Membrane Distillation of Saline Water Contaminated with Oil and Surfactants
Abstract
:1. Introduction
- (i)
- (ii)
- (iii)
- (iv)
- (v)
- (vi)
2. Materials and Methods
- (i)
- Baltic Sea water,
- (ii)
- Baltic Sea contaminated by oil and SDS,
- (iii)
- High salinity solutions (NaCl) contaminated by SDS and oil.
3. Results and Discussion
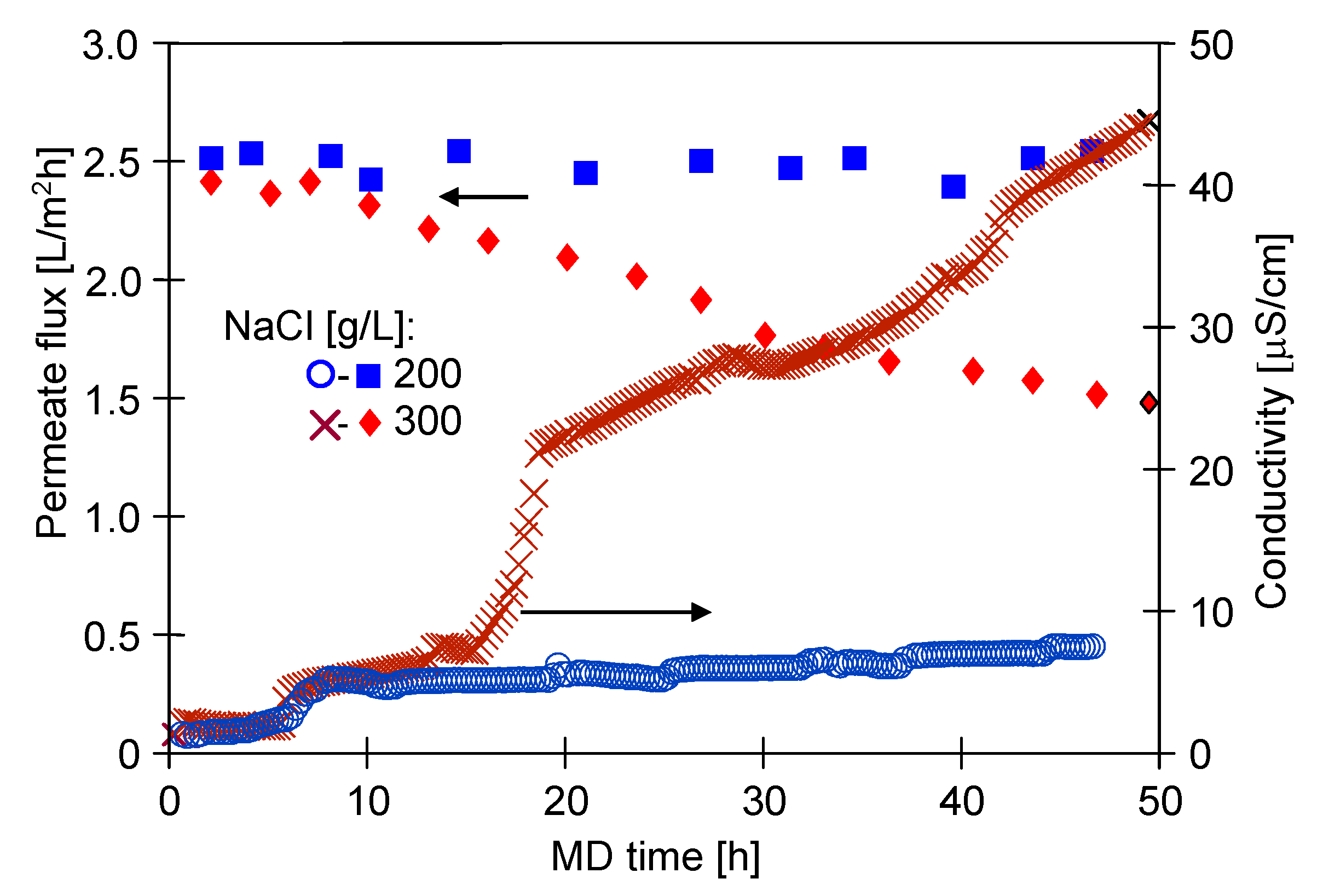
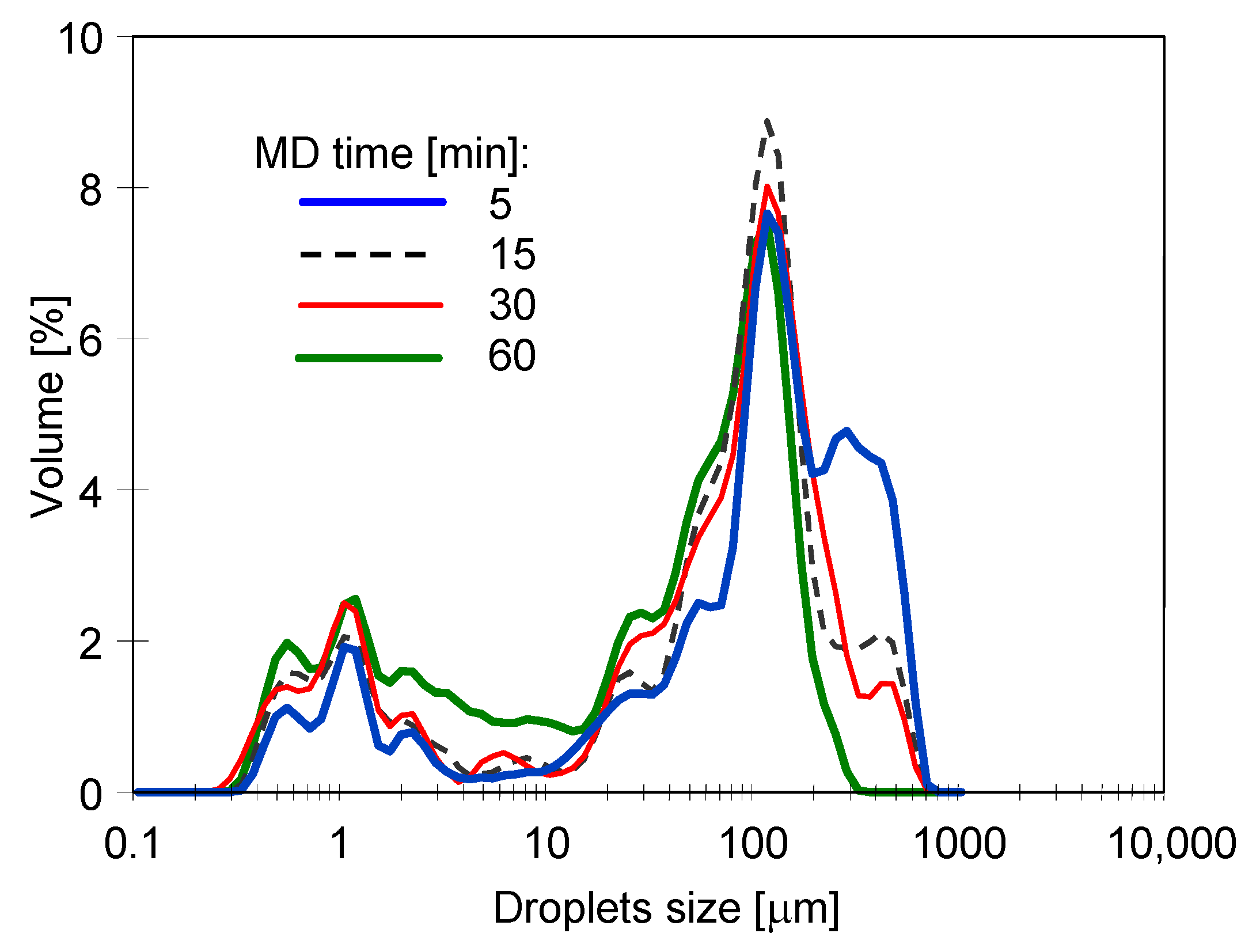
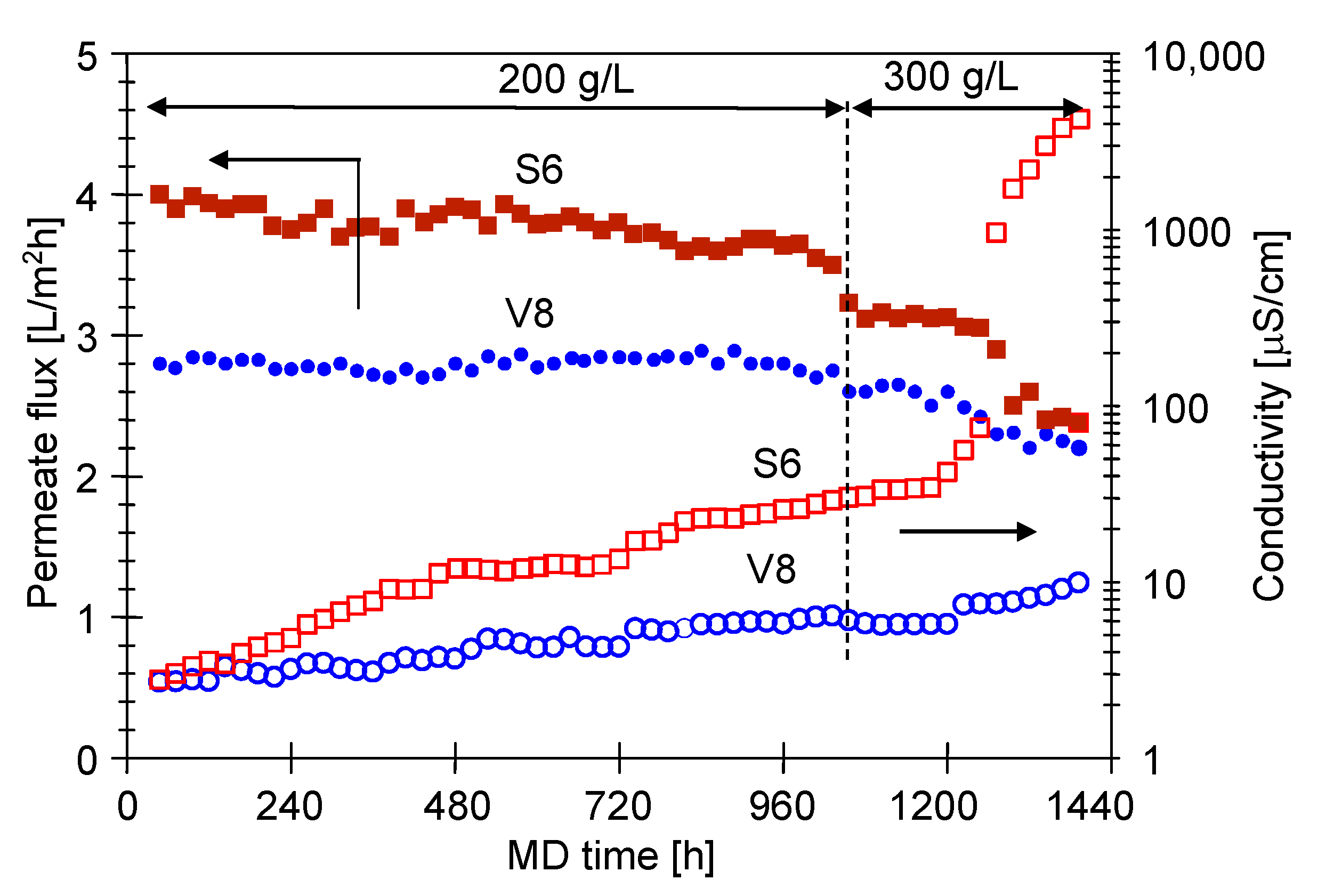
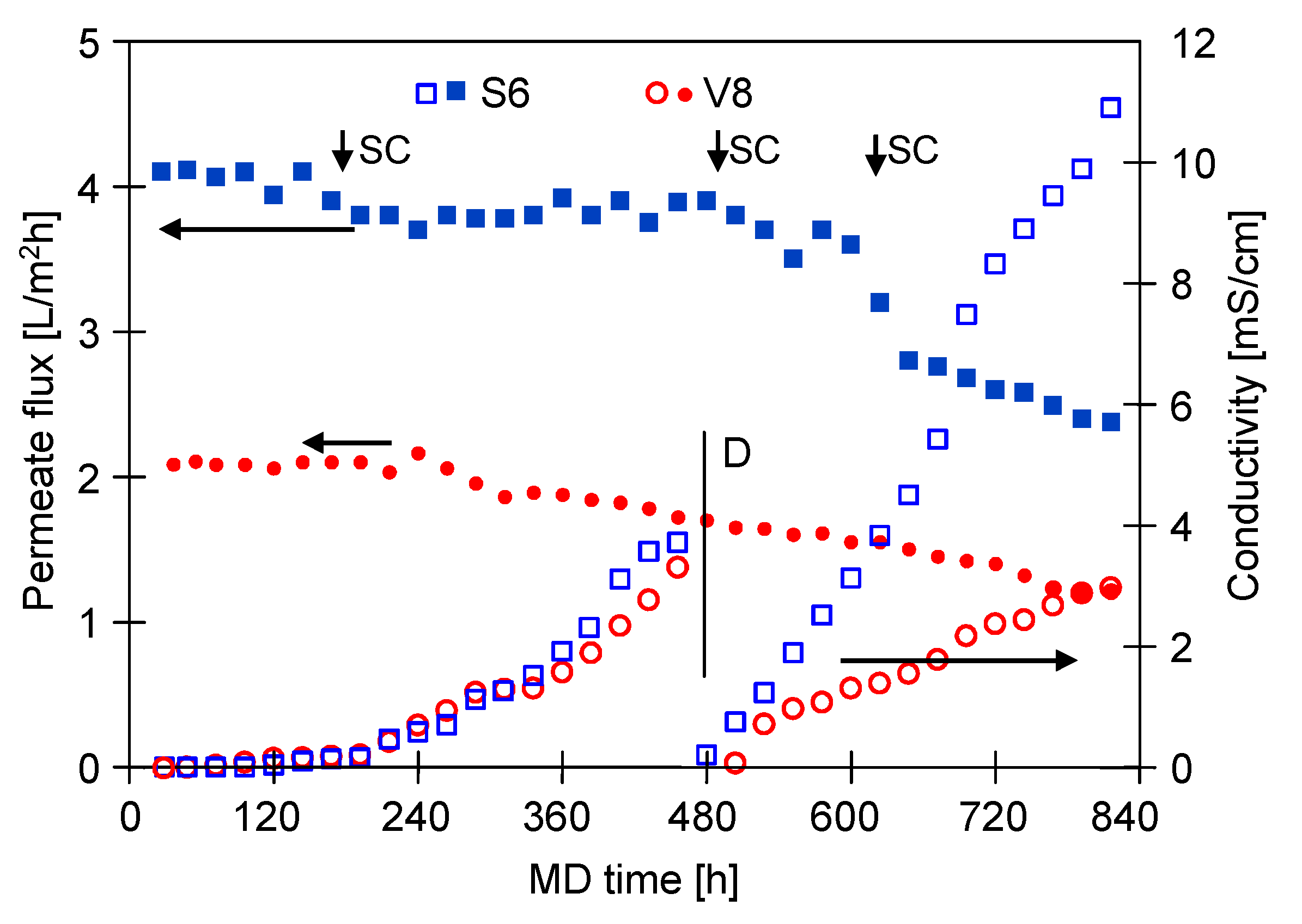
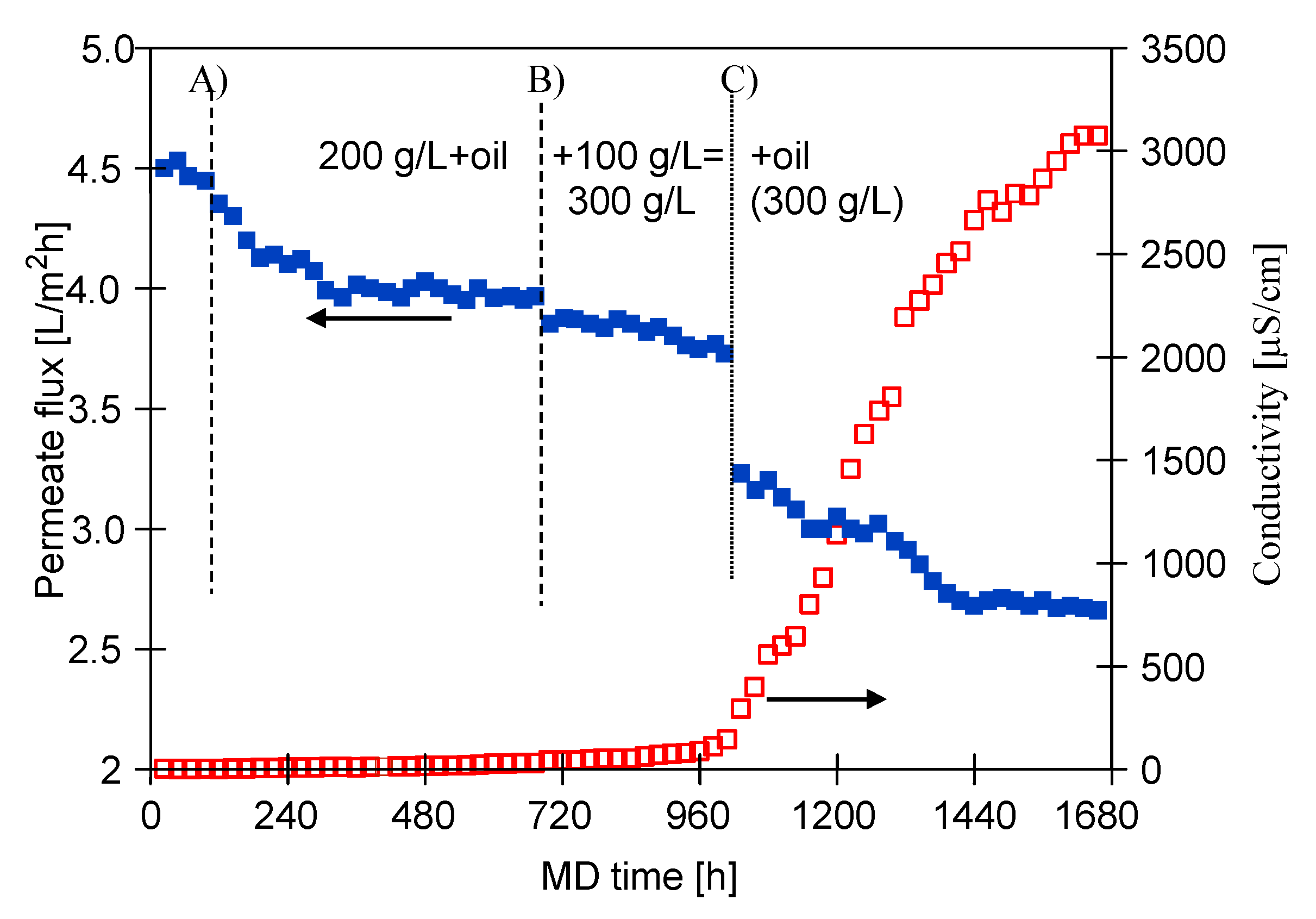
3.1. MD Process Efficiency
3.1.1. The Capillary Module
3.1.2. The Submerged Module
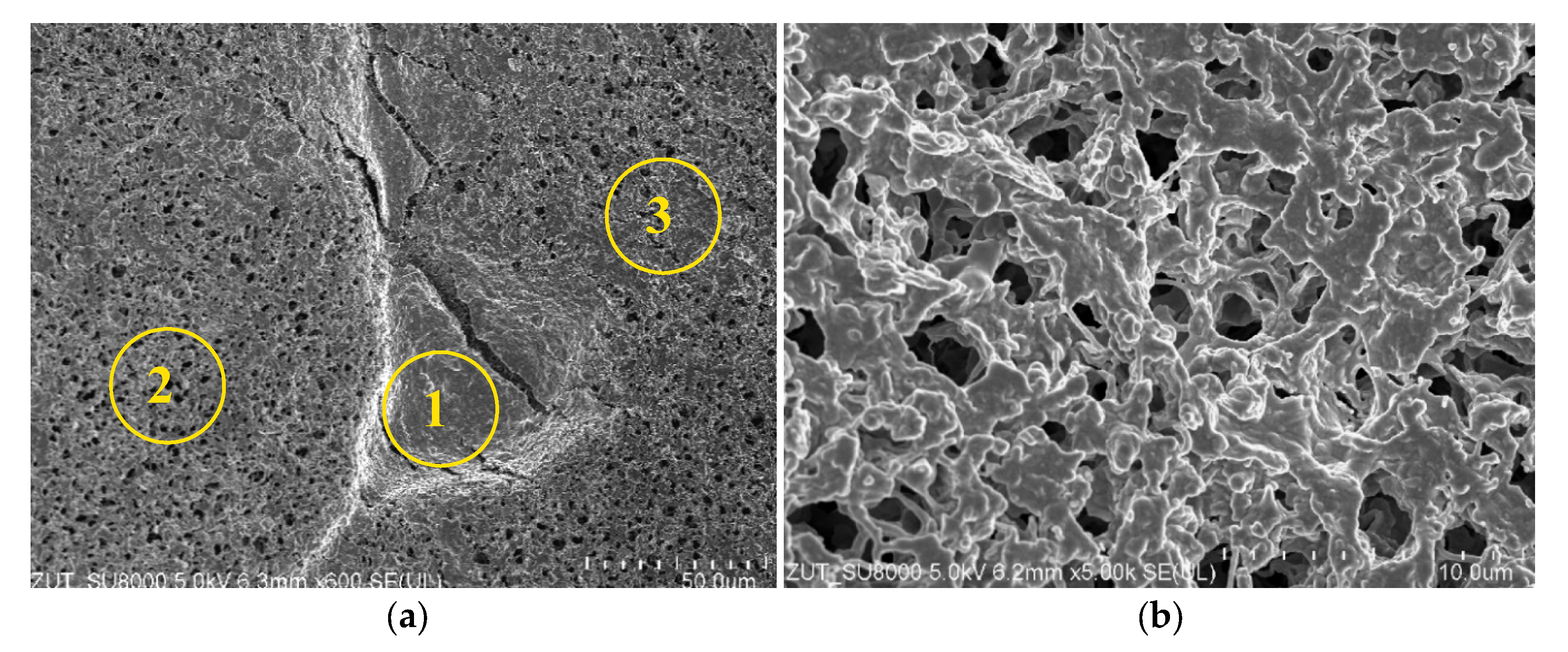
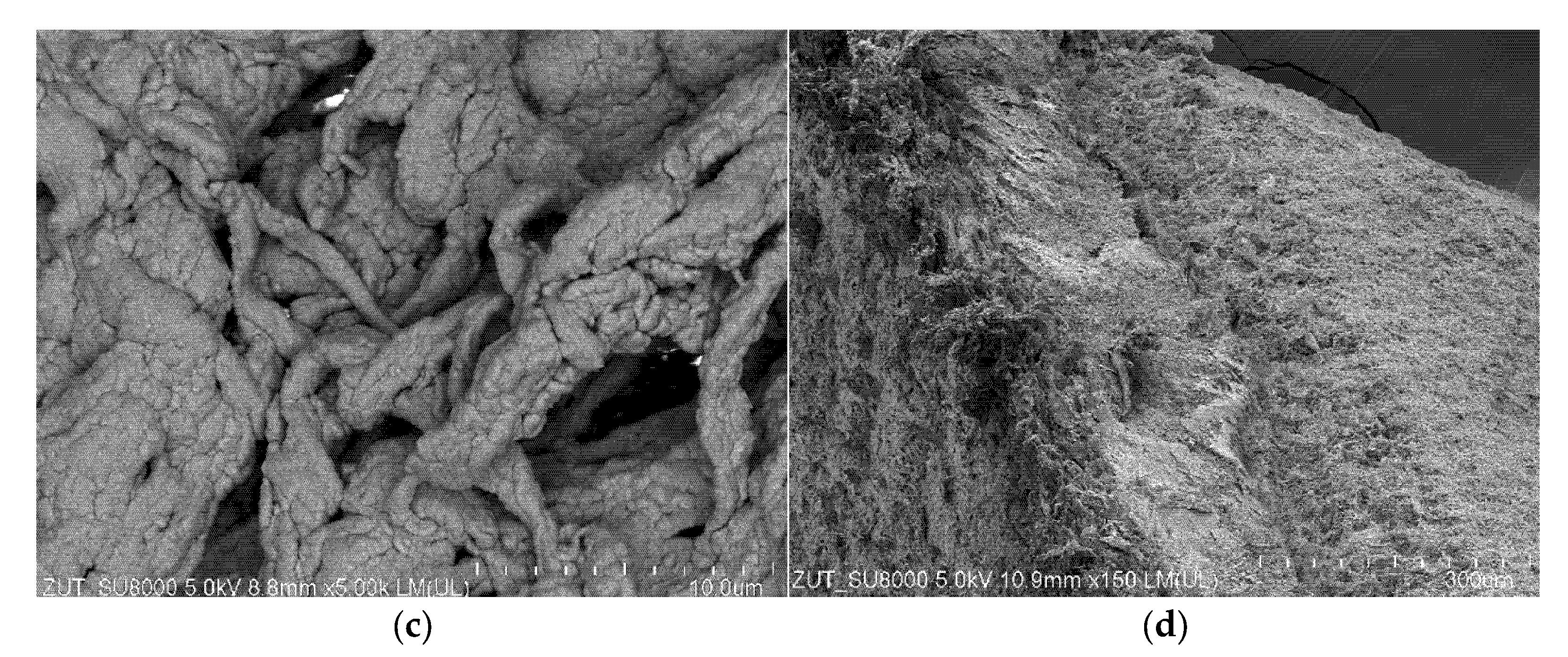
3.2. Membranes Scaling
3.2.1. The Capillary Module
3.2.2. The Submerged Module
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Belessiotis, V.; Kalogirou, S.; Delyannis, E. Membrane Distillation. In Thermal Solar Desalination; Elsevier: Amsterdam, The Netherlands, 2016; pp. 191–251. ISBN 978-0-12-809656-7. [Google Scholar]
- Soukane, S.; Elcik, H.; Alpatova, A.; Orfi, J.; Ali, E.; AlAnsary, H.; Ghaffour, N. Scaling Sets the Limits of Large Scale Membrane Distillation Modules for the Treatment of High Salinity Feeds. J. Clean. Prod. 2021, 287, 125555. [Google Scholar] [CrossRef]
- Zhao, S.; Jiang, C.; Fan, J.; Hong, S.; Mei, P.; Yao, R.; Liu, Y.; Zhang, S.; Li, H.; Zhang, H.; et al. Hydrophilicity Gradient in Covalent Organic Frameworks for Membrane Distillation. Nat. Mater. 2021, 20, 1551–1558. [Google Scholar] [CrossRef]
- Liu, L.; Xiao, Z.; Liu, Y.; Li, X.; Yin, H.; Volkov, A.; He, T. Understanding the Fouling/Scaling Resistance of Superhydrophobic/Omniphobic Membranes in Membrane Distillation. Desalination 2021, 499, 114864. [Google Scholar] [CrossRef]
- Elcik, H.; Fortunato, L.; Alpatova, A.; Soukane, S.; Orfi, J.; Ali, E.; AlAnsary, H.; Leiknes, T.; Ghaffour, N. Multi-Effect Distillation Brine Treatment by Membrane Distillation: Effect of Antiscalant and Antifoaming Agents on Membrane Performance and Scaling Control. Desalination 2020, 493, 114653. [Google Scholar] [CrossRef]
- Al-Furaiji, M.; Arena, J.T.; Ren, J.; Benes, N.; Nijmeijer, A.; McCutcheon, J.R. Triple-Layer Nanofiber Membranes for Treating High Salinity Brines Using Direct Contact Membrane Distillation. Membranes 2019, 9, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Wang, Z.; Jennings, G.K.; Lin, S. Probing Pore Wetting in Membrane Distillation Using Impedance: Early Detection and Mechanism of Surfactant-Induced Wetting. Environ. Sci. Technol. Lett. 2017, 4, 505–510. [Google Scholar] [CrossRef]
- Choi, Y.; Naidu, G.; Jeong, S.; Vigneswaran, S.; Lee, S.; Wang, R.; Fane, A.G. Experimental Comparison of Submerged Membrane Distillation Configurations for Concentrated Brine Treatment. Desalination 2017, 420, 54–62. [Google Scholar] [CrossRef]
- Guan, Y.; Li, J.; Cheng, F.; Zhao, J.; Wang, X. Influence of Salt Concentration on DCMD Performance for Treatment of Highly Concentrated NaCl, KCl, MgCl2 and MgSO4 Solutions. Desalination 2015, 355, 110–117. [Google Scholar] [CrossRef]
- Bush, J.A.; Vanneste, J.; Cath, T.Y. Membrane Distillation for Concentration of Hypersaline Brines from the Great Salt Lake: Effects of Scaling and Fouling on Performance, Efficiency, and Salt Rejection. Sep. Purif. Technol. 2016, 170, 78–91. [Google Scholar] [CrossRef] [Green Version]
- Ge, J.; Peng, Y.; Li, Z.; Chen, P.; Wang, S. Membrane Fouling and Wetting in a DCMD Process for RO Brine Concentration. Desalination 2014, 344, 97–107. [Google Scholar] [CrossRef]
- Minier-Matar, J.; Hussain, A.; Janson, A.; Benyahia, F.; Adham, S. Field Evaluation of Membrane Distillation Technologies for Desalination of Highly Saline Brines. Desalination 2014, 351, 101–108. [Google Scholar] [CrossRef]
- Chen, Y.-R.; Xin, R.; Huang, X.; Zuo, K.; Tung, K.-L.; Li, Q. Wetting-Resistant Photothermal Nanocomposite Membranes for Direct Solar Membrane Distillation. J. Membr. Sci. 2021, 620, 118913. [Google Scholar] [CrossRef]
- Hou, D.; Yuan, Z.; Tang, M.; Wang, K.; Wang, J. Effect and Mechanism of an Anionic Surfactant on Membrane Performance during Direct Contact Membrane Distillation. J. Membr. Sci. 2020, 595, 117495. [Google Scholar] [CrossRef]
- Madalosso, H.B.; Machado, R.; Hotza, D.; Marangoni, C. Membrane Surface Modification by Electrospinning, Coating, and Plasma for Membrane Distillation Applications: A State-of-the-Art Review. Adv. Eng. Mater. 2021, 23, 2001456. [Google Scholar] [CrossRef]
- Macedonio, F.; Ali, A.; Poerio, T.; El-Sayed, E.; Drioli, E.; Abdel-Jawad, M. Direct Contact Membrane Distillation for Treatment of Oilfield Produced Water. Sep. Purif. Technol. 2014, 126, 69–81. [Google Scholar] [CrossRef]
- Costa, F.C.R.; Ricci, B.C.; Teodoro, B.; Koch, K.; Drewes, J.E.; Amaral, M.C.S. Biofouling in Membrane Distillation Applications—A Review. Desalination 2021, 516, 115241. [Google Scholar] [CrossRef]
- Sparenberg, M.-C.; Hanot, B.; Molina-Fernández, C.; Luis, P. Experimental Mass Transfer Comparison between Vacuum and Direct Contact Membrane Distillation for the Concentration of Carbonate Solutions. Sep. Purif. Technol. 2021, 275, 119193. [Google Scholar] [CrossRef]
- Kharraz, J.A.; Farid, M.U.; Jassby, D.; An, A.K. A Systematic Study on the Impact of Feed Composition and Substrate Wettability on Wetting and Fouling of Omniphobic and Janus Membranes in Membrane Distillation. J. Membr. Sci. 2022, 641, 119873. [Google Scholar] [CrossRef]
- Yao, M.; Tijing, L.D.; Naidu, G.; Kim, S.-H.; Matsuyama, H.; Fane, A.G.; Shon, H.K. A Review of Membrane Wettability for the Treatment of Saline Water Deploying Membrane Distillation. Desalination 2020, 479, 114312. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, P.; Li, R.; Li, X. Characterization and Mitigation of the Fouling of Flat-Sheet Ceramic Membranes for Direct Filtration of the Coagulated Domestic Wastewater. J. Hazard. Mater. 2020, 385, 121557. [Google Scholar] [CrossRef] [PubMed]
- Usman, H.S.; Touati, K.; Rahaman, M.S. An Economic Evaluation of Renewable Energy-Powered Membrane Distillation for Desalination of Brackish Water. Renew. Energy 2021, 169, 1294–1304. [Google Scholar] [CrossRef]
- Ravi, J.; Othman, M.H.D.; Matsuura, T.; Ro’il Bilad, M.; El-badawy, T.H.; Aziz, F.; Ismail, A.F.; Rahman, M.A.; Jaafar, J. Polymeric Membranes for Desalination Using Membrane Distillation: A Review. Desalination 2020, 490, 114530. [Google Scholar] [CrossRef]
- Gryta, M. Surface Modification of Polypropylene Membrane by Helium Plasma Treatment for Membrane Distillation. J. Membr. Sci. 2021, 628, 119265. [Google Scholar] [CrossRef]
- Gryta, M. Application of Polypropylene Membranes Hydrophilized by Plasma for Water Desalination by Membrane Distillation. Desalination 2021, 515, 115187. [Google Scholar] [CrossRef]
- Gryta, M.; Tomczak, W. Stability of Ar/O2 Plasma-Treated Polypropylene Membranes Applied for Membrane Distillation. Membranes 2021, 11, 531. [Google Scholar] [CrossRef] [PubMed]
- Chul Woo, Y.; Chen, Y.; Tijing, L.D.; Phuntsho, S.; He, T.; Choi, J.-S.; Kim, S.-H.; Kyong Shon, H. CF4 Plasma-Modified Omniphobic Electrospun Nanofiber Membrane for Produced Water Brine Treatment by Membrane Distillation. J. Membr. Sci. 2017, 529, 234–242. [Google Scholar] [CrossRef]
- Pedram, S.; Mortaheb, H.R.; Arefi-Khonsari, F. Plasma Treatment of Polyethersulfone Membrane for Benzene Removal from Water by Air Gap Membrane Distillation. Environ. Technol. 2018, 39, 157–171. [Google Scholar] [CrossRef] [Green Version]
- Jeong, S.; Shin, B.; Jo, W.; Kim, H.-Y.; Moon, M.-W.; Lee, S. Nanostructured PVDF Membrane for MD Application by an O2 and CF4 Plasma Treatment. Desalination 2016, 399, 178–184. [Google Scholar] [CrossRef]
- Elmarghany, M.; El-Shazly, A.H.; Rajabzadeh, S.; Salem, M.S.; Shouman, M.A.; Nabil Sabry, M.; Matsuyama, H.; Nady, N. Triple-Layer Nanocomposite Membrane Prepared by Electrospinning Based on Modified PES with Carbon Nanotubes for Membrane Distillation Applications. Membranes 2020, 10, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, X.; Liu, Z.; Hu, Y. Amphiphobic Surface Modification of Electrospun Nanofibrous Membranes for Anti-Wetting Performance in Membrane Distillation. Desalination 2018, 432, 23–31. [Google Scholar] [CrossRef]
- Li, K.; Hou, D.; Fu, C.; Wang, K.; Wang, J. Fabrication of PVDF Nanofibrous Hydrophobic Composite Membranes Reinforced with Fabric Substrates via Electrospinning for Membrane Distillation Desalination. J. Environ. Sci. 2019, 75, 277–288. [Google Scholar] [CrossRef]
- Contreras-Martínez, J.; García-Payo, C.; Khayet, M. Electrospun Nanostructured Membrane Engineering Using Reverse Osmosis Recycled Modules: Membrane Distillation Application. Nanomaterials 2021, 11, 1601. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Zhu, Z.; Zhong, L.; Zhang, W.; Wang, W. Design of Firm-Pore Superhydrophobic Fibrous Membrane for Advancing the Durability of Membrane Distillation. Desalination 2021, 519, 115185. [Google Scholar] [CrossRef]
- Kusuma, N.C.; Purwanto, M.; Sudrajat, M.A.; Jaafar, J.; Othman, M.H.D.; Aziz, M.H.A.; Raharjo, Y.; Qtaishat, M.R. Fabrication and Characterization of Modified PVDF Hollow Fiber Membrane Coated with Hydrophobic Surface Modifying Macromolecules for Desalination Application. J. Environ. Chem. Eng. 2021, 9, 105582. [Google Scholar] [CrossRef]
- Lee, H.K.; Kim, W.; Kim, Y.M.; Kwon, Y.-N. Surface Modification of Polyvinylidene Fluoride Membrane for Enhanced Wetting Resistance. Appl. Surf. Sci. 2019, 491, 32–42. [Google Scholar] [CrossRef]
- Jiao, L.; Yan, K.; Wang, J.; Lin, S.; Li, G.; Bi, F.; Zhang, L. Low Surface Energy Nanofibrous Membrane for Enhanced Wetting Resistance in Membrane Distillation Process. Desalination 2020, 476, 114210. [Google Scholar] [CrossRef]
- Hussein, S.S.; Ibrahim, S.S.; Toma, M.A.; Alsalhy, Q.F.; Drioli, E. Novel Chemical Modification of Polyvinyl Chloride Membrane by Free Radical Graft Copolymerization for Direct Contact Membrane Distillation (DCMD) Application. J. Membr. Sci. 2020, 611, 118266. [Google Scholar] [CrossRef]
- Rastegarpanah, A.; Mortaheb, H.R. Surface Treatment of Polyethersulfone Membranes for Applying in Desalination by Direct Contact Membrane Distillation. Desalination 2016, 377, 99–107. [Google Scholar] [CrossRef]
- Zhang, J.; Li, N.; Wang, D.; Li, J.; Chen, Y.; Wang, Z. Omniphobic Palygorskite Coated Janus Membrane with Enhanced Fouling and Wetting Resistance for Direct Contact Membrane Distillation. Desalination 2021, 505, 114986. [Google Scholar] [CrossRef]
- Tang, M.; Zheng, L.; Hou, D.; Jia, X.; Wang, J. Microstructure Design and Construction of Anti-Wetting and Anti-Fouling Multifunctional Janus Membrane for Robust Membrane Distillation. Chem. Eng. J. 2022, 430, 132973. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhong, L.; Chen, X.; Zheng, W.; Zuo, J.; Zeng, G.; Wang, W. Monolithic and Self-Roughened Janus Fibrous Membrane with Superhydrophilic/Omniphobic Surface for Robust Antifouling and Antiwetting Membrane Distillation. J. Membr. Sci. 2020, 615, 118499. [Google Scholar] [CrossRef]
- Feng, D.; Chen, Y.; Wang, Z.; Lin, S. Janus Membrane with a Dense Hydrophilic Surface Layer for Robust Fouling and Wetting Resistance in Membrane Distillation: New Insights into Wetting Resistance. Environ. Sci. Technol. 2021, 55, 14156–14164. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lu, K.-J.; Liang, C.Z.; Chung, T.-S. Mechanically Strong Janus Tri-Bore Hollow Fiber Membranes with Asymmetric Pores for Anti-Wetting and Anti-Fouling Membrane Distillation. Chem. Eng. J. 2022, 429, 132455. [Google Scholar] [CrossRef]
- Qing, W.; Wu, Y.; Li, X.; Shi, X.; Shao, S.; Mei, Y.; Zhang, W.; Tang, C.Y. Omniphobic PVDF Nanofibrous Membrane for Superior Anti-Wetting Performance in Direct Contact Membrane Distillation. J. Membr. Sci. 2020, 608, 118226. [Google Scholar] [CrossRef]
- Lin, S.; Nejati, S.; Boo, C.; Hu, Y.; Osuji, C.O.; Elimelech, M. Omniphobic Membrane for Robust Membrane Distillation. Environ. Sci. Technol. Lett. 2014, 1, 443–447. [Google Scholar] [CrossRef]
- Kalla, S. Use of Membrane Distillation for Oily Wastewater Treatment—A Review. J. Environ. Chem. Eng. 2021, 9, 104641. [Google Scholar] [CrossRef]
- Bodell, B.R. Silicon Rubber Vapour Diffusion in Saline Water Distillation. U.S. Patent 3,361,645, 3 June 1963. [Google Scholar]
- Wae AbdulKadir, W.A.F.; Ahmad, A.L.; Seng, O.B.; Che Lah, N.F. Biomimetic Hydrophobic Membrane: A Review of Anti-Wetting Properties as a Potential Factor in Membrane Development for Membrane Distillation (MD). J. Ind. Eng. Chem. 2020, 91, 15–36. [Google Scholar] [CrossRef]
- Tan, Y.Z.; Velioglu, S.; Han, L.; Joseph, B.D.; Unnithan, L.G.; Chew, J.W. Effect of Surfactant Hydrophobicity and Charge Type on Membrane Distillation Performance. J. Membr. Sci. 2019, 587, 117168. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Y.; Sun, X.; Duddu, R.; Lin, S. Mechanism of Pore Wetting in Membrane Distillation with Alcohol vs. Surfactant. J. Membr. Sci. 2018, 559, 183–195. [Google Scholar] [CrossRef]
- Rezaei, M.; Warsinger, D.M.; Lienhard, J.H., V; Duke, M.C.; Matsuura, T.; Samhaber, W.M. Wetting Phenomena in Membrane Distillation: Mechanisms, Reversal, and Prevention. Water Res. 2018, 139, 329–352. [Google Scholar] [CrossRef]
- Afsari, M.; Shon, H.K.; Tijing, L.D. Janus Membranes for Membrane Distillation: Recent Advances and Challenges. Adv. Colloid Interface Sci. 2021, 289, 102362. [Google Scholar] [CrossRef] [PubMed]
- Rajwade, K.; Barrios, A.C.; Garcia-Segura, S.; Perreault, F. Pore Wetting in Membrane Distillation Treatment of Municipal Wastewater Desalination Brine and Its Mitigation by Foam Fractionation. Chemosphere 2020, 257, 127214. [Google Scholar] [CrossRef]
- Reza Shirzad Kebria, M.; Rahimpour, A. Membrane Distillation: Basics, Advances, and Applications. In Advances in Membrane Technologies; Abdelrasoul, A., Ed.; IntechOpen: London, UK, 2020; ISBN 978-1-78984-806-9. [Google Scholar]
- Horseman, T.; Yin, Y.; Christie, K.S.; Wang, Z.; Tong, T.; Lin, S. Wetting, Scaling, and Fouling in Membrane Distillation: State-of-the-Art Insights on Fundamental Mechanisms and Mitigation Strategies. ACS EST Eng. 2021, 1, 117–140. [Google Scholar] [CrossRef]
- Chamani, H.; Woloszyn, J.; Matsuura, T.; Rana, D.; Lan, C.Q. Pore Wetting in Membrane Distillation: A Comprehensive Review. Prog. Mater. Sci. 2021, 122, 100843. [Google Scholar] [CrossRef]
- Varjani, S.; Rakholiya, P.; Yong Ng, H.; Taherzadeh, M.J.; Hao Ngo, H.; Chang, J.-S.; Wong, J.W.C.; You, S.; Teixeira, J.A.; Bui, X.-T. Bio-Based Rhamnolipids Production and Recovery from Waste Streams: Status and Perspectives. Bioresour. Technol. 2021, 319, 124213. [Google Scholar] [CrossRef] [PubMed]
- Moldes, A.B.; Rodríguez-López, L.; Rincón-Fontán, M.; López-Prieto, A.; Vecino, X.; Cruz, J.M. Synthetic and Bio-Derived Surfactants Versus Microbial Biosurfactants in the Cosmetic Industry: An Overview. Int. J. Mol. Sci. 2021, 22, 2371. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.; Trybala, A.; Starov, V.; Pinfield, V.J. Effect of Synthetic Surfactants on the Environment and the Potential for Substitution by Biosurfactants. Adv. Colloid Interface Sci. 2021, 288, 102340. [Google Scholar] [CrossRef]
- Shaban, S.M.; Kang, J.; Kim, D.-H. Surfactants: Recent Advances and Their Applications. Compos. Commun. 2020, 22, 100537. [Google Scholar] [CrossRef]
- Atta, D.Y.; Negash, B.M.; Yekeen, N.; Habte, A.D. A State-of-the-Art Review on the Application of Natural Surfactants in Enhanced Oil Recovery. J. Mol. Liq. 2021, 321, 114888. [Google Scholar] [CrossRef]
- Esfandyari, H.; Moghani Rahimi, A.; Esmaeilzadeh, F.; Davarpanah, A.; Mohammadi, A.H. Amphoteric and Cationic Surfactants for Enhancing Oil Recovery from Carbonate Oil Reservoirs. J. Mol. Liq. 2021, 322, 114518. [Google Scholar] [CrossRef]
- Pan, F.; Zhang, Z.; Zhang, X.; Davarpanah, A. Impact of Anionic and Cationic Surfactants Interfacial Tension on the Oil Recovery Enhancement. Powder Technol. 2020, 373, 93–98. [Google Scholar] [CrossRef]
- Gong, L.; Liao, G.; Luan, H.; Chen, Q.; Nie, X.; Liu, D.; Feng, Y. Oil Solubilization in Sodium Dodecylbenzenesulfonate Micelles: New Insights into Surfactant Enhanced Oil Recovery. J. Colloid Interface Sci. 2020, 569, 219–228. [Google Scholar] [CrossRef]
- Zulkifli, N.N.; Mahmood, S.M.; Akbari, S.; Manap, A.A.A.; Kechut, N.I.; Elrais, K.A. Evaluation of New Surfactants for Enhanced Oil Recovery Applications in High-Temperature Reservoirs. J. Pet. Explor Prod. Technol 2020, 10, 283–296. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Xu, P.; Wang, H. Interplay of the Factors Affecting Water Flux and Salt Rejection in Membrane Distillation: A State-of-the-Art Critical Review. Water 2020, 12, 2841. [Google Scholar] [CrossRef]
- Kinoshita, K.; Parra, E.; Needham, D. Adsorption of Ionic Surfactants at Microscopic Air-Water Interfaces Using the Micropipette Interfacial Area-Expansion Method: Measurement of the Diffusion Coefficient and Renormalization of the Mean Ionic Activity for SDS. J. Colloid Interface Sci. 2017, 504, 765–779. [Google Scholar] [CrossRef] [PubMed]
- Umar, A.A.; Saaid, I.B.M.; Sulaimon, A.A.; Pilus, R.B.M. A Review of Petroleum Emulsions and Recent Progress on Water-in-Crude Oil Emulsions Stabilized by Natural Surfactants and Solids. J. Pet. Sci. Eng. 2018, 165, 673–690. [Google Scholar] [CrossRef]
- Schroën, K.; de Ruiter, J.; Berton-Carabin, C. The Importance of Interfacial Tension in Emulsification: Connecting Scaling Relations Used in Large Scale Preparation with Microfluidic Measurement Methods. ChemEngineering 2020, 4, 63. [Google Scholar] [CrossRef]
- Chew, N.G.P.; Zhao, S.; Loh, C.H.; Permogorov, N.; Wang, R. Surfactant Effects on Water Recovery from Produced Water via Direct-Contact Membrane Distillation. J. Membr. Sci. 2017, 528, 126–134. [Google Scholar] [CrossRef]
- Eykens, L.; De Sitter, K.; Dotremont, C.; De Schepper, W.; Pinoy, L.; Van Der Bruggen, B. Wetting Resistance of Commercial Membrane Distillation Membranes in Waste Streams Containing Surfactants and Oil. Appl. Sci. 2017, 7, 118. [Google Scholar] [CrossRef] [Green Version]
- Han, L.; Tan, Y.Z.; Netke, T.; Fane, A.G.; Chew, J.W. Understanding Oily Wastewater Treatment via Membrane Distillation. J. Membr. Sci. 2017, 539, 284–294. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhong, L.; Horseman, T.; Liu, Z.; Zeng, G.; Li, Z.; Lin, S.; Wang, W. Superhydrophobic-Omniphobic Membrane with Anti-Deformable Pores for Membrane Distillation with Excellent Wetting Resistance. J. Membr. Sci. 2021, 620, 118768. [Google Scholar] [CrossRef]
- Huang, Y.-X.; Wang, Z.; Jin, J.; Lin, S. Novel Janus Membrane for Membrane Distillation with Simultaneous Fouling and Wetting Resistance. Environ. Sci. Technol. 2017, 51, 13304–13310. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Wang, Y.; Liao, Y.; You, X.; Yao, L.; Razaqpur, A.G. Effects of Different Surfactant Properties on Anti-Wetting Behaviours of an Omniphobic Membrane in Membrane Distillation. J. Membr. Sci. 2021, 634, 119433. [Google Scholar] [CrossRef]
- McGaughey, A.L.; Gustafson, R.D.; Childress, A.E. Effect of Long-Term Operation on Membrane Surface Characteristics and Performance in Membrane Distillation. J. Membr. Sci. 2017, 543, 143–150. [Google Scholar] [CrossRef]
- Gryta, M.; Barancewicz, M. Influence of Morphology of PVDF Capillary Membranes on the Performance of Direct Contact Membrane Distillation. J. Membr. Sci. 2010, 358, 158–167. [Google Scholar] [CrossRef]
- Gryta, M. Resistance of Polypropylene Membrane to Oil Fouling during Membrane Distillation. Membranes 2021, 11, 552. [Google Scholar] [CrossRef] [PubMed]
- Gryta, M.; Grzechulska-Damszel, J.; Markowska, A.; Karakulski, K. The Influence of Polypropylene Degradation on the Membrane Wettability during Membrane Distillation. J. Membr. Sci. 2009, 326, 493–502. [Google Scholar] [CrossRef]
- Tiselius, P.; Magnusson, K. Toxicity of Treated Bilge Water: The Need for Revised Regulatory Control. Mar. Pollut. Bull. 2017, 114, 860–866. [Google Scholar] [CrossRef]
- Nowrouzi, I.; Manshad, A.K.; Mohammadi, A.H. Effects of Ions and Dissolved Carbon Dioxide in Brine on Wettability Alteration, Contact Angle and Oil Production in Smart Water and Carbonated Smart Water Injection Processes in Carbonate Oil Reservoirs. Fuel 2019, 235, 1039–1051. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Darwish, N.; Hilal, N. Membrane Distillation: A Comprehensive Review. Desalination 2012, 287, 2–18. [Google Scholar] [CrossRef]
- Macedonio, F. Direct Contact Membrane Distillation (DCMD). In Encyclopedia of Membranes; Drioli, E., Giorno, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–3. ISBN 978-3-642-40872-4. [Google Scholar]
- Zmievskii, Y.G. Determination of Critical Pressure in Membrane Distillation Process. Pet. Chem. 2015, 55, 308–314. [Google Scholar] [CrossRef]
- Gryta, M. Capillary Polypropylene Membranes for Membrane Distillation. Fibers 2018, 7, 1. [Google Scholar] [CrossRef] [Green Version]
- Sulaimon, A.A.; Adeyemi, B.J. Effects of Interfacial Tension Alteration on the Destabilization of Water-Oil Emulsions. In Science and Technology Behind Nanoemulsions; Karakuş, S., Ed.; IntechOpen: London, UK, 2018; ISBN 978-1-78923-570-8. [Google Scholar]
- Costa, C.; Medronho, B.; Filipe, A.; Mira, I.; Lindman, B.; Edlund, H.; Norgren, M. Emulsion Formation and Stabilization by Biomolecules: The Leading Role of Cellulose. Polymers 2019, 11, 1570. [Google Scholar] [CrossRef] [Green Version]
- Qtaishat, M.R.; Matsuura, T. Modelling of Pore Wetting in Membrane Distillation Compared with Pervaporation. In Pervaporation, Vapour Permeation and Membrane Distillation; Elsevier: Amsterdam, The Netherlands, 2015; pp. 385–413. ISBN 978-1-78242-246-4. [Google Scholar]
- Gryta, M. The Application of Submerged Modules for Membrane Distillation. Membranes 2020, 10, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, S.; McClements, D.J. Influence of Disperse Phase Transfer on Properties of Nanoemulsions Containing Oil Droplets with Different Compositions and Physical States. Food Biophys. 2019, 14, 355–364. [Google Scholar] [CrossRef]
- Velioğlu, S.; Han, L.; Chew, J.W. Understanding Membrane Pore-Wetting in the Membrane Distillation of Oil Emulsions via Molecular Dynamics Simulations. J. Membr. Sci. 2018, 551, 76–84. [Google Scholar] [CrossRef]
- Mahmoudvand, M.; Javadi, A.; Pourafshary, P. Brine Ions Impacts on Water-Oil Dynamic Interfacial Properties Considering Asphaltene and Maltene Constituents. Colloids Surf. A Physicochem. Eng. Asp. 2019, 579, 123665. [Google Scholar] [CrossRef]
- Jha, N.K.; Iglauer, S.; Sangwai, J.S. Effect of Monovalent and Divalent Salts on the Interfacial Tension of n-Heptane against Aqueous Anionic Surfactant Solutions. J. Chem. Eng. Data 2018, 63, 2341–2350. [Google Scholar] [CrossRef]
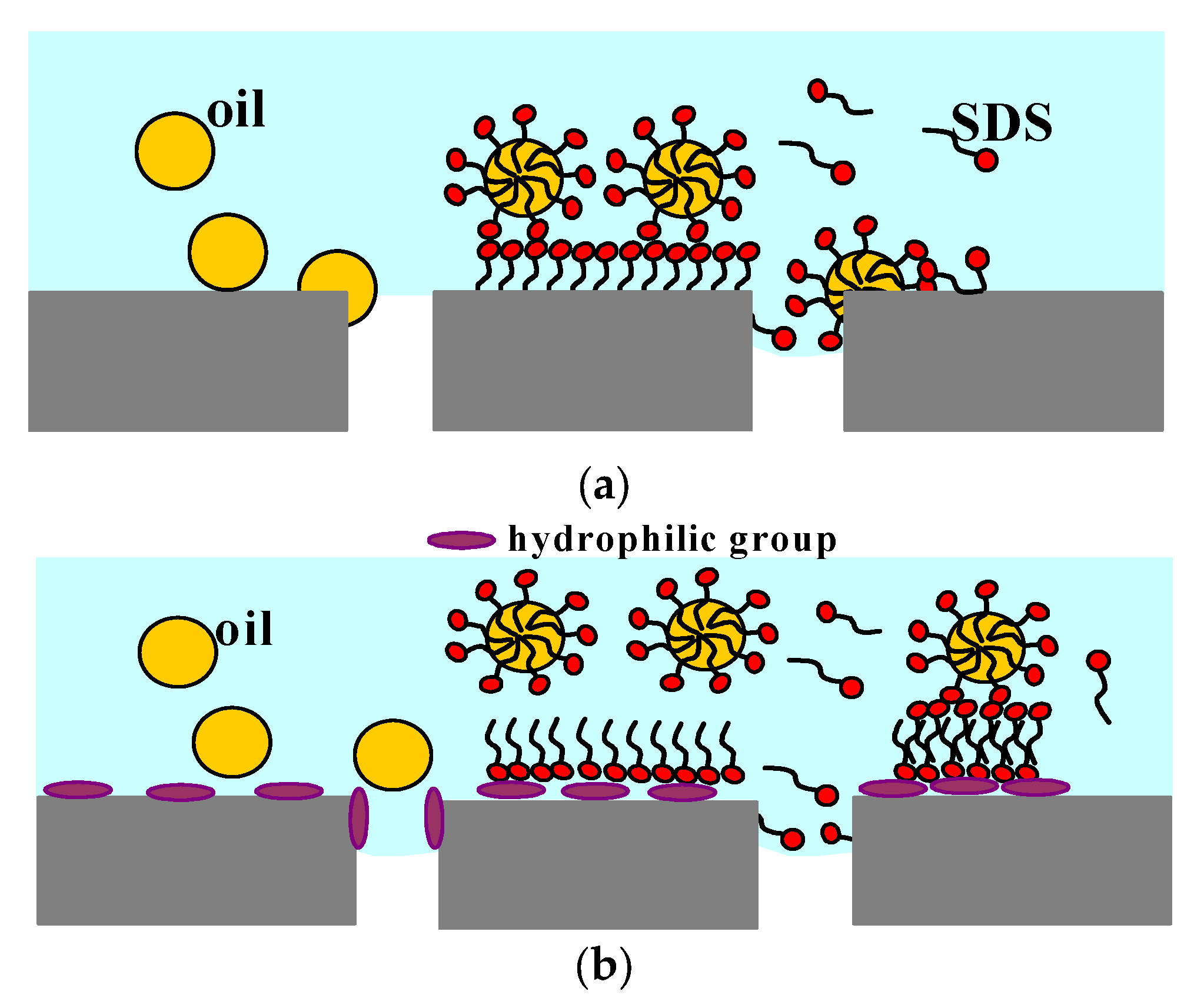
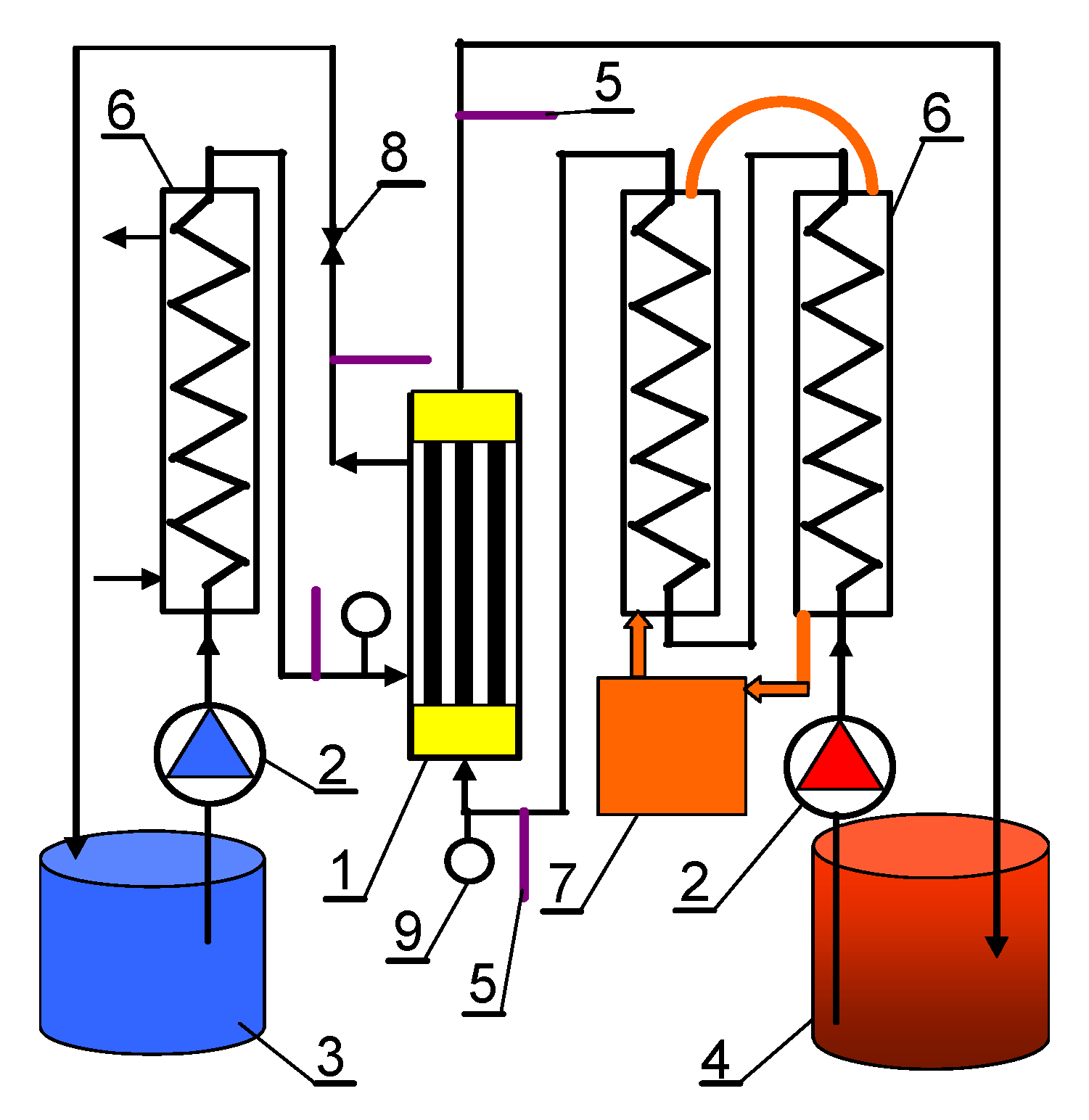



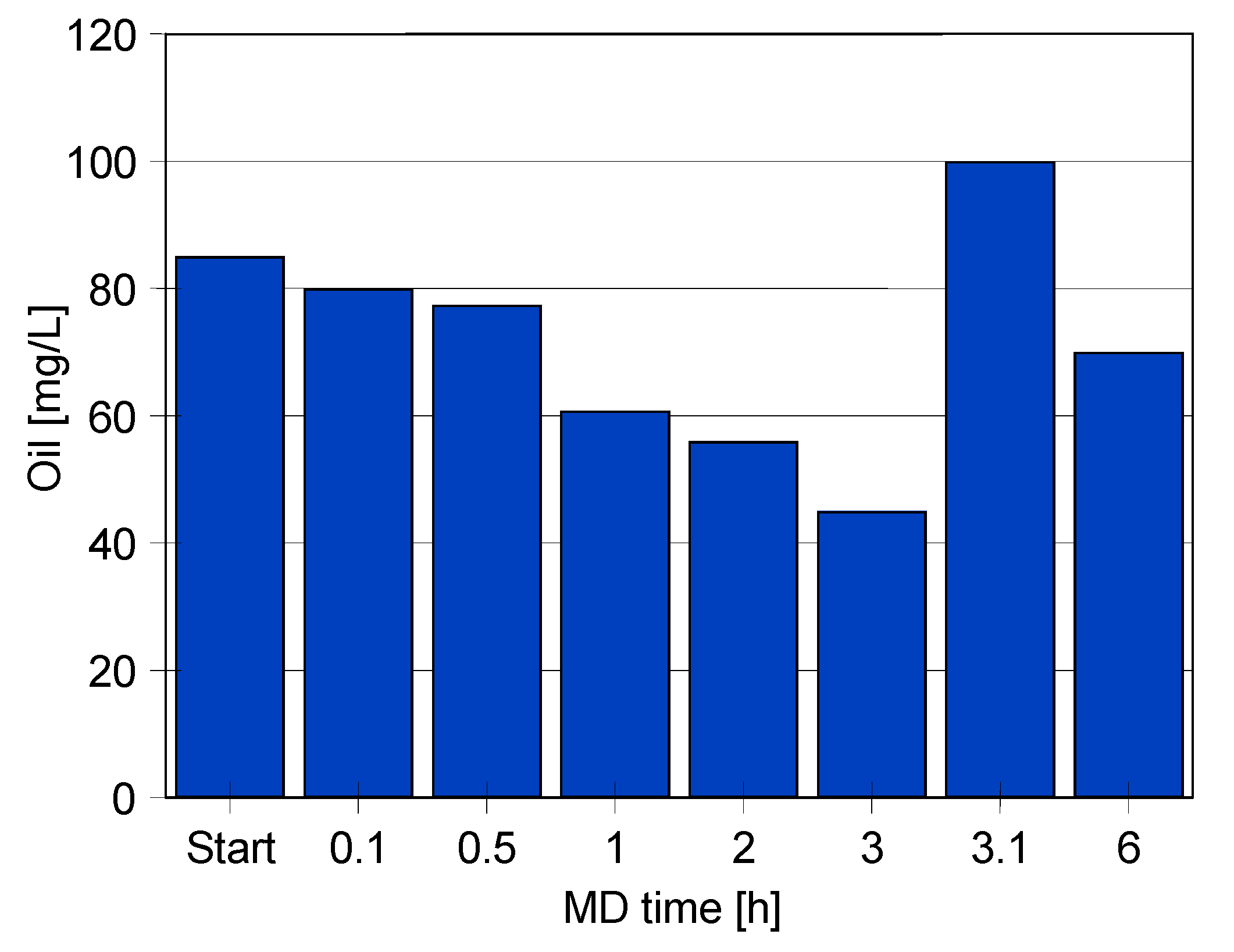
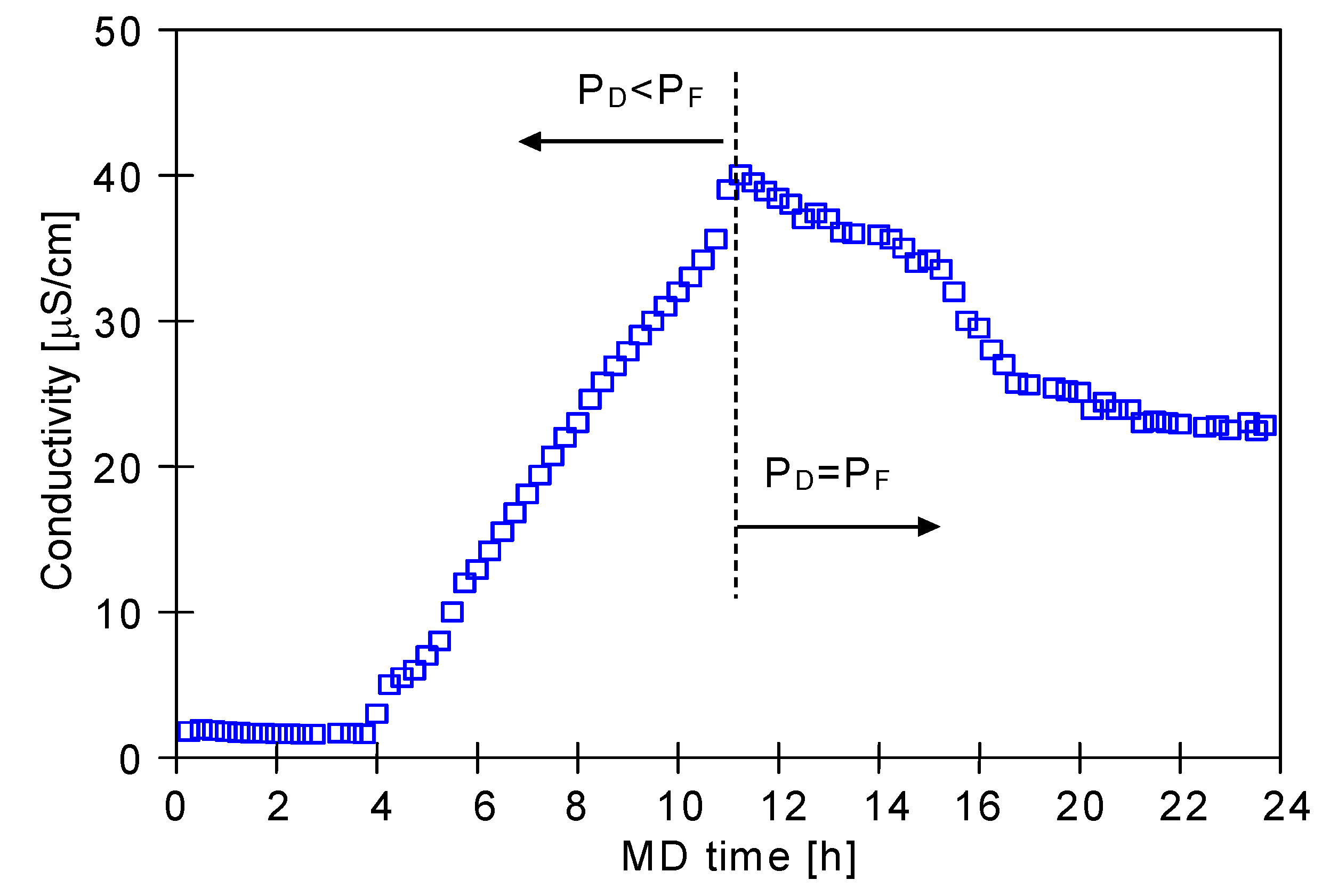





| Module | Membrane | No. of Capillaries | Length(m) | Area (Inside) (m2) | Housing Diameter (m) |
|---|---|---|---|---|---|
| CMD | Accurel PP V8/2 HF | 3 | 0.095 | 49.2 × 10–3 | 0.02 |
| SMD-V8 | Accurel PP V8/2 HF | 1 | 0.25 | 43.2 × 10–3 | not applicable |
| SMD-S6 | Accurel PP S6/2 | 3 | 0.25 | 42.4 × 10–3 | not applicable |
| Ion | Na+ | Cl− | SO42− | Mg2+ | Ca2+ | K+ | PO43− |
|---|---|---|---|---|---|---|---|
| Concentration (mg/L) | 2329 | 3720 | 538 | 504 | 137 | 78 | 7.5 |
| Area | Si | S | Cl | Ca | K | Na | C |
|---|---|---|---|---|---|---|---|
| 1 | 26.40 | 9.00 | 30.60 | 14.70 | 1.20 | 2.10 | 16.00 |
| 2 | 0.26 | 0.10 | 0.07 | 0.28 | 0 | 0.03 | 99.26 |
| 3 | 2.90 | 0.80 | 2.30 | 0.50 | 0.60 | 1.30 | 91.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomczak, W.; Gryta, M. Membrane Distillation of Saline Water Contaminated with Oil and Surfactants. Membranes 2021, 11, 988. https://doi.org/10.3390/membranes11120988
Tomczak W, Gryta M. Membrane Distillation of Saline Water Contaminated with Oil and Surfactants. Membranes. 2021; 11(12):988. https://doi.org/10.3390/membranes11120988
Chicago/Turabian StyleTomczak, Wirginia, and Marek Gryta. 2021. "Membrane Distillation of Saline Water Contaminated with Oil and Surfactants" Membranes 11, no. 12: 988. https://doi.org/10.3390/membranes11120988
APA StyleTomczak, W., & Gryta, M. (2021). Membrane Distillation of Saline Water Contaminated with Oil and Surfactants. Membranes, 11(12), 988. https://doi.org/10.3390/membranes11120988







