Recent Developments in Nanoporous Graphene Membranes for Organic Solvent Nanofiltration: A Short Review
Abstract
:1. Introduction
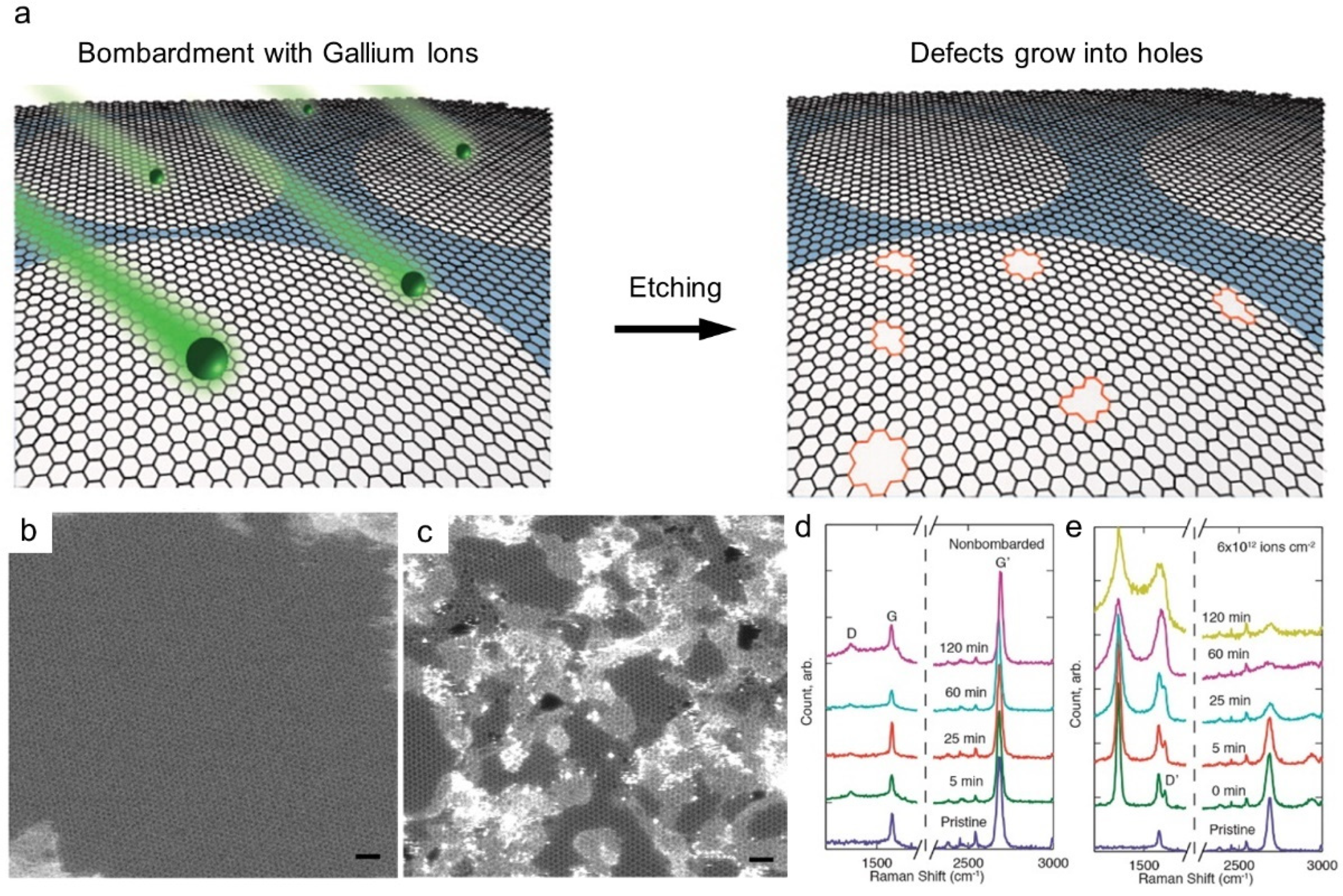
2. Methods for Introducing Nanopores in Graphene
3. OSN Performance of NG-Based Membranes
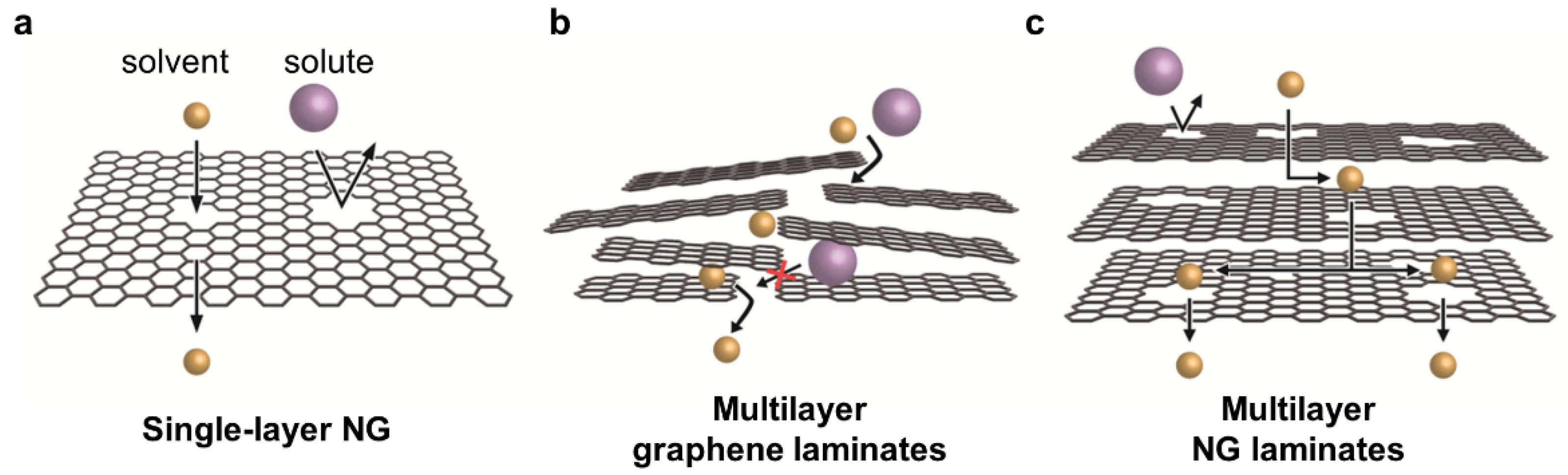
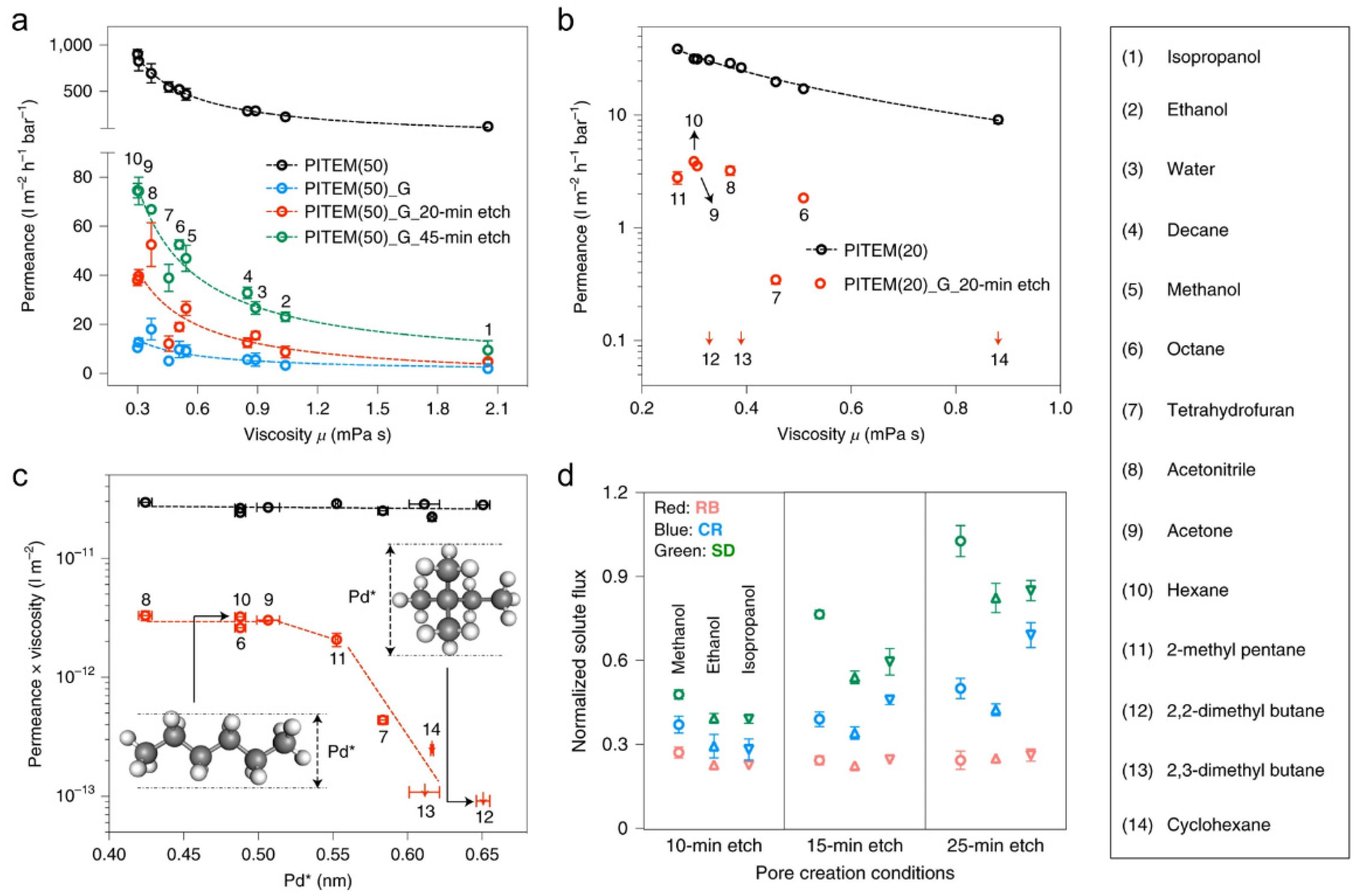
3.1. Single-Layer NG
3.2. Multilayer Graphene Laminates
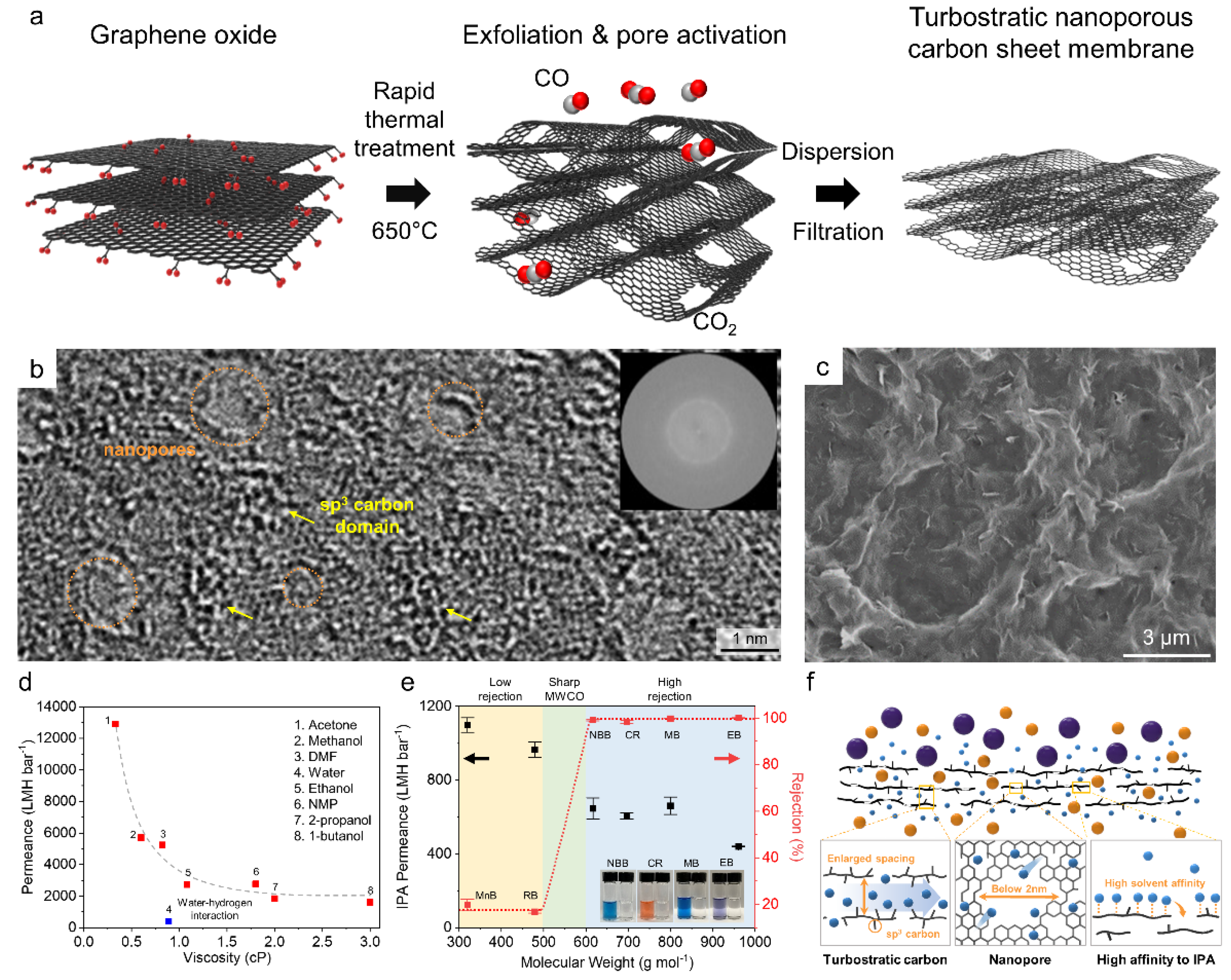
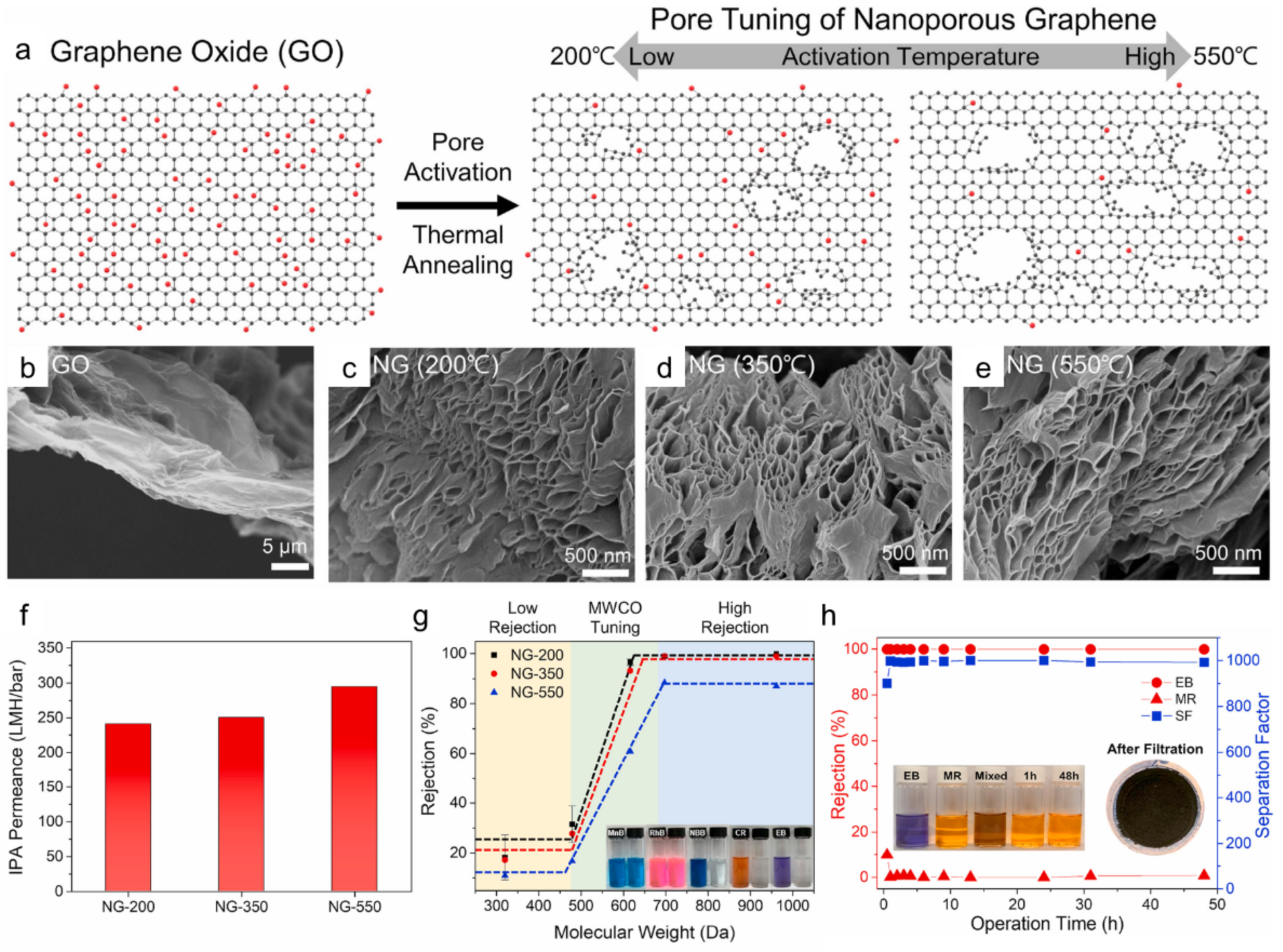
3.3. Multilayer NG Laminates
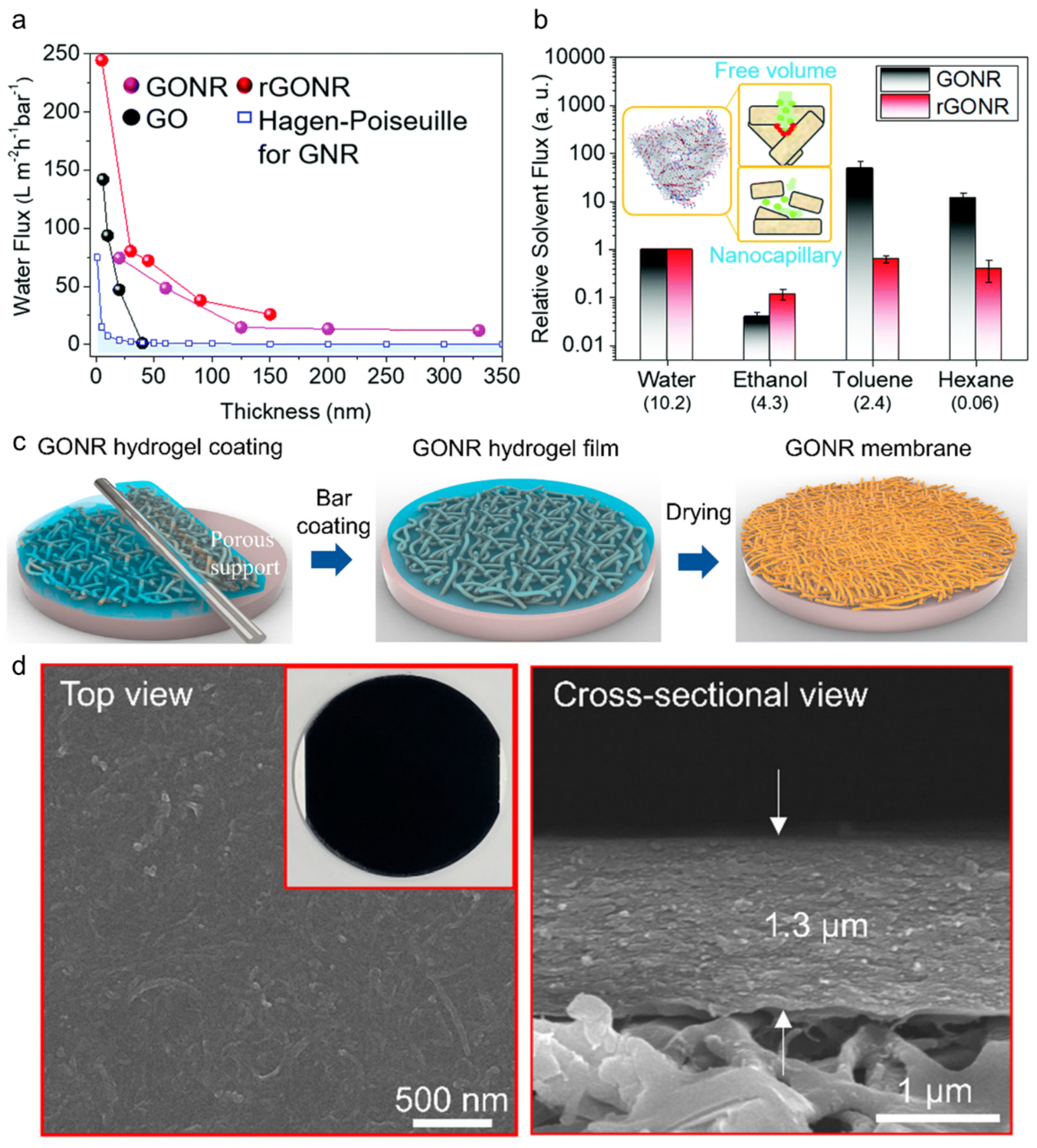
3.4. Graphene Nanoribbon Membrane (Nanopore Made of Entangled GN Nanoribbons)
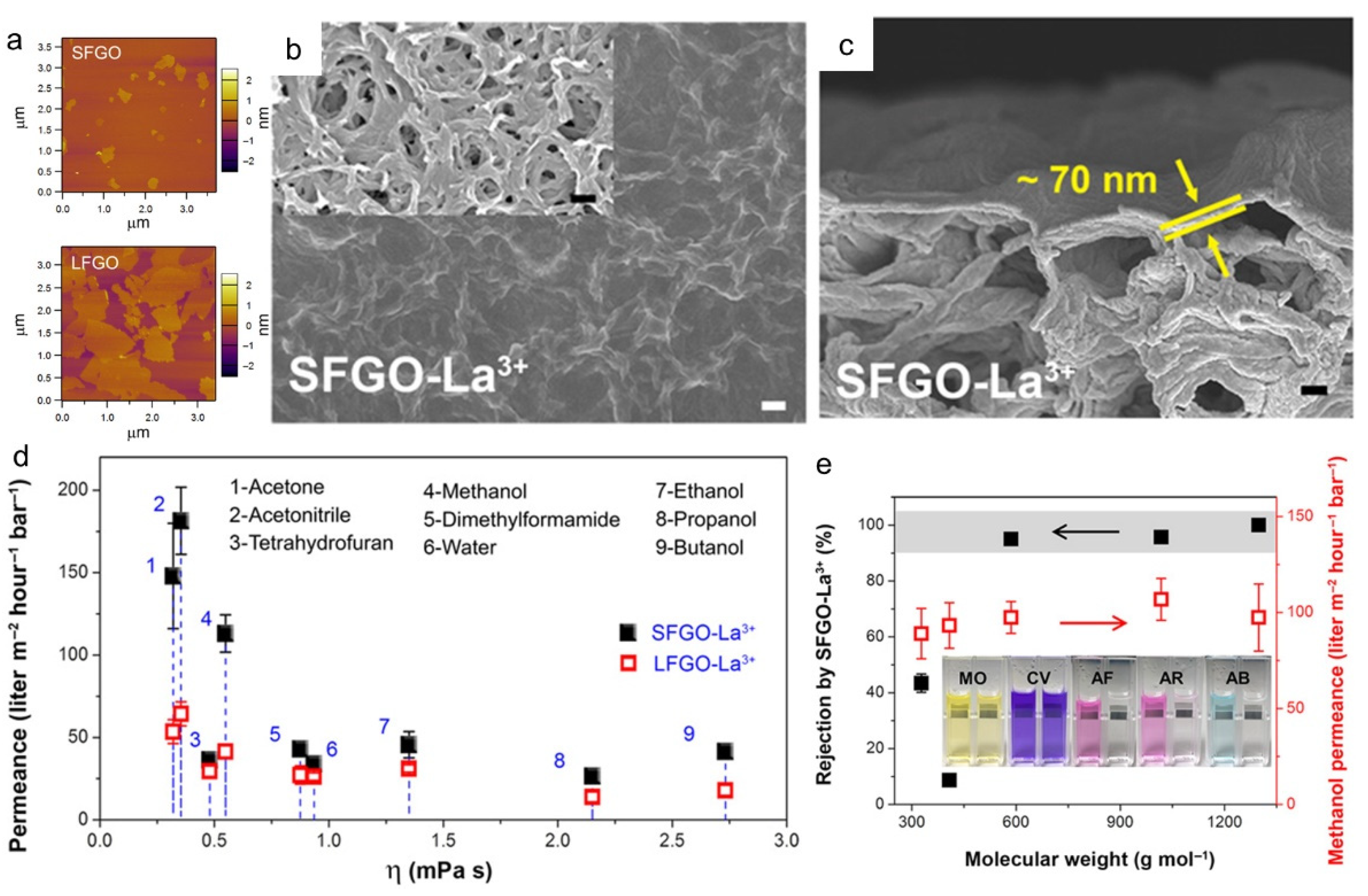
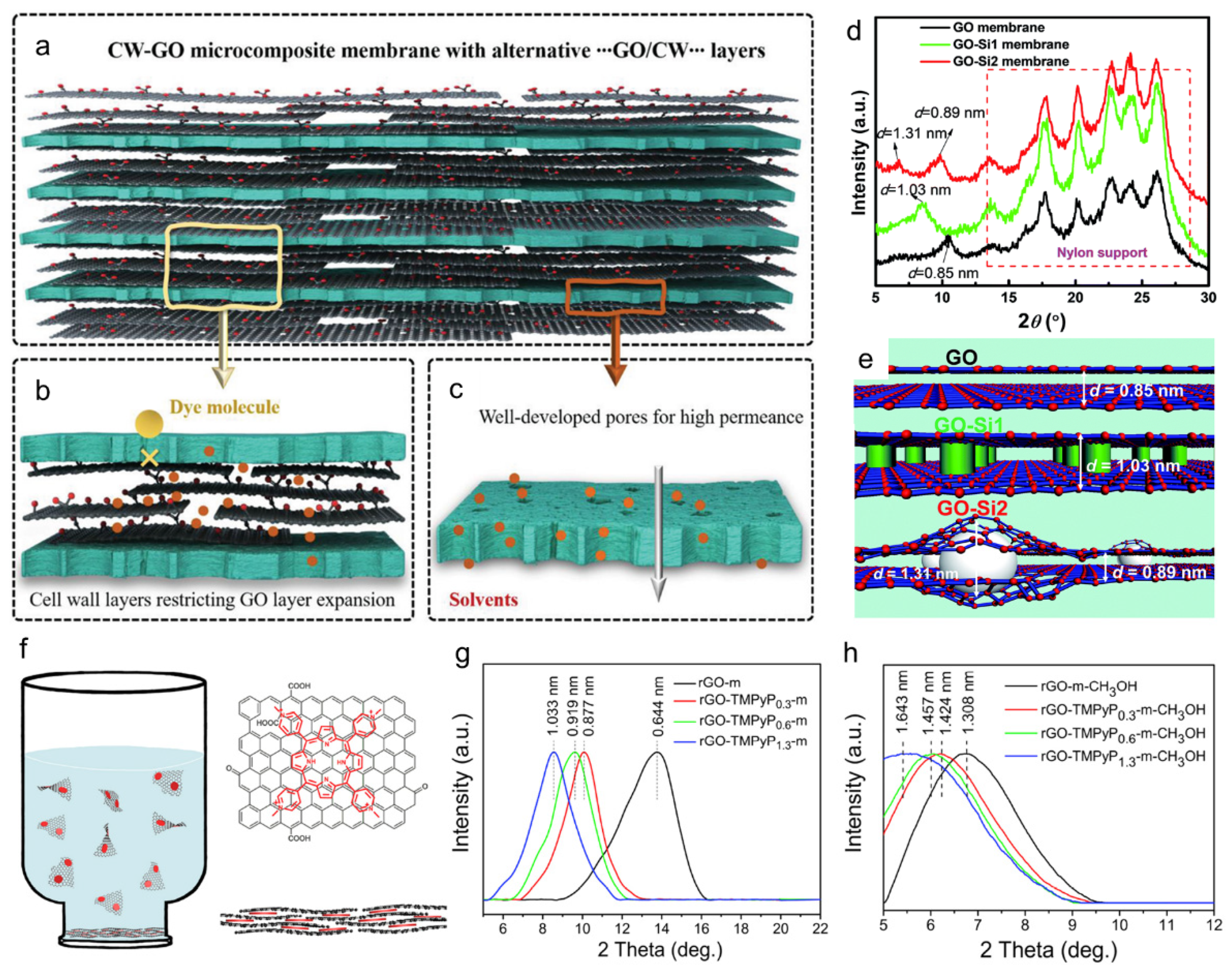
3.5. Graphene-Based Hybrid Membranes
4. Conclusions and Prospects
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Marchetti, P.; Solomon, M.F.J.; Szekely, G.; Livingston, A.G. Molecular Separation with Organic Solvent Nanofiltration: A Critical Review. Chem. Rev. 2014, 114, 10735–10806. [Google Scholar] [CrossRef]
- Nie, L.A.; Chuah, C.Y.; Bae, T.H.; Lee, J.M. Graphene-Based Advanced Membrane Applications in Organic Solvent Nanofiltration. Adv. Funct. Mater. 2021, 31, 2006949. [Google Scholar] [CrossRef]
- Frank, O.; Tsoukleri, G.; Parthenios, J.; Papagelis, K.; Riaz, I.; Jalil, R.; Novoselov, K.S.; Galiotis, C. Compression Behavior of Single-Layer Graphenes. ACS. Nano 2010, 4, 3131–3138. [Google Scholar] [CrossRef] [Green Version]
- Hod, O.; Scuseria, G.E. Electromechanical Properties of Suspended Graphene Nanoribbons. Nano Lett. 2009, 9, 2619–2622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.; Zhang, M.; Li, C.; Shi, G.Q. Graphene-Based Membranes for Molecular Separation. J. Phys. Chem. Lett. 2015, 6, 2806–2815. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Wei, X.D.; Kysar, J.W.; Hone, J. Measurement of the Elastic Properties and Intrinsic Strength of Monolayer Graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Akbari, A.; Sheath, P.; Martin, S.T.; Shinde, D.B.; Shaibani, M.; Banerjee, P.C.; Tkacz, R.; Bhattacharyya, D.; Majumder, M. Large-area Graphene-based Nanofiltration Membranes by Shear Alignment of Discotic Nematic Liquid Crystals of Graphene Oxide. Nat. Commun. 2016, 7, 10891. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Kim, S.S.; Kim, J.H.; Kang, J.; Choi, E.; Choi, S.E.; Kim, J.P.; Kwon, O.; Kim, D.W. Graphene Oxide Nanoribbon Hydrogel: Viscoelastic Behavior and Use as a Molecular Separation Membrane. ACS Nano 2020, 14, 12195–12202. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Choi, Y.; Kang, J.H.; Choi, E.; Choi, S.E.; Kwon, O.; Kim, D.W. Scalable Fabrication of Deoxygenated Graphene Oxide Nanofiltration Membrane by Continuous Slot-die Coating. J. Membr. Sci. 2020, 612, 118454. [Google Scholar] [CrossRef]
- Kwon, O.; Choi, Y.; Choi, E.; Kim, M.; Woo, Y.C.; Kim, D.W. Fabrication Techniques for Graphene Oxide-Based Molecular Separation Membranes: Towards Industrial Application. Nanomaterials 2021, 11, 757. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.K.; Carbone, P.; Wang, F.C.; Kravets, V.G.; Su, Y.; Grigorieva, I.V.; Wu, H.A.; Geim, A.K.; Nair, R.R. Precise and Ultrafast Molecular Sieving Through Graphene Oxide Membranes. Science 2014, 343, 752–754. [Google Scholar] [CrossRef] [Green Version]
- Morelos-Gomez, A.; Cruz-Silva, R.; Muramatsu, H.; Ortiz-Medina, J.; Araki, T.; Fukuyo, T.; Tejima, S.; Takeuchi, K.; Hayashi, T.; Terrones, M.; et al. Effective NaCl and Dye Rejection of Hybrid Graphene Oxide/Graphene Layered Membranes. Nat. Nanotechnol. 2017, 12, 1083–1088. [Google Scholar] [CrossRef]
- Yang, Q.; Su, Y.; Chi, C.; Cherian, C.T.; Huang, K.; Kravets, V.G.; Wang, F.C.; Zhang, J.C.; Pratt, A.; Grigorenko, A.N.; et al. Ultrathin Graphene-based Membrane with Precise Molecular Sieving and Ultrafast Solvent Permeation. Nat. Mater. 2017, 16, 1198–1202. [Google Scholar] [CrossRef] [PubMed]
- Bunch, J.S.; Verbridge, S.S.; Alden, J.S.; van der Zande, A.M.; Parpia, J.M.; Craighead, H.G.; McEuen, P.L. Impermeable Atomic Membranes from Graphene Sheets. Nano Lett. 2008, 8, 2458–2462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berry, V. Impermeability of Graphene and its Applications. Carbon 2013, 62, 1–10. [Google Scholar] [CrossRef]
- Abraham, J.; Vasu, K.S.; Williams, C.D.; Gopinadhan, K.; Su, Y.; Cherian, C.T.; Dix, J.; Prestat, E.; Haigh, S.J.; Grigorieva, I.V.; et al. Tunable Sieving of Ions using Graphene Oxide Membranes. Nat. Nanotechnol. 2017, 12, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Shi, G.S.; Shen, J.; Peng, B.Q.; Zhang, B.W.; Wang, Y.Z.; Bian, F.G.; Wang, J.J.; Li, D.Y.; Qian, Z.; et al. Ion Sieving in Graphene Oxide Membranes via Cationic Control of Interlayer Spacing. Nature 2017, 550, 415–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.W.; Yoon, H.W.; Yoon, S.M.; Yoo, B.M.; Ahn, B.K.; Cho, Y.H.; Shin, H.J.; Yang, H.; Paik, U.; Kwon, S.; et al. Selective Gas Transport Through Few-Layered Graphene and Graphene Oxide Membranes. Science 2013, 342, 91–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Williams, C.M.; Boutilier, M.S.H.; Kidambi, P.R.; Karnik, R. Single-Layer Graphene Membranes Withstand Ultrahigh Applied Pressure. Nano Lett. 2017, 17, 3081–3088. [Google Scholar] [CrossRef]
- Huang, S.Q.; Dakhchoune, M.; Luo, W.; Oveisi, E.; He, G.W.; Rezaei, M.; Zhao, J.; Alexander, D.T.L.; Zuttel, A.; Strano, M.S.; et al. Single-layer Graphene Membranes by Crack-free Transfer for Gas Mixture Separation. Nat. Commun. 2018, 9, 2632. [Google Scholar] [CrossRef]
- Sun, P.Z.; Yang, Q.; Kuang, W.J.; Stebunov, Y.V.; Xiong, W.Q.; Yu, J.; Nair, R.R.; Katsnelson, M.I.; Yuan, S.J.; Grigorieva, I.V.; et al. Limits on Gas Impermeability of Graphene. Nature 2020, 579, 229–232. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Kravets, V.G.; Wong, S.L.; Waters, J.; Geim, A.K.; Nair, R.R. Impermeable Barrier Films and Protective Coatings based on Reduced Graphene Oxide. Nat. Commun. 2014, 5, 4843. [Google Scholar] [CrossRef]
- Liang, S.W.; Song, Y.M.; Zhang, Z.M.; Mu, B.W.; Li, R.; Li, Y.; Yang, H.; Wang, M.D.; Pan, F.S.; Jiang, Z.Y. Construction of Graphene Oxide Membrane through Non-covalent Cross-linking by Sulfonated Cyclodextrin for Ultra-permeable Butanol Dehydration. J. Membr. Sci. 2021, 621, 118938. [Google Scholar] [CrossRef]
- Shin, Y.; Taufique, M.F.N.; Devanathan, R.; Cutsforth, E.C.; Lee, J.; Liu, W.; Fifield, L.S.; Gotthold, D.W. Highly Selective Supported Graphene Oxide Membranes for Water-Ethanol Separation. Sci. Rep. 2019, 9, 2251. [Google Scholar] [CrossRef] [PubMed]
- O’Hern, S.C.; Boutilier, M.S.H.; Idrobo, J.C.; Song, Y.; Kong, J.; Laoui, T.; Atieh, M.; Karnik, R. Selective Ionic Transport through Tunable Subnanometer Pores in Single-Layer Graphene Membranes. Nano Lett. 2014, 14, 1234–1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Surwade, S.P.; Smirnov, S.N.; Vlassiouk, I.V.; Unocic, R.R.; Veith, G.M.; Dai, S.; Mahurin, S.M. Water Desalination using Nanoporous Single-layer Graphene. Nat. Nanotechnol. 2015, 10, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Koenig, S.P.; Wang, L.D.; Pellegrino, J.; Bunch, J.S. Selective Molecular Sieving through Porous Graphene. Nat. Nanotechnol. 2012, 7, 728–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischbein, M.D.; Drndic, M. Electron Beam Nanosculpting of Suspended Graphene Sheets. Appl. Phys. Lett. 2008, 93, 113107. [Google Scholar] [CrossRef] [Green Version]
- Celebi, K.; Buchheim, J.; Wyss, R.M.; Droudian, A.; Gasser, P.; Shorubalko, I.; Kye, J.I.; Lee, C.; Park, H.G. Ultimate Permeation Across Atomically Thin Porous Graphene. Science 2014, 344, 289–292. [Google Scholar] [CrossRef]
- Yang, Y.B.; Yang, X.D.; Liang, L.; Gao, Y.Y.; Cheng, H.Y.; Li, X.M.; Zou, M.C.; Cao, A.Y.; Ma, R.Z.; Yuan, Q.; et al. Large-area Graphene-nanomesh/carbon-nanotube Hybrid Membranes for Ionic and Molecular Nanofiltration. Science 2019, 364, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Choi, J.; Kim, D.; Jung, H.T. Enhanced Water Permeation based on Nanoporous Multilayer Graphene Membranes: The Role of Pore Size and Density. J. Mater. Chem. A 2016, 4, 17773–17781. [Google Scholar] [CrossRef]
- Jang, J.; Nam, Y.T.; Kim, D.; Kim, Y.J.; Kim, D.W.; Jung, H.T. Turbostratic Nanoporous Carbon Sheet Membrane for Ultrafast and Selective Nanofiltration in Viscous Green Solvents. J. Mater. Chem. A 2020, 8, 8292–8299. [Google Scholar] [CrossRef]
- Xu, Y.X.; Lin, Z.Y.; Zhong, X.; Huang, X.Q.; Weiss, N.O.; Huang, Y.; Duan, X.F. Holey Graphene Frameworks for Highly Efficient Capacitive Energy Storage. Nat. Commun. 2014, 5, 4554. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.W.; Murali, S.; Stoller, M.D.; Ganesh, K.J.; Cai, W.W.; Ferreira, P.J.; Pirkle, A.; Wallace, R.M.; Cychosz, K.A.; Thommes, M.; et al. Carbon-Based Supercapacitors Produced by Activation of Graphene. Science 2011, 332, 1537–1541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, Z.J.; Zhao, Q.K.; Li, T.Y.; Yan, J.; Ren, Y.M.; Feng, J.; Wei, T. Easy Synthesis of Porous Graphene Nanosheets and their use in Supercapacitors. Carbon 2012, 50, 1699–1703. [Google Scholar] [CrossRef]
- Bieri, M.; Blankenburg, S.; Kivala, M.; Pignedoli, C.A.; Ruffieux, P.; Mullen, K.; Fasel, R. Surface-Supported 2D Heterotriangulene Polymers. Chem. Commun. 2011, 47, 10239–10241. [Google Scholar] [CrossRef]
- Wang, J.T.; Chen, P.P.; Shi, B.B.; Guo, W.W.; Jaroniec, M.; Qiao, S.Z. A Regularly Channeled Lamellar Membrane for Unparalleled Water and Organics Permeation. Angew. Chem. Int. Ed. 2018, 57, 6814–6818. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Zhang, H.Q.; Wu, X.L.; Zhang, J.K.; Wang, J.T.; Li, Y.F. Novel Thin-film Nanocomposite Membranes Filled with Multi-functional Ti3C2Tx Nanosheets for Task-specific Solvent Transport. Compos. Part A 2017, 100, 139–149. [Google Scholar] [CrossRef]
- Shao, D.D.; Zhang, Q.X.; Wang, L.; Wang, Z.Y.; Jing, Y.X.; Cao, X.L.; Zhang, F.; Sun, S.P. Enhancing Interfacial Adhesion of MXene Nanofiltration Membranes via Pillaring Carbon Nanotubes for Pressure and Solvent Stable Molecular Sieving. J. Membr. Sci. 2021, 623, 119033. [Google Scholar] [CrossRef]
- Ran, J.; Zhang, P.P.; Chu, C.Q.; Cui, P.; Ai, X.Y.; Pan, T.; Wu, Y.Y.; Xu, T.W. Ultrathin Lamellar MoS2 Membranes for Organic Solvent Nanofiltration. J. Membr. Sci. 2020, 602, 117963. [Google Scholar] [CrossRef]
- Tham, H.M.; Japip, S.; Chung, T.S. WS2 Deposition on Cross-linked Polyacrylonitrile with Synergistic Transformation to Yield Organic Solvent Nanofiltration Membranes. J. Membr. Sci. 2019, 588, 117219. [Google Scholar] [CrossRef]
- Jiang, S.D.; Koh, A.Y.K.; Chong, K.H.; Zhang, S. Opening Organic Solvent Pathways by Molybdenum Disulfide in Mixed Matrix Membranes for Molecular Separation. J. Membr. Sci. 2019, 585, 60–66. [Google Scholar] [CrossRef]
- Liu, J.T.; Han, G.; Zhao, D.L.; Lu, K.J.; Gao, J.; Chung, T.S. Self-standing and Flexible Covalent Organic Framework (COF) Membranes for Molecular Separation. Sci. Adv. 2020, 6, eabb1110. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, W.P.; Fang, Q.Y.; Zuo, K.C.; Hou, G.M.; Ai, Q.; Li, Q.L.; Ci, L.J.; Lou, J. High Performance Hierarchically Nanostructured Graphene Oxide/Covalent Organic Framework Hybrid Membranes for Stable Organic Solvent Nanofiltration. Appl. Mater. Today 2020, 20, 100791. [Google Scholar] [CrossRef]
- Manoranjan, N.; Zhang, F.; Wang, Z.Y.; Dong, Y.P.; Fang, W.X.; Zhang, Y.T.; Zhu, Y.Z.; Jin, J. A Single-Walled Carbon Nanotube/Covalent Organic Framework Nanocomposite Ultrathin Membrane with High Organic Solvent Resistance for Molecule Separation. ACS Appl. Mater. Interfaces 2020, 12, 53096–53103. [Google Scholar] [CrossRef]
- Lei, X.T.; Tay, S.W.; Ong, P.J.; Hong, L. Organic Dye Solution Nanofiltration by 2D Zn-TCPP(Fe) Membrane—Leverage of Chemical and Fluid Dynamic Effects. J. Ind. Eng. Chem. 2019, 78, 410–420. [Google Scholar] [CrossRef]
- Ang, E.H.; Velioglu, S.; Chew, J.W. Tunable Affinity Separation Enables Ultrafast Solvent Permeation through Layered Double Hydroxide Membranes. J. Membr. Sci. 2019, 591, 117318. [Google Scholar] [CrossRef]
- Ang, E.H.; Chew, J.W. Two-Dimensional Transition-Metal Dichalcogenide-Based Membrane for Ultrafast Solvent Permeation. Chem. Mater. 2019, 31, 10002–10007. [Google Scholar] [CrossRef]
- Chen, C.; Wang, J.M.; Liu, D.; Yang, C.; Liu, Y.C.; Ruoff, R.S.; Lei, W.W. Functionalized Boron Nitride Membranes with Ultrafast Solvent Transport Performance for Molecular Separation. Nat. Commun. 2018, 9, 1902. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Iyengar, S.A.; Karnik, R. Molecular Size-dependent Subcontinuum Solvent Permeation and Ultrafast Nanofiltration Across Nanoporous Graphene Membranes. Nat. Nanotechnol. 2021, 16, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Hua, D.; Chung, T.S. Polyelectrolyte Functionalized Lamellar Graphene Oxide Membranes on Polypropylene Support for Organic Solvent Nanofiltration. Carbon 2017, 122, 604–613. [Google Scholar] [CrossRef]
- Li, Y.Y.; Li, C.; Li, S.X.; Su, B.W.; Han, L.H.; Mandal, B. Graphene Oxide (GO)-interlayered Thin-film Nanocomposite (TFN) Membranes with High Solvent Resistance for Organic Solvent Nanofiltration (OSN). J. Mater. Chem. A 2019, 7, 13315–13330. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Zhang, M.C.; Liu, G.P.; Jin, W.Q.; Li, X.Y. Fungal Cell Wall-Graphene Oxide Microcomposite Membrane for Organic Solvent Nanofiltration. Adv. Funct. Mater. 2021, 31, 2100110. [Google Scholar] [CrossRef]
- Wang, S.F.; Mahalingam, D.; Sutisna, B.; Nunes, S.P. 2D-dual-spacing Channel Membranes for High Performance Organic Solvent Nanofiltration. J. Mater. Chem. A 2019, 7, 11673–11682. [Google Scholar] [CrossRef] [Green Version]
- Gao, T.T.; Wu, H.B.; Tao, L.; Qu, L.T.; Li, C. Enhanced Stability and Separation Efficiency of Graphene Oxide Membranes in Organic Solvent Nanofiltration. J. Mater. Chem. A 2018, 6, 19563–19569. [Google Scholar] [CrossRef]
- Gao, T.T.; Huang, L.; Li, C.; Xu, G.C.; Shi, G.Q. Graphene Membranes with Tuneable Nanochannels by Intercalating Self-Assembled Porphyrin Molecules for Organic Solvent Nanofiltration. Carbon 2017, 124, 263–270. [Google Scholar] [CrossRef]
- Li, B.F.; Cui, Y.; Japip, S.; Thong, Z.W.; Chung, T.S. Graphene Oxide (GO) Laminar Membranes for Concentrating Pharmaceuticals and Food Additives in Organic Solvents. Carbon 2018, 130, 503–514. [Google Scholar] [CrossRef]
- Akbari, A.; Meragawi, S.E.; Martin, S.T.; Corry, B.; Shamsaei, E.; Easton, C.D.; Bhattacharyya, D.; Majumder, M. Solvent Transport Behavior of Shear Aligned Graphene Oxide Membranes and Implications in Organic Solvent Nanofiltration. ACS Appl. Mater. Interfaces 2018, 10, 2067–2074. [Google Scholar] [CrossRef] [PubMed]
- Ai, X.Y.; Zhang, P.P.; Dou, Y.; Wu, Y.Y.; Pan, T.; Chu, C.Q.; Cui, P.; Ran, J. Graphene Oxide Membranes with Hierarchical Structures Used for Molecule Sieving. Sep. Purif. Technol. 2020, 230, 115879. [Google Scholar] [CrossRef]
- Liu, M.L.; Guo, J.L.; Japip, S.; Jia, T.Z.; Shao, D.D.; Zhang, S.; Li, W.J.; Wang, J.; Cao, X.L.; Sun, S.P. One-step Enhancement of Solvent Transport, Stability and Photocatalytic Properties of Graphene Oxide/polyimide Membranes with Multifunctional Cross-linkers. J. Mater. Chem. A 2019, 7, 3170–3178. [Google Scholar] [CrossRef]
- Kang, J.; Choi, Y.; Kim, J.P.; Kim, J.H.; Kim, J.Y.; Kwon, O.; Kim, D.I.; Kim, D.W. Thermally-induced Pore Size Tuning of Multilayer Nanoporous Graphene for Organic Solvent Nanofiltration. J. Membr. Sci. 2021, 637, 119620. [Google Scholar] [CrossRef]
- Cohen-Tanugi, D.; Grossman, J.C. Water Desalination across Nanoporous Graphene. Nano Lett. 2012, 12, 3602–3608. [Google Scholar] [CrossRef] [PubMed]
- Sint, K.; Wang, B.; Kral, P. Selective Ion Passage through Functionalized Graphene Nanopores. J. Am. Chem. Soc. 2008, 130, 16448–16449. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.Z.; Hu, Y.Y.; Koehler, S.; Cai, L.H.; Wen, J.J.; Tan, X.J.; Xu, W.W.L.; Sheng, Q.; Hou, X.; Xue, J.M.; et al. Ultrafast Nanofiltration through Large-Area Single-Layered Graphene Membranes. ACS Appl. Mater. Interfaces 2017, 9, 9239–9244. [Google Scholar] [CrossRef]
- Nam, Y.T.; Kim, S.J.; Kang, K.M.; Jung, W.B.; Kim, D.W.; Jung, H.T. Enhanced Nanofiltration Performance of Graphene-based Membranes on Wrinkled Polymer Supports. Carbon 2019, 148, 370–377. [Google Scholar] [CrossRef]
- Huang, K.; Liu, G.P.; Shen, J.; Chu, Z.Y.; Zhou, H.L.; Gu, X.H.; Jin, W.Q.; Xu, N.P. High-Efficiency Water-Transport Channels using the Synergistic Effect of a Hydrophilic Polymer and Graphene Oxide Laminates. Adv. Funct. Mater. 2015, 25, 5809–5815. [Google Scholar] [CrossRef]
- Kim, D.W.; Kim, Y.H.; Jeong, H.S.; Jung, H.T. Direct Visualization of Large-area Graphene Domains and Boundaries by Optical Birefringency. Nat. Nanotechnol. 2012, 7, 29–34. [Google Scholar] [CrossRef]
- Ibrahim, A.F.M.; Lin, Y.S. Synthesis of Graphene Oxide Membranes on Polyester Substrate by Spray coating for Gas Separation. Chem. Eng. Sci. 2018, 190, 312–319. [Google Scholar] [CrossRef]
- Dong, Y.H.; Cheng, Y.X.; Xu, G.H.; Cheng, H.W.; Huang, K.J.; Duan, J.L.; Mo, D.; Zeng, J.; Bai, J.; Sun, Y.M.; et al. Selectively Enhanced Ion Transport in Graphene Oxide Membrane/PET Conical Nanopore System. ACS Appl. Mater. Interfaces 2019, 11, 14960–14969. [Google Scholar] [CrossRef]
- Tsou, C.H.; An, Q.F.; Lo, S.C.; De Guzman, M.; Hung, W.S.; Hu, C.C.; Lee, K.R.; Lai, J.Y. Effect of Microstructure of Graphene Oxide Fabricated through Different Self-assembly Techniques on 1-butanol Dehydration. J. Membr. Sci. 2015, 477, 93–100. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, D.W.; Jung, H.T.; Choi, J.W. Controlled Lithium Dendrite Growth by a Synergistic Effect of Multilayered Graphene Coating and an Electrolyte Additive. Chem. Mater. 2015, 27, 2780–2787. [Google Scholar] [CrossRef]
- Kim, D.; Kim, D.W.; Lim, H.K.; Jeon, J.; Kim, H.; Jung, H.T.; Lee, H. Intercalation of Gas Molecules in Graphene Oxide Inter layer: The Role of Water. J. Phys. Chem. C 2014, 118, 11142–11148. [Google Scholar] [CrossRef]
- Park, J.W.; Tatavarty, R.; Kim, D.W.; Jung, H.T.; Gu, M.B. Immobilization-Free Screening of Aptamers Assisted by Graphene Oxide. Chem. Commun. 2012, 48, 2071–2073. [Google Scholar] [CrossRef]
- Huang, L.; Li, Y.R.; Zhou, Q.Q.; Yuan, W.J.; Shi, G.Q. Graphene Oxide Membranes with Tunable Semipermeability in Organic Solvents. Adv. Mater. 2015, 27, 3797–3802. [Google Scholar] [CrossRef]
- Zheng, S.X.; Tu, Q.S.; Wang, M.N.; Urban, J.J.; Mi, B.X. Correlating Interlayer Spacing and Separation Capability of Graphene Oxide Membranes in Organic Solvents. ACS Nano 2020, 14, 6013–6023. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Goh, K.; Wang, Y.; Lee, J.; Huang, Y.J.; Karahan, H.E.; Zhou, K.; Guiver, M.D.; Bae, T.H. Realizing Mmall-Flake Graphene Oxide Membranes for Ultrafast Size-Dependent Organic Solvent Nanofiltration. Sci. Adv. 2020, 6, eaaz9184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen-Tanugi, D.; Lin, L.C.; Grossman, J.C. Multilayer Nanoporous Graphene Membranes for Water Desalination. Nano Lett. 2016, 16, 1027–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.W.; Kim, I.; Jang, J.; Nam, Y.T.; Park, K.; Kwon, K.O.; Cho, K.M.; Choi, J.; Kim, D.; Kang, K.M.; et al. One Dimensional Building Blocks for Molecular Separation: Laminated Graphitic Nanoribbons. Nanoscale 2017, 9, 19114–19123. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.X.; Sun, H.; Yang, H.Y.; Li, X.T.; Ji, S.L.; An, Q.F. Hollow Polyhedron-Modified Graphene Oxide Membranes for Organic Solvent Nanofiltration with Enhanced Permeance. ACS Appl. Nano Mater. 2020, 3, 5874–5880. [Google Scholar] [CrossRef]
- Mahalingam, D.K.; Wang, S.F.; Nunes, S.P. Stable Graphene Oxide Cross-Linked Membranes for Organic Solvent Nanofiltration. Ind. Eng. Chem. Res. 2019, 58, 23106–23113. [Google Scholar] [CrossRef]
- Nam, Y.T.; Choi, J.; Kang, K.M.; Kim, D.W.; Jung, H.T. Enhanced Stability of Laminated Graphene Oxide Membranes for Nanofiltration via Interstitial Amide Bonding. ACS Appl. Mater. Interfaces 2016, 8, 27376–27382. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kim, D.W.; Cho, K.M.; Kang, K.M.; Choi, J.; Kim, D.; Jung, H.T. Ultrathin Graphene Oxide Membranes on Freestanding Carbon Nanotube Supports for Enhanced Selective Permeation in Organic Solvents. Sci. Rep. 2018, 8, 1959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Type | Material | Permeance (a) | Rejection (b) | MWCO (c) | Fabrication Method | Filtered Solvent | REF |
|---|---|---|---|---|---|---|---|
| MXene | Ti3C2Tx | 982.7 | AY79 (100%) | 992 | Vacuum filtration | Isopropanol | [37] |
| PAN/PEI-Ti3C2Tx-NH2 | 3 | PEG800 (96%) | 200 | Drop casting | Isopropanol | [38] | |
| Ti3C2Tx/CNTs-CTAB | 60.8 | CR (>95%) | 697 | Vacuum filtration | Ethanol | [39] | |
| TMD | S-MoS2 | 636 | MnB (99%) MB (100%) | 320 | Vacuum filtration | Isopropanol | [40] |
| D-MoS2 | 109 | MnB (98%) BF (100%) | 320 | Vacuum filtration | Isopropanol | [40] | |
| WS2-NMP-15 | 44.4 | EB (99%) RBB (91.5%) | 627 | Pressure-assisted filtration | Ethanol | [41] | |
| (3PEI/5PSS-2.5MoS2)1.5 | 3.4 | MnB (93.4%) | 320 | Layer by layer | Ethanol | [42] | |
| COF | TAPA-TFP | 127.3 | BBR (94.8%) | 826 | Interfacial reaction | Ethanol | [43] |
| GO/COF | 50.8 | MnB (99%) CR (99.8%) | 320 | Vacuum filtration | Ethanol | [44] | |
| SWCNT/COF | 60.5 | BBG (93%) | 854 | In-situ growth | Acetone | [45] | |
| MOF | Zn-TCPP (Fe) | 140 | BBG (90%) | 854 | Vacuum filtration | Isopropanol | [46] |
| LDH | Mg-AlLDH | 651 | AF (99.6%) MO (98.3%) | 327 | Vacuum filtration | Acetone | [47] |
| NiS2/Ni-AlLDH | 2464 | MO (99.9%) | 327 | Vacuum filtration | Acetone | [48] | |
| BN | FBN-2 | 330 † | CR (>99%) | 697 | Vacuum filtration | Ethanol | [49] |
| FBN-8 | 240 † | MnB (93%) | 320 | Vacuum filtration | Methanol | [49] | |
| Single-layer NG | Defect-sealed NG | 170.7 | RB (97.3%) | 826 | CVD | Methanol | [50] |
| 50.9 | RB (95.9%) | 826 | CVD | Ethanol | [50] | ||
| Multilayer graphene | TPP/GO/HPEI | 8.5 | AB (95%) | 1299 | Pressure-assisted filtration | Ethanol | [51] |
| PA/cGO/cross-linked PI | 4.9 | EY (100%) RhB (99.3%) | 479 | Immersion | Ethanol | [52] | |
| CW-GO | 58.1 | EB (96%) | 248 | Vacuum filtration | Methanol | [53] | |
| GO-Si2 | 290 | RB (91.9%) CR (95.8%) | 697 | Vacuum filtration | Methanol | [54] | |
| GO-0.5BA-T | 4 | AF (95.8%) | 586 | Vacuum filtration | Methanol | [55] | |
| rGO-TMPyP0.6-44 | 5.3 | EB (>99.9%) AF (92.2%) | 586 | Vacuum filtration | Methanol | [56] | |
| GO/EDA | 0.5 | VB (95.3%) | 837 | Pressure-assisted filtration | Isopropanol | [57] | |
| Shear-aligned GO | 130 | RhB (91%) | 974 | Gravure printing | Isopropanol | [58] | |
| Micro & nanosized GO | 109.5 | MnB (98%) FB (91%) | 338 | Vacuumfiltration | Isopropanol | [59] | |
| PI/GO | 107.8 | OII (96.3%) RB (99.9%) | 350 | Pressure-assisted filtration | Acetonitrile | [60] | |
| Multilayer NG | TNCS | 1839 | NBB (99%) | 600 | Vacuum filtration | Isopropanol | [32] |
| NG-200 | 241 | EB (99.9%) CR (98.9%) | 616 | Vacuum filtration | Isopropanol | [61] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nam, Y.-T.; Kang, J.-H.; Jang, J.-D.; Bae, J.-H.; Jung, H.-T.; Kim, D.-W. Recent Developments in Nanoporous Graphene Membranes for Organic Solvent Nanofiltration: A Short Review. Membranes 2021, 11, 793. https://doi.org/10.3390/membranes11100793
Nam Y-T, Kang J-H, Jang J-D, Bae J-H, Jung H-T, Kim D-W. Recent Developments in Nanoporous Graphene Membranes for Organic Solvent Nanofiltration: A Short Review. Membranes. 2021; 11(10):793. https://doi.org/10.3390/membranes11100793
Chicago/Turabian StyleNam, Yoon-Tae, Jun-Hyeok Kang, Jae-Dong Jang, Jun-Hyuk Bae, Hee-Tae Jung, and Dae-Woo Kim. 2021. "Recent Developments in Nanoporous Graphene Membranes for Organic Solvent Nanofiltration: A Short Review" Membranes 11, no. 10: 793. https://doi.org/10.3390/membranes11100793
APA StyleNam, Y.-T., Kang, J.-H., Jang, J.-D., Bae, J.-H., Jung, H.-T., & Kim, D.-W. (2021). Recent Developments in Nanoporous Graphene Membranes for Organic Solvent Nanofiltration: A Short Review. Membranes, 11(10), 793. https://doi.org/10.3390/membranes11100793








