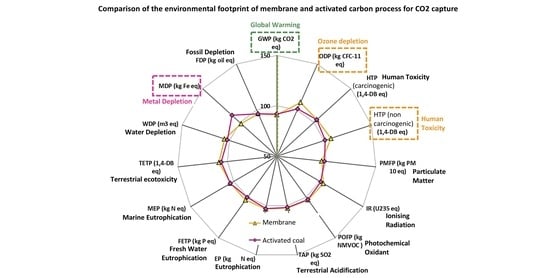
3.2. Impact Assessment
Figure 3 compares the four scenarios considered in terms of their impacts. In this graph, the reference scenario corresponding to the actual plant without CCU is set to 100% and the other scenarios are reported in terms of their relative environmental impacts.
Globally, one observes that for all the selected categories of impacts, the results are very close to the reference case or worse; no significant improvement is observed in any category.
Concerning GWP, which is the main concern of the present study, the potential impacts for the four scenarios are in a nutshell. Chemical absorption, membranes and adsorption on activated coal reach 98.2, 97.2 and 97.8% of the reference case, respectively. This negligible and non-significant gain results from two factors: the energy required to run CC facilities, which erases the benefice of the CO
2 recycling, and the relatively low amount of CO
2 captured and reused onsite. Indeed, the CC facilities were designed to capture 1000 kg/h of CO
2, which hardly represents 2% of the standard emissions of the coal-fired power plant. At the same time, the production of Prod A is increased by the CO
2 recycling by around 12%, which means that a substantial improvement in term of GWP could be expected, after rescaling to the functional unit. However, it appears that the extra energy required to operate the CC facilities strongly mitigates the results. This is clearly illustrated on
Figure 4, which represents the amount of CO
2 generated by the power plant to produce 1 kWh
e with (mean value) and without carbon capture. This graph shows that the implementation of CC facilities results in net CO
2 emissions.
Similar conclusions can be derived regarding the particulate matter formation potential (PMFP) category, which is directly linked to the emissions associated to the production of energy with the coal-fired power plant. The category photochemical oxidant formation potential (POFP) is also strongly linked to the energy production through the SOx emissions resulting from the combustion of coal, but one has to keep in mind that the absolute value of the indicator for this category is relatively weak due to the strong de-SOx purification process operated.
Absorption with MEA exhibits significantly higher results than the other processes regarding the eutrophication potential (EP), freshwater eutrophication potential (FETP), terrestrial eutrophication potential (TETP), ozone depletion potential (ODP) and terrestrial acidification potential (TAP). These results are in agreement with the literature [
30]. ODP category is strongly affected by the ethylene oxide associated to the production of MEA, and by the degradation of MEA during CO
2 separation, which represents 22% and 78% of the impacts associated to the absorption process in this category, respectively. The degradation of MEA also strongly affects EP, TETP and TAP categories. The end-of-life treatment of the solvent rather impacts FETP and EP.
The membrane technology is slightly better than MEA absorption in most categories of impacts, which is consistent with the results published in [
31]. The ozone depletion potential (ODP) is strongly impacted by emissions of chlorofluorocarbides and hydrofluorocarbides during the incineration of the membranes and to a lesser extent by the emissions associated de production of the membranes. These emissions represent 52% and 28% of the impacts associated to the membrane process in this category, respectively. The production of the membranes is also responsible for emissions of hydrogen fluoride, which affects the human toxicity potential category.
Adsorption on activated coal, which appears to be the most attractive alternative, presents impacts relatively close to the reference case in most categories. The differences mainly come from the extra energy required to operate the facility. To our knowledge no dedicated literature investigated the environmental assessment of this technology.
Those results are globally disappointing and especially in terms of global warming potential. The integration of the CC facilities improves the production of Prod A but offers no benefice in terms of CO2 emissions and more or less significantly increases the impacts in all the other categories for the given functional unit.
3.3. Parametrical Study
The previous calculations were conducted strictly meeting the performances criteria of the CC facilities previously presented. As those criteria led to unsatisfying results regarding the goal of the study, we decided to conduct a parametrical study to try to find a compromise. For each process, we identified one key parameter, which could be modified without compromising the separation process. The principal results are presented below.
A significant part of the impacts associated to the MEA process comes from MEA and its degradation products released during CO2 separation. Direct releases of MEA originate from the purge implemented in the process to maintain a constant quality of the solvent. Consequently, reducing the purge rate has a positive impact regarding the MEA releases and the global MEA consumption of the process, but at the same time it decreases the efficiency of the process because of the accumulation of degraded products in the solvent. We conducted calculations for a reduction of 15% in the purge rate and correspondingly the consumption of extra MEA was reduced by 5%, the CO2 recovery and the CO2 purity fell to 73% and 85%, respectively. Those values are still satisfying but unfortunately in terms environmental impacts, the benefice does not exceed 5% of reduction for ozone depletion potential and freshwater ecotoxicity potential and is not significant for the other categories, especially regarding GWP.
The impacts associated to the membrane process mainly come from the energy consumption during separation and from the materials (polymers) of the membranes. We investigated thinner membranes switching from 1.5 μm to 0.07 μm for the Pebax 1657 and from 1 to 0.03 μm for the PEO, which resulted in significant material savings. Reducing the membranes thickness maintain a good efficiency of the process but implies a drop of 11% in the CO2 purity.
Impacts are globally unchanged except for ozone depletion and human toxicity (canc.), which are reduced by about 5%.
For this process, the impacts are mainly associated to the energy required to heat the bed and to a lesser extent to the activated coal itself. We tried to reduce the amount of activated coal from 10%, which implied a drop of 13% in the CO2 recovery. As expected, this modification hardly reduces the environmental impacts.
The previous results clearly showed that a significant decrease in the different categories of impacts, and especially in GWP, could not be reached with a simple tuning of processes operating parameters. Nevertheless, we further thought about the minimal performances criteria of the separation processes established at the beginning of the study. The minimal CO2 production capacity and the minimal CO2 recovery rate are imposed by technical constraints that cannot be modified. However, it appeared that the CO2 purity, which was first set to 95% to ensure a standard high purity, could be reduced without damage to the process A. Process A actually requires a very clean CO2 to ensure the high purity of Prod A required by its market. But this purity is disconnected from the concentration of CO2 in the feed gas. On the actual plant, process A is fed with a CO2 stream, which is purified from unwanted components (dust, SOx and NOx) but which is highly diluted: the CO2 molar fraction does not exceed 40%. Consequently, as the tail gas collected on the power plant is highly purified prior to CO2 separation, it could be eligible for process A with a molar fraction considerably lower than the 95% considered previously. Two additional scenarios, with membrane process and chemical adsorption on activated coal, were then considered with a CO2 molar fraction of 40%. Chemical absorption with MEA, which appeared to be the less promising process in terms of environmental impacts, was not considered in the following.
Reducing the purity requirements to 40% considerably simplify the membrane separation process, as only one stage of Pebax 1657, without recycling loop, is enough to reach this concentration. The total surface of membranes and the energy required to operate the process are drastically reduced (by about 70% and 60%, respectively). The separation process with activated coal is also deeply impacted: a smaller quantity of coal is required (66% less), which leads to a strong cut in the energy required to heat the bed and run the process (60% less). The main characteristics of the new processes are reported in
Table 7.
The results for these two alternatives are reported in
Figure 5. This graph clearly shows a significant drop of the results in all the considered categories of impacts, compared to the previous calculations with a CO
2 purity of 95%. With a CO
2 purity of 40%, both membranes and activated coal options lie within the same range of impacts as the reference case. Impacts on GWP are now significantly lower than the reference case, with a 9% reduction for the membranes and an 8% reduction for the activated coal. This drop is mainly attributed to the saving in the energy required during CO
2 separation. Consequently, a significant decrease in the particulate matter formation potential impacts, which are directly linked to the energy consumption, is observed as well. For all the other categories, the impacts remain higher than for the reference case, though they are drastically reduced in comparison to scenarios with a CO
2 purity of 95%.
For all the categories considered, except for GWP and particulate matter formation potential, the membranes have higher impacts than the activated coal.