Effect of Membrane Properties on the Carbonation of Anion Exchange Membrane Fuel Cells
Abstract
1. Introduction
2. Experimental
2.1. Electrode Preparation
2.2. Anion Exchange Membrane Fuel Cell (AEMFC) Assembly and Break-In Procedure
2.3. AEMFC Carbon Dioxide Measurements
2.4. Wide Angle X-ray Scattering Analysis
2.5. Neutron Imaging Cell and Operation
3. Results and Discussion
3.1. Influence of Anion Exchange Membrane (AEM) Thickness on AEMFC Performance with 400 ppm Cathode CO2
3.2. Effect of AEM Chemical Structure on the Performance of AEMFCs Operating with 400 ppm CO2
3.3. Water Distribution of Carbonated AEMFC by Operando Neutron Imaging
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, G.; Mandal, M.; Peng, X.; Yang-Neyerlin, A.C.; Pivovar, B.S.; Mustain, W.E.; Kohl, P.A.; Soc, J.E.; Huang, G.; Mandal, M.; et al. Composite Poly(norbornene) Anion Conducting Membranes for Achieving Durability, Water Management and High Power (3.4 W/cm2) in Hydrogen/Oxygen Alkaline Fuel Cells. J. Electrochem. Soc. 2019, 166, F637–F644. [Google Scholar] [CrossRef]
- Hassan, N.U.; Mandal, M.; Huang, G.; Firouzjaie, H.A.; Kohl, P.A.; Mustain, W.E. Achieving High-Performance and 2000 h Stability in Anion Exchange Membrane Fuel Cells by Manipulating Ionomer Properties and Electrode Optimization. Adv. Energy Mater 2020, 10, 2001986. [Google Scholar] [CrossRef]
- Alia, S.M.; Ngo, C.; Shulda, S.; Ha, M.; Dameron, A.A.; Weker, J.N.; Neyerlin, K.C.; Kocha, S.S.; Pylypenko, S.; Pivovar, B.S. Exceptional Oxygen Reduction Reaction Activity and Durability of Platinum − Nickel Nanowires through Synthesis and Post-Treatment Optimization. ACS Omega 2017, 2, 1408–1418. [Google Scholar] [CrossRef]
- Mustain, W.E.; Chatenet, M.; Page, M.; Kim, Y.S. Durability challenges of anion exchange membrane fuel cells. Energy Environ. Sci 2020, 13, 2805–2838. [Google Scholar] [CrossRef]
- Peng, X.; Kulkarni, D.; Huang, Y.; Omasta, T.J.; Ng, B.; Zheng, Y.; Wang, L.; Lamanna, J.M.; Hussey, D.S.; Varcoe, J.R.; et al. Using operando techniques to understand and design high performance and stable alkaline membrane fuel cells. Nat. Commun. 2020, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gülzow, E.; Schulze, M. Long-term operation of AFC electrodes with CO2 containing gases. J. Power Sources 2004, 127, 243–251. [Google Scholar] [CrossRef]
- Tewari, V.A.; Sambhy, M.; Urquidi Macdonald, A. Sen Quantification of carbon dioxide poisoning in air breathing alkaline fuel cells. J. Power Sources 2006, 153, 1–10. [Google Scholar] [CrossRef]
- Adams, L.A.; Poynton, S.D.; Tamain, C.; Slade, R.C.T.; Varcoe, J.R. A carbon dioxide tolerant aqueous-electrolyte-free anion-exchange membrane alkaline fuel cell. ChemSusChem 2008, 1, 79–81. [Google Scholar] [CrossRef]
- Zheng, Y.; Omasta, T.J.; Peng, X.; Wang, L.; Varcoe, J.R.; Pivovar, S.; Mustain, W.E. Quantifying and elucidating the effect of CO2 on the thermodynamics, kinetics and charge transport of AEMFCs. Energy Environ. Sci. 2019, 12, 2806–2819. [Google Scholar] [CrossRef]
- Zheng, Y.; Huang, G.; Wang, L.; Varcoe, J.R.; Kohl, P.A.; Mustain, E. Effect of reacting gas flowrates and hydration on the carbonation of anion exchange membrane fuel cells in the presence of CO2. J. Power Sources 2020, 467, 228350. [Google Scholar] [CrossRef]
- Gerhardt, M.R.; Pant, L.M.; Weber, A.Z. Along-the-Channel Impacts of Water Management and Carbon-Dioxide Contamination in Hydroxide-Exchange-Membrane Fuel Cells: A Modeling Study. J. Electrochem. Soc. 2019, 166, F3180–F3192. [Google Scholar] [CrossRef]
- Wrubel, J.A.; Peracchio, A.A.; Cassenti, B.N.; Grew, K.N.; Chiu, W.K.S. Anion Exchange Membrane Fuel Cell Performance in the Presence of Carbon Dioxide: An Investigation into the Self-Purging Mechanism. J. Electrochem. Soc. 2019, 166, 810–820. [Google Scholar] [CrossRef]
- Ziv, N.; Mustain, W.E.; Dekel, D.R. The Effect of Ambient Carbon Dioxide on Anion-Exchange Membrane Fuel Cells. ChemSusChem 2018, 11, 1136–1150. [Google Scholar] [CrossRef] [PubMed]
- Siroma, Z.; Watanabe, S.; Yasuda, K.; Fukuta, K.; Yanagi, H. Mathematical Modeling of the Concentration Profile of Carbonate Ions in an Anion Exchange Membrane Fuel Cell. ECS Trans. 2010, 33, 1935–1943. [Google Scholar] [CrossRef]
- Matsui, Y.; Saito, M.; Tasaka, A.; Inaba, M. Influence of Carbon Dioxide on the Performance of Anion-Exchange Membrane Fuel Cells. ECS Trans. 2010, 25, 105–110. [Google Scholar] [CrossRef]
- Inaba, M.; Matsui, Y.; Saito, M.; Tasaka, A.; Fukuta, K.; Watanabe, S.; Yanagi, H. Effects of Carbon Dioxide on the Performance of Anion-Exchange Membrane Fuel Cells. Electrochemistry 2011, 5, 322–325. [Google Scholar] [CrossRef]
- Yanagi, H.; Fukuta, K. Anion Exchange Membrane and Ionomer for Alkaline Membrane Fuel Cells (AMFCs). ECS Trans. 2008, 16, 257–262. [Google Scholar] [CrossRef]
- Rigdon, W.A.; Omasta, T.J.; Lewis, C.; Hickner, M.A.; Varcoe, J.R.; Renner, J.N.; Ayers, K.E.; Mustain, W.E. Carbonate Dynamics and Opportunities With Low Temperature, Anion Exchange Membrane-Based Electrochemical Carbon Dioxide Separators. J. Electrochem. Energy Convers. Storage 2017, 14, 1–8. [Google Scholar] [CrossRef]
- Gerhardt, M.R.; Pant, L.M.; Shiau, H.-S.; Weber, A.Z. Modeling Water Management and Carbon-Dioxide Contamination Effects in Anion-Exchange Membrane Fuel Cells. ECS Trans. 2018, 86, 15–24. [Google Scholar] [CrossRef]
- Krewer, U.; Weinzierl, C.; Ziv, N.; Dekel, D.R. Impact of carbonation processes in anion exchange membrane fuel cells. Electrochim. Acta 2018, 263, 433–446. [Google Scholar] [CrossRef]
- Wrubel, J.A.; Peracchio, A.A.; Cassenti, B.N.; Myles, T.D.; Grew, K.N.; Chiu, W.K.S. Anion Exchange Membrane Ionic Conductivity in the Presence of Carbon Dioxide under Fuel Cell Operating Conditions. J. Electrochem. Soc. 2017, 164, F1063–F1073. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, C.; Zhang, X.; Chen, L.; Jiang, L.; Meng, Y.; Wang, X. A green approach for preparing anion exchange membrane based on cardo polyetherketone powders. J. Power Sources 2014, 272, 211–217. [Google Scholar] [CrossRef]
- Lee, H.C.; Liu, K.L.; Tsai, L.D.; Lai, J.Y.; Chao, C.Y. Anion exchange membranes based on novel quaternized block copolymers for alkaline direct methanol fuel cells. RSC Adv. 2014, 4, 10944. [Google Scholar] [CrossRef]
- Merle, G.; Chairuna, A.; Van De Ven, E.; Nijmeijer, K. An easy method for the preparation of anion exchange membranes: Graft-polymerization of ionic liquids in porous supports. J. Appl. Polym. Sci. 2013, 129, 1143–1150. [Google Scholar] [CrossRef]
- Ponce-Gonzalez, J.; Whelligan, D.K.; Wang, L.; Bance-Soualhi, R.; Wang, Y.; Peng, Y.; Peng, H.; Apperley, D.C.; Sarode, H.N.; Pandey, T.P.; et al. High performance aliphatic-heterocyclic benzyl-quaternary ammonium radiation-grafted anion-exchange membranes. Energy Environ. Sci. 2016, 9, 3724–3735. [Google Scholar] [CrossRef]
- Ren, X.; Price, S.C.; Jackson, A.C.; Pomerantz, N.; Beyer, F.L. Highly Conductive Anion Exchange Membrane for High Power Density Fuel-Cell Performance. ACS Appl. Mater. Interfaces 2014, 6, 13330–13333. [Google Scholar] [CrossRef]
- Jheng, L.; Hsu, S.L.; Lin, B.; Hsu, Y. Quaternized polybenzimidazoles with imidazolium cation moieties for anion exchange membrane fuel cells. J. Memb. Sci. 2014, 460, 160–170. [Google Scholar] [CrossRef]
- Li, Z.; He, X.; Jiang, Z.; Yin, Y.; Zhang, B.; He, G.; Tong, Z.; Wu, H.; Jiao, K. Enhancing Hydroxide Conductivity and Stability of Anion Exchange Membrane by Blending Quaternary Ammonium Functionalized Polymers. Electrochim. Acta 2017, 240, 486–494. [Google Scholar] [CrossRef]
- He, X.; Liu, J.; Zhu, H.; Zheng, Y.; Chen, D. Novel quaternary ammonium functional addition-type norbornene copolymer as hydroxide-conductive and durable anion exchange membrane for direct methanol fuel cells. RSC Adv. 2015, 5, 63215–63225. [Google Scholar] [CrossRef]
- Lin, X.; Gong, M.; Liu, Y.; Wu, L.; Li, Y.; Liang, X.; Li, Q.; Xu, T. A convenient, efficient and green route for preparing anion exchange membranes for potential application in alkaline fuel cells. J. Memb. Sci. 2013, 425–426, 190–199. [Google Scholar] [CrossRef]
- Lu, W.; Shao, Z.G.; Zhang, G.; Zhao, Y.; Li, J.; Yi, B. Preparation and characterization of imidazolium-functionalized poly (ether sulfone) as anion exchange membrane and ionomer for fuel cell application. Int. J. Hydrogen Energy 2013, 38, 9285–9296. [Google Scholar] [CrossRef]
- Lee, K.H.; Cho, D.H.; Kim, Y.M.; Moon, S.J.; Seong, J.G.; Shin, D.W.; Sohn, J.-Y.; Kim, J.F.; Lee, Y.M. Highly conductive and durable poly(arylene ether sulfone) anion exchange membrane with end-group cross-linking. Energy Environ. Sci. 2017, 10, 275–285. [Google Scholar] [CrossRef]
- Tripathi, B.P.; Kumar, M.; Shahi, V.K. Organic-inorganic hybrid alkaline membranes by epoxide ring opening for direct methanol fuel cell applications. J. Memb. Sci. 2010, 360, 90–101. [Google Scholar] [CrossRef]
- Mandal, M.; Huang, G.; Hassan, N.U.; Peng, X.; Gu, T.; Brooks-starks, A.H.; Bahar, B.; Mustain, W.E.; Kohl, P.A. The Importance of Water Transport in High Conductivity and High-Power Alkaline Fuel Cells. J. Electrochem. Soc. 2020, 167, 054501. [Google Scholar] [CrossRef]
- Wang, L.; Peng, X.; Mustain, W.E.; Varcoe, J.R. Radiation-grafted anion-exchange membranes: The switch from low- to high-density polyethylene leads to remarkably enhanced fuel cell performance. Energy Environ. Sci. 2019, 12, 1575–1579. [Google Scholar] [CrossRef]
- Mandal, M.; Huang, G.; Kohl, P.A. Anionic multiblock copolymer membrane based on vinyl addition polymerization of norbornenes: Applications in anion-exchange membrane fuel cells. J. Memb. Sci. 2019, 570–571, 394–402. [Google Scholar] [CrossRef]
- Chen, W.; Mandal, M.; Huang, G.; Wu, X.; He, G.; Kohl, P.A. Highly Conducting Anion-Exchange Membranes Based on Cross-Linked Poly(norbornene): Ring Opening Metathesis Polymerization. ACS Appl. Energy Mater. 2019, 2, 2458–2468. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Y.; Setzler, B.P.; Rojas-Carbonell, S.; Ben Yehuda, C.; Amel, A.; Page, M.; Wang, L.; Hu, K.; Shi, L.; et al. Poly(aryl piperidinium) membranes and ionomers for hydroxide exchange membrane fuel cells. Nat. Energy 2019, 4, 392. [Google Scholar] [CrossRef]
- Omasta, T.J.; Park, A.M.; Lamanna, J.M.; Zhang, Y.; Peng, X.; Wang, L.; Jacobson, D.L.; Varcoe, J.R.; Hussey, D.S.; Pivovar, B.S.; et al. Beyond Catalysis and Membranes: Visualizing and Solving the Challenge of Electrode Water Accumulation and Flooding in AEMFCs. Energy Environ. Sci. 2018, 11, 551–558. [Google Scholar] [CrossRef]
- Omasta, T.J.; Wang, L.; Peng, X.; Lewis, C.A.; Varcoe, J.R.; Mustain, W.E. Importance of balancing membrane and electrode water in anion exchange membrane fuel cells. J. Power Sources 2018, 375, 205–213. [Google Scholar] [CrossRef]
- Omasta, T.J.; Peng, X.; Miller, H.A.; Vizza, F.; Wang, L.; Varcoe, J.R.; Dekel, D.R.; Mustain, W.E. Beyond 1.0 W cm−2 Performance without Platinum: The Beginning of a New Era in Anion Exchange Membrane Fuel Cells. J. Electrochem. Soc. 2018, 165, J3039–J3044. [Google Scholar] [CrossRef]
- Poynton, S.D.; Slade, R.C.T.; Omasta, T.J.; Mustain, W.E.; Escudero-Cid, R.; Ocón, P.; Varcoe, J.R. Preparation of radiation-grafted powders for use as anion exchange ionomers in alkaline polymer electrolyte fuel cells. J. Mater. Chem. A 2014, 2, 5124–5130. [Google Scholar] [CrossRef]
- Wang, L.; Brink, J.J.; Liu, Y.; Herring, A.M.; Ponce-González, J.; Whelligan, D.K.; Varcoe, J.R. Non-fluorinated pre-irradiation-grafted (peroxidated) LDPE-based anion-exchange membranes with high performance and stability. Energy Environ. Sci. 2017, 10, 2154–2167. [Google Scholar] [CrossRef]
- Lamanna, J.; Hussey, D.S.; Baltic, E.; Jacobson, D.L. Neutron and X-ray Tomography ( NeXT ) system for simultaneous, dual modality tomography. Rev. Sci. Instrum. 2017, 88, 113702. [Google Scholar] [CrossRef] [PubMed]
- Hussey, D.S.; Jacobson, D.L.; Arif, M.; Huffman, P.R.; Williams, R.E.; Cook, J.C. New neutron imaging facility at the NIST. Nucl. Instruments Methods Phys. Res. A 2005, 542, 9–15. [Google Scholar] [CrossRef]
- Hussey, D.S.; Lamanna, J.M.; Baltic, E.; Jacobson, D.L. Neutron imaging detector with 2 μm spatial resolution based on event reconstruction of neutron capture in gadolinium oxysulfide scintillators. Nucl. Inst. Methods Phys. Res. A 2017, 866, 9–12. [Google Scholar] [CrossRef]
- Liu, F.; Yi, B.; Xing, D.; Yu, J.; Zhang, H. Nafion / PTFE composite membranes for fuel cell applications. J. Memb. Sci. 2003, 212, 213–223. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiao, K.; Du, Q.; Yin, Y.; Li, X. Gas diffusion layer deformation and its effect on the transport characteristics and performance of proton exchange membrane fuel cell. Int. J. Hydrogen Energy 2013, 38, 12891–12903. [Google Scholar] [CrossRef]
- Liu, J.G.; Zhao, T.S.; Liang, Z.X.; Chen, R. Effect of membrane thickness on the performance and efficiency of passive direct methanol fuel cells. J. Power Sources 2006, 153, 61–67. [Google Scholar] [CrossRef]
- Mandal, M.; Huang, G.; Kohl, P.A. Highly Conductive Anion-Exchange Membranes Based on Cross-Linked Poly(norbornene): Vinyl Addition Polymerization. ACS Appl. Energy Mater. 2019, 2, 2447–2457. [Google Scholar] [CrossRef]
- Zheng, Y.; Ash, U.; Pandey, R.P.; Ozioko, A.G.; Ponce-gonzález, J.; Handl, M.; Weissbach, T.; Varcoe, J.R.; Holdcroft, S.; Matthew, W.; et al. Water uptake study of anion exchange membranes. Macromolecules 2018, 51, 3264–3278. [Google Scholar] [CrossRef]
- Divekar, A.G.; Yang-Neyerlin, A.C.; Antunes, C.M.; Strasser, D.J.; Motz, A.R.; Seifert, S.S.; Zuo, X.; Pivovar, B.S.; Herring, A.M. In-depth understanding of the CO2 limitation of air fed anion exchange membrane fuel cells. Sustain. Energy Fuels 2020, 4, 1801–1811. [Google Scholar] [CrossRef]
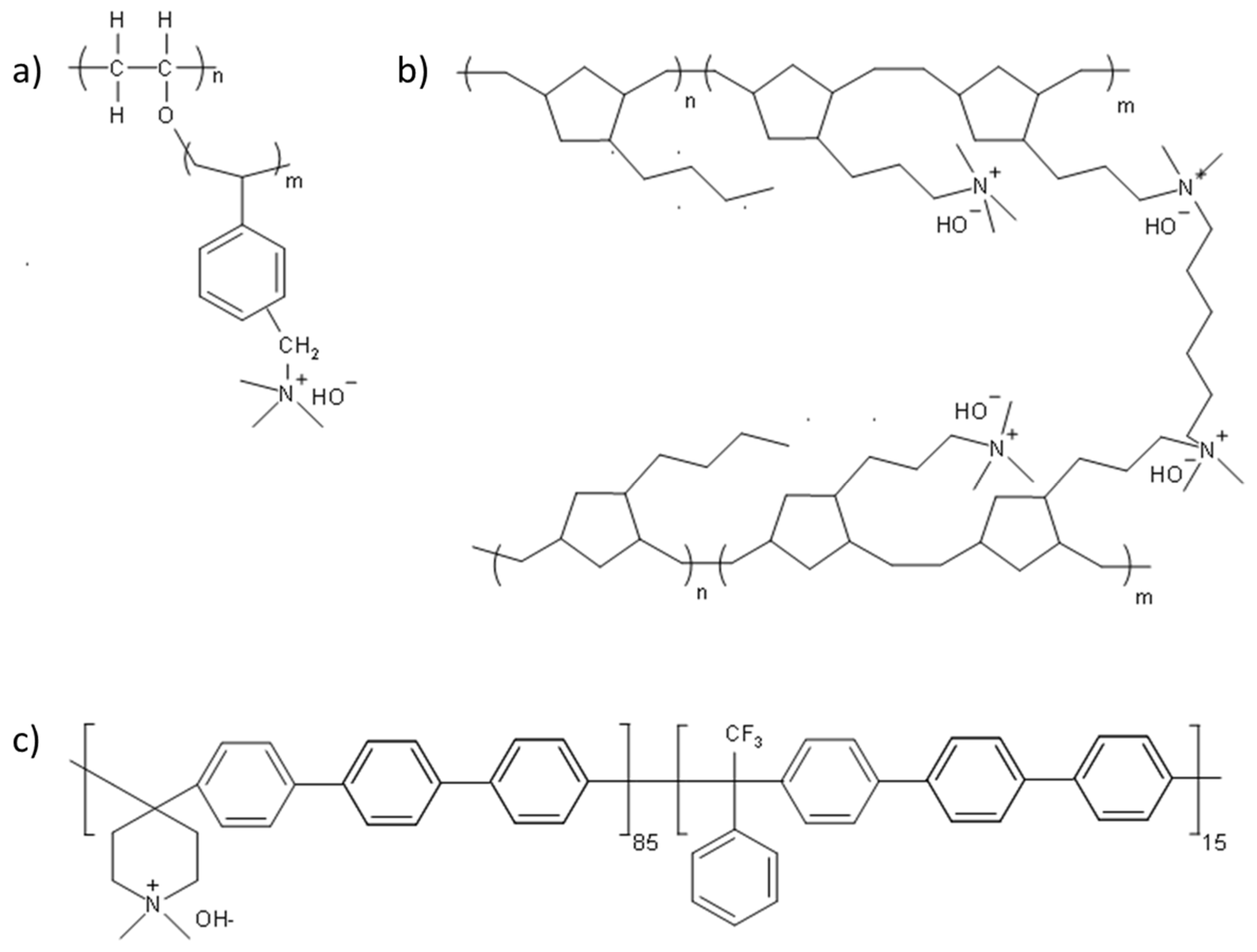
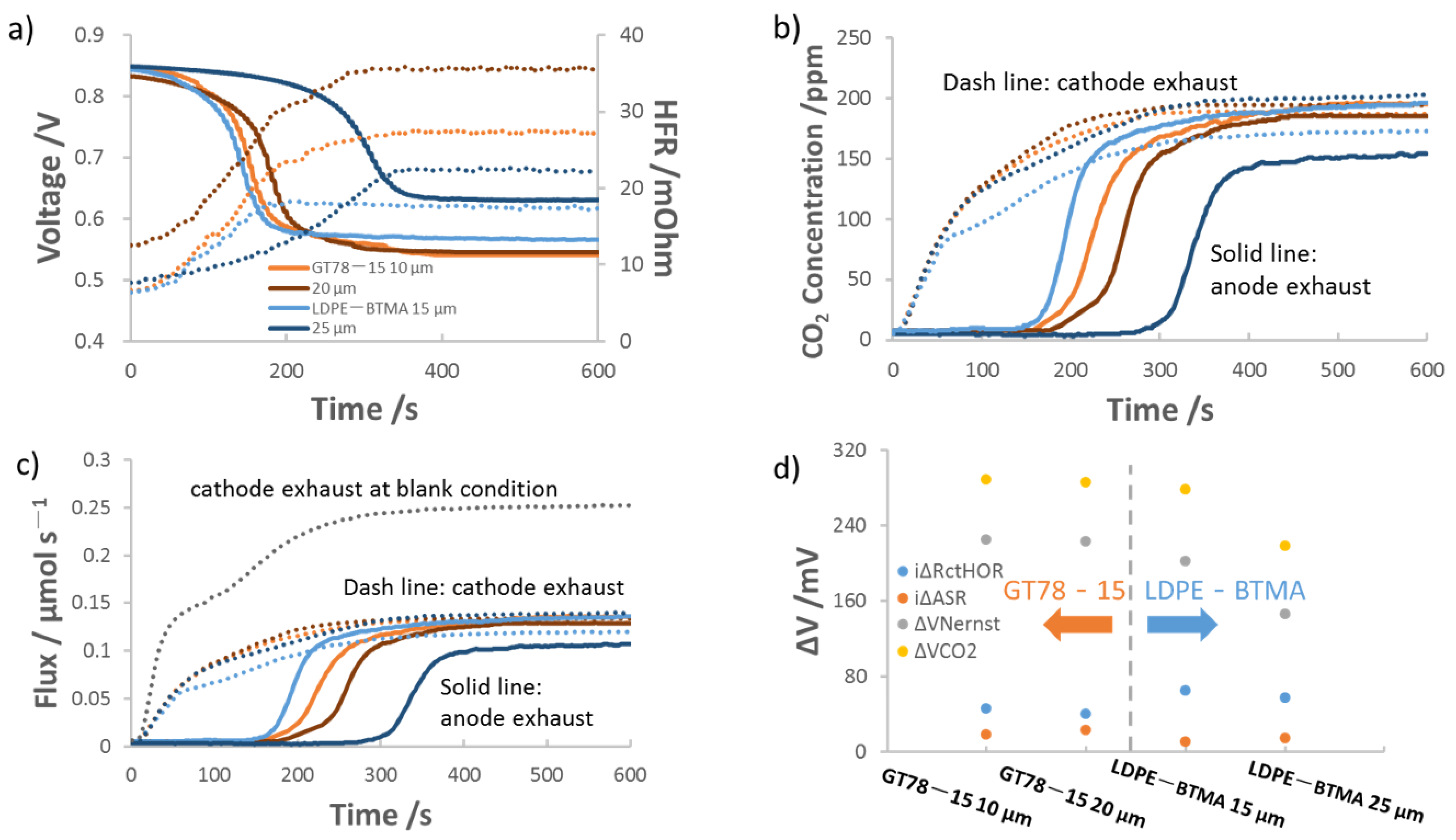
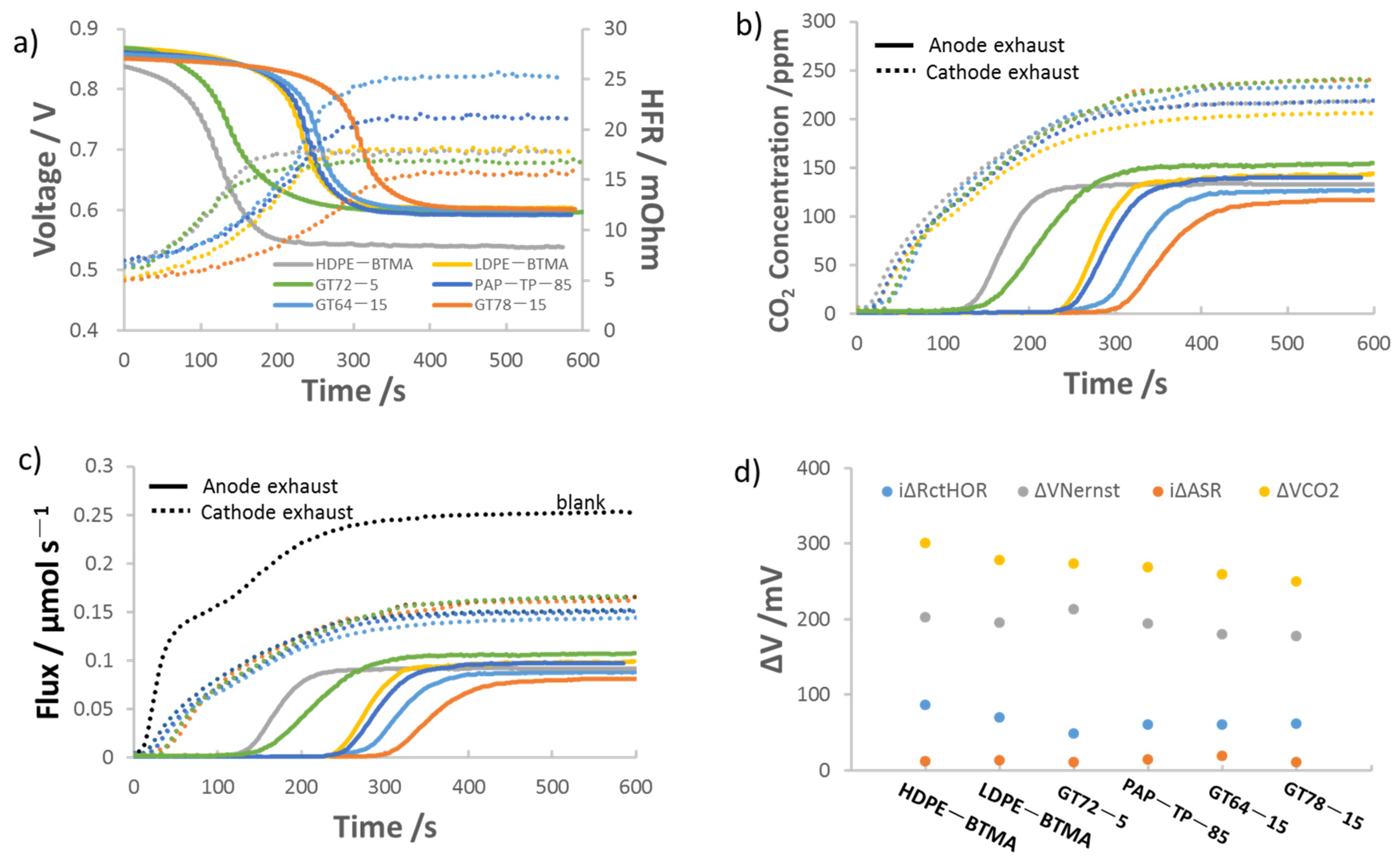
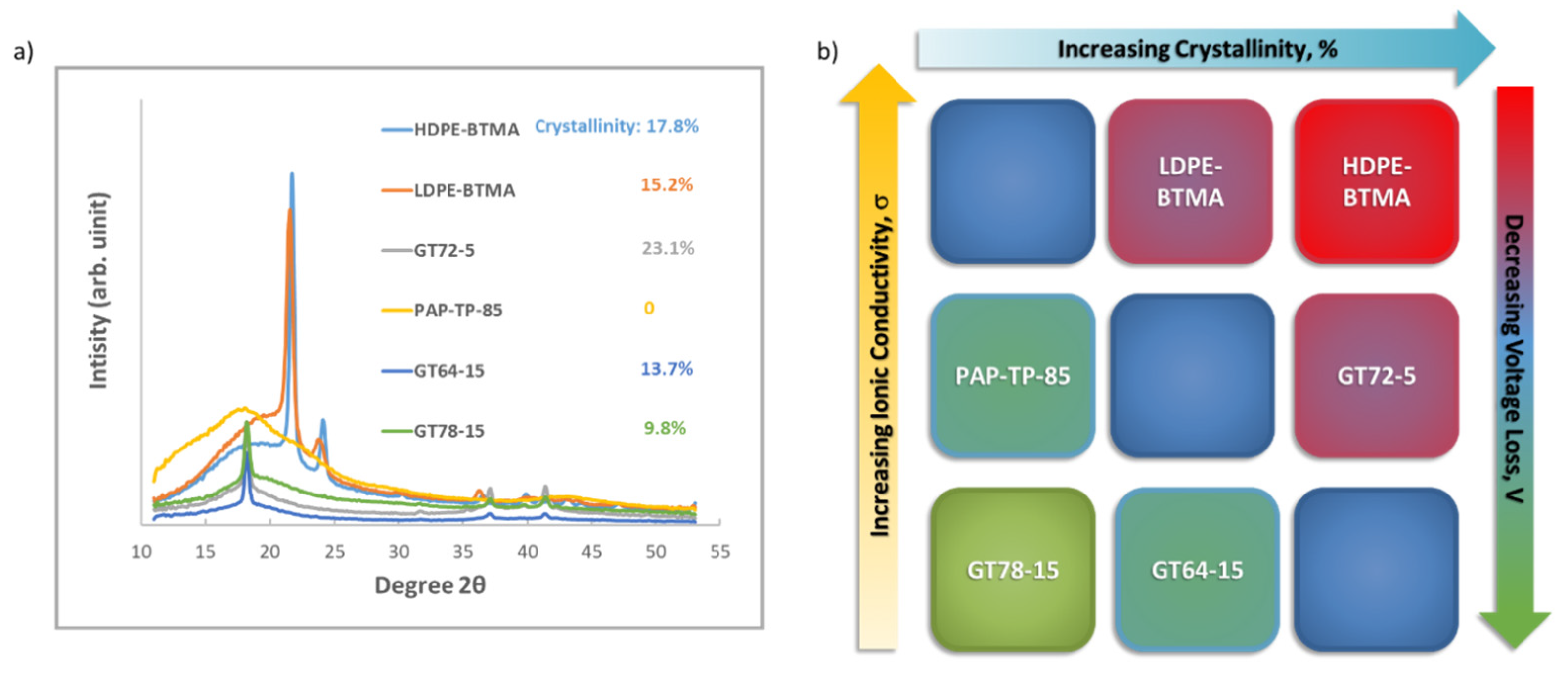

| AEM | Cross-Linking Ratio (%) | IEC a (mmol/g) | Thickness b (μm) | WU c (%) | λH2O d | Swelling e (%) | σ f, OH−, 80 °C (mS cm−1) | Young’s Modulus (MPa) g | Stress at Break (MPa) g |
|---|---|---|---|---|---|---|---|---|---|
| LDPE-BTMA [35] | NR | 2.54 ± 0.21 | 15 | 149 ± 16 | 32 ± 3 | 27 ± 10 | 208 ± 6 | 248 ± 31 | 23 ± 6 |
| 2.87 ± 0.05 | 25 | 104 ± 9 | 18 ± 2 | 22 ± 2 | 145 ± 8 | 386 ± 83 | 29 ± 5 | ||
| HDPE-BTMA [35] | NR | 2.44 ± 0.04 | 15 | 155 ± 15 | 35 ± 2 | 38 ± 7 | 214 ± 2 | NR | 35 |
| PAP-TP-85 [38] | NR | 2.2 | 15 | 60 | 15.13 | 8 | 175 | 425 | 50 |
| GT72-5 [1,37] | 5 | 3.44 | 10 | 96 | 15.24 | 35 | 175 | NR | NR |
| GT78-15 [1,37] | 15 | 3.62 | 10, 20 | 65 | 9.98 | 50 | 138 | NR | 28 |
| GT64-15 [1,37] | 15 | 3.28 | 10 | 29 | 8.81 | 14 | 142 | 175 | NR |
| GT78-15 | LDPE-BTMA | |||
|---|---|---|---|---|
| 10 μm | 20 μm | 15 μm | 25 μm | |
| AEMFC carbonate NCO2/µmol | 19 ± 1 | 24 ± 1 | 21 ± 1 | 32 ± 1 |
| ΔASR/mΩ·cm2 | 0.91 | 116 | 54 | 73 |
| ΔRctHOR/mΩ | 46 | 40 | 65 | 58 |
| Degree of Carbonation, DOC (%) | 27 | 21 | 41 | 44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Irizarry Colón, L.N.; Ul Hassan, N.; Williams, E.R.; Stefik, M.; LaManna, J.M.; Hussey, D.S.; Mustain, W.E. Effect of Membrane Properties on the Carbonation of Anion Exchange Membrane Fuel Cells. Membranes 2021, 11, 102. https://doi.org/10.3390/membranes11020102
Zheng Y, Irizarry Colón LN, Ul Hassan N, Williams ER, Stefik M, LaManna JM, Hussey DS, Mustain WE. Effect of Membrane Properties on the Carbonation of Anion Exchange Membrane Fuel Cells. Membranes. 2021; 11(2):102. https://doi.org/10.3390/membranes11020102
Chicago/Turabian StyleZheng, Yiwei, Lyzmarie Nicole Irizarry Colón, Noor Ul Hassan, Eric R. Williams, Morgan Stefik, Jacob M. LaManna, Daniel S. Hussey, and William E. Mustain. 2021. "Effect of Membrane Properties on the Carbonation of Anion Exchange Membrane Fuel Cells" Membranes 11, no. 2: 102. https://doi.org/10.3390/membranes11020102
APA StyleZheng, Y., Irizarry Colón, L. N., Ul Hassan, N., Williams, E. R., Stefik, M., LaManna, J. M., Hussey, D. S., & Mustain, W. E. (2021). Effect of Membrane Properties on the Carbonation of Anion Exchange Membrane Fuel Cells. Membranes, 11(2), 102. https://doi.org/10.3390/membranes11020102







