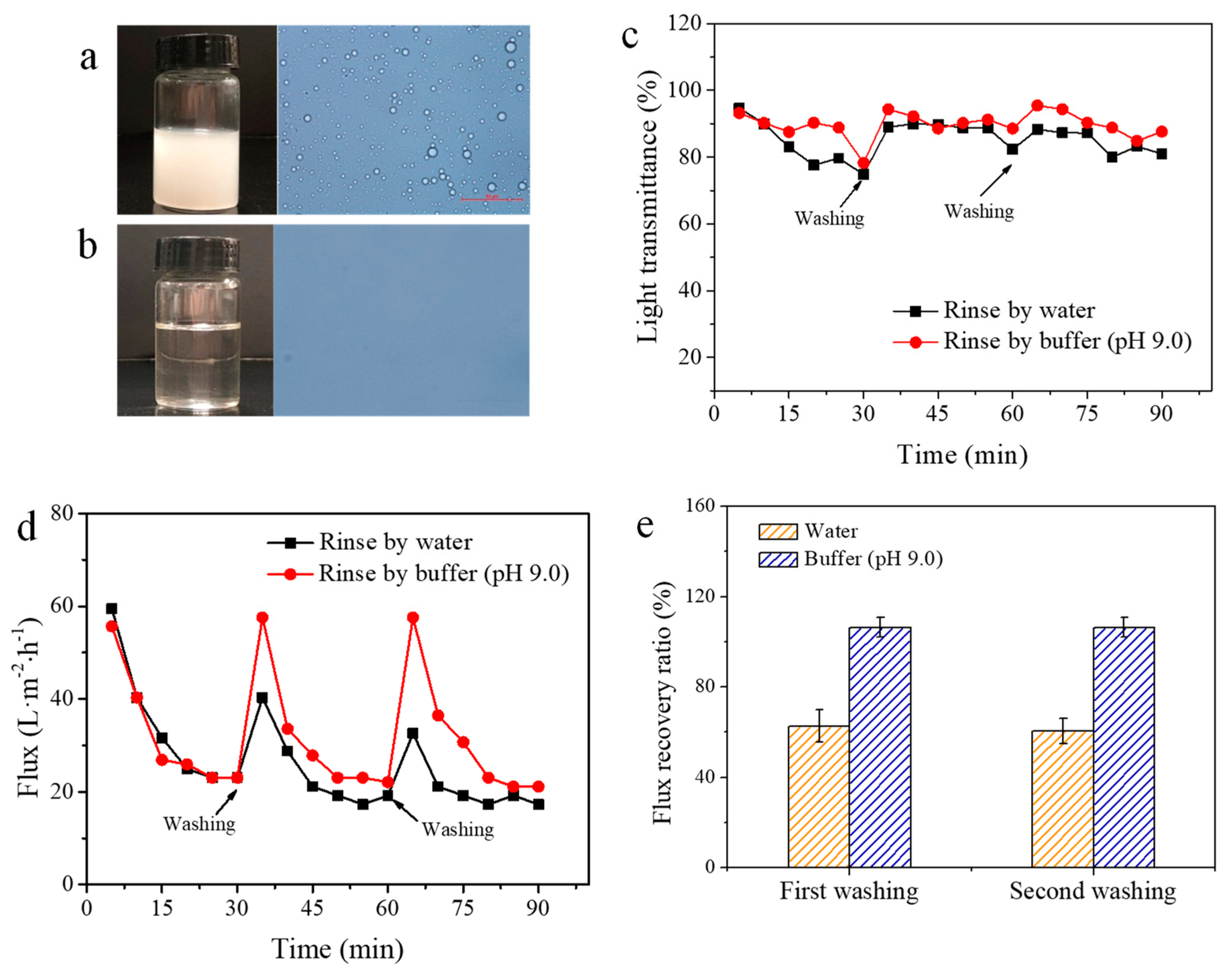
3.2. Adsorption and Desorption of Reactive Dyes on the PP-g-pDMAEMA Membrane
3.3. Adsorption and Desorption of Surfactants
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmed, F.E.; Lalia, B.S.; Hilal, N.; Hashaikeh, R. Underwater superoleophobic cellulose/electrospun PVDF–HFP membranes for efficient oil/water separation. Desalination 2014, 344, 48–54. [Google Scholar] [CrossRef]
- Chu, Z.; Feng, Y.; Seeger, S. Oil/Water Separation with Selective Superantiwetting/Superwetting Surface Materials. Angew. Chem. Int. Ed. 2015, 54, 2328–2338. [Google Scholar] [CrossRef] [PubMed]
- Kota, A.K.; Kwon, G.; Choi, W.; Mabry, J.M.; Tuteja, A. Hygro-responsive membranes for effective oil–water separation. Nat. Commun. 2012, 3, 1025. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, Y.; Liu, X.; Wang, D.; Li, J.; Jiang, L.; Jin, J. Salt-Induced Fabrication of Superhydrophilic and Underwater Superoleophobic PAA-g-PVDF Membranes for Effective Separation of Oil-in-Water Emulsions. Angew. Chem. Int. Ed. 2014, 53, 856–860. [Google Scholar] [CrossRef]
- Zhang, W.B.; Shi, Z.; Zhang, F.; Liu, X.; Jin, J.; Jiang, L. Superhydrophobic and Superoleophilic PVDF Membranes for Effec-tive Separation of Water-in-Oil Emulsions with High Flux. Adv. Mater. 2013, 25, 2071–2076. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.L.; Bai, R. Membrane fouling and cleaning in microfiltration of activated sludge wastewater. J. Membr. Sci. 2003, 216, 279–290. [Google Scholar] [CrossRef]
- Zhao, Q.; Hou, J.; Shen, J.; Liu, J.; Zhang, Y. Long-lasting antibacterial behavior of a novel mixed matrix water purification membrane. J. Mater. Chem. A 2015, 3, 18696–18705. [Google Scholar] [CrossRef]
- Liu, J.; Wang, L.; Wang, N.; Guo, F.; Hou, L.; Chen, Y.; Liu, J.; Zhao, Y.; Jiang, L. A Robust Cu(OH)2 Nanoneedles Mesh with Tunable Wettability for Nonaqueous Multiphase Liquid Separation. Small 2017, 13, 1600499. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Zhao, X.; Wang, W.; Gong, X. Durable Self-Cleaning Surfaces with Superhydrophobic and Highly Oleophobic Properties. Langmuir 2019, 35, 8404–8412. [Google Scholar] [CrossRef]
- Wang, Y.; Gong, X. Superhydrophobic Coatings with Periodic Ring Structured Patterns for Self-Cleaning and Oil-Water Separation. Adv. Mater. Interfaces 2017, 4, 1700190. [Google Scholar] [CrossRef]
- Otanicar, T.; Patel, J.; Sarica, E. Mechanical milling of thermoresponsive poly(N-isopropylacrylamide) hydrogel for parti-cle-oriented oil–water separation. J. Appl. Polym. Sci. 2020, 137, 48771. [Google Scholar] [CrossRef]
- Wang, D.-C.; Yang, X.; Yu, H.-Y.; Gu, J.; Qi, D.; Yao, J.; Ni, Q. Smart nonwoven fabric with reversibly dual-stimuli responsive wettability for intelligent oil-water separation and pollutants removal. J. Hazard. Mater. 2020, 383, 121123. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.T.; Liu, Y.L. pH-Induced switches of the oil- and water-selectivity of crosslinked polymeric membranes for gravi-ty-driven oil–water separation. J. Mater. Chem. A 2016, 4, 13543–13548. [Google Scholar] [CrossRef]
- Lü, T.; Qi, D.; Zhang, D.; Fu, K.; Li, Y.; Zhao, H. Fabrication of recyclable multi-responsive magnetic nanoparticles for emulsified oil-water separation. J. Clean. Prod. 2020, 255, 120293. [Google Scholar] [CrossRef]
- Li, R.Q.; Zhang, G.L.; Wang, J.F.; Li, J.Q.; Zhang, C.H.; Wang, P.L. Superwetting pH-Responsive Polyaniline Coatings: To-ward Versatile Separation of Complex Oil–Water Mixtures. Langmuir 2020, 36, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, A.W.; Cox, H.J.; Barrientos-Palomo, S.N.; Sharples, G.J.; Badyal, J.P.S. Bioinspired multifunctional polymer–nanoparticle–surfactant complex nanocomposite surfaces for antibacterial oil–water separation. Colloids Surf. A 2019, 560, 352–359. [Google Scholar] [CrossRef]
- Wu, J.D.; Wei, W.; Li, S.H.; Zhong, Q.; Liu, F.; Zheng, J.H.; Wang, J.P. The effect of membrane surface charges on demulsifi-cation and fouling resistance during emulsion separation. J. Membr. Sci. 2018, 563, 126–133. [Google Scholar] [CrossRef]
- Wu, J.S.; Liu, C.H.; Chu, K.H.; Suen, S.Y. Removal of cationic dye methyl violet 2B from water by cation exchange mem-branes. J. Membr. Sci. 2008, 309, 239–245. [Google Scholar] [CrossRef]
- Ma, Q.L.; Cheng, H.F.; Fane, A.G.; Wang, R.; Zhang, H. Recent Development of Advanced Materials with Special Wettability for Selective Oil/Water Separation. Small 2016, 12, 2186–2202. [Google Scholar] [CrossRef]
- Zhu, Y.Z.; Wang, D.; Jiang, L.; Jin, J. Recent progress in developing advanced membranes for emulsified oil/water separa-tion. NPG Asia Mater. 2014, 6, e101. [Google Scholar] [CrossRef]
- Ponnamma, D.; Nair, S.S.; Parangusan, H.; Hassan, M.K.; Adham, S.; Karim, A.; Al-Maadeed, M.A.A. White Graphene-Cobalt Oxide Hybrid Filler Reinforced Polystyrene Nanofibers for Selective Oil Absorption. Polymers 2019, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Dong, Y.; Liu, Y.; Meng, X. Modification of Polyurethane Sponge Based on the Thiol–Ene Click Reaction and Its Application for Oil/Water Separation. Polymers 2019, 11, 2072. [Google Scholar] [CrossRef] [PubMed]
- Qian, D.L.; Chen, D.G.; Li, N.J.; Xu, Q.F.; Li, H.; He, J.H.; Lu, J.M. TiO2/sulfonated graphene oxide/Ag nanoparticle mem-brane: In situ separation and photodegradation of oil/water emulsions. J. Membr. Sci. 2018, 554, 16–25. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, S.; Zhang, J.; Zhong, W.; Huang, X. Cu2−xSe/CdS composite photocatalyst with enhanced visible light photocatalysis activity. Appl. Surf. Sci. 2019, 478, 762–769. [Google Scholar] [CrossRef]
- Li, F.; Yu, Z.; Shi, H.; Yang, Q.; Chen, Q.; Pan, Y.; Zeng, G.; Yan, L. A Mussel-inspired method to fabricate reduced graphene oxide/g-C 3 N 4 composites membranes for catalytic decomposition and oil-in-water emulsion separation. Chem. Eng. J. 2017, 322, 33–45. [Google Scholar] [CrossRef]
- Zhang, Z.-M.; Gan, Z.-Q.; Bao, R.-Y.; Ke, K.; Liu, Z.-Y.; Yang, M.-B.; Yang, W. Green and robust superhydrophilic electrospun stereocomplex polylactide membranes: Multifunctional oil/water separation and self-cleaning. J. Membr. Sci. 2020, 593, 117420. [Google Scholar] [CrossRef]
- Zhou, C.; Cheng, J.; Hou, K.; Zhu, Z.; Zheng, Y. Preparation of CuWO4@Cu2O film on copper mesh by anodization for oil/water separation and aqueous pollutant degradation. Chem. Eng. J. 2017, 307, 803–811. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, Q.; Gu, H.; He, Z.; Xu, W.; Zhang, J.; Liu, Y.; Xiong, L.; Zheng, L.; Feng, Y. Giant Microgels with CO2-Induced On–Off, Selective, and Recyclable Adsorption for Anionic Dyes. ACS Appl. Mater. Interfaces 2018, 10, 38073–38083. [Google Scholar] [CrossRef]
- Wei, W.; Sun, M.Y.; Zhang, L.; Zhao, S.F.; Wu, J.D.; Wang, J.P. Underwater oleophobic PTFE membrane for efficient and re-usable emulsion separation and the influence of surface wettability and pore size. Sep. Purif. Technol. 2017, 189, 32–39. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Zhang, X.; Wei, W.; Zhang, H.; Wang, Y.; Cai, G.; Wu, J. pH-Sensitive Membranes with Smart Cleaning Capability for Efficient Emulsion Separation and Pollutant Removal. Membranes 2021, 11, 193. https://doi.org/10.3390/membranes11030193
Zhang J, Zhang X, Wei W, Zhang H, Wang Y, Cai G, Wu J. pH-Sensitive Membranes with Smart Cleaning Capability for Efficient Emulsion Separation and Pollutant Removal. Membranes. 2021; 11(3):193. https://doi.org/10.3390/membranes11030193
Chicago/Turabian StyleZhang, Jiaming, Xiansheng Zhang, Wei Wei, Huiling Zhang, Yunfei Wang, Guoqiang Cai, and Jindan Wu. 2021. "pH-Sensitive Membranes with Smart Cleaning Capability for Efficient Emulsion Separation and Pollutant Removal" Membranes 11, no. 3: 193. https://doi.org/10.3390/membranes11030193
APA StyleZhang, J., Zhang, X., Wei, W., Zhang, H., Wang, Y., Cai, G., & Wu, J. (2021). pH-Sensitive Membranes with Smart Cleaning Capability for Efficient Emulsion Separation and Pollutant Removal. Membranes, 11(3), 193. https://doi.org/10.3390/membranes11030193