Pilot Tests on the Treatment of Bath Wastewater by a Membrane Bioreactor
Abstract
1. Introduction
2. Test Device and Method
2.1. Wastewater Source and Water Quality
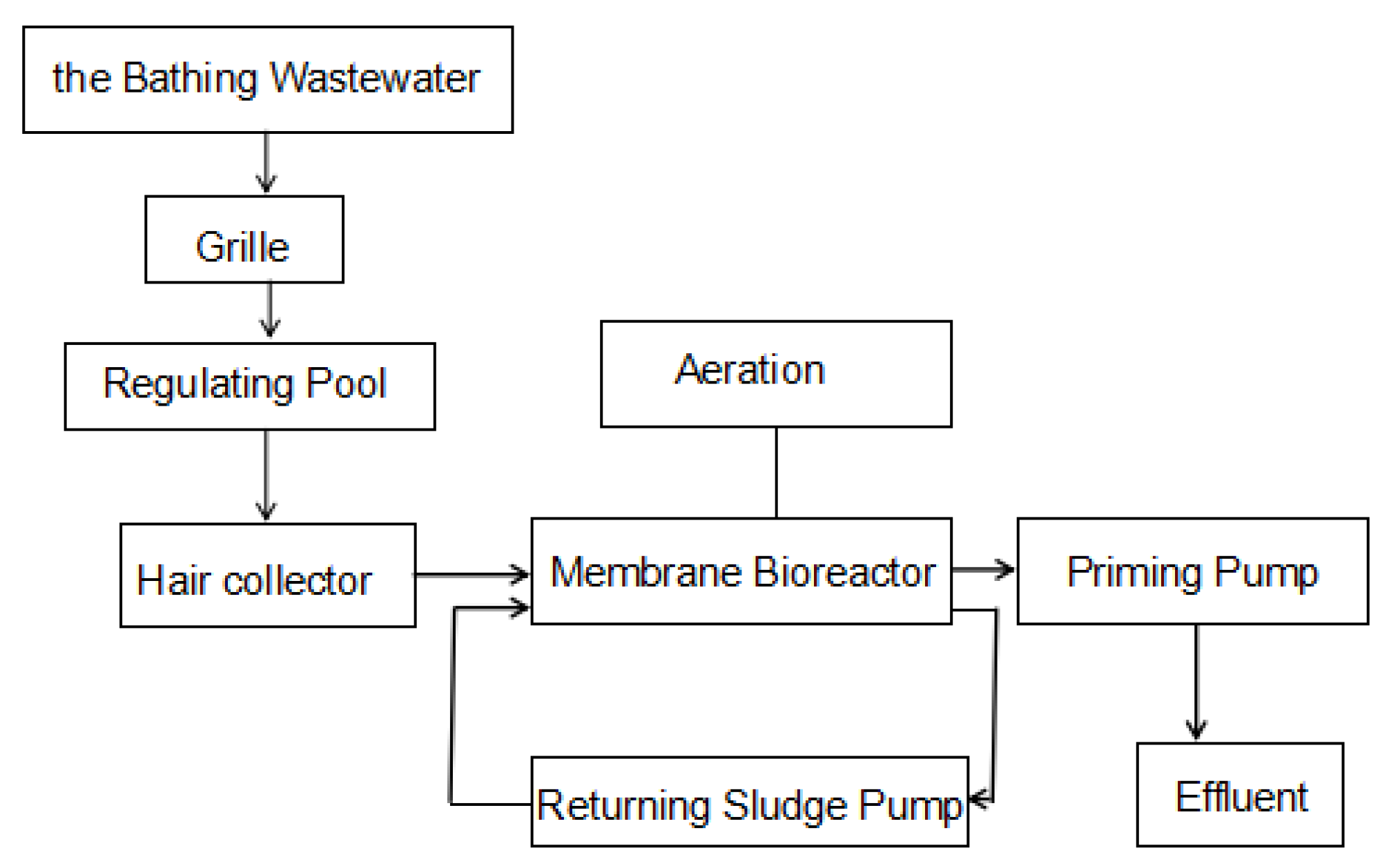
2.2. Testing Device and Procedure
3. Initial Experimental Section
3.1. Process of Inoculation
3.2. Methods of Cultivation for Activated Sludge
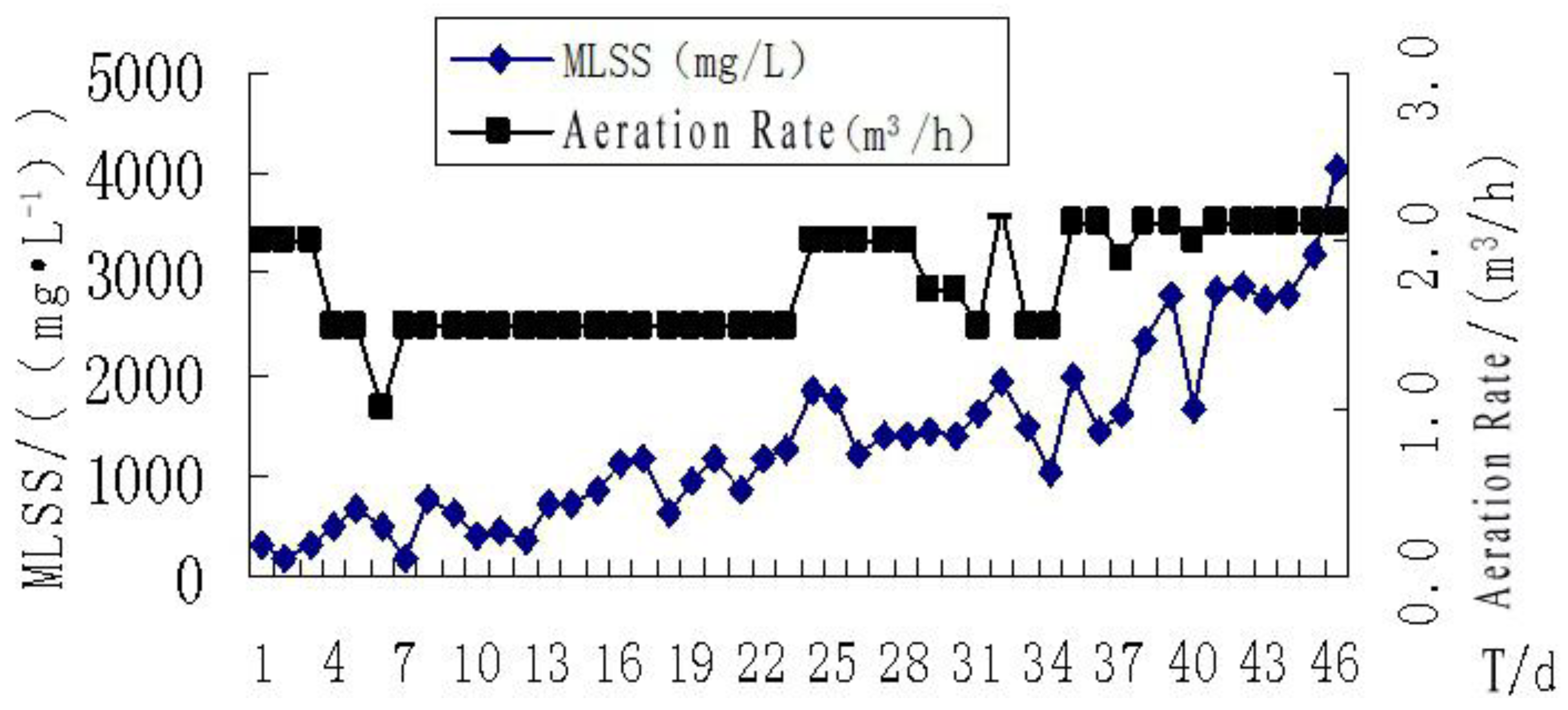
3.3. The Sludge Growth Curve and Results Analysis
4. Stable Experimental Section
4.1. Test and Analysis Methods
4.2. Results and Discussion
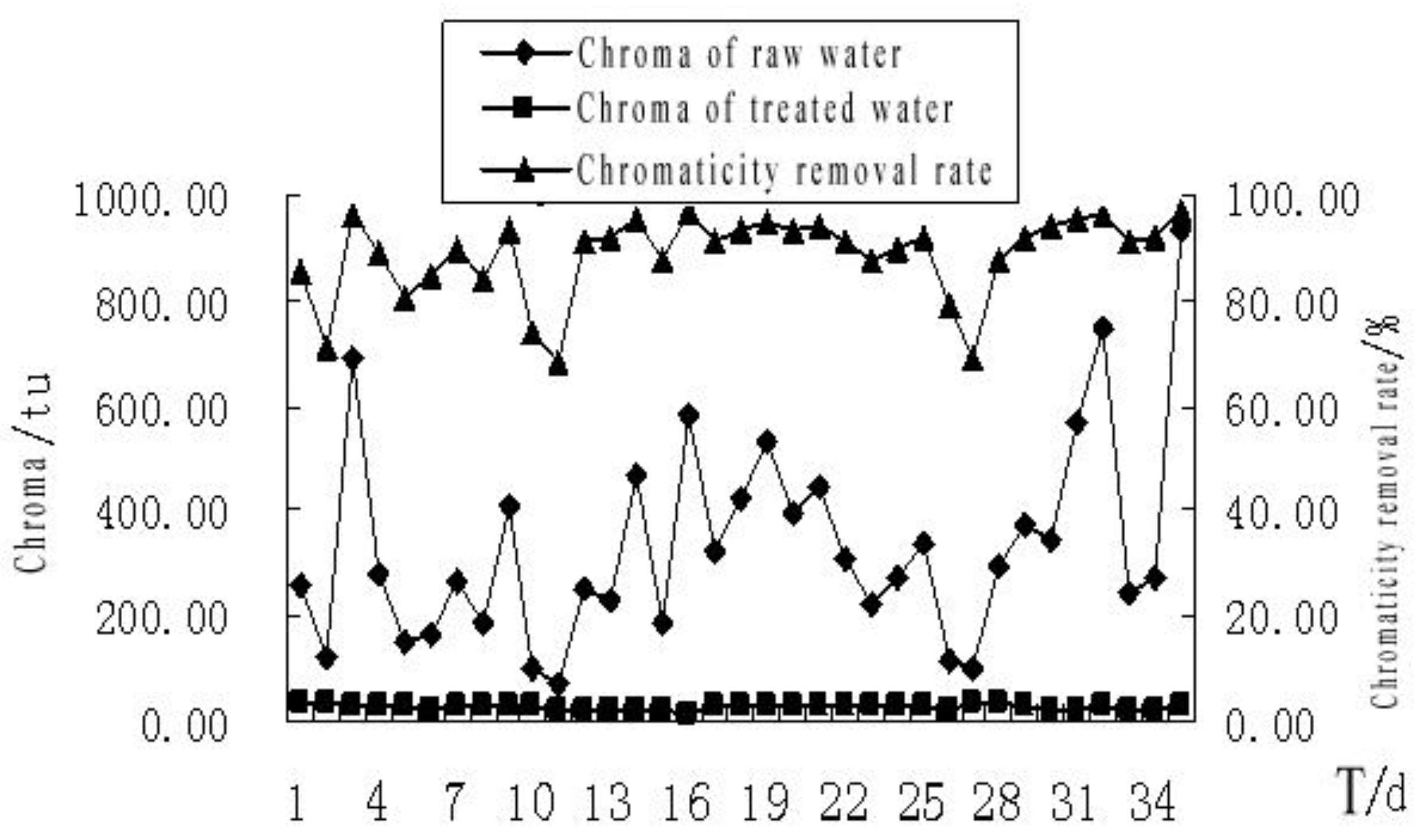
4.2.1. The Effect of MBR Treatment on the Chromaticity of the Bath Wastewater
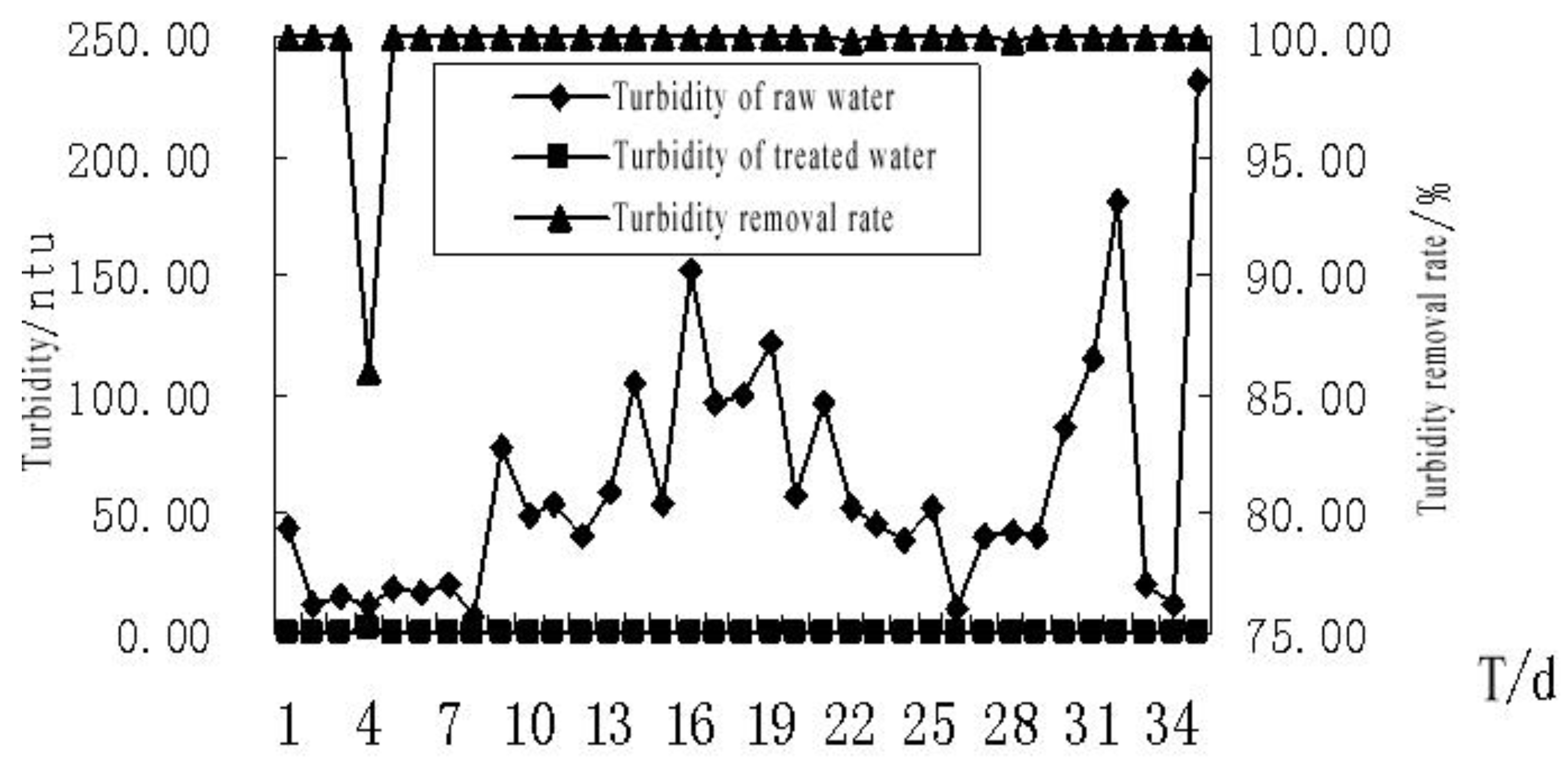
4.2.2. The Effect of MBR Treatment on the Turbidity of the Bath Wastewater
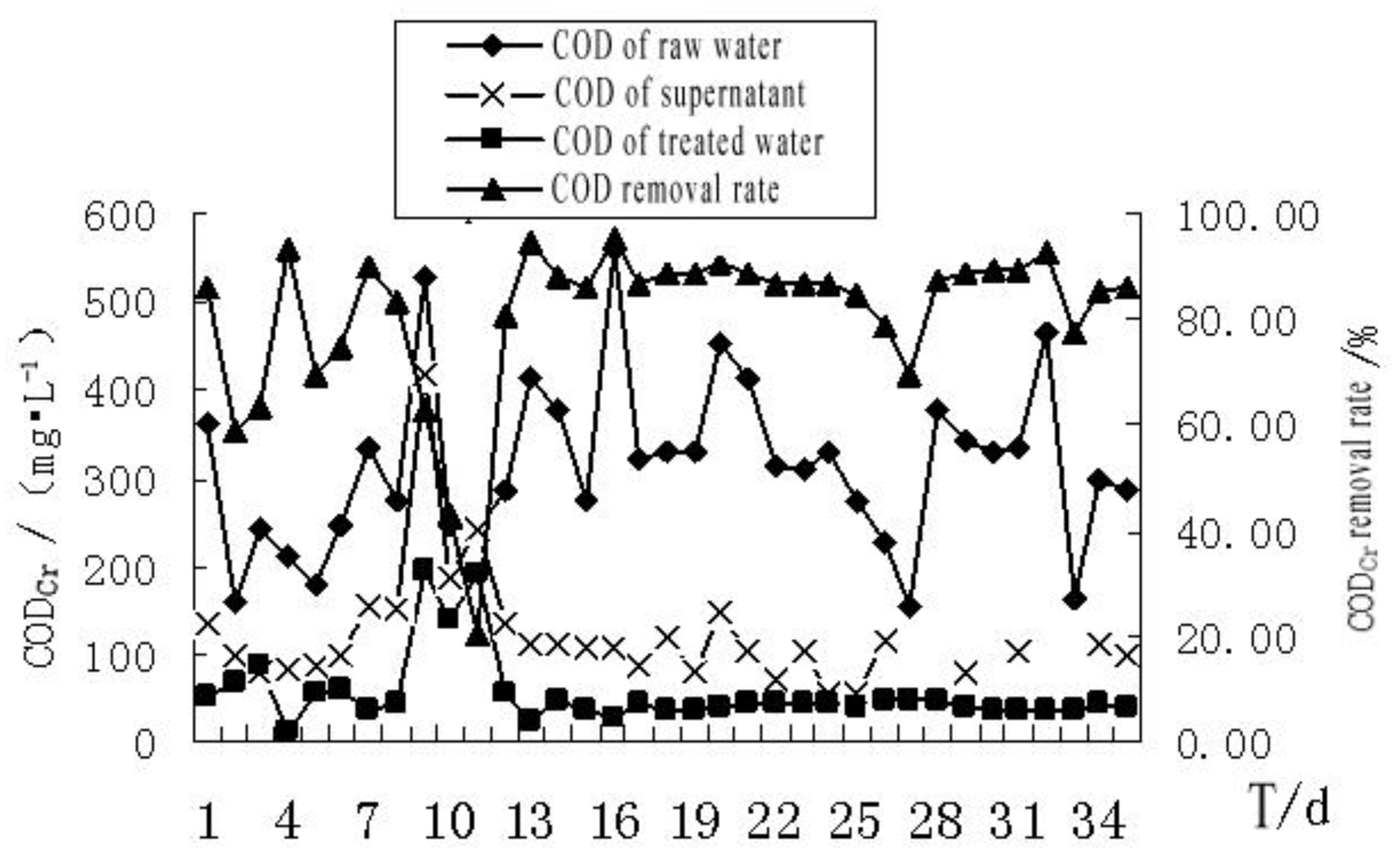
4.2.3. The Effect of MBR Treatment on COD of the Bath Wastewater
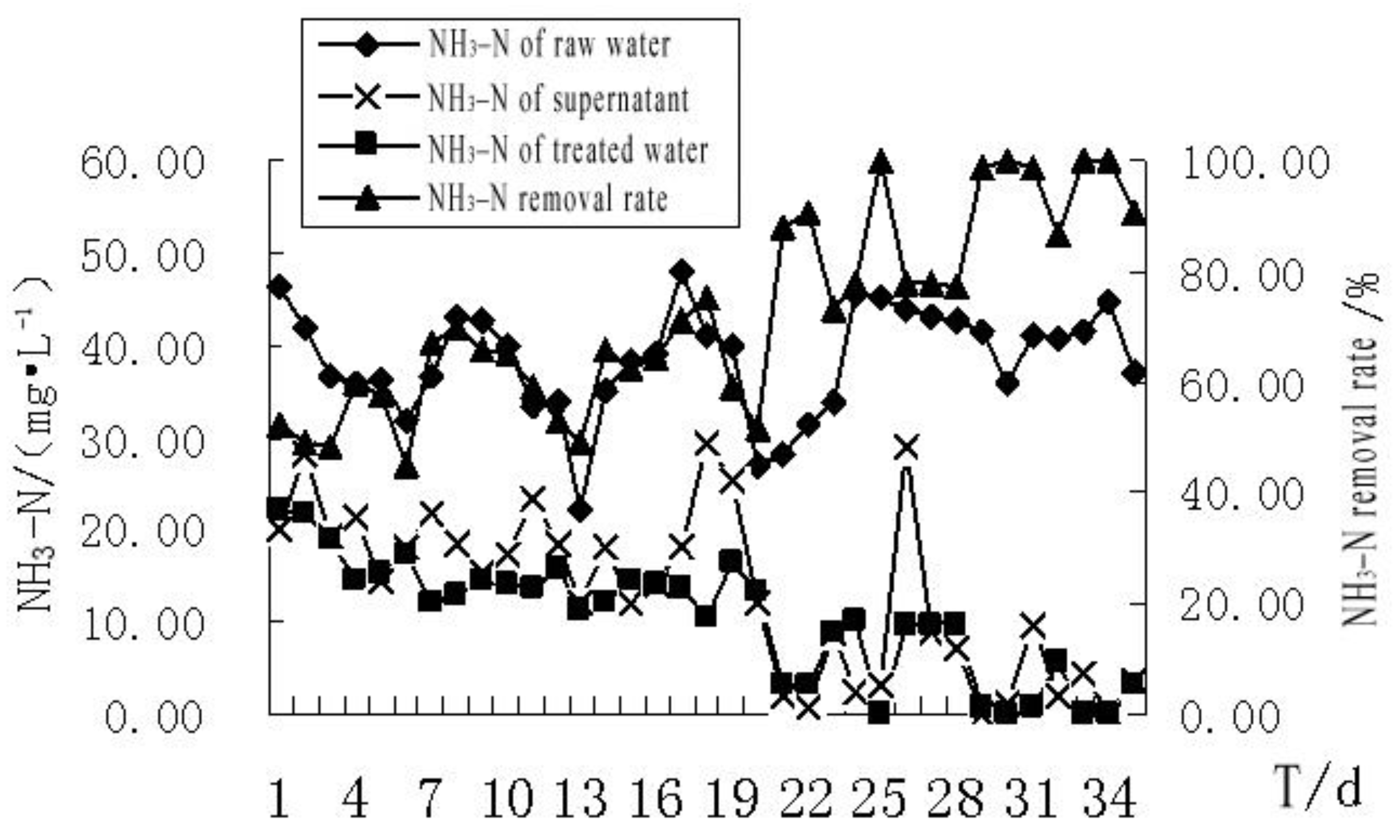
4.2.4. The Effect of MBR Treatment on NH3–N Removal from the Bath Wastewater
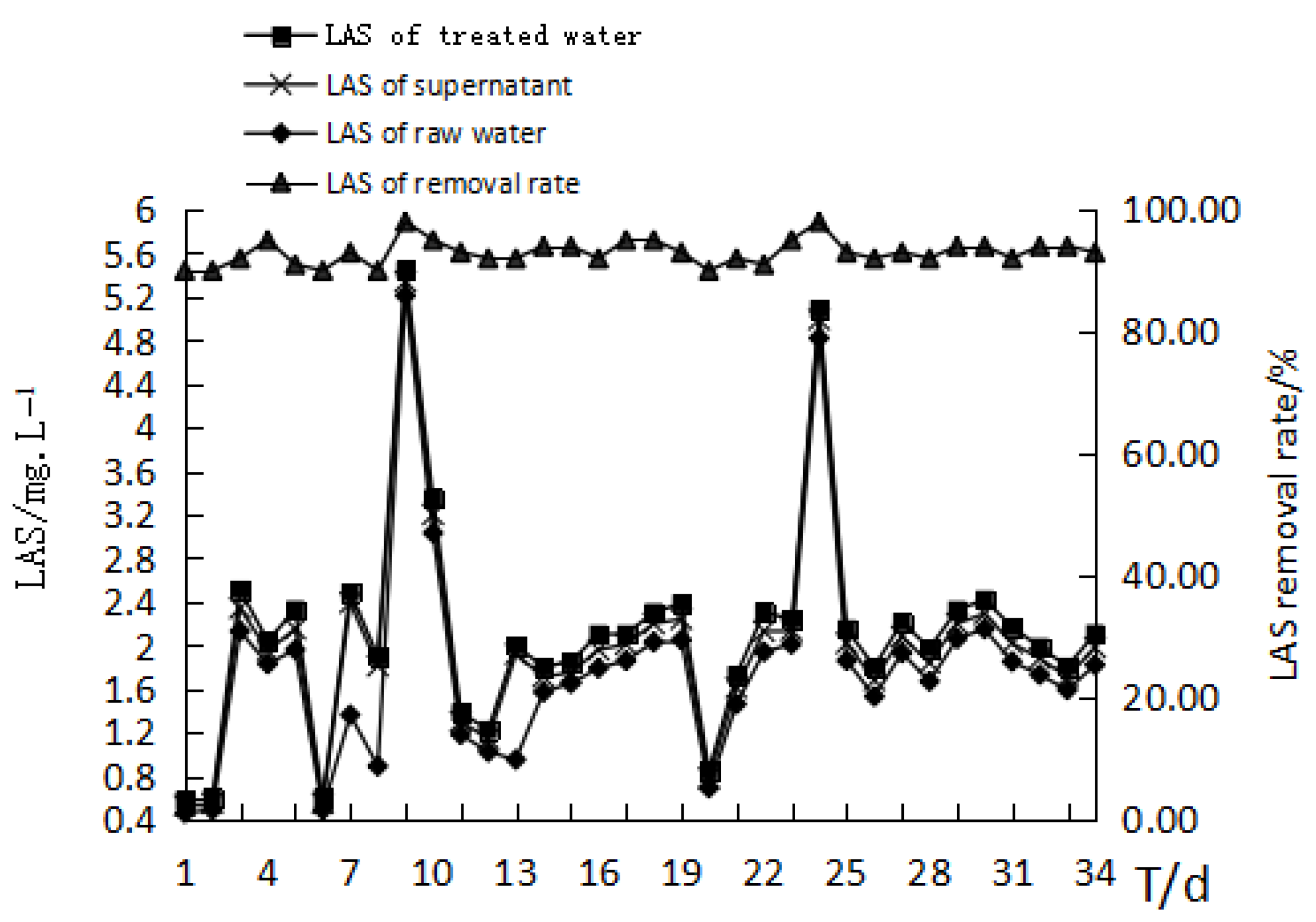
4.2.5. The Effect of MBR Treatment on LAS Removal from the Bath Wastewater
5. Discussion on Costs and Benefits
5.1. Discussion on Costs
- (1)
- Electricity. The power consumption was 40 KW/h-mo, corresponding to 0.55 CNY/(KW-h) or 0.73 CNY/m3.
- (2)
- Labor. The salary at the current rate is 180 CNY/day. As the project was tested in the laboratory, with a scale up to about 200 times the current lab scale, the cost would be about 0.90 CNY/m3.
- (3)
- Reagent cost. The concentration of sodium hypochlorite as an additive was 10 mg/L. With a price of 8 CNY/kg, the reagent cost would be about 0.08 CNY/m3.
- (4)
- Materials depreciation. The lifetime of the membrane could be up to five years, and the cost of the MBR membrane is CNY 600. Thus, the material cost of treating a cubic meter of water and the membrane replacement cost is CNY 0.33. The lifetime of the system equipment is about 10 years. Thus, the production of each cubic meter of water by the depreciation expense is CNY 0.22. The lifetime of the civil system is about 20 years. Thus, the depreciation expense of each cubic meter of water production is CNY 0.16.
- (5)
- The sum of the above costs is 2.42 CNY/m3. If mass produced, the cost will be even lower.
5.2. Comparison of Reuse Costs
6. Conclusions
- (1)
- MBR is effective in treating bath wastewater. The effluent COD, NH3–N and LAS were <50, <10 and <0.12 mg/L, while the turbidity was <0.5 ntu and the chroma average was 26.4 tu. The quality of the effluent water meets the requirements of the urban miscellaneous water standard of China (GB/T 18920-2002) and conforms to the water reuse standard.
- (2)
- Although the COD and NH3–N of the treated water varied to some degree, the effluent was stable, suggesting that the sludge system of the MBR has very good ability to resist the impact of loading variation. The results suggest that MBR may also be used to treat sewage water whose water quality is inferior to the bath wastewater to meet the reuse standard.
- (3)
- Processing bath wastewater in the concentrated water area by MBR could reduce the cost of wastewater treatment operations and save water resources. As such, the economic benefits and social benefits are obvious.
- (4)
- Future studies should be focused on the following aspects. (1) Developing membrane materials with the capacity of high temperature resistance, contamination resistance, acid and alkali resistance properties and lowering the cost. (2) Developing membrane modules that can give full play to the membrane properties and can be developed on a large scale. (3) Determining the mechanism of membrane fouling, and finding the best way of prolonging the service life of the membrane. (4) Elucidating the mechanism of performance, considering the factors which influence the membrane separation process fully and reducing the parameters that need to be experimentally determined in the model. (5) Future development should also be combined with the use of various membrane separation technologies, the combination of membrane separation technology and conventional environmental processing units, higher separation performance and simple operation process systems.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dadmand, F.; Naji-Azimi, Z.; Farimani, N.M.; Davary, K. Sustainable allocation of water resources in water-scarcity conditions using robust fuzzy stochastic programming. J. Clean. Prod. 2020, 276, 123812. [Google Scholar] [CrossRef]
- Li, N.; Wang, H. Analysis on the evaluation of sustainable utilization of water resources by extension evaluation method. Arab. J. Geosci. 2020, 13, 813. [Google Scholar] [CrossRef]
- Blanke, A.; Rozelle, S.; Lohmar, B.; Wang, J.; Huang, J. Water saving technology and saving water in China. Agric. Water Manag. 2007, 87, 139–150. [Google Scholar] [CrossRef]
- Hou, L.; Zhang, L.; Li, G.; He, Q. Sustainable Development Strategy for the Water Resource Economy in Qinba Mountain Area. Strategic Study CAE 2020. [Google Scholar] [CrossRef]
- Eyni, A.; Skardi, M.J.E.; Kerachian, R. A Regret-Based Behavioral Model for Shared Water Resources Management: Application of the Correlated Equilibrium Concept ScienceDirect. Sci. Total Environ. 2020, 759, 143892. [Google Scholar] [CrossRef]
- Naidu, D.S.; Rao, P.J. Study on Sustainable Management of Groundwater Resources in Greater Visakhapatnam Municipal Corporation, Visakhapatnam District, India—A Hydro Informatics Approach. In Proceedings of International Conference on Remote Sensing for Disaster Management; Springer: Cham, Switzerland, 2019; pp. 719–728. [Google Scholar]
- Ma, L. Explore the role of sustainable utilization of water resources and water resources management. IOP Conf. Ser. 2020, 560, 012057. [Google Scholar] [CrossRef]
- Hart, B.; O’Donnell, E.; Horne, A. Sustainable water resources development in northern Australia: The need for coordination, integration and representation. Int. J. Water Resour. Dev. 2020, 36, 777–799. [Google Scholar] [CrossRef]
- Elfithri, R.; Mokhtar, M.B.; Zakaria, S. The need for awareness raising, advocacy, and capacity building in Integrated Water Resources Management toward sustainable development: A case study in Malaysia. World Water Policy 2019, 5, 43–54. [Google Scholar] [CrossRef]
- Dong, W.; Lian, Y.; Zhang, Y. Startup of Formatting Biological Membrane in Denitrifying Filter at Low Temperature. In Sustainable Development of Water Resources and Hydraulic Engineering in China; Springer: Cham, Switzerland, 2019; pp. 275–287. [Google Scholar]
- Jinlin, C. Application of decentralized sewage treatment technology in sewage treatment. Mod. Salt Chem. Ind. 2019. [Google Scholar]
- Fu, J. China’s sewage treatment industry status quo and improvement measures. IOP Conf. Ser. 2020, 514, 032064. [Google Scholar] [CrossRef]
- Zipf, M.S.; Pinheiro, I.G.; Conegero, M.G. Simplified greywater treatment systems: Slow filters of sand and slate waste followed by granular activated carbon. J. Environ. Manag. 2016, 176, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Dalahmeh, S.S.; Lalander, C.; Pell, M.; Vinnerås, B.; Jönsson, H. Quality of greywater treated in biochar filter and risk assessment of gastroenteritis due to household exposure during maintenance and irrigation. J. Appl. Microbiol. 2016, 121, 1427–1443. [Google Scholar] [CrossRef] [PubMed]
- Tsoumachidou, S.; Velegraki, T.; Antoniadis, A.; Poulios, I. Greywater as a sustainable water source: A photocatalytic treatment technology under artificial and solar illumination. J. Environ. Manag. 2016, 195, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Ayekoe, C.Y.P.; Robert, D.; Lancine, D.G. Combination of coagulation-flocculation and heterogeneous photocatalysis for improving the removal of humic substances in real treated water from Agbo River. Catal. Today 2017, 281, 2–13. [Google Scholar] [CrossRef]
- Liberman, N.; Shandalov, S.; Forgacs, C.; Oron, G.; Brenner, A. Use of MBR to sustain active biomass for treatment of low organic load grey water. Clean Technol. Environ. Policy 2016, 18, 1219–1224. [Google Scholar] [CrossRef]
- Ding, A.; Liang, H.; Li, G.; Szivak, I.; Traber, J.; Pronk, W. A low energy gravity-driven membrane bioreactor system for grey water treatment: Permeability and removal performance of organics. J. Membr. Sci. 2017, 542, 408–417. [Google Scholar] [CrossRef]
- Chrispim, M.C.; Nolasco, M.A. Greywater treatment using a moving bed biofilm reactor at a university campus in Brazil. J. Clean. Prod. 2017, 142, 290–296. [Google Scholar] [CrossRef]
- Bani-Melhem, K.; Smith, E. Grey water treatment by a continuous process of an electrocoagulation unit and a submerged membrane bioreactor system. Chem. Eng. J. 2012, 198, 201–210. [Google Scholar] [CrossRef]
- De Sanctis, M.; Del Moro, G.; Levantesi, C.; Luprano, M.L.; Di Iaconi, C. Integration of an innovative biological treatment with physical or chemical disinfection for wastewater reuse. Sci. Total Environ. 2016, 543, 206–213. [Google Scholar] [CrossRef]
- Du, M.; Ding, Y.; Qiu, L. Water quality control during treatment of bathing wastewater. J. Harbin Univ. Archit. 2002, 35. [Google Scholar]
- Cui, F.-Y.; Ren, G. Pilot study of process of bathing wastewater treatment for reuse. J. Harbin Inst. Technol. 2006, 4. [Google Scholar]
- Sun, K.; Shi, Y. Experimental Study on the Treatment of Bath Wastewater in the Campus by Membrane Bioreactor. Adv. Mater. Res. 2014, 926–930, 124–127. [Google Scholar] [CrossRef]
- Guo, J.; Wang, R.; Xia, S. Comparison of Nitrogen Removal in an AN/AO and an AO Submerged Membrane Bioreactor in Treating Bathing Wastewater. In Proceedings of the 2008 2nd International Conference on Bioinformatics and Biomedical Engineering, Shanghai, China, 16–18 May 2008; pp. 2728–2733. [Google Scholar]
- Bai, H.; Xing, G.; Xu, B.; Tian, B.; Wang, X. Submerged Composite Membrane Bio-reactor for Treatment and Reuse of Bathing Wastewater. China Water Wastewater 2004, 20, 90–92. [Google Scholar]
- Zhang, Y.; Xing, G.; Zhu, W.; Sun, B. Engineering application of membrane bioreactor for the treatment of bathing wastewater. Ind. Water Treat. 2003, 23, 60–61. [Google Scholar]
- Li, K. Experimental Study on In-Situ Treatment and Reuse BathingWastewater by Electrocoagulation-Flotation; Dalian University of Technology: Dalian, China, 2018. [Google Scholar]
- Yang, Y.; Zhang, Y.; Yang, L.; Zhang, X.; Qu, H. UF-RO Integrated Technology for Bathroom Wastewater Treatment and Reuse. Environ. Sci. Technol. 2010, 33, 129–132. [Google Scholar]
- Zhang, S.; Wang, K.Y.; Chung, T.S.; Chen, H.; Jean, Y.C.; Amy, G. Well-constructed cellulose acetate membranes for forward osmosis: Minimized internal concentration polarization with an ultra-thin selective layer. J Membr. Sci. 2010, 360, 522–535. [Google Scholar] [CrossRef]
- Paris, S.; Celine, S. Greywater recycling in Vietnam–Application of the HUBER MBR process. Desalination 2010, 250, 1027–1030. [Google Scholar] [CrossRef]
- Lin, H.; Liao, B.Q.; Chen, J.; Gao, W.; Wang, L.; Wang, F.; Lu, X. New insights into membrane fouling in a submerged anaerobic membrane bioreactor based on characterization of cake sludge and bulk sludge. Bioresour. Technol. 2011, 102, 2373–2379. [Google Scholar] [CrossRef]
- Wang, C.; Chen, W.N.; Hu, Q.Y. Dynamic fouling behavior and cake layer structure changes in nonwoven membrane bioreactor for bath wastewater treatment. Chem. Eng. J. 2015, 264, 462–469. [Google Scholar] [CrossRef]
- Xinhua, Z.; Ke, X.; Xilin, L. Experimental study on enhanced adsorptive biomembrane for the treatment of bathing sewage. Ind. Water Treat. 2015, 35, 41–44. [Google Scholar]
- Xiyue, W.; Dongfang, L.; Zhendong, W. Experimental Study on Treatment of Bath Wastewater by Coagulation-AF-BAF Combination Process. Technol. Water Treat. 2019, 45, 115–119. [Google Scholar]
- Serdarevic, A.; Dzubur, A.; Muhibic, T. Role and Efficiency of MBR Technology for Wastewater Treatment. In International Symposium on Innovative and Interdisciplinary Applications of Advanced Technologies; Springer: Cham, Switzerland, 2020; pp. 229–237. [Google Scholar]
- Taesopapong, S.; Ratanatamskul, C. Innovative Eco Biofilter/Membrane Bioreactor (MBR) Technology for Community Wastewater Recycling. IOP Conf. Ser. Earth Environ. Sci. 2020, 427, 012011. [Google Scholar] [CrossRef]
- Sun, K. The MBR Process University Campus Sewage Recycling Experimental Study. Ph.D. Thesis, North China Institute of Water Conservancy and Hydropower, Zhengzhou, China, 2007. [Google Scholar]
- Paul, P. Comparison of phenomenological membrane bioreactor activated sludge biological models with alternative versions based on time series input-output approaches. Desalination Water Treat. 2011, 35, 110–117. [Google Scholar] [CrossRef]
- Datta, T.; Racz, L.; Kotay, S.M.; Goel, R. Seasonal variations of nitrifying community in trickling filter-solids contact (TF/SC) activated sludge systems. Bioresour. Technol. 2011, 102, 2272–2279. [Google Scholar] [CrossRef] [PubMed]
- Regmi, P.; Thomas, W.; Schafran, G.; Bott, C.; Rutherford, B.; Waltrip, D. Nitrogen removal assessment through nitrification rates and media biofilm accumulation in an IFAS process demonstration study. Water Res. 2011, 45, 6699–6708. [Google Scholar] [CrossRef]
- Kim, H.S.; Gellner, J.W.; Boltz, J.P.; Freudenberg, R.G.; Gunsch, C.K.; Schuler, A.J. Effects of integrated fixed film activated sludge media on activated sludge settling in biological nutrient removal systems. Water Res. 2010, 44, 1553–1561. [Google Scholar] [CrossRef]
- Ollivier, P.R.; Bahrou, A.S.; Church, T.M.; Hanson, T.E. Aeration controls the reduction and methylation of tellurium by the aerobic, tellurite-resistant marine yeast Rhodotorula mucilaginosa. Appl. Environ. Microbiol. 2011, 77, 4610–4617. [Google Scholar] [CrossRef]
- The state environmental protection administration, the water and wastewater monitoring analysis method of editorial board. In Water and Wastewater Monitoring Analysis Method, 4th ed.; Environmental Science Press: Beijing, China, 2002.
- Gao, T.; Gu, G. Water Pollution Control Engineering, 2nd ed.; Higher Education Press: Beijing, China, 2010. [Google Scholar]
- Li, S.; Cui, C.; Wu, J.; Huang, J. Treatment of bathing waste water by membrane bioreactor. J. Harbin Univ. Commer. 2005, 21, 711–714. [Google Scholar]
- Harada, H.; Momonoi, K.; Yamazaki, S.; Takizawa, S. Application of anaerobic UF membrane reactor for treatment of a wastewater containing high strength particulate organics. Water Sci. Technol. 1994, 30, 307–319. [Google Scholar] [CrossRef]
- Zhang, B.; Yamamoto, K.; Ohgaki, S.; Kamiko, N. Floc size distribution and bacterial activities in membrane separation activated sludge processes for small scale wastewater treatment/reclamation. Water Sci. Technol. 1997, 35, 37–44. [Google Scholar] [CrossRef]
- Chen, F.; Fan, Z.; Wang, C.; Huang, X.; Wen, X.; Liang, H.; Wen, J. Long-term Operation Performance of Full-scale Submerged Membrane Bioreactor for Bath Wastewater Treatment. China Water Wastewater 2006, 22, 67–69. [Google Scholar]
- Xing, C.H.; Tardieu, E.; Qian, Y.; Wen, X.H. Ultrafiltration membrane bioreactor for urban wastewater reclamation. J. Membr. Sci. 2000, 177, 73–82. [Google Scholar] [CrossRef]
- Huang, X.; Liu, R.; Qian, Y. Behaviour of soluble microbial products in a membrane bioreactor. Process. Biochem. 2000, 36, 401–406. [Google Scholar] [CrossRef]
- Liu, R.; Huang, X.; Liu, R.; Qian, Y. A comparison between a submerged membrane bioreactor and a conventional activated sludge process. Chin. J. Environ. Sci. 2001, 22, 20–24. [Google Scholar]
- Zhang, Y.; Xing, G.; Zhu, W.; Sun, B.; Zhao, X. Pilot-scale study on membrane bioreactor for treatment of bathing wastewater. China Water Wastewater 2003, 12, 49–50. [Google Scholar]
- Ueda, T.; Hata, K.; Kikuoka, Y. Treatment of domestic sewage from rural settlements by a membrane bioreactor. Water Sci. Technol. 1996, 34, 189–196. [Google Scholar] [CrossRef]
| Water Quality Index | Concentrations | Mean |
|---|---|---|
| COD (Chemical oxygen demand) (mg/L) | 155–562 | 314 |
| NH3–N (Ammonia nitrogen content index) (mg/L) | 22.2–47.8 | 38.4 |
| Turbidity (ntu) | 7.0–231.0 | 62.1 |
| Chrominance (tu) | 71–938 | 334 |
| LAS (Linear alkylbenzene sulfonates) (mg/L) | 0.42–5.21 | 1.8 |
| pH | 7.3–8.5 | 7.9 |
| Reuse Cost (CNY/m3) | Processes | References | Notes |
|---|---|---|---|
| 2.29 | coagulation, filtration, adsorption | [14] | |
| 1.61 | submerged composite membrane bioreactor | [26] | |
| 1.44 | biological contact oxidization and membrane bioreactor | [27] | No depreciation charge |
| 2.6 | electrocoagulation–flotation | [28] | |
| 2.42 | MBR | this paper |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; Zhong, S.; Li, Z. Pilot Tests on the Treatment of Bath Wastewater by a Membrane Bioreactor. Membranes 2021, 11, 85. https://doi.org/10.3390/membranes11020085
Shi Y, Zhong S, Li Z. Pilot Tests on the Treatment of Bath Wastewater by a Membrane Bioreactor. Membranes. 2021; 11(2):85. https://doi.org/10.3390/membranes11020085
Chicago/Turabian StyleShi, Yan, Songtao Zhong, and Zhaohui Li. 2021. "Pilot Tests on the Treatment of Bath Wastewater by a Membrane Bioreactor" Membranes 11, no. 2: 85. https://doi.org/10.3390/membranes11020085
APA StyleShi, Y., Zhong, S., & Li, Z. (2021). Pilot Tests on the Treatment of Bath Wastewater by a Membrane Bioreactor. Membranes, 11(2), 85. https://doi.org/10.3390/membranes11020085






