Study the Use of Activated Carbon and Bone Char on the Performance of Gravity Sand-Bag Water Filter
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Fourier Transform Infra-Red Spectrometry (FTIR)
2.2.2. Brunauer-Emmett-Teller (BET)
2.2.3. Zeta Potential Measurement
2.3. Static Adsorption Study
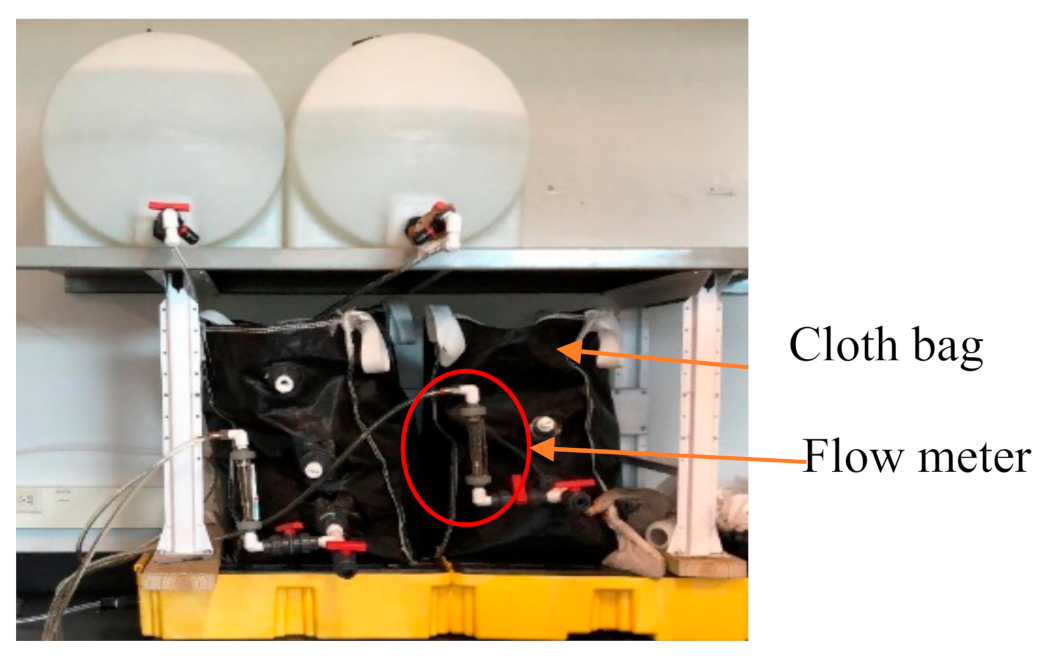
2.4. Biosand Bag Filtration Unit
3. Results and Discussion
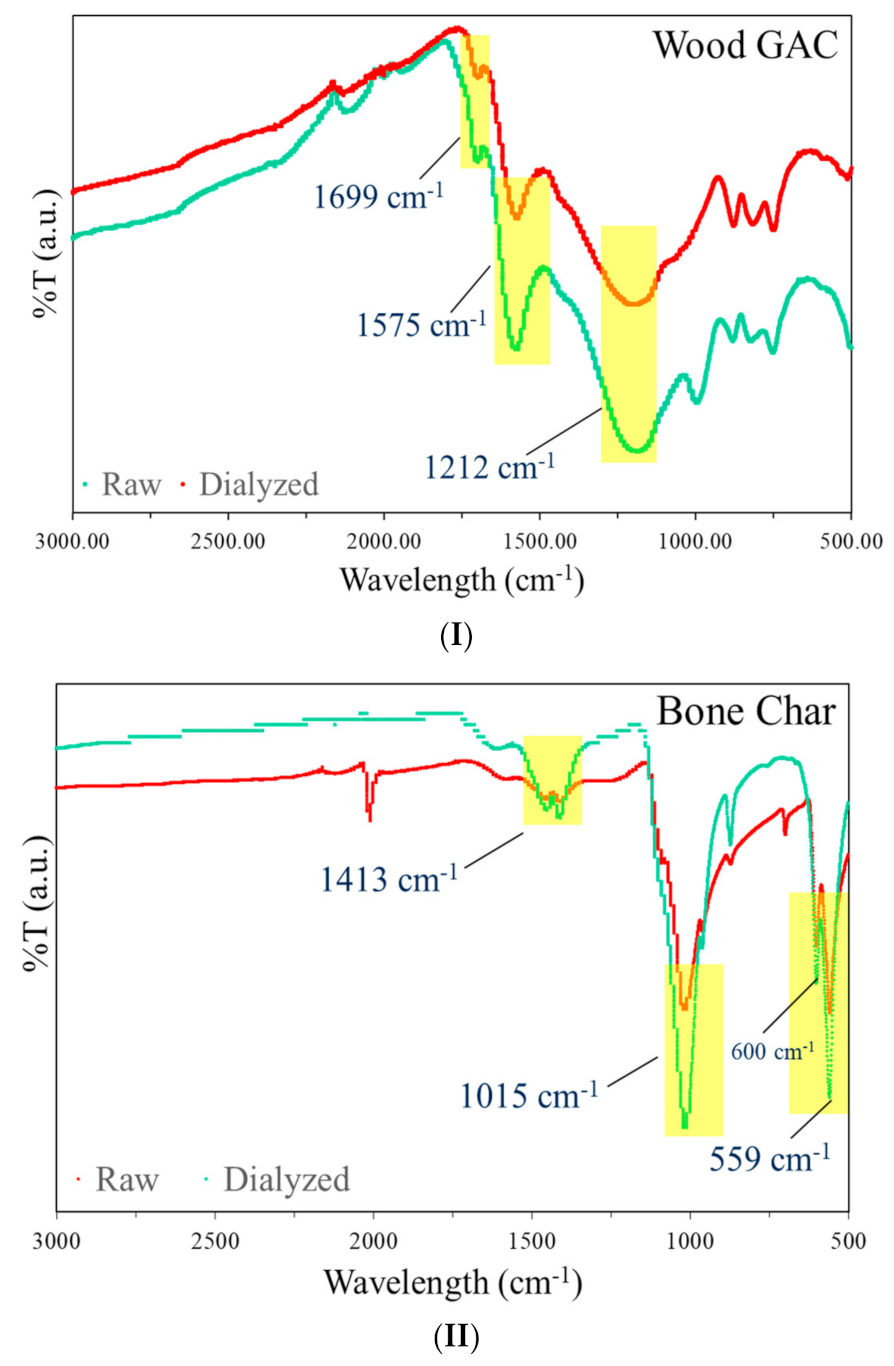
3.1. FTIR Analysis of GAC and BC
3.2. Surface Area Analysis
3.3. Zeta Potential Measurement
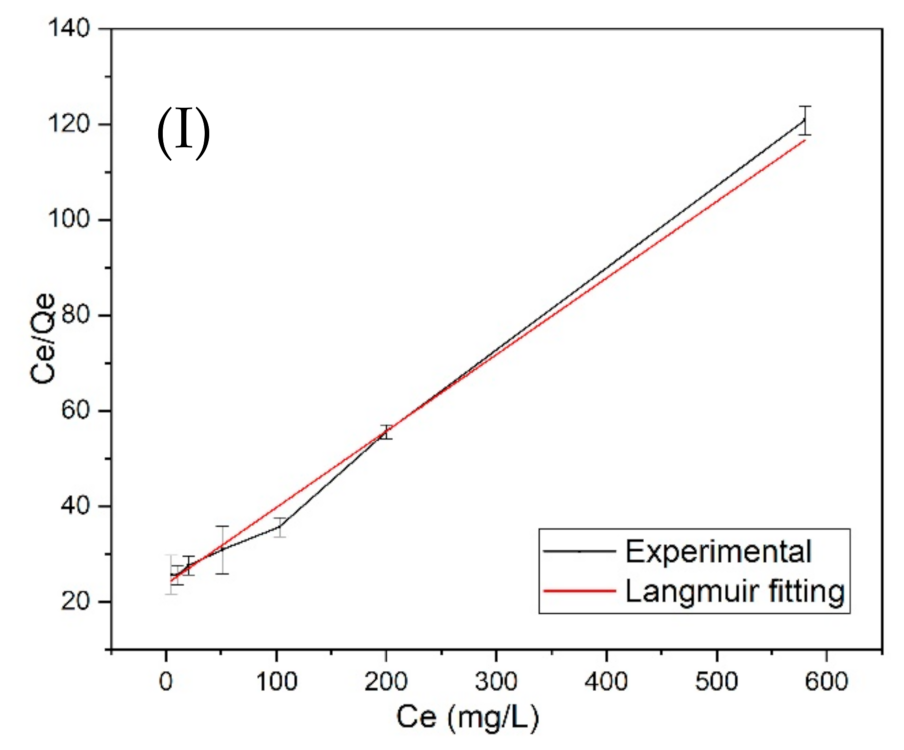
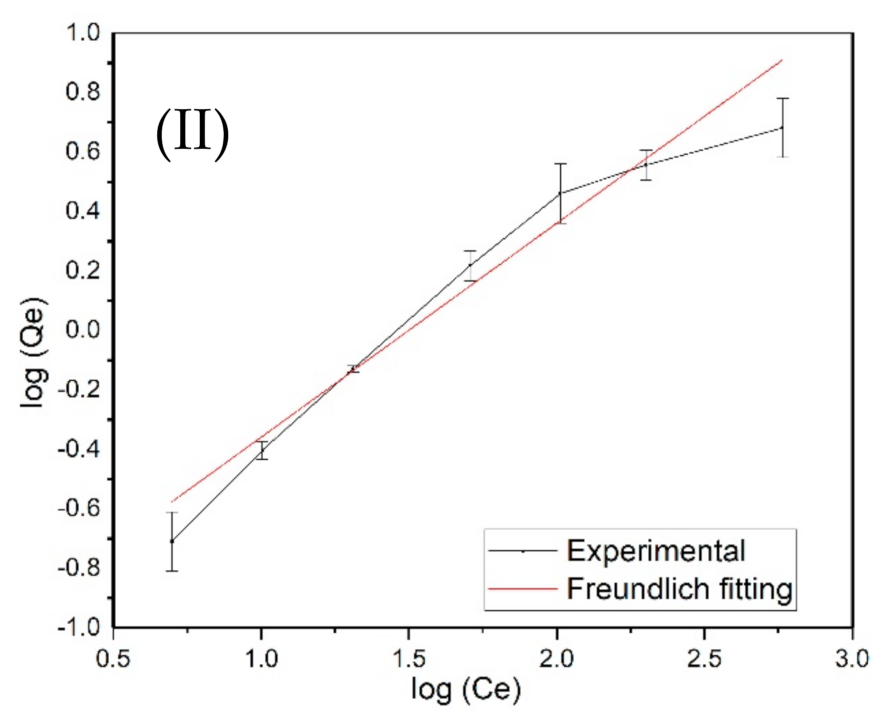
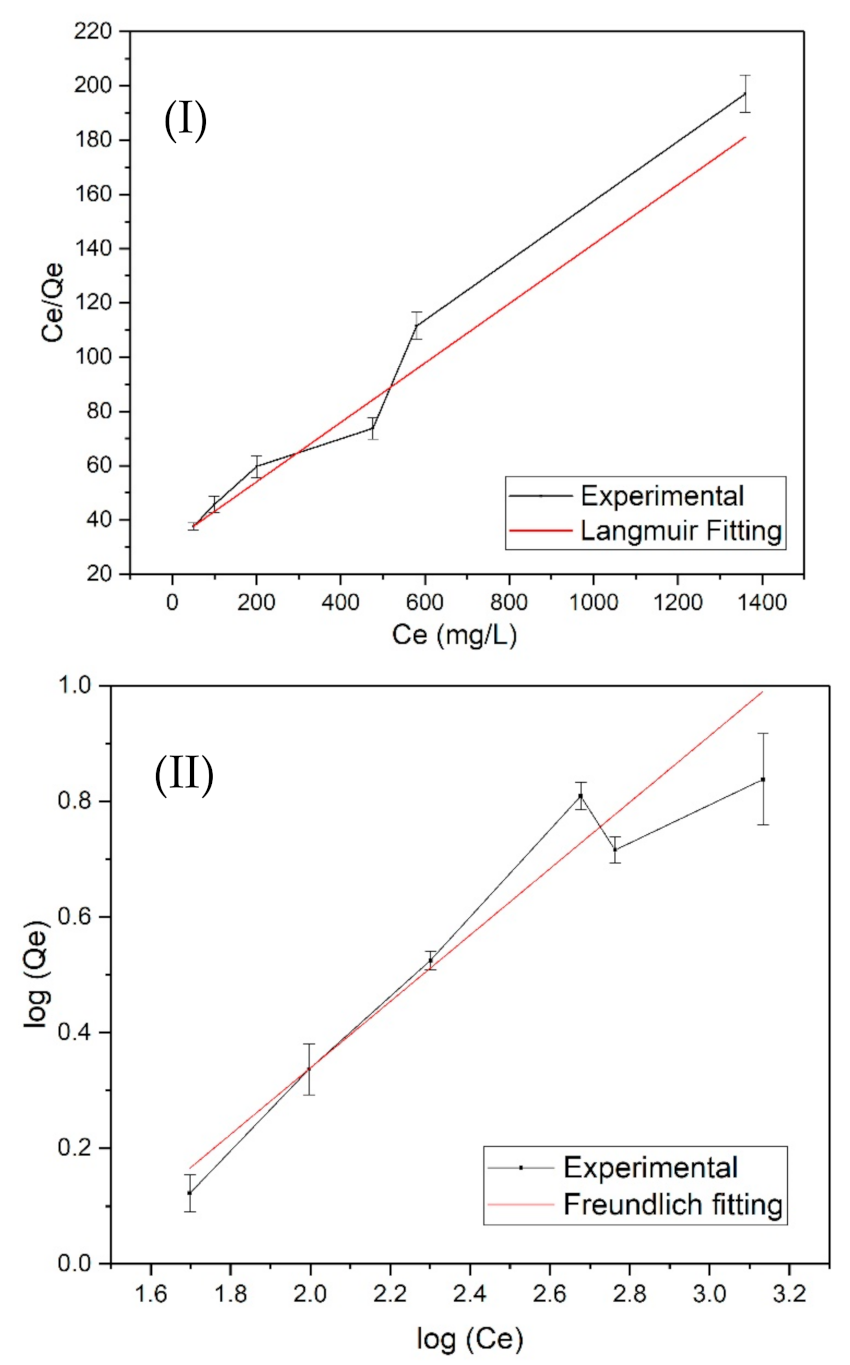
3.4. Langmuir and Freundlich Adsorption Isotherm Models
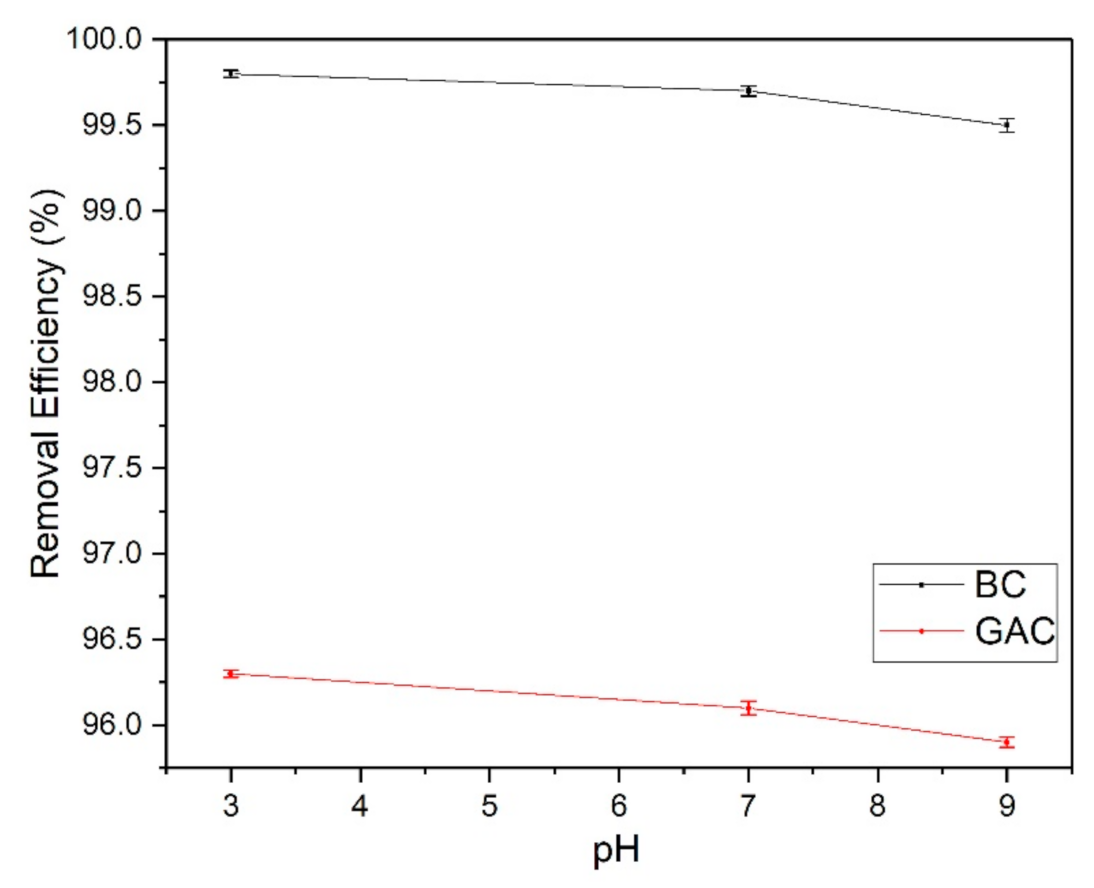
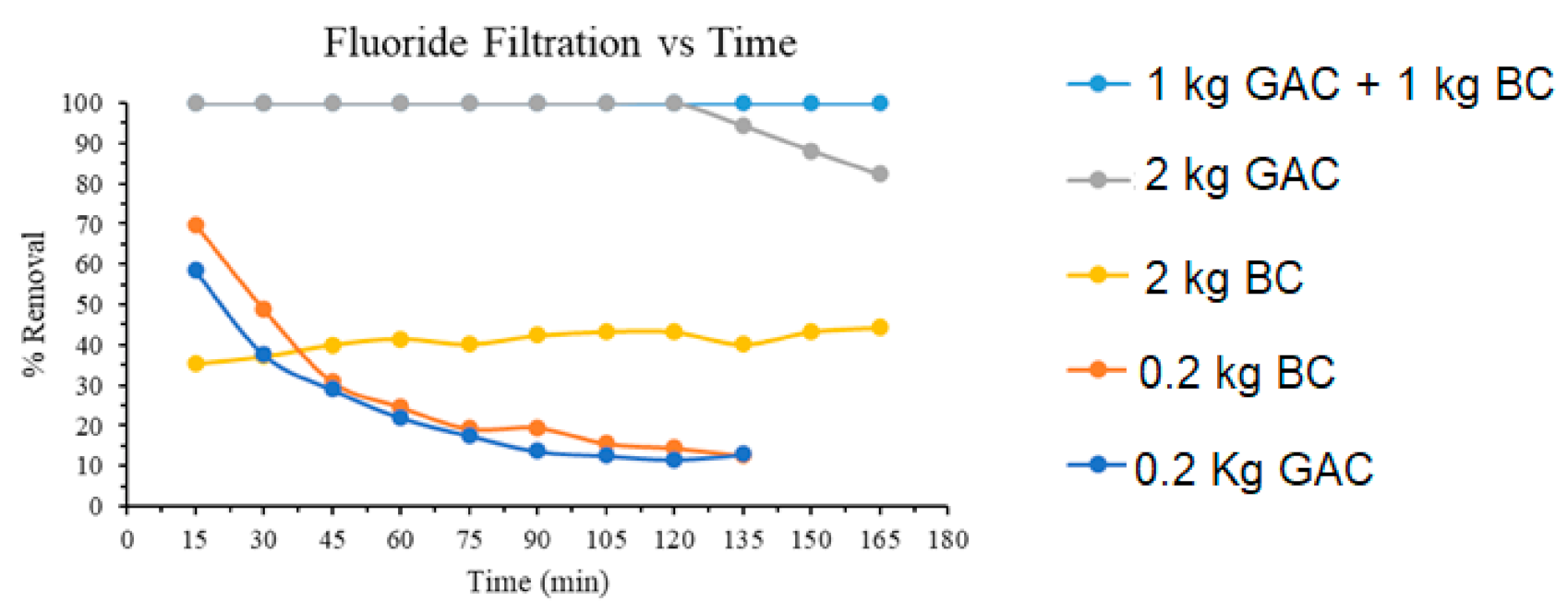
3.5. Sand-Bag Gravity Filtration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alkurdi, S.S.A.; Al-Juboori, R.A.; Bundschuh, J.; Hamawand, I. Bone char as a green sorbent for removing health threatening fluoride from drinking water. Environ. Int. 2019, 127, 704–719. [Google Scholar] [CrossRef] [PubMed]
- WHO; UNICEF. Almost 2 Billion People Depend on Health Care Facilities without Basic Water Services; WHO: Geneva, Switzerland; UNICEF: New York, NY, USA, 2020. [Google Scholar]
- Medellin-Castillo, N.A.; Leyva-Ramos, R.; Ocampo-Perez, R.; Garcia de la Cruz, R.F.; Aragon-Piña, A.; Martinez-Rosales, J.M.; Guerrero-Coronado, R.M.; Fuentes-Rubio, L. Adsorption of Fluoride from Water Solution on Bone Char. Ind. Eng. Chem. Res. 2007, 46, 9205–9212. [Google Scholar] [CrossRef]
- Sharma, P.R.; Chattopadhyay, A.; Zhan, C.; Sharma, S.K.; Geng, L.; Hsiao, B.S.J.C. Lead removal from water using carboxycellulose nanofibers prepared by nitro-oxidation method. Cellulose 2018, 25, 1961–1973. [Google Scholar] [CrossRef]
- Sharma, P.R.; Chattopadhyay, A.; Sharma, S.K.; Hsiao, B.S.J.I.; Research, E.C. Efficient removal of UO22+ from water using carboxycellulose nanofibers prepared by the nitro-oxidation method. Ind. Eng. Chem. Res. 2017, 56, 13885–13893. [Google Scholar] [CrossRef]
- Sharma, P.R.; Zheng, B.; Sharma, S.K.; Zhan, C.; Wang, R.; Bhatia, S.R.; Hsiao, B.S. High aspect ratio carboxycellulose nanofibers prepared by nitro-oxidation method and their nanopaper properties. ACS Appl. Nano Mater. 2018, 1, 3969–3980. [Google Scholar] [CrossRef]
- Sharma, P.R.; Sharma, S.K.; Borges, W.; Chen, H.; Hsiao, B.S. Remediation of UO22+ from water by nitro-oxidized carboxycellulose nanofibers: Performance and mechanism. In Contaminants in Our Water: Identification and Remediation Methods; ACS Publications: Washington, DC, USA, 2020; pp. 269–283. [Google Scholar]
- Chen, H.; Sharma, S.K.; Sharma, P.R.; Chi, K.; Fung, E.; Aubrecht, K.; Keroletswe, N.; Chigome, S.; Hsiao, B.S.J.C. Nitro-oxidized carboxycellulose nanofibers from moringa plant: Effective bioadsorbent-for mercury removal. Cellulose 2021, 28, 8611–8628. [Google Scholar] [CrossRef]
- Zhan, C.; Sharma, P.R.; Geng, L.; Sharma, S.K.; Wang, R.; Joshi, R.; Hsiao, B.S. Structural characterization of carboxyl cellulose nanofibers extracted from underutilized sources. Sci. China Ser. E Technol. Sci. 2019, 62, 971–981. [Google Scholar] [CrossRef]
- Sharma, P.R.; Chattopadhyay, A.; Sharma, S.K.; Geng, L.; Amiralian, N.; Martin, D.; Hsiao, B.S. Engineering, Nanocellulose from spinifex as an effective adsorbent to remove cadmium (II) from water. ACS Sustain. Chem. Eng. 2018, 6, 3279–3290. [Google Scholar] [CrossRef]
- Sharma, S.K.; Sharma, P.R.; Chen, H.; Johnson, K.; Zhan, C.; Wang, R.; Hsiao, B. Cellulose-Supported Nanosized Zinc Oxide: Highly Efficient Bionanomaterial for Removal of Arsenic from Water. In Contaminants in Our Water: Identification and Remediation Methods; American Chemical Society: Washington, DC, USA, 2020; Volume 1352, pp. 253–267. [Google Scholar]
- Chen, H.; Sharma, S.K.; Sharma, P.R.; Yeh, H.; Johnson, K.; Hsiao, B.S. Arsenic(III) Removal by Nanostructured Dialdehyde Cellulose–Cysteine Microscale and Nanoscale Fibers. ACS Omega 2019, 4, 22008–22020. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.R.; Sharma, S.K.; Nolan, M.; Li, W.; Kundal, L.; Hsiao, B.S. Sequential Oxidation on Wood and Its Application in Pb2+ Removal from Contaminated Water. Polysaccharides 2021, 2, 245–256. [Google Scholar] [CrossRef]
- Sharma, P.R.; Sharma, S.K.; Lindström, T.; Hsiao, B.S. Nanocellulose-Enabled Membranes for Water Purification: Perspectives. Adv. Sustain. Syst. 2020, 4, 1900114. [Google Scholar] [CrossRef]
- Zhan, C.; Li, Y.; Sharma, P.R.; He, H.; Sharma, S.K.; Wang, R.; Hsiao, B.S. A study of TiO2 nanocrystal growth and environmental remediation capability of TiO2/CNC nanocomposites. RSC Adv. 2019, 9, 40565–40576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, P.R.; Joshi, R.; Sharma, S.K.; Hsiao, B.S. A Simple Approach to Prepare Carboxycellulose Nanofibers from Untreated Biomass. Biomacromolecules 2017, 18, 2333–2342. [Google Scholar] [CrossRef] [PubMed]
- Azizi Samir, M.A.S.; Alloin, F.; Dufresne, A. Review of Recent Research into Cellulosic Whiskers, Their Properties and Their Application in Nanocomposite Field. Biomacromolecules 2005, 6, 612–626. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.R. Microcolony and biofilm formation as a survival strategy for bacteria. J. Theor. Biol. 2008, 251, 24–34. [Google Scholar] [CrossRef] [Green Version]
- Orandi, S.; Lewis, D.M.; Moheimani, N.R. Biofilm establishment and heavy metal removal capacity of an indigenous mining algal-microbial consortium in a photo-rotating biological contactor. J. Ind. Microbiol. Biotechnol. 2012, 39, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Doke, K.M.; Khan, E.M. Equilibrium, kinetic and diffusion mechanism of Cr(VI) adsorption onto activated carbon derived from wood apple shell. Arab. J. Chem. 2017, 10, S252–S260. [Google Scholar] [CrossRef] [Green Version]
- Yorgun, S.; Yıldız, D. Preparation and characterization of activated carbons from Paulownia wood by chemical activation with H3PO4. J. Taiwan Inst. Chem. Eng. 2015, 53, 122–131. [Google Scholar] [CrossRef]
- Reynel-Avila, H.E.; Mendoza-Castillo, D.I.; Bonilla-Petriciolet, A. Relevance of anionic dye properties on water decolorization performance using bone char: Adsorption kinetics, isotherms and breakthrough curves. J. Mol. Liq. 2016, 219, 425–434. [Google Scholar] [CrossRef]
- Şahin, Ö.; Saka, C. Preparation and characterization of activated carbon from acorn shell by physical activation with H2O–CO2 in two-step pretreatment. Bioresour. Technol. 2013, 136, 163–168. [Google Scholar] [CrossRef]
- Fito, J.; Said, H.; Feleke, S.; Worku, A. Fluoride removal from aqueous solution onto activated carbon of Catha edulis through the adsorption treatment technology. Environ. Syst. Res. 2019, 8, 25. [Google Scholar] [CrossRef]
- Suneetha, M.; Sundar, B.S.; Ravindhranath, K. Removal of fluoride from polluted waters using active carbon derived from barks of Vitex negundo plant. J. Anal. Sci. Technol. 2015, 6, 15. [Google Scholar] [CrossRef] [Green Version]
- Dehghani, M.H.; Farhang, M.; Alimohammadi, M.; Afsharnia, M.; McKay, G. Adsorptive removal of fluoride from water by activated carbon derived from CaCl2-modified Crocus sativus leaves: Equilibrium adsorption isotherms, optimization, and influence of anions. Chem. Eng. Commun. 2018, 205, 955–965. [Google Scholar] [CrossRef]
- Kaseva, M.E. Optimization of regenerated bone char for fluoride removal in drinking water: A case study in Tanzania. J. Water Health 2006, 4, 139–147. [Google Scholar] [CrossRef] [Green Version]
- Mutchimadilok, Y.; Smittakorn, S.; Mongkolnchai-arunya, S.; Durnford, D. Defluoridation with Locally Produced Thai Bone Char. Adv. Environ. Chem. 2014, 2014, 483609. [Google Scholar] [CrossRef] [Green Version]
- Nigri, E.M.; Cechinel, M.A.P.; Mayer, D.A.; Mazur, L.P.; Loureiro, J.M.; Rocha, S.D.F.; Vilar, V.J.P. Cow bones char as a green sorbent for fluorides removal from aqueous solutions: Batch and fixed-bed studies. Environ. Sci. Pollut. Res. 2017, 24, 2364–2380. [Google Scholar] [CrossRef] [PubMed]
- Yadav, K.K.; Gupta, N.; Kumar, V.; Khan, S.A.; Kumar, A. A review of emerging adsorbents and current demand for defluoridation of water: Bright future in water sustainability. Environ. Int. 2018, 111, 80–108. [Google Scholar] [CrossRef] [PubMed]
- Sahira, J.; Mandira, A.; Prasad, P.; Ram, P.R. Effects of activating agents on the activated carbons prepared from Lapsi seed stone. Res. J. Chem. Sci. 2013, 3, 19–24. [Google Scholar]
- Amalraj, A.; Pius, A. Removal of fluoride from drinking water using aluminum hydroxide coated activated carbon prepared from bark of Morinda tinctoria. Appl. Water Sci. 2017, 7, 2653–2665. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Jiang, Y.; Qin, C.; Yang, S.; Song, X.; Wang, S.; Li, K.J.C. Enzyme-assisted mechanical grinding for cellulose nanofibers from bagasse: Energy consumption and nanofiber characteristics. Int. J. Environ. Res. Public Health 2018, 25, 7065–7078. [Google Scholar] [CrossRef]
| Surface Area (m2/g) | Total Pore Volume (cm3/g) | Average Pore Diameter (nm) | Surface Charge (mV) | Reference | |
|---|---|---|---|---|---|
| Harwood GAC | 739.0 | 0.237 | 2.321 | −2.9 | This work |
| Mixed BC | 74.9 | 0.86 | 6.32 | 4.5 | This work |
| Fija Fluor BC | 104 | 0.30 | 11.1 | NA | [3] |
| Adsorbent | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|
| Qm (mg/g) | K | R2 | KF | n | R2 | |
| GAC | 6.23 | 0.16036 | 0.9946 | −1.0822 | 1.430 | 0.997 |
| BC | 9.13 | 0.10949 | 0.970 | −0.7228 | 1.896 | 0.9664 |
| Adsorbents | Maximum Adsorption Capacity (mg/g) | Removal Efficiency%/ Fluoride Concentration (mg/L) | References |
|---|---|---|---|
| Activated charcoal of Catha edulis | 18 | 80–85/1–10 | [24] |
| Activated carbon of Vitex negundo | 1.02 | 99–50/1–12 | [25] |
| Activated carbon of Lapsi seeds | - | 50–99/1–25 | [31] |
| Crocus sativus leaves | - | 85/6.5 | [26] |
| Bark of Morinda tinctoria | 26 | - | [32] |
| Modified sludge adsorbent | 1.5 | 81/1–5 | [33] |
| Cattle BC | 11.9 | - | [1] |
| GAC | 6.23 | 100/5 | This work |
| BC | 9.13 | 40/5 | This work |
| GAC + BC | - | 100/5 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fung, E.; Johnson, K.I.; Li, W.; Borges, W.; Chi, K.; Sharma, S.K.; Madan, Y.; Sharma, P.R.; Hsiao, B.S. Study the Use of Activated Carbon and Bone Char on the Performance of Gravity Sand-Bag Water Filter. Membranes 2021, 11, 868. https://doi.org/10.3390/membranes11110868
Fung E, Johnson KI, Li W, Borges W, Chi K, Sharma SK, Madan Y, Sharma PR, Hsiao BS. Study the Use of Activated Carbon and Bone Char on the Performance of Gravity Sand-Bag Water Filter. Membranes. 2021; 11(11):868. https://doi.org/10.3390/membranes11110868
Chicago/Turabian StyleFung, Eric, Ken I. Johnson, Wenqi Li, William Borges, Kai Chi, Sunil K. Sharma, Yogita Madan, Priyanka R. Sharma, and Benjamin S. Hsiao. 2021. "Study the Use of Activated Carbon and Bone Char on the Performance of Gravity Sand-Bag Water Filter" Membranes 11, no. 11: 868. https://doi.org/10.3390/membranes11110868
APA StyleFung, E., Johnson, K. I., Li, W., Borges, W., Chi, K., Sharma, S. K., Madan, Y., Sharma, P. R., & Hsiao, B. S. (2021). Study the Use of Activated Carbon and Bone Char on the Performance of Gravity Sand-Bag Water Filter. Membranes, 11(11), 868. https://doi.org/10.3390/membranes11110868









