Comparative Study on Water Vapour Resistance of Poly(lactic acid) Films Prepared by Blending, Filling and Surface Deposit
Abstract
:1. Introduction
2. Experimental
2.1. Materials
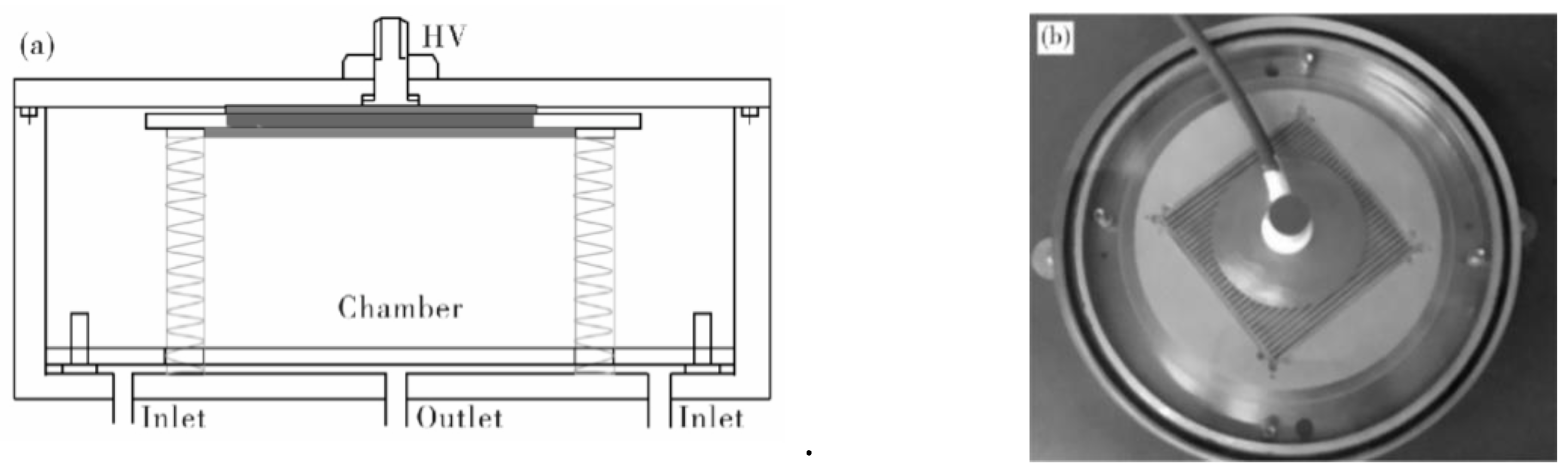
2.2. Film Manufacting and Depositing Methods
2.3. Performance Testing and Characterization Methods
2.3.1. Mechanical Property Test
2.3.2. Friction Coefficient Test
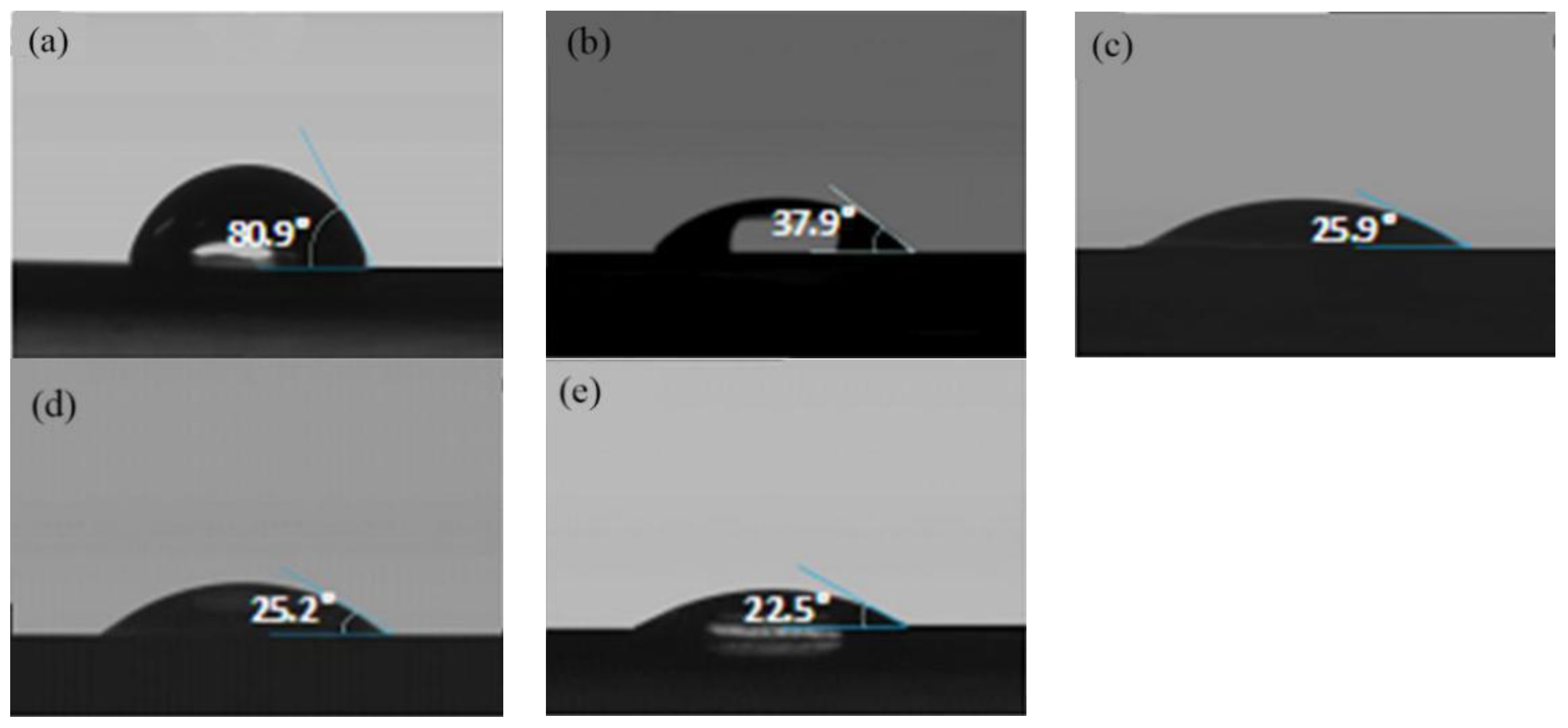
2.3.3. Contact Angle Test
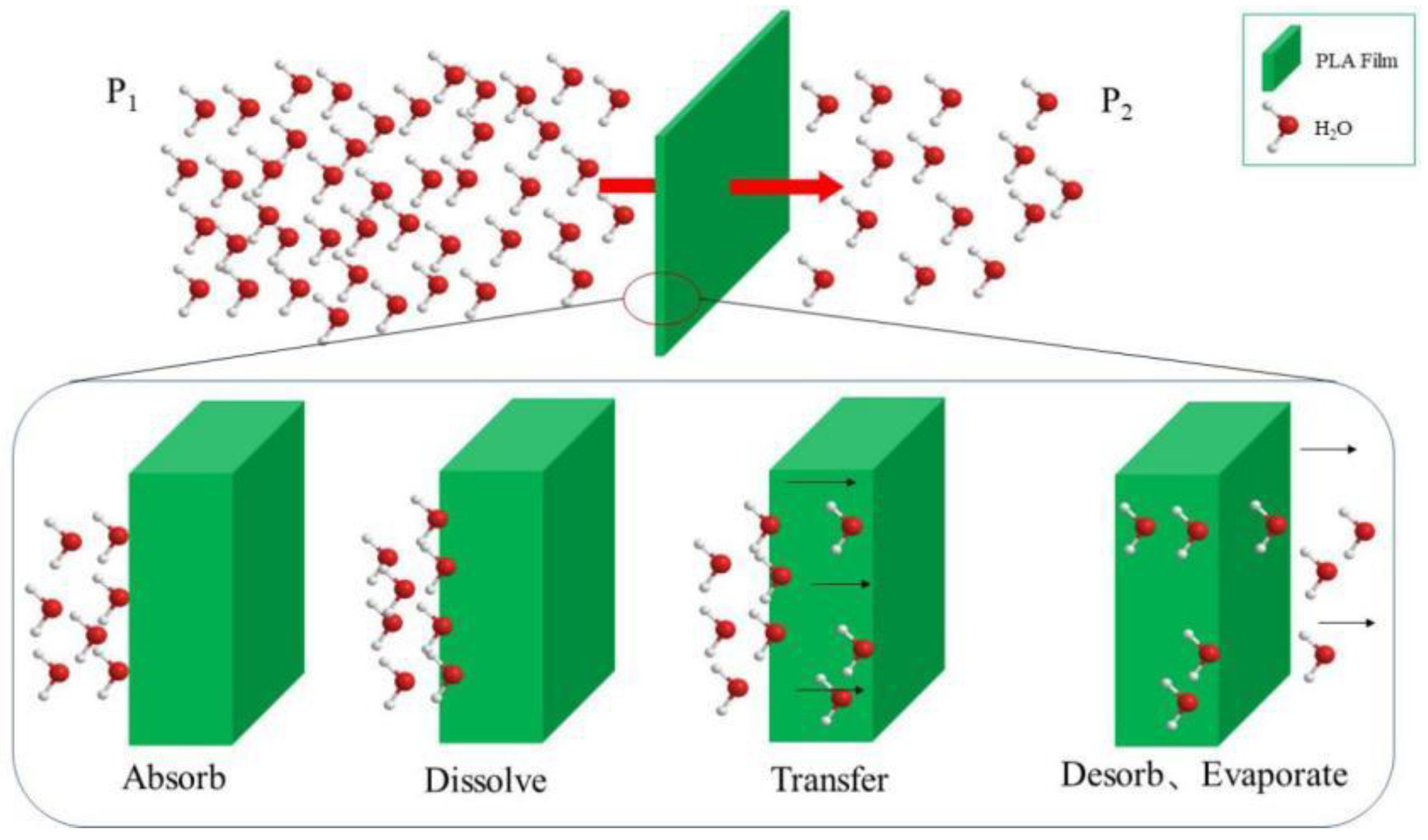
2.3.4. Moisture Barrier Property Test
3. Results and Discussion
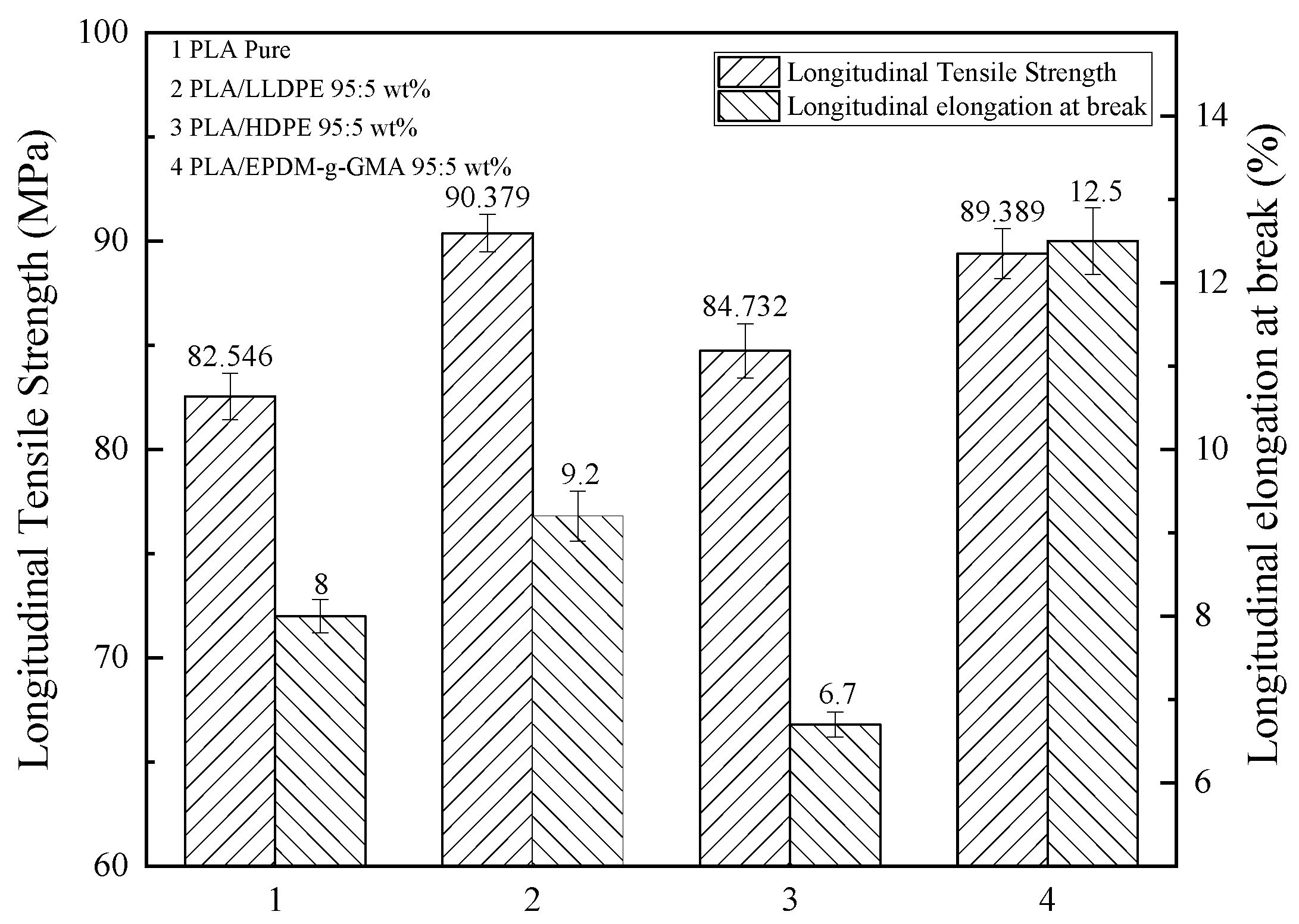
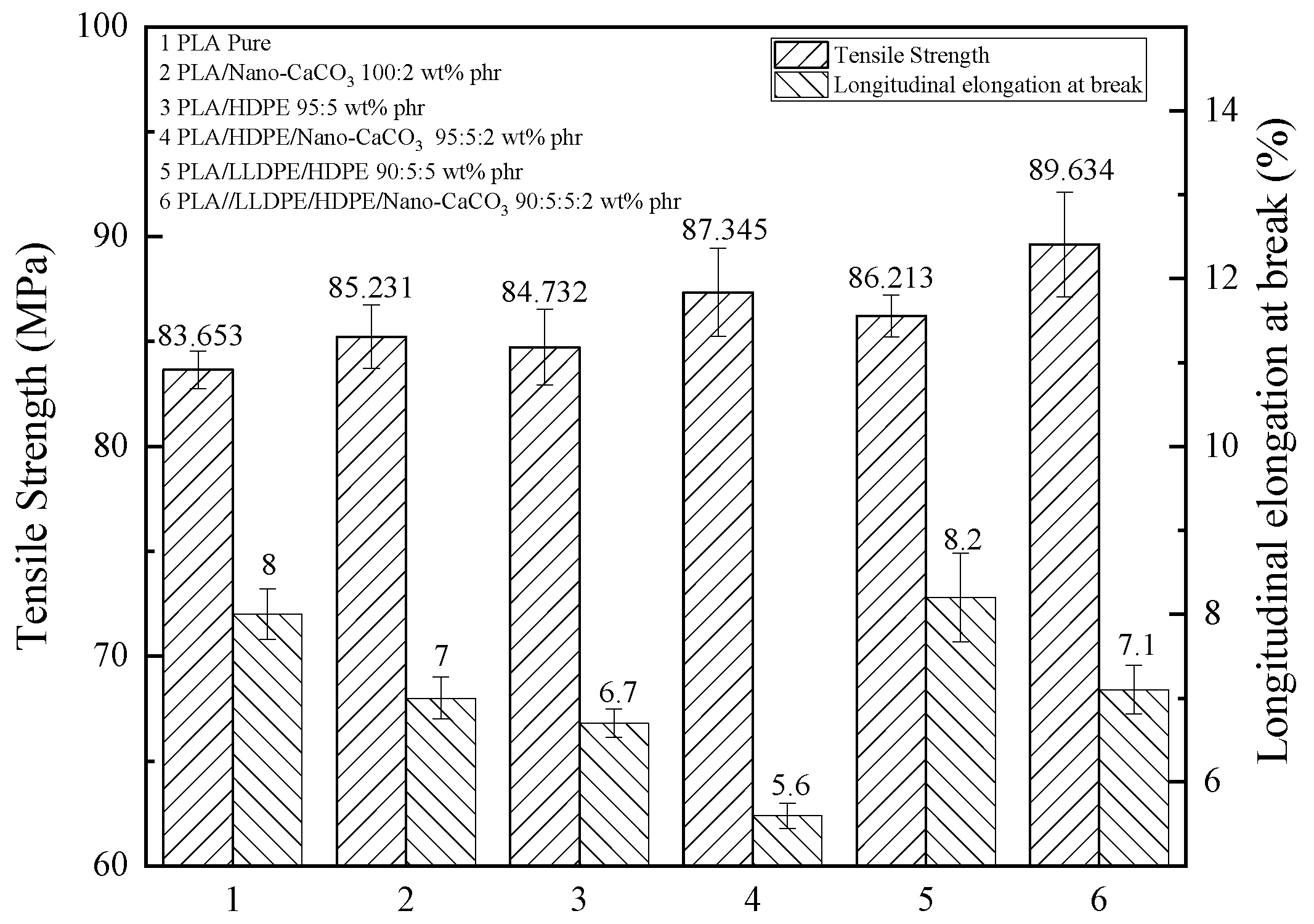
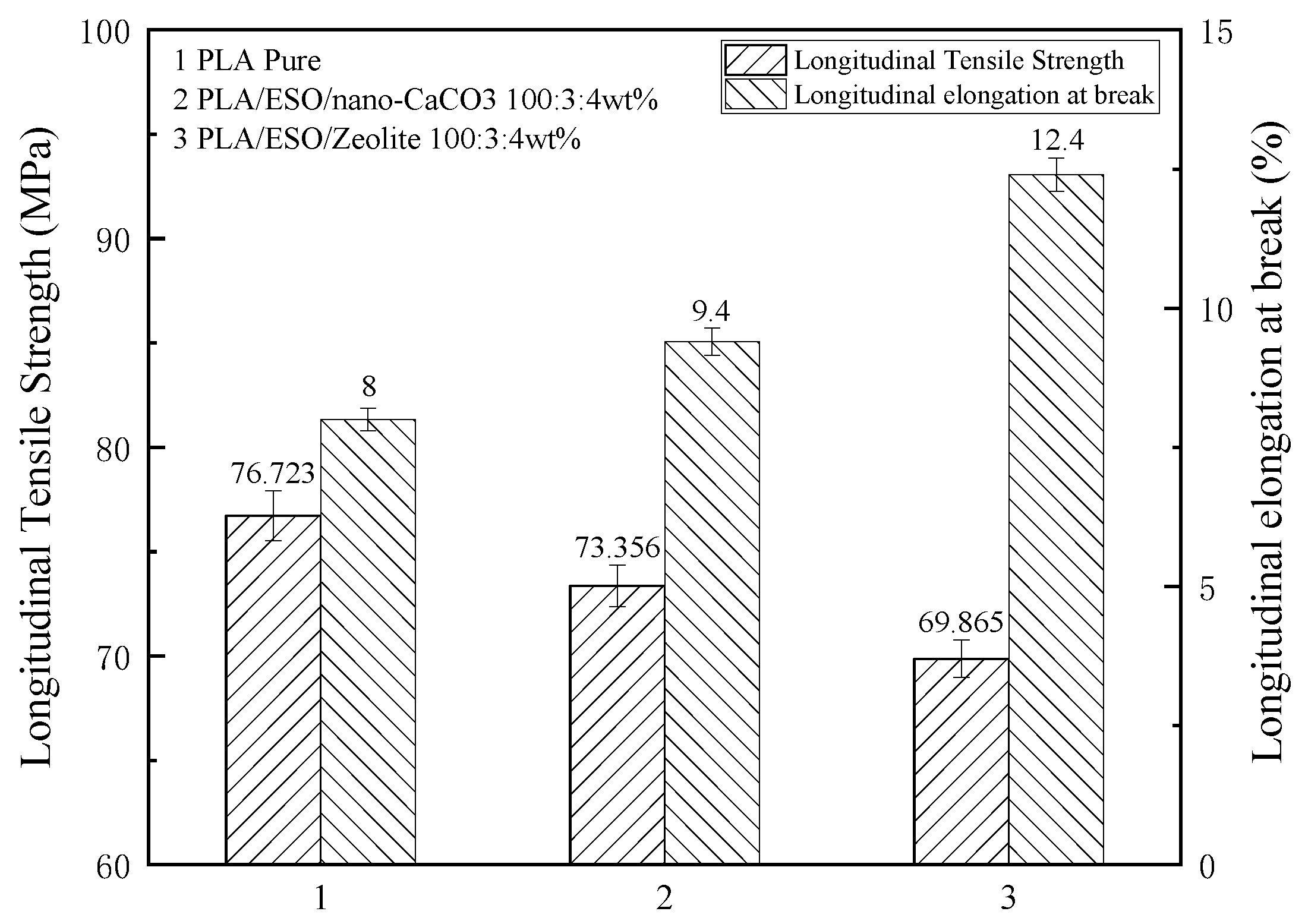
3.1. The Mechanical Properties of Modified PLA Films
3.2. The Surface Properties of Modified PLA Films
3.3. The Water Vapour Resistance of Modified PLA Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, F.; Zhang, C.; Weng, Y. Improvement of the Gas Barrier Properties of PLA/OMMT Films by Regulating the Interlayer Spacing of OMMT and the Crystallinity of PLA. ACS Omega 2020, 5, 18675–18684. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, E.L.; Paul, U.C.; Tran, T.N.; Suarato, G.; Ceseracciu, L.; Marras, S.; d’Arcy, R.; Athanassiou, A. Sustainable Active Food Packaging from Poly(Lactic Acid) and Cocoa Bean Shells. ACS Appl. Mater. Interfaces 2019, 11, 31317–31327. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Núñez, J.R.; Madera-Santana, T.J.; Burrola-Núñez, H.; Martínez-Encinas, E.G. Composite Materials Based on PLA and Its Applications in Food Packaging. In Composites Materials for Food Packaging; Cirillo, G., Kozlowski, M.A., Spizzirri, U.G., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018; pp. 355–399. ISBN 978-1-119-16024-3. [Google Scholar]
- Hu, H.; Zhang, R.; Shi, L.; Ying, W.B.; Wang, J.; Zhu, J. Modification of Poly(Butylene 2,5-Furandicarboxylate) with Lactic Acid for Biodegradable Copolyesters with Good Mechanical and Barrier Properties. Ind. Eng. Chem. Res. 2018, 57, 11020–11030. [Google Scholar] [CrossRef]
- Bosiers, L.; Engelmann, S. Thermoformed Packaging Made of PLA. Kunststoffe Plast Europe 2003, 93, 21–23+48. [Google Scholar]
- Le Gars, M.; Dhuiège, B.; Delvart, A.; Belgacem, M.N.; Missoum, K.; Bras, J. High-Barrier and Antioxidant Poly(Lactic Acid)/Nanocellulose Multilayered Materials for Packaging. ACS Omega 2020, 5, 22816–22826. [Google Scholar] [CrossRef]
- Zhang, T.; Yu, Q.; Fang, L.; Wang, J.; Wu, T.; Song, P. All-Organic Multilayer Coatings for Advanced Poly(Lactic Acid) Films with High Oxygen Barrier and Excellent Antifogging Properties. ACS Appl. Polym. Mater. 2019, 1, 3470–3476. [Google Scholar] [CrossRef]
- Sharma, S. Polylactic Acid (PLA) and Its Composites: An Eco-Friendly Solution for Packaging. In Sustainable Food Packaging Technology; Athanassiou, A., Ed.; Wiley: Hoboken, NJ, USA, 2021; pp. 107–132. ISBN 978-3-527-34556-4. [Google Scholar]
- Li, C.; Jiang, T.; Wang, J.; Peng, S.; Wu, H.; Shen, J.; Guo, S.; Zhang, X.; Harkin-Jones, E. Enhancing the Oxygen-Barrier Properties of Polylactide by Tailoring the Arrangement of Crystalline Lamellae. ACS Sustain. Chem. Eng. 2018, 6, 6247–6255. [Google Scholar] [CrossRef]
- Rocca-Smith, J.R.; Pasquarelli, R.; Lagorce-Tachon, A.; Rousseau, J.; Fontaine, S.; Aguié-Béghin, V.; Debeaufort, F.; Karbowiak, T. Toward Sustainable PLA-Based Multilayer Complexes with Improved Barrier Properties. ACS Sustain. Chem. Eng. 2019, 7, 3759–3771. [Google Scholar] [CrossRef]
- Xu, P.-P.; Zhang, S.-M.; Huang, H.-D.; Xu, L.; Zhong, G.-J.; Li, Z.-M. Highly Efficient Three-Dimensional Gas Barrier Network for Biodegradable Nanocomposite Films at Extremely Low Loading Levels of Graphene Oxide Nanosheets. Ind. Eng. Chem. Res. 2020, 59, 5818–5827. [Google Scholar] [CrossRef]
- Messin, T.; Follain, N.; Guinault, A.; Sollogoub, C.; Gaucher, V.; Delpouve, N.; Marais, S. Structure and Barrier Properties of Multinanolayered Biodegradable PLA/PBSA Films: Confinement Effect via Forced Assembly Coextrusion. ACS Appl. Mater. Interfaces 2017, 9, 29101–29112. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Xiao, A.; Yu, B.; Bhat, G.; Zhu, F. Effect of PCL and Compatibilizer on the Tensile and Barrier Properties of PLA/PCL Films. pk 2017, 41, 181. [Google Scholar] [CrossRef]
- Yu, H.-Y.; Zhang, H.; Song, M.-L.; Zhou, Y.; Yao, J.; Ni, Q.-Q. From Cellulose Nanospheres, Nanorods to Nanofibers: Various Aspect Ratio Induced Nucleation/Reinforcing Effects on Polylactic Acid for Robust-Barrier Food Packaging. ACS Appl. Mater. Interfaces 2017, 9, 43920–43938. [Google Scholar] [CrossRef]
- Thurber, C.M.; Xu, Y.; Myers, J.C.; Lodge, T.P.; Macosko, C.W. Accelerating Reactive Compatibilization of PE/PLA Blends by an Interfacially Localized Catalyst. ACS Macro Lett. 2015, 4, 30–33. [Google Scholar] [CrossRef]
- Xu, Y.; Loi, J.; Delgado, P.; Topolkaraev, V.; McEneany, R.J.; Macosko, C.W.; Hillmyer, M.A. Reactive Compatibilization of Polylactide/Polypropylene Blends. Ind. Eng. Chem. Res. 2015, 54, 6108–6114. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Hong, S.-I.; Ha, C.-S. Tensile, Water Vapor Barrier and Antimicrobial Properties of PLA/Nanoclay Composite Films. LWT Food Sci. Technol. 2009, 42, 612–617. [Google Scholar] [CrossRef]
- Mele, G.; Bloise, E.; Cosentino, F.; Lomonaco, D.; Avelino, F.; Marcianò, T.; Massaro, C.; Mazzetto, S.E.; Tammaro, L.; Scalone, A.G.; et al. Influence of Cardanol Oil on the Properties of Poly(Lactic Acid) Films Produced by Melt Extrusion. ACS Omega 2019, 4, 718–726. [Google Scholar] [CrossRef]
- Satam, C.C.; Irvin, C.W.; Lang, A.W.; Jallorina, J.C.R.; Shofner, M.L.; Reynolds, J.R.; Meredith, J.C. Spray-Coated Multilayer Cellulose Nanocrystal—Chitin Nanofiber Films for Barrier Applications. ACS Sustain. Chem. Eng. 2018, 6, 10637–10644. [Google Scholar] [CrossRef]
- Meriçer, Ç.; Minelli, M.; Angelis, M.G.D.; Giacinti Baschetti, M.; Stancampiano, A.; Laurita, R.; Gherardi, M.; Colombo, V.; Trifol, J.; Szabo, P.; et al. Atmospheric Plasma Assisted PLA/Microfibrillated Cellulose (MFC) Multilayer Biocomposite for Sustainable Barrier Application. Ind. Crop. Prod. 2016, 93, 235–243. [Google Scholar] [CrossRef]
- Mattioli, S.; Peltzer, M.; Fortunati, E.; Armentano, I.; Jiménez, A.; Kenny, J.M. Structure, Gas-Barrier Properties and Overall Migration of Poly(Lactic Acid) Films Coated with Hydrogenated Amorphous Carbon Layers. Carbon 2013, 63, 274–282. [Google Scholar] [CrossRef]
- Hirvikorpi, T.; Laine, R.; Vähä-Nissi, M.; Kilpi, V.; Salo, E.; Li, W.-M.; Lindfors, S.; Vartiainen, J.; Kenttä, E.; Nikkola, J.; et al. Barrier Properties of Plastic Films Coated with an Al2O3 Layer by Roll-to-Toll Atomic Layer Deposition. Thin Solid Film. 2014, 550, 164–169. [Google Scholar] [CrossRef]
- Wardman, R.H.; Abdrabbo, A. Effect of Plasma Treatment on the Spreading of Micro Drops through Polylactic Acid (Pla) and Polyester (Pet) Fabrics. AUTEX Res. J. 2010, 10, 7. [Google Scholar]
- Wei, H.Y. Study on Deposition of High Barrier Plastic Film by Atomic Layer at Atmospheric Pressure. Master’s Thesis, Qilu University of Technology, Jinan, China. Available online: https://www.wanfangdata.com.cn/index.html (accessed on 19 December 2018).
- Ye, D.Q.; Hu, H.B. Discussion on the Application of flexible Packaging Materials in Milk Powder Packaging. Plast. Packag. 2010, 20, 20–25. [Google Scholar]
- Mouhamad, Y.; Mokarian-Tabari, P.; Clarke, N.; Jones, R.A.L.; Geoghegan, M. Dynamics of Polymer Film Formation during Spin Coating. J. Appl. Phys. 2014, 116, 123513. [Google Scholar] [CrossRef]
- Langereis, E.; Creatore, M.; Heil, S.B.S.; van de Sanden, M.C.M.; Kessels, W.M.M. Plasma-Assisted Atomic Layer Deposition of Al2O3 Moisture Permeation Barriers on Polymers. Appl. Phys. Lett. 2006, 89, 081915. [Google Scholar] [CrossRef]






| Film Material | Thickness (μm) | Longitudinal Tensile Strength (MPa) | Longitudinal Elongation at Break (%) | Transverse Tensile Strength (MPa) | Transverse Elongation at Break (%) |
|---|---|---|---|---|---|
| Pure PLA blown film | 35 ± 15 | 82.546 | 8.0 | 48.819 | 5.7 |
| Bio-oriented PLA film | 40 ± 5 | 120.8 | 73.4 | 138.1 | 122.1 |
| Direction | Longitudinal | Inner Side | Outer Side |
|---|---|---|---|
| Pure PLA | Static | 0.503 | 0.470 |
| Dynamic | 0.466 | 0.444 | |
| PLA/LLDPE (95:5 wt%) | Static | 0.593 | 0.302 |
| Dynamic | 0.531 | 0.211 | |
| PLA/HDPE (95:5 wt%) | Static | 0.553 | 0.330 |
| Dynamic | 0.499 | 0.292 | |
| PLA/EPDM-g-GMA (95:5 wt%) | Static | 0.337 | 0.315 |
| Dynamic | 0.330 | 0.316 |
| Direction | Longitudinal | Inner Side | Outer Side |
|---|---|---|---|
| Pure PLA | Static | 0.503 | 0.470 |
| Dynamic | 0.466 | 0.444 | |
| PLA/Nano-CaCO3 (100:2 wt%) | Static | 0.593 | 0.302 |
| Dynamic | 0.531 | 0.211 | |
| PLA/HDPE (95:5 wt%) | Static | 0.553 | 0.330 |
| Dynamic | 0.499 | 0.292 | |
| PLA/HDPE/LLDPE (90:5:5 wt%) | Static | 0.490 | 0.359 |
| Dynamic | 0.459 | 0.328 | |
| PLA/HDPE/Nano-CaCO3 (95:5:2 wt%) | Static | 0.623 | 0.327 |
| Dynamic | 0.558 | 0.232 | |
| PLA/HDPE/LLDPE/Nano-CaCO3 (90:5:5:2 wt%) | Static | 0.605 | 0.275 |
| Dynamic | 0.529 | 0.224 |
| Direction | Longitudinal | Inner Side | Outer Side |
|---|---|---|---|
| Pure PLA | Static | 0.503 | 0.470 |
| Dynamic | 0.466 | 0.444 | |
| PLA/ESO/Nano-CaCO3 (100:3:4 wt%) | Static | 0.456 | 0.420 |
| Dynamic | 0.421 | 0.400 | |
| PLA/ESO/zeolite (100:3:4 wt%) | Static | 0.535 | 0.490 |
| Dynamic | 0.501 | 0.467 |
| Film Material | WVTR/(g/m2, 24 h), 38 °C, (90–0)%RH |
|---|---|
| Pure PLA | 263.10 ± 13.64 |
| PLA/LLDPE (95:5 wt%) | 165.44 ± 8.34 |
| PLA/HDPE (95:5 wt%) | 122.12 ± 5.22 |
| PLA/EPDM-g-GMA (95:5 wt%) | 88.81 ± 6.43 |
| PLA/ESO/nano-CaCO3(100:3:4 wt%) | 65.88 ± 4.36 |
| PLA/ESO/Zeolite (100:3:4 wt%) | 67.62 ± 3.54 |
| Deposite Degree/Cycle | WVTR (g/m2, 24 h), 38 °C, (90–10)%RH |
|---|---|
| 0 | 80.040 ± 8.336 |
| 100 | 16.352 ± 2.210 |
| 200 | 0.683 ± 0.086 |
| 300 | 0.617 ± 0.115 |
| 400 | 0.505 ± 0.054 |
| 500 | 0.511 ± 0.093 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Shen, Q.; Guo, C.; Guo, H. Comparative Study on Water Vapour Resistance of Poly(lactic acid) Films Prepared by Blending, Filling and Surface Deposit. Membranes 2021, 11, 915. https://doi.org/10.3390/membranes11120915
Wang S, Shen Q, Guo C, Guo H. Comparative Study on Water Vapour Resistance of Poly(lactic acid) Films Prepared by Blending, Filling and Surface Deposit. Membranes. 2021; 11(12):915. https://doi.org/10.3390/membranes11120915
Chicago/Turabian StyleWang, Shuo, Qinyu Shen, Chuanyan Guo, and Hongge Guo. 2021. "Comparative Study on Water Vapour Resistance of Poly(lactic acid) Films Prepared by Blending, Filling and Surface Deposit" Membranes 11, no. 12: 915. https://doi.org/10.3390/membranes11120915
APA StyleWang, S., Shen, Q., Guo, C., & Guo, H. (2021). Comparative Study on Water Vapour Resistance of Poly(lactic acid) Films Prepared by Blending, Filling and Surface Deposit. Membranes, 11(12), 915. https://doi.org/10.3390/membranes11120915






