Development of a Short-Cut Combined Magnetic Coagulation–Sequence Batch Membrane Bioreactor for Swine Wastewater Treatment
Abstract
1. Introduction
2. Materials and Methods
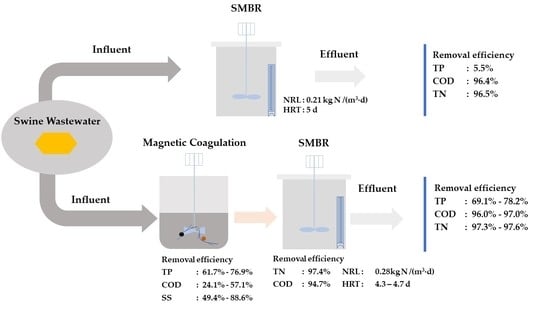
2.1. Setup and Experimental Design of the Combined Process
2.2. Raw Swine Wastewater and Operational Parameters of Magnetic Coagulation
2.3. Operation of the SMBR
2.4. Analysis Methods
2.5. Data Analysis
3. Results and Discussion
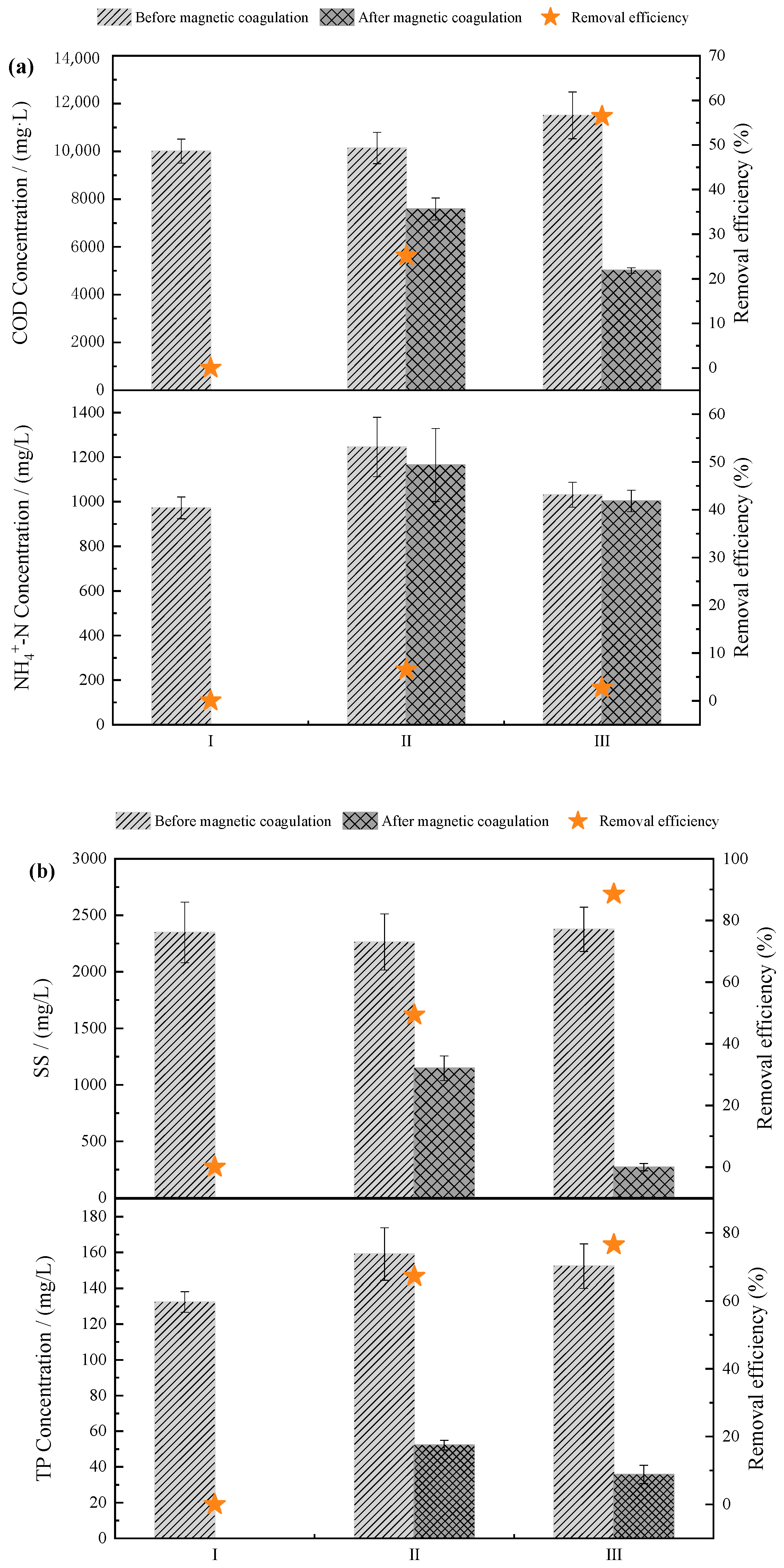
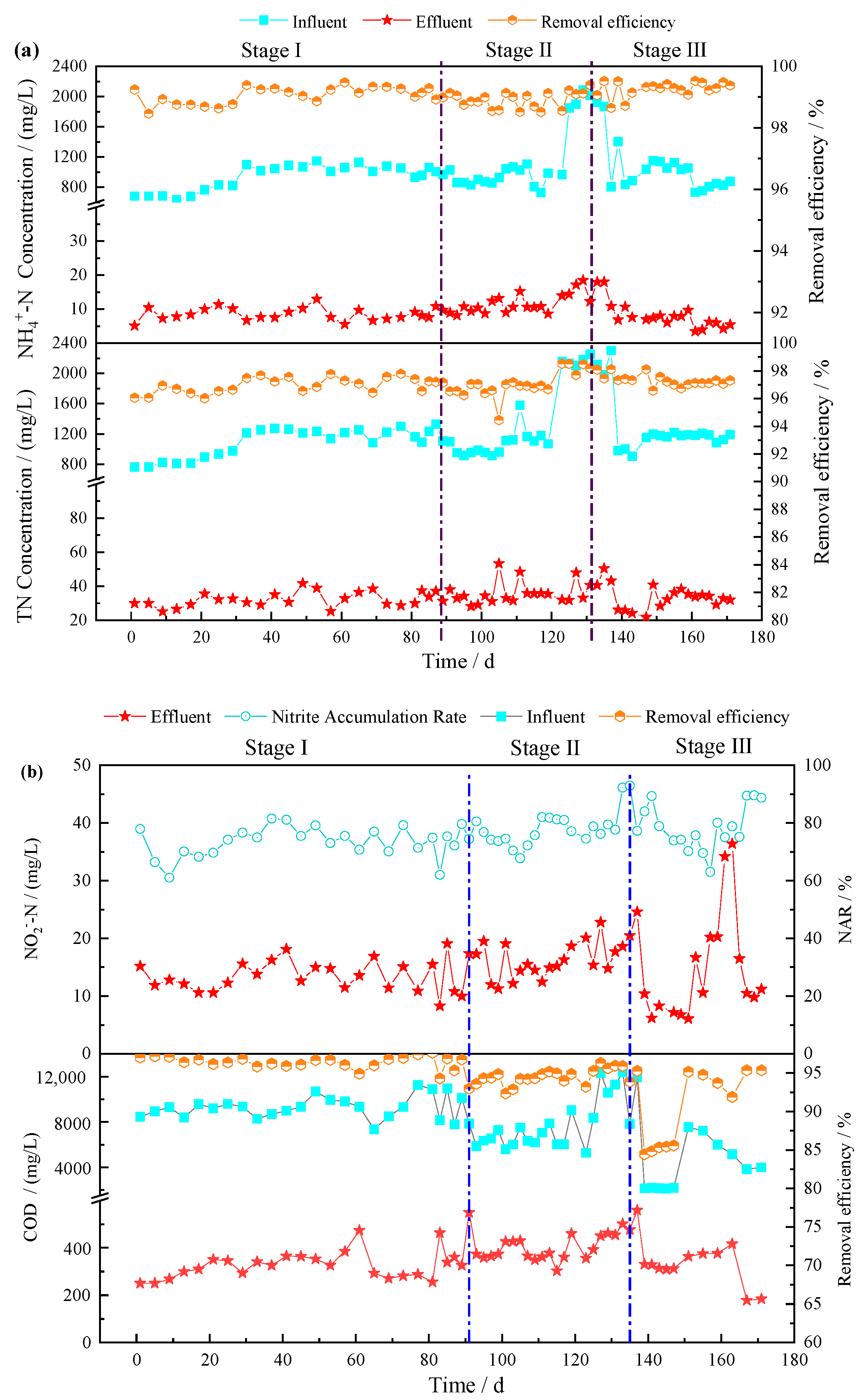
3.1. Treatment Performance of the Combined MC-SMBR Process
3.1.1. Pre-Treatment of MC
3.1.2. Treatment Performance of the SMBR
3.1.3. Total Treatment Performance
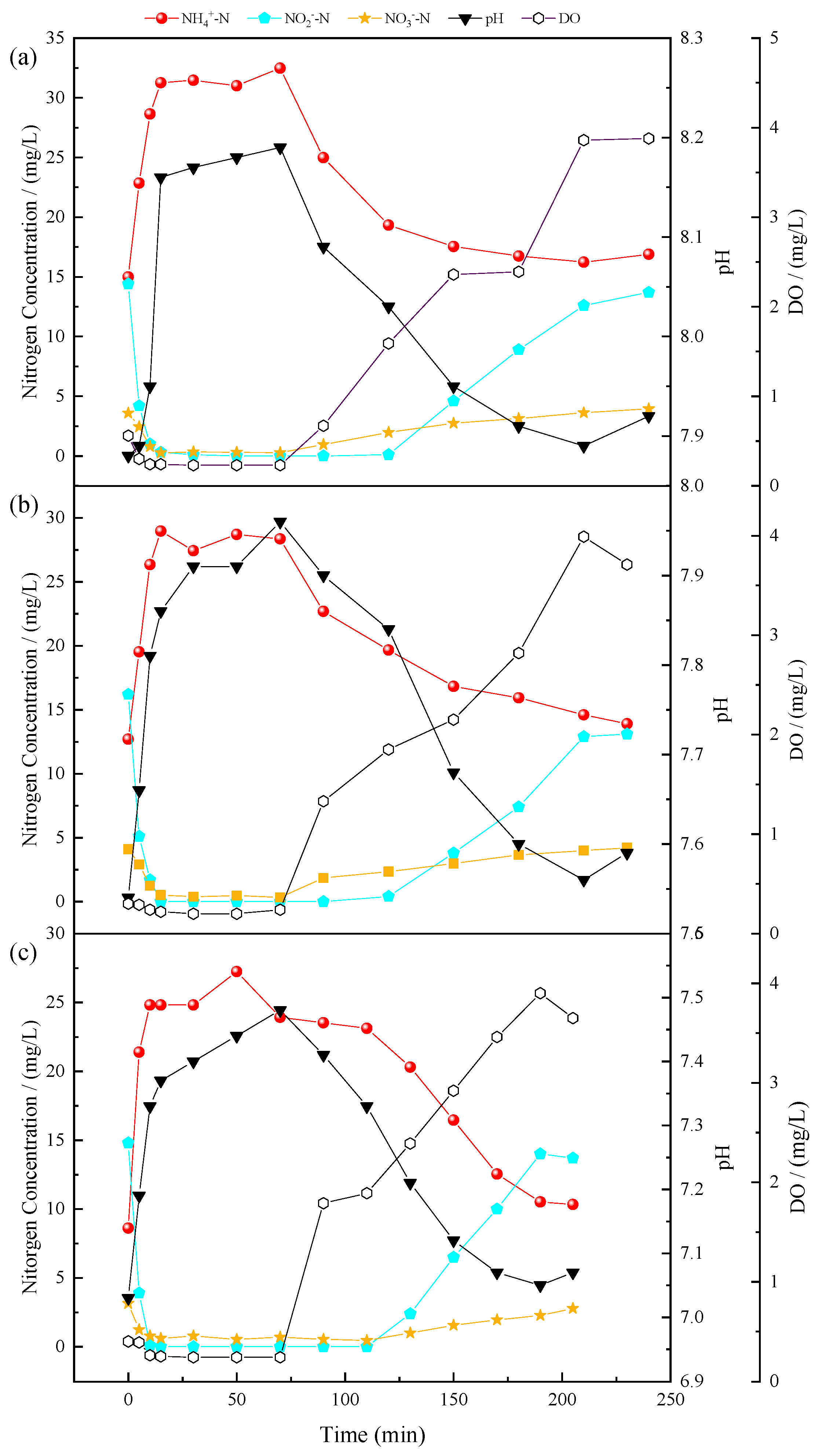
3.2. Evolution of Nitrogen Compounds in Typical Cycles of the SMBR
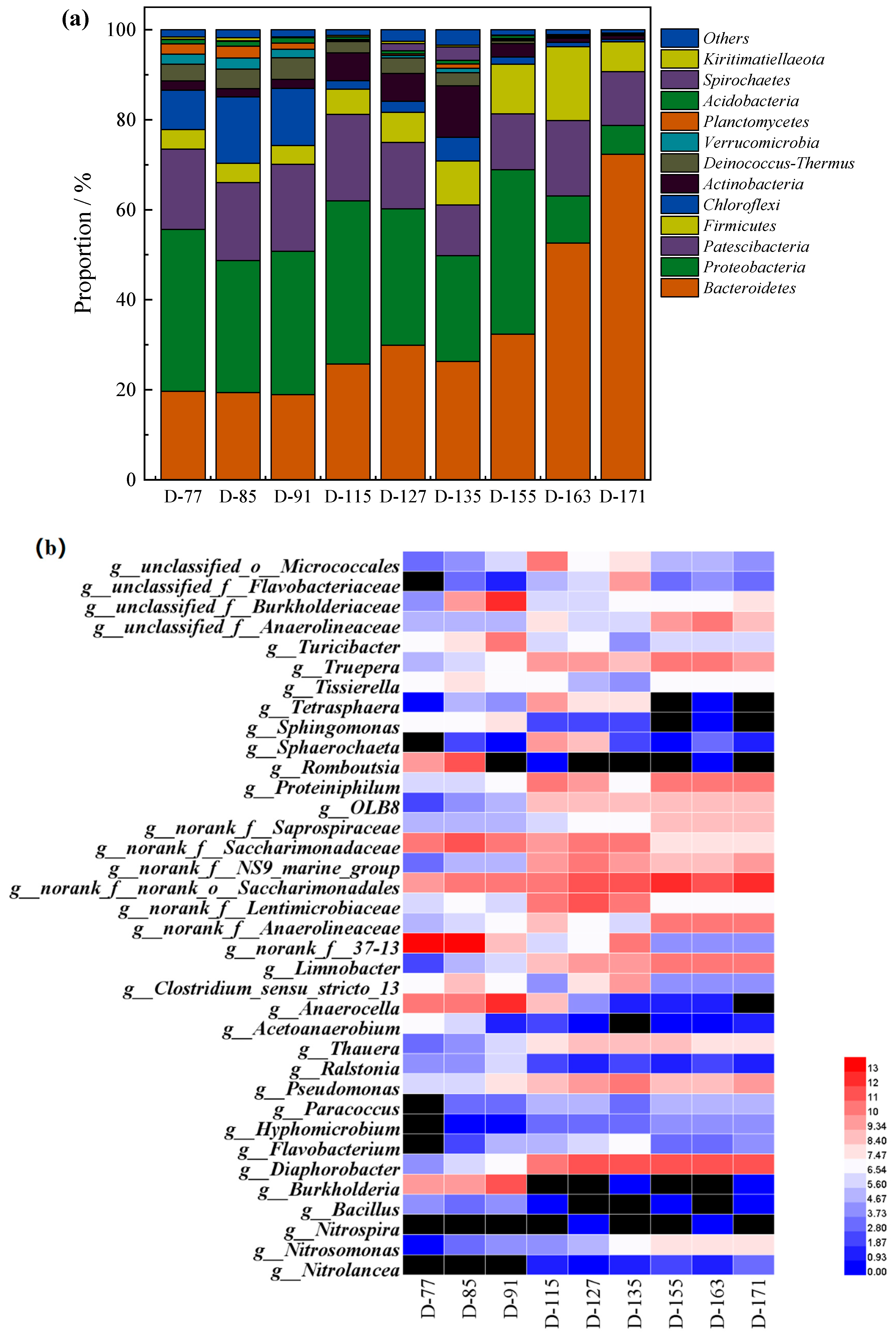
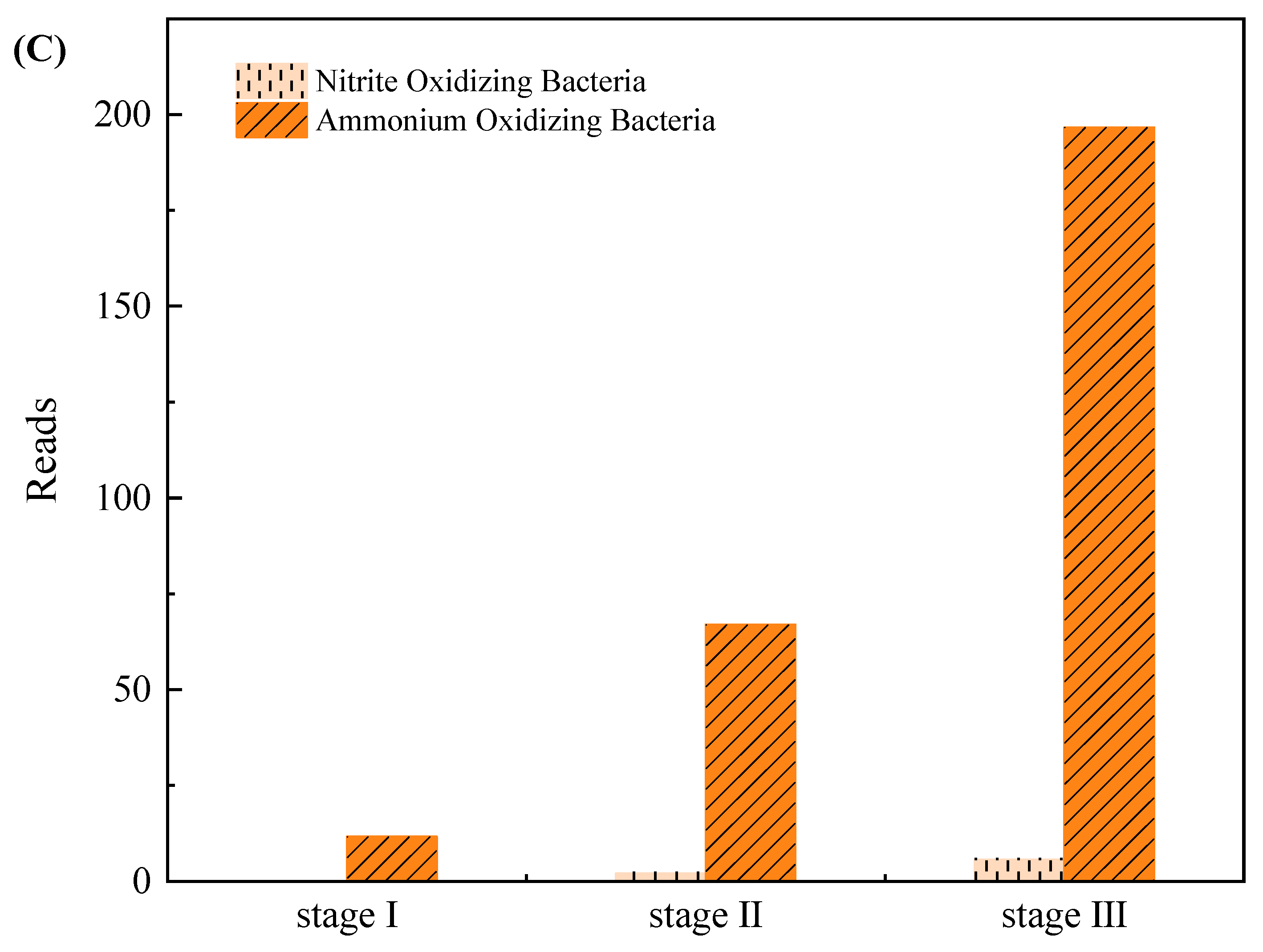
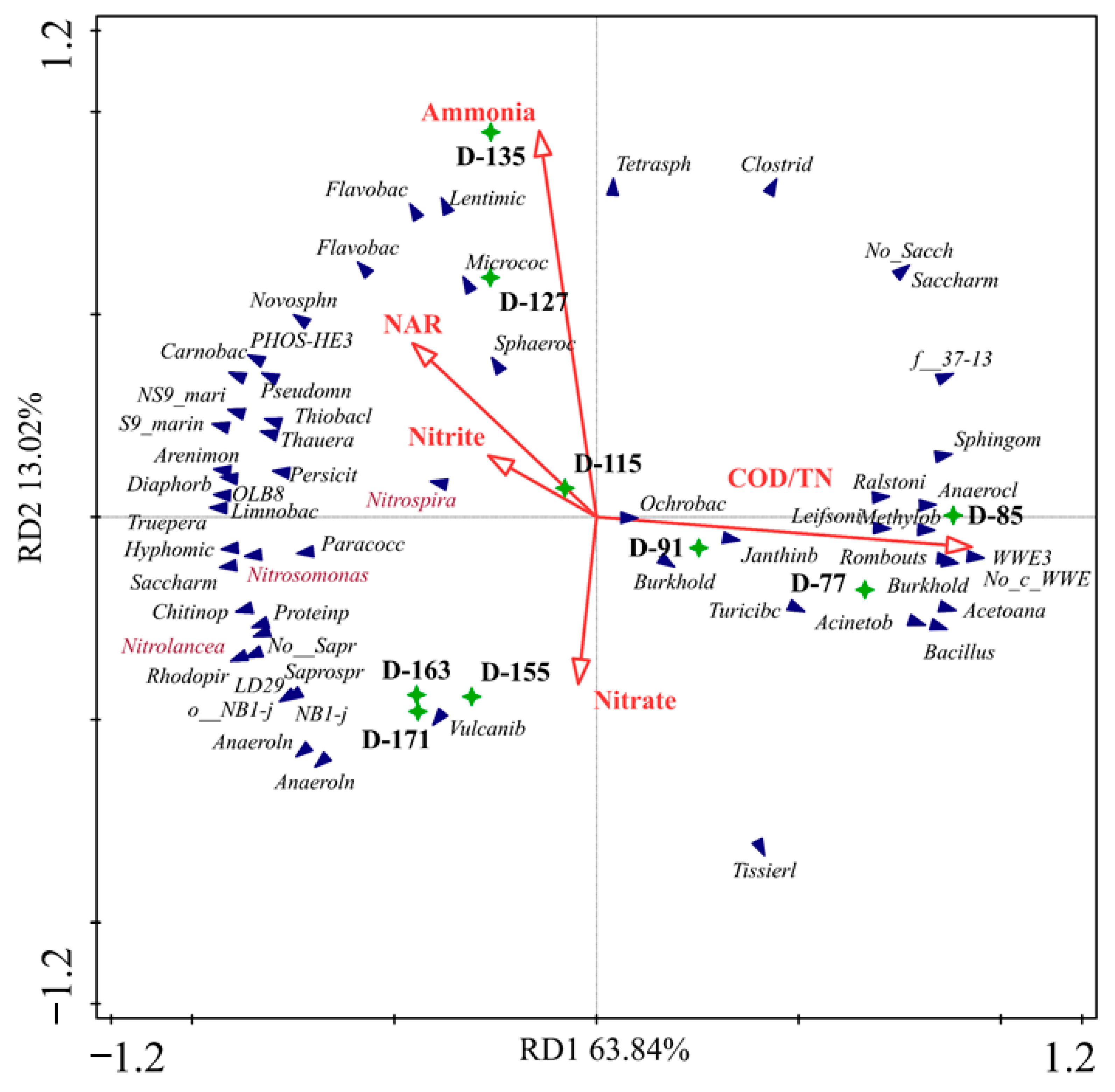
3.3. Microbial Community Evolution in the SMBR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ministry of Ecology and Environment of People’s Republic of China. Bulletin of the Second National Census of Pollution Sources in China. Available online: http://www.mee.gov.cn/xxgk2018/xxgk/xxgk01/202006/W020200610353985963290.pdf (accessed on 10 September 2020).
- Yu, J.J.; Hu, H.C.; Wu, X.D.; Zhou, T.; Liu, Y.H.; Ruan, R.; Zheng, H.L. Coupling of biochar-mediated absorption and algal-bacterial system to enhance nutrients recovery from swine wastewater. Sci. Total Environ. 2020, 701, 134935. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.H.; Narindri, B.; Chu, H.; Whang, L.M. Recent advancement on biological technologies and strategies for resource recovery from swine wastewater. Bioresour. Technol. 2020, 303, 122861. [Google Scholar] [CrossRef] [PubMed]
- Hai, R.T.; He, Y.Q.; Wang, X.H.; Li, Y. Simultaneous removal of nitrogen and phosphorus from swine wastewater in a sequencing batch biofilm reactor. Chin. J. Chem. Eng. 2015, 23, 303–308. [Google Scholar] [CrossRef]
- Lourinho, G.; Brito, P.S.D. Electrolytic Treatment of Swine Wastewater: Recent Progress and Challenges. Waste Biomass Valorization 2020, 1–24. [Google Scholar] [CrossRef]
- Wang, X.; Yang, R.; Zhang, Z.; Wu, J.; Chen, S. Mass balance and bacterial characteristics in an in-situ full-scale swine wastewater treatment system occurring anammox process. Bioresour. Technol. 2019, 292, 122005. [Google Scholar] [CrossRef]
- Nagarajan, D.; Kusmayadi, A.; Yen, H.-W.; Dong, C.-D.; Lee, D.-J.; Chang, J.-S. Current advances in biological swine wastewater treatment using microalgae-based processes. Bioresour. Technol. 2019, 289, 121718. [Google Scholar] [CrossRef]
- Sandefur, H.N.; Asgharpour, M.; Mariott, J.; Gottberg, E.; Vaden, J.; Matlock, M.; Hestekin, J. Recovery of nutrients from swine wastewater using ultrafiltration: Applications for microalgae cultivation in photobioreactors. Ecol. Eng. 2016, 94, 75–81. [Google Scholar] [CrossRef]
- Jiang, M.M.; Westerholm, M.; Qiao, W.; Wandera, S.M.; Dong, R.J. High rate anaerobic digestion of swine wastewater in an anaerobic membrane bioreactor. Energy 2020, 193, 677–687. [Google Scholar] [CrossRef]
- Ding, X.; Zhao, J.; Chen, Y.; Hu, B.; Ma, B. Comparison of nitrogen and phosphorus removal performances for traditional and modified A2/O process. Chin. J. Environ. Eng. 2018, 12, 1480–1489. [Google Scholar]
- He, L.-Y.; He, L.-K.; Liu, Y.-S.; Zhang, M.; Zhao, J.-L.; Zhang, Q.-Q.; Ying, G.-G. Microbial diversity and antibiotic resistome in swine farm environments. Sci. Total Environ. 2019, 685, 197–207. [Google Scholar] [CrossRef]
- Kelly, P.T.; He, Z. Nutrients removal and recovery in bioelectrochemical systems: A review. Bioresour. Technol. 2014, 153, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Sui, Q.; Jiang, C.; Yu, D.; Chen, M.; Zhang, J.; Wang, Y.; Wei, Y. Performance of a sequencing-batch membrane bioreactor (SMBR) with an automatic control strategy treating high-strength swine wastewater. J. Hazard. Mater. 2018, 342, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Deng, L.; Zheng, D.; Wang, L.; Liu, Y. Separation of swine wastewater into different concentration fractions and its contribution to combined anaerobic–aerobic process. J. Environ. Manag. 2016, 168, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Xu, G. Research on Pretreatment Technology of Diary Farm Wastewater; Yangzhou University: Yangzhou, China, 2019. [Google Scholar]
- Zeng, Z.; Zheng, P.; Shi, C.; Zhang, M.; Shan, S. A challenge in anaerobic digestion of swine wastewater: Recalcitrance and enhanced-degradation of dietary fibres. Biodegradation 2019, 30, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Ritigala, T.; Demissie, H.; Chen, Y.; Zheng, J.; Zheng, L.; Zhu, J.; Fan, H.; Li, J.; Wang, D.; Weragoda, S.K.; et al. Optimized pre-treatment of high strength food waste digestate by high content aluminum-nanocluster based magnetic coagulation. J. Environ. Sci. 2021, 104, 430–443. [Google Scholar]
- Lv, M.; Zhang, Z.H.; Zeng, J.Y.; Liu, J.F.; Sun, M.C.; Yadav, R.S.; Feng, Y.J. Roles of magnetic particles in magnetic seeding coagulation-flocculation process for surface water treatment. Sep. Purif. Technol. 2019, 212, 337–343. [Google Scholar] [CrossRef]
- Luo, G.; Zhang, C.; Zheng, L.; Wei, Y.; Yu, D. Optimization and evaluation of magnetic coagulation process pretreating swine manure biogas slurry. Chin. J. Environ. Eng. 2019, 13, 414–423. [Google Scholar]
- Luo, L.; Nguyen, A.V. A review of principles and applications of magnetic flocculation to separate ultrafine magnetic particles. Sep. Purif. Technol. 2016, 172, 85–99. [Google Scholar] [CrossRef]
- Wang, S.; Cheng, F.; Guo, X.; Shen, S. Mechanism analysis of magnetic powder function in deep phosphorus removal by magnetic coagulation. Chin. J. Environ. Eng. 2019, 13, 302–309. [Google Scholar]
- Zheng, L.; Jiao, Y.; Chen, M.; Wang, Z.; Zhang, H.; Zhenjun, W.; Huang, G.; Yu, W.; Wei, Y. The pollutants removal in municipal wastewater treatment by magnetic coagulation technology. Acta Sci. Circumstantiae 2020, 40, 2118–2127. [Google Scholar]
- Wang, C.R.; Ren, X.; Li, W.X.; Hou, Z.F.; Ke, C.; Geng, Q. Magnetic Flocculation Technology for Copper and Zinc Ions Removal from the Tin Smelting Wastewater. Appl. Mech. Mater. 2013, 295–298, 1284–1288. [Google Scholar] [CrossRef]
- Sui, Q.; Chen, Y.; Yu, D.; Wang, T.; Hai, Y.; Zhang, J.; Chen, M.; Wei, Y. Fates of intracellular and extracellular antibiotic resistance genes and microbial community structures in typical swine wastewater treatment processes. Environ. Int. 2018, 133, 105183. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Wei, Y.; Chen, M. In-situ restoring nitrogen removal for the combined partial nitritation-anammox process deteriorated by nitrate build-up. Biochem. Eng. J. 2015, 98, 127–136. [Google Scholar] [CrossRef]
- Gonzalez-Tineo, P.A.; Duran-Hinojosa, U.; Delgadillo-Mirquez, L.R.; Meza-Escalante, E.R.; Gortares-Moroyoqui, P.; Ulloa-Mercado, R.G.; Serrano-Palacios, D. Performance improvement of an integrated anaerobic-aerobic hybrid reactor for the treatment of swine wastewater. J. Water Process Eng. 2020, 34, 101164. [Google Scholar] [CrossRef]
- Lü, J.; Yuan, Z.; Li, W.; Wei, Y.; Fan, S. Experimental study of phosphorus removal from oil-bearing cleaning wastewater by coagulation sedimentation. Ind. Water Treat. 2019, 39, 37–41. [Google Scholar]
- Oberson, A.; Tagmann, H.U.; Langmeier, M.; Dubois, D.; Mäder, P.; Frossard, E. Fresh and residual phosphorus uptake by ryegrass from soils with different fertilization histories. Plant Soil 2010, 334, 391–407. [Google Scholar] [CrossRef][Green Version]
- Oginni, O.; Yakaboylu, G.A.; Singh, K.; Sabolsky, E.M.; Unal-Tosun, G.; Jaisi, D.; Khanal, S.; Shah, A. Phosphorus adsorption behaviors of MgO modified biochars derived from waste woody biomass resources. J. Environ. Chem. Eng. 2020, 8, 103723. [Google Scholar] [CrossRef]
- Kim, D.; Min, K.J.; Lee, K.; Yu, M.S.; Park, K.Y. Effects of pH, molar ratios and pre-treatment on phosphorus recovery through struvite crystallization from effluent of anaerobically digested swine wastewater. Environ. Eng. Res. 2017, 22, 12–18. [Google Scholar] [CrossRef]
- Xiang, L.J. Application of magnetic coagulation process for slightly polluted river water treatment. Chin. J. Environ. Eng. 2014, 8, 2901–2905. [Google Scholar]
- Kim, J.H.; Chen, M.; Kishida, N.; Sudo, R. Integrated real-time control strategy for nitrogen removal in swine wastewater treatment using sequencing batch reactors. Water Res. 2004, 38, 3340–3348. [Google Scholar] [CrossRef]
- Han, Z.; Chen, S.; Lin, X.; Yu, H.; Duan, L.A.; Ye, Z.; Jia, Y.; Zhu, S.; Liu, D. Performance and membrane fouling of a step-fed submerged membrane sequencing batch reactor treating swine biogas digestion slurry. J. Environ. Sci. Health Part A 2017, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Chen, L.P.; Guan, D.T.; Zhang, Z.G.; Tian, X.J.; Wang, A.M.; Wang, G.T.; Yao, Q.; Peng, D.; Li, J.Y. On-site nutrient recovery and removal from source-separated urine by phosphorus precipitation and short-cut nitrification-denitrification. Chemosphere 2017, 175, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhou, W.N.; Wan, R.; Luo, J.Y.; Su, Y.L.; Huang, H.N.; Chen, Y.G. Increasing municipal wastewater BNR by using the preferred carbon source derived from kitchen wastewater to enhance phosphorus uptake and short-cut nitrification-denitrification. Chem. Eng. J. 2018, 344, 556–564. [Google Scholar] [CrossRef]
- Li, J.B.; Ye, W.; Wei, D.; Ngo, H.H.; Guo, W.S.; Qiao, Y.M.; Xu, W.Y.; Du, B.; Wei, Q. System performance and microbial community succession in a partial nitrification biofilm reactor in response to salinity stress. Bioresour. Technol. 2018, 270, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Ngo, H.H.; Guo, W.S.; Xu, W.Y.; Du, B.; Wei, Q. Partial nitrification granular sludge reactor as a pretreatment for anaerobic ammonium oxidation (Anammox): Achievement, performance and microbial community. Bioresour. Technol. 2018, 269, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Shao, M.F.; Ye, L. 454 Pyrosequencing reveals bacterial diversity of activated sludge from 14 sewage treatment plants. Isme J. 2012, 6, 1137–1147. [Google Scholar] [CrossRef] [PubMed]
- Michael, D.; Franke-Whittle, I.H.; Heribert, I.; Yona, C.; Yitzhak, H.J.F.M.E. Molecular analysis of bacterial community succession during prolonged compost curing. J. FEMS Microbiol. Ecol. 2008, 65, 133–144. [Google Scholar]

| Stage | COD | NH4+-N | TN | PO3−-P | TP | SS | pH | T (°C) |
|---|---|---|---|---|---|---|---|---|
| I | 9227.2 ± 1012.8 | 943.4 ± 162.5 | 1097.0 ± 184.5 | 114.3 ± 8.6 | 132.4 ± 5.8 | 2348.33 ± 268.3 | 8.1–8.5 | 25–27 |
| II | 10,141.3 ± 1292.3 | 1245.7 ± 271.2 | 1514.9 ± 126.4 | 140.2 ± 15.2 | 159.1 ± 14.7 | 2263.7 ± 248.4 | 8.0–8.5 | 25–27 |
| III | 11,507.3 ± 1817.4 | 1031.3 ± 181.1 | 1201.5 ± 145.7 | 134.4 ± 13.9 | 152.4 ± 12.4 | 2375 ± 196.3 | 8.1–8.4 | 25–28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Sui, Q.; Yu, D.; Zheng, L.; Chen, M.; Ritigala, T.; Wei, Y. Development of a Short-Cut Combined Magnetic Coagulation–Sequence Batch Membrane Bioreactor for Swine Wastewater Treatment. Membranes 2021, 11, 83. https://doi.org/10.3390/membranes11020083
Chen Y, Sui Q, Yu D, Zheng L, Chen M, Ritigala T, Wei Y. Development of a Short-Cut Combined Magnetic Coagulation–Sequence Batch Membrane Bioreactor for Swine Wastewater Treatment. Membranes. 2021; 11(2):83. https://doi.org/10.3390/membranes11020083
Chicago/Turabian StyleChen, Yanlin, Qianwen Sui, Dawei Yu, Libing Zheng, Meixue Chen, Tharindu Ritigala, and Yuansong Wei. 2021. "Development of a Short-Cut Combined Magnetic Coagulation–Sequence Batch Membrane Bioreactor for Swine Wastewater Treatment" Membranes 11, no. 2: 83. https://doi.org/10.3390/membranes11020083
APA StyleChen, Y., Sui, Q., Yu, D., Zheng, L., Chen, M., Ritigala, T., & Wei, Y. (2021). Development of a Short-Cut Combined Magnetic Coagulation–Sequence Batch Membrane Bioreactor for Swine Wastewater Treatment. Membranes, 11(2), 83. https://doi.org/10.3390/membranes11020083