Facile Fabrication of Multi-Hydrogen Bond Self-Assembly Poly(MAAc-co-MAAm) Hydrogel Modified PVDF Ultrafiltration Membrane to Enhance Anti-Fouling Property
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Membrane Modification
2.3. Membrane Characterization
2.4. Evaluation of Gel Layer Stability
2.5. Fouling Properties
2.5.1. Static Adsorption of Protein
2.5.2. Dynamic Fouling Experiments
3. Results and Discussion
3.1. Surface Characterization of Membranes
3.2. Morphology of Membranes
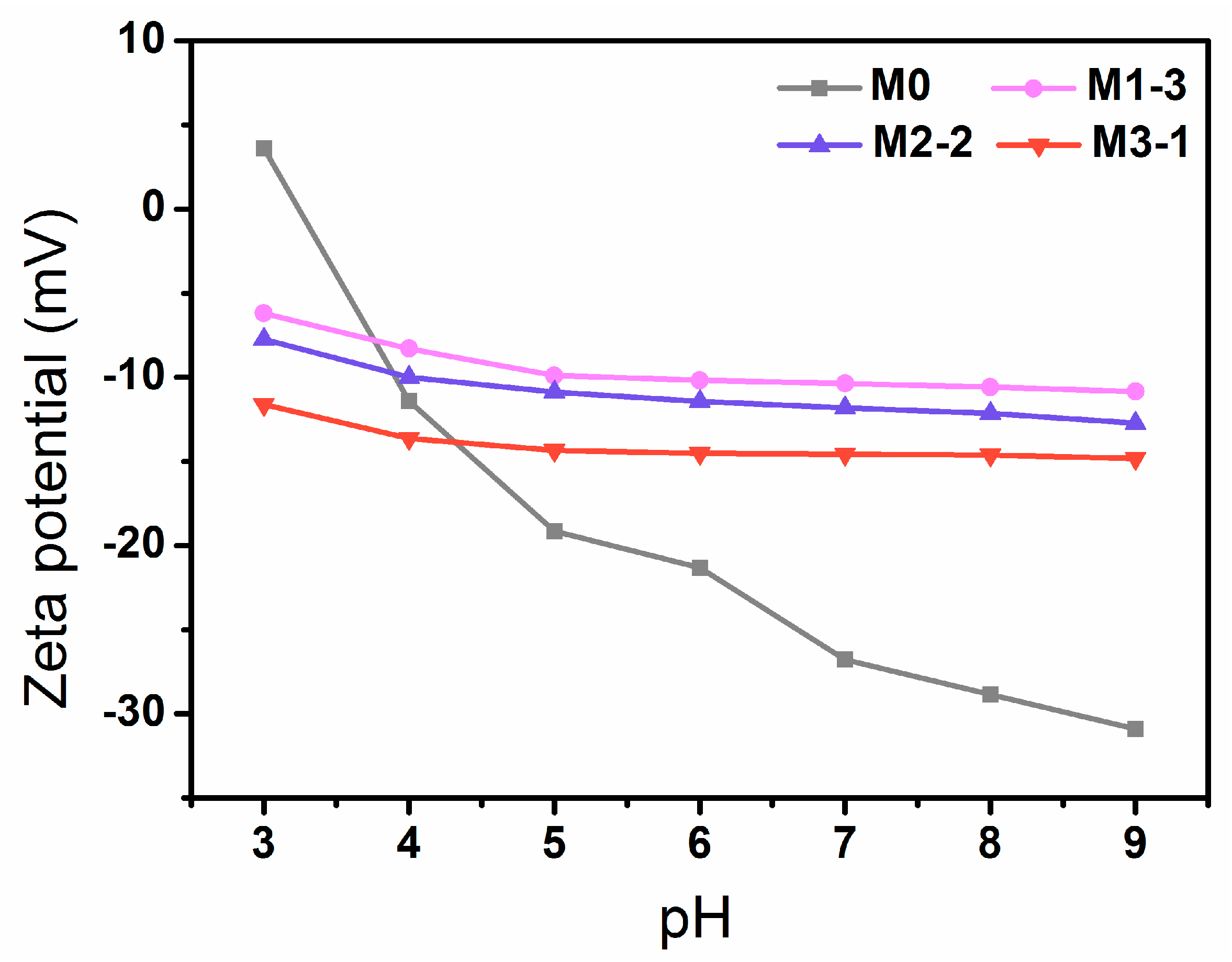
3.3. Zeta Potential of Membranes
3.4. Surface Hydrophilicity and Swelling Degree
3.5. Stability of the Grafted Functional Layer
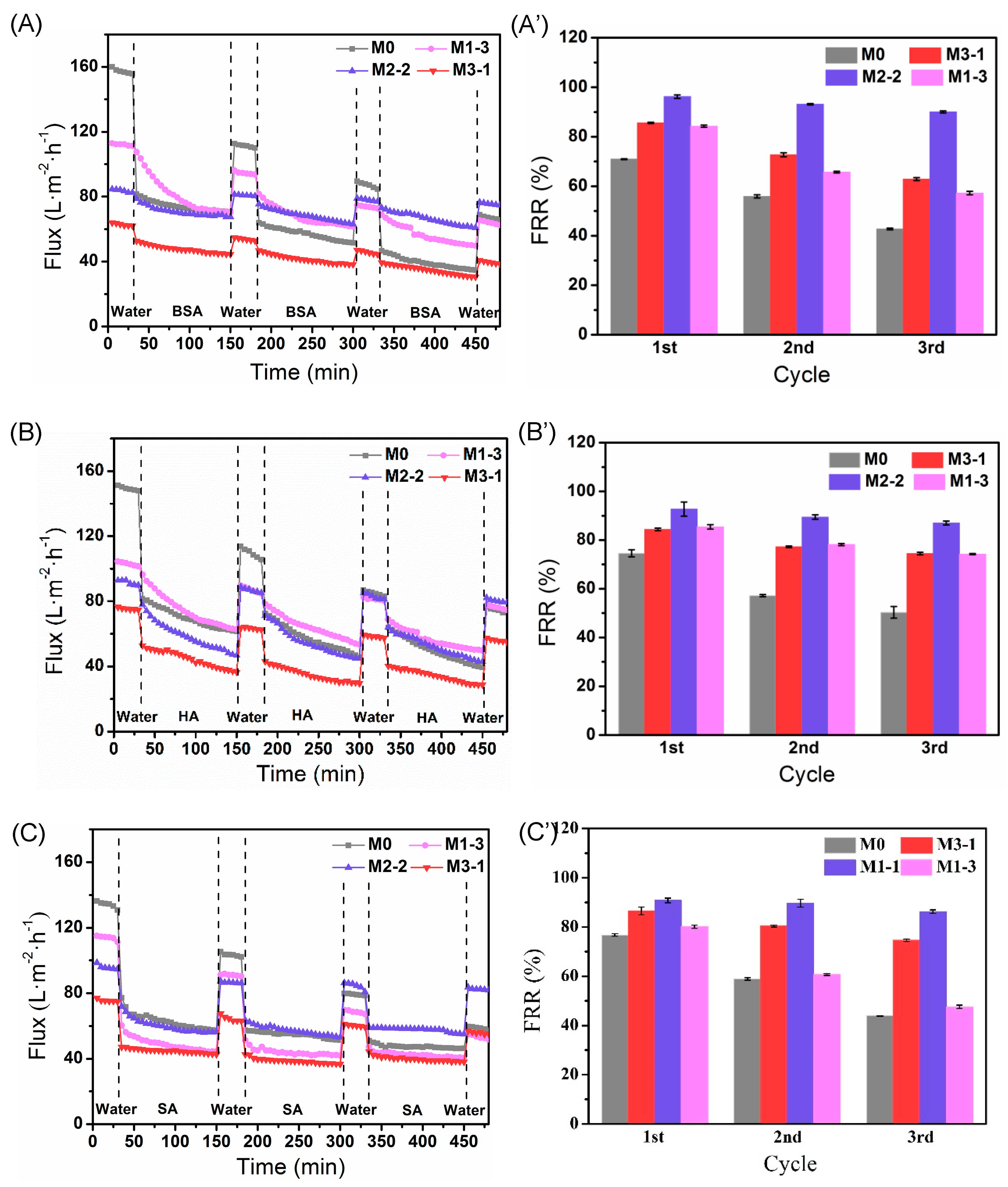
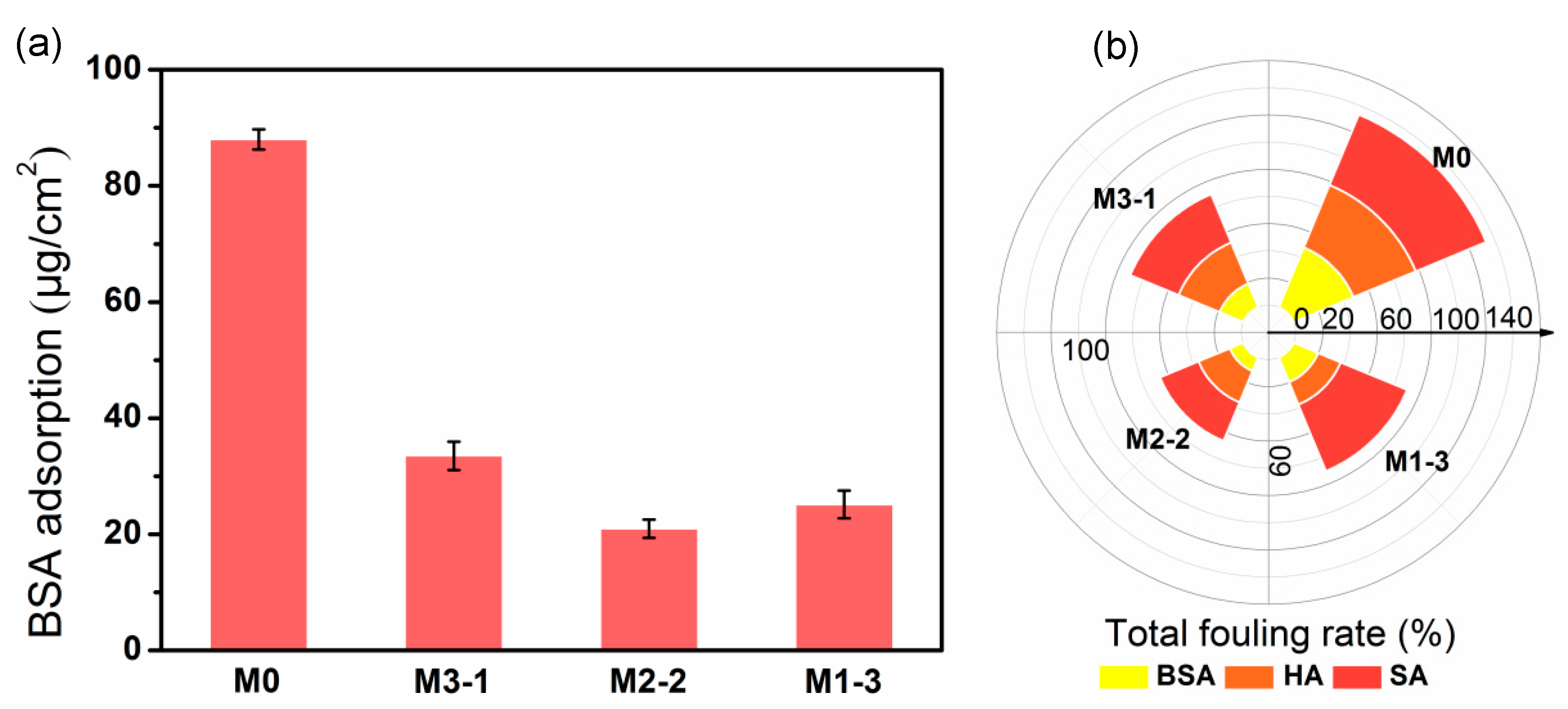
3.6. Antifouling Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lively, R.P.; Sholl, D.S. From water to organics in membrane separations. Nat. Mater. 2017, 16, 276–279. [Google Scholar] [CrossRef]
- Teow, Y.H.; Ooi, B.S.; Ahmad, A.L.; Lim, J.K. Investigation of anti-fouling and UV-cleaning properties of PVDF/TiO2 mixed-matrix membrane for humic acid removal. Membranes 2021, 11, 16. [Google Scholar] [CrossRef]
- Shahkaramipour, N.; Tran, T.N.; Ramanan, S.; Lin, H.Q. Membranes with surface-enhanced antifouling purification. Membranes 2017, 7, 13. [Google Scholar] [CrossRef] [Green Version]
- Nady, N. PES surface modification rsing green chemistry: New generation of antifouling membranes. Membranes 2016, 6, 23. [Google Scholar] [CrossRef] [Green Version]
- Ranjani, M.; Yoo, D.J.; Gnanakumar, G. Sulfonated Fe3O4@SiO2 nanorods incorporated sPVdF anocomposite membranes for DMFC applications. J. Membr. Sci. 2018, 555, 497–506. [Google Scholar] [CrossRef]
- Cui, Z.Y.; Tang, X.X.; Li, W.; Liu, H.N.; Zhang, J.; Wang, H.; Li, J.X. EVOH in situ fibrillation and its effect of strengthening, toughening and hydrophilic modification on PVDF hollow fiber microfiltration membrane via TIPS process. J. Mater. Sci. 2019, 54, 5971–5987. [Google Scholar] [CrossRef]
- Liu, Y.P.; Gao, J.W.; Ge, Y.H.; Yu, S.C.; Liu, M.H.; Gao, C.J. A combined interfacial polymerization and in-situ sol-gel strategy to construct composite nanofiltration embrane with improved pore size distribution and anti-protein-fouling property. J. Membr. Sci. 2021, 623, 119097. [Google Scholar] [CrossRef]
- Yang, Y.; Qiao, S.; Jin, R.F.; Zhou, J.T.; Quan, X. Fouling control mechanisms in filtrating natural organic matters by electro-enhanced carbon nanotubes hollow fiber membranes. J. Membr. Sci. 2018, 553, 54–62. [Google Scholar] [CrossRef]
- Fan., L.; Harris, J.L.; Roddick, F.A.; Booker, N.A. Influence of the characteristics of natural organic matter on the fouling of microfiltration membranes. Water Res. 2002, 35, 4455–4463. [Google Scholar] [CrossRef]
- Zularisam, A.W.; Ismail, A.F.; Salim, R. Behaviours of natural organic matter in membrane filtration for surface water treatment—A review. Desalination 2006, 194, 211–231. [Google Scholar] [CrossRef] [Green Version]
- Bu., F.; Gao, B.Y.; Yue, Q.Y.; Shen, X.; Wang, W.Y. Characterization of dissolved organic matter and membrane fouling in coagulation-ultrafiltration process treating micro-polluted surface water. J. Environ. Sci. 2019, 75, 318–324. [Google Scholar] [CrossRef]
- Yuan, W.; Kocic, A.; Zydney, A.L. Analysis of humic acid fouling during microfiltration using a pore blockage–cake filtration model. J. Membr. Sci. 2002, 198, 51–62. [Google Scholar] [CrossRef]
- Guan, Y.F.; Qian, C.; Chen, W.; Huang, B.C.; Wang, Y.J.; Yu, H.Q. Interaction between humic acid and protein in membrane fouling process: A spectroscopic insight. Water Res. 2018, 145, 146–152. [Google Scholar] [CrossRef]
- Hashino, M.; Hirami, K.; Katagiri, T.; Kubota, N.; Ohmukai, Y.; Ishigami, T.; Maruyama, T.; Matsuyama, H. Effects of three natural organic matter types on cellulose acetate butyrate microfiltration membrane fouling. J. Membr. Sci. 2011, 379, 233–238. [Google Scholar] [CrossRef]
- Breite, D.; Went, M.; Prager, A.; Schulze, A. Tailoring membrane surface charges: A novel study on electrostatic interactions during membrane fouling. Polymers 2015, 7, 2017–2030. [Google Scholar] [CrossRef] [Green Version]
- Mazlan, N.M.; Marchetti, P.; Maples, H.A.; Gu, B.; Karan, S.; Bismarck, A.; Livingston, A.G. Organic fouling behaviour of structurally and chemically different forward osmosis membranes-A study of cellulose triacetate and thin film composite membranes. J. Membr. Sci. 2016, 520, 247–261. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.Y.; Fang, L.F.; Cheng, L.; Jeon, S.; Kato, N.; Matsuyama, H. Improved antifouling properties of membranes by simple introduction of zwitterionic copolymers via electrostatic adsorption. J. Membr. Sci. 2018, 564, 672–681. [Google Scholar] [CrossRef]
- Deng, B.; Yu, M.; Yang, X.X.; Zhang, B.W.; Li, L.F.; Xie, L.D.; Li, J.Y.; Lu, X.F. Antifouling microfiltration membranes prepared from acrylic acid or methacrylic acid grafted poly(vinylidene fluoride) powder synthesized via pre-irradiation induced graft polymerization. J. Membr. Sci. 2010, 350, 252–258. [Google Scholar] [CrossRef]
- Chen, F.T.; Shi, X.X.; Chen, X.B.; Chen, W.X. Preparation and characterization of amphiphilic copolymer PVDF-g-PMABS and its application in improving hydrophilicity and protein fouling resistance of PVDF membrane. Appl. Surf. Sci. 2018, 427, 787–797. [Google Scholar] [CrossRef]
- Fu, X.; Zhu, L.P.; Songmiao Liang, S.M.; Jin, Y.; Yang, S.G. Sulfonated poly(α,β,β-trifluorostyrene)-doped PVDF ultrafiltration membrane with enhanced hydrophilicity and antifouling property. J. Membr. Sci. 2020, 603, 118046. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, Z.; Kaufman, Y.; Bernstein, R.J. Surface and anti-fouling properties of a polyampholyte hydrogel grafted onto a polyethersulfone membrane. J. Colloid Interf. Sci. 2018, 517, 155–165. [Google Scholar] [CrossRef]
- Lien, C.C.; Yeh, L.C.; Venault, A.; Tsai, S.C.; Hsu, C.H.; Dizon, G.V.; Huang, Y.T.; Higuchi, A.; Chang, Y. Controlling the zwitterionization degree of alternate copolymers for minimizing biofouling on PVDF membranes. J. Membr. Sci. 2018, 565, 119–130. [Google Scholar] [CrossRef]
- Fu, W.G.; Pei, T.F.; Mao, Y.Y.; Li, G.X.; Zhao, Y.P.; Chen, L. Highly hydrophilic poly(vinylidene fluoride) ultrafiltration membranes modified by poly(N-acryloyl glycinamide) hydrogel based on multi-hydrogen bond self-assembly for reducing protein fouling. J. Membr. Sci. 2019, 572, 453–463. [Google Scholar] [CrossRef]
- Birkner, M.; Ulbricht, M. Ultrafiltration membranes with markedly different pH- and ion-responsivity by photografted zwitterionic polysulfobetain or polycarbobetain. J. Membr. Sci. 2015, 494, 57–67. [Google Scholar] [CrossRef]
- Bertolasi, V.; Pretto, L.; Gilli, G.; Gilli, P. π-Bond cooperativity and anticooperativity effects in resonance-assisted hydrogen bonds (RAHBs). Acta Cryst. 2006, 62, 850–863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.X.; Lin, B.B.; Zhao, K.Y.; Wei, J.F.; Guo, J.; Cui, W.K.; Jiang, S.; Yang, Y.P.; Li, J.X. A free-standing calcium alginate/polyacrylamide hydrogel nanofiltration membrane with high anti-fouling performance: Preparation and characterization. Desalination 2015, 365, 234–241. [Google Scholar] [CrossRef]
- Song, L.Z.; Zhang, Z.J.; Song, S.Z.; Gao, Z.M. Preparation and characterization of the modified polyvinylidene flouride (PVDF) hollow fibre microfiltation membrane. J. Mater. Sei. Technol. 2007, 23, 55–60. [Google Scholar]
- Wang, Y.J.; Zhang, X.N.; Song, Y.H.; Zhao, Y.P.; Chen, L.; Su, F.M.; Li, L.B.; Wu, Z.L.; Zheng, Q. Ultrastiff and tough supramolecular hydrogels with a dense and robust hydrogen bond network. Chem. Mater. 2019, 31, 1430–1440. [Google Scholar] [CrossRef]
- Zhou, Q.; Lei, X.P.; Li, J.H.; Yan, B.F.; Zhang, Q.Q. Antifouling, adsorption and reversible flux properties of zwitterionic grafted PVDF membrane prepared via physisorbed free radical polymerization. Desalination 2014, 337, 6–15. [Google Scholar] [CrossRef]
- Zhu, J.; Zhao, X.Z.; He, C.J. Zwitterionic SiO2 nanoparticles as novel additives to improve the antifouling properties of PVDF membranes. RSC Adv. 2015, 5, 53653–53659. [Google Scholar] [CrossRef]
- Li, Y.; Huang, S.B.; Zhou, S.F.; Faned, A.G.; Zhang, Y.Q.; Zhao, S.F. Effective dye purification using tight ceramic ultrafiltration membrane. J. Membr. Sci. 2018, 556, 154–163. [Google Scholar]
- Zhu, L.J.; Liu, F.; Yu, X.M.; Xue, L.X. Poly(Lactic Acid) hemodialysis membranes with poly(lactic acid)-block-poly(2-hydroxyethyl methacrylate) copolymer as additive: Preparation, characterization, and performance. ACS Appl. Mater. Inter. 2015, 7, 17748–17755. [Google Scholar] [CrossRef] [PubMed]
- Zhang., F.; Zhang, W.B.; Yu, Y.; Deng, B.; Li, J.Y.; Jin, J. Sol-gel preparation of PAA-g-PVDF/TiO2 nanocomposite hollow fiber membranes with extremely high water flux and improved anti-fouling property. J. Membr. Sci. 2013, 432, 25–32. [Google Scholar] [CrossRef]
- Chen, X.; Bi, S.Y.; Shi, C.C.; He, Y.; Zhao, L.Z.; Chen, L. Temperature-sensitive membranes prepared from blends of poly(vinylidene fluoride) and poly(N-isopropylacrylamides) microgels. Colloid Polym. Sci. 2013, 291, 2419–2428. [Google Scholar] [CrossRef]
- Dylla-Spears, R.; Wong, L.; Shen, N.; Steele, W.; Menapace, J.; Miller, P.; Feit, M.; Suratwala, T. Adsorption of silica colloids onto like-charged silica surfaces of different roughness. Colloids Surf. A 2017, 520, 85–96. [Google Scholar] [CrossRef] [Green Version]
- Shen, X.; Yin, X.B.; Zhao, Y.P.; Chen, L. Antifouling enhancement of PVDF membrane tethered with polyampholyte hydrogel layers. Polym. Eng. Sci. 2015, 55, 1367–1373. [Google Scholar] [CrossRef]
- Chang, Y.; Ko, C.Y.; Shih, Y.J.; Quémener, D.; Deratani, A.; Wei, T.C.; Wang, D.M.; Lai, J.Y. Surface grafting control of PEGylated poly(vinylidene fluoride) antifouling membrane via surface-initiated radical graft copolymerization. J. Membr. Sci. 2009, 345, 160–169. [Google Scholar] [CrossRef]
- Shen, X.; Liu, P.; Xia, S.B.; Liu, J.J.; Wang, R.; Zhao, H.; Liu, Q.J.; Xu, J.; Wang, F. Anti-Fouling and anti-bacterial modification of poly(vinylidene fluoride) membrane by blending with the capsaicin-based copolymer. Polymers 2019, 11, 323. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.Z.; Liu, C.K. Irreversible fouling control of PVDF ultrafiltration membrane with “fouled surface” for mimetic sewage treatment. RSC Adv. 2016, 6, 94184–94192. [Google Scholar] [CrossRef]
- Katsoufidou, K.S.; Sioutopoulos, D.C.; Yiantsios, S.G.; Karabelas, A.J. UF membrane fouling by mixtures of humic acids and sodium alginate: Fouling mechanisms and reversibility. Desalination 2010, 264, 220–227. [Google Scholar] [CrossRef]
- Kimura, K.; Yamamura, H.; Watanabe, Y. Irreversible fouling in MF/UF membranes caused by natural organic matters (NOMs) isolated from different origins. Sep. Sci. Technol. 2006, 41, 1331–1344. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, J.; Chu, H.; Zhou, X.; Wei, Y. Effect of modified attapulgite addition on the performance of a PVDF ultrafiltration membrane. Desalination 2014, 344, 71–78. [Google Scholar] [CrossRef]
- Shen, L.G.; Wang, H.Z.; Zhang, Y.C.; Li, R.J.; Fabien, B.; Yu, G.Y.; Lin, H.J.; Li, B.Q. New strategy of grafting hydroxyethyl acrylate (HEA) via γ ray radiation to modify polyvinylidene fluoride (PVDF) membrane: Thermodynamic mechanisms of the improved antifouling performance. Sep. Purif. Technol. 2018, 207, 83–91. [Google Scholar] [CrossRef]

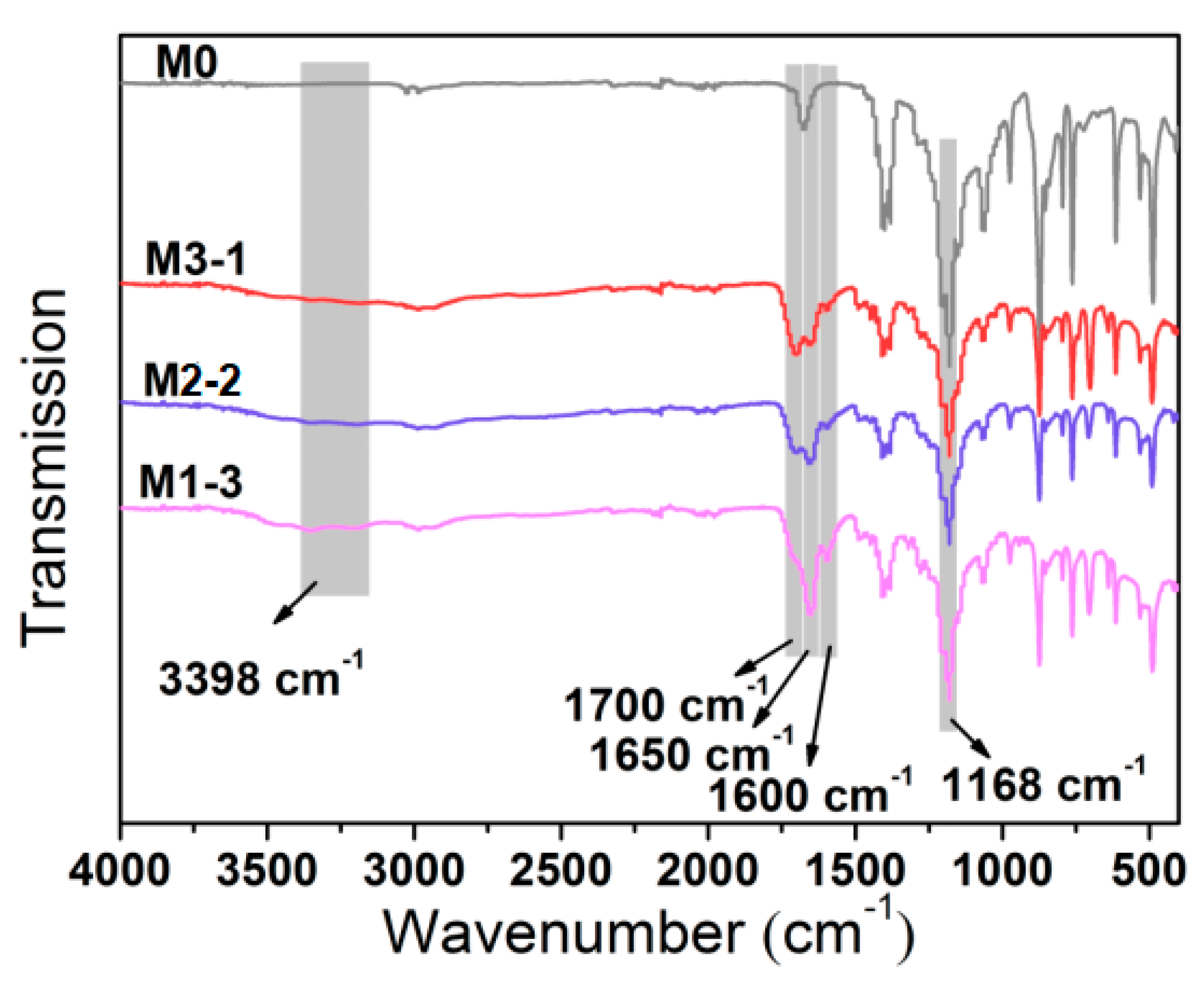

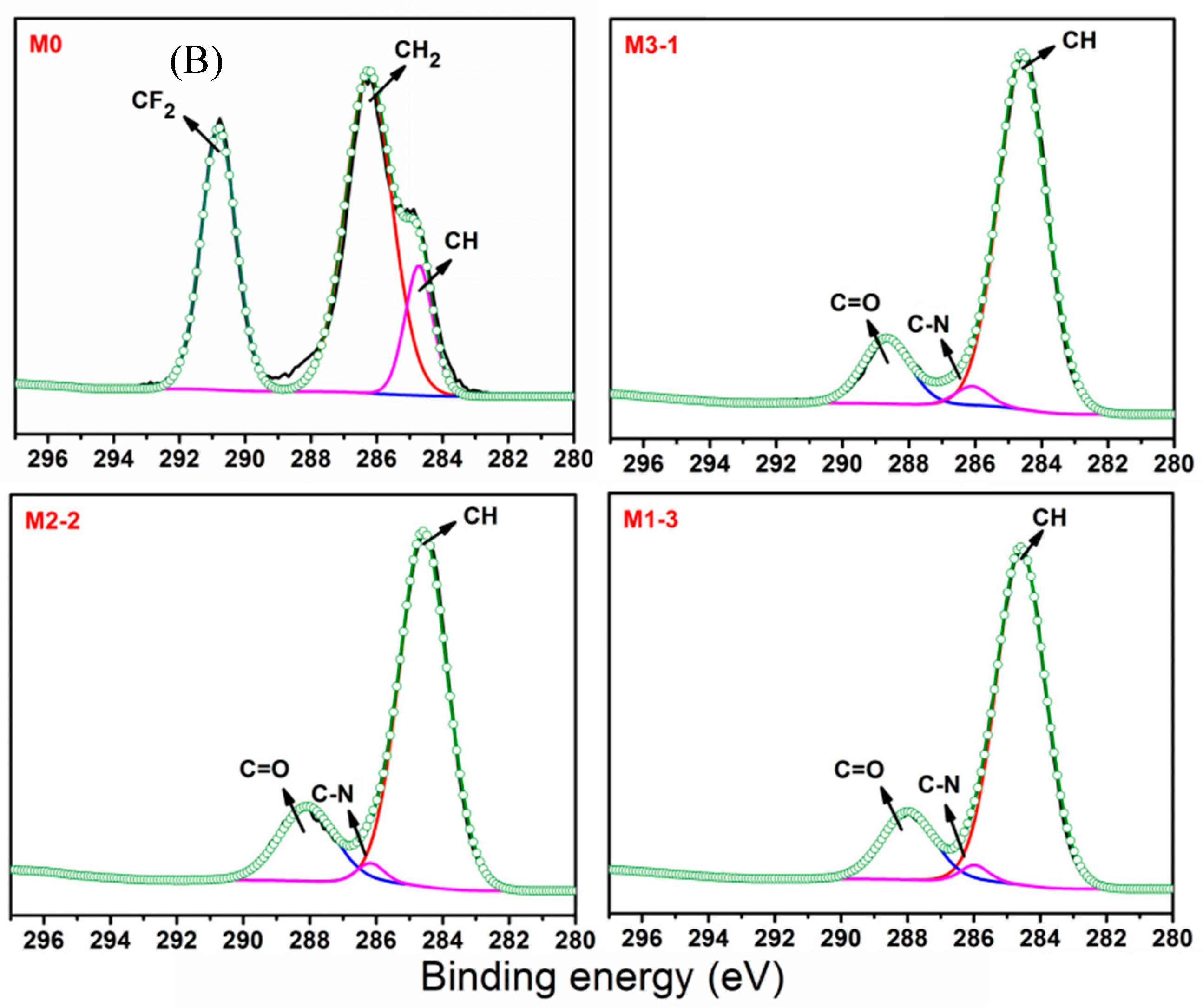
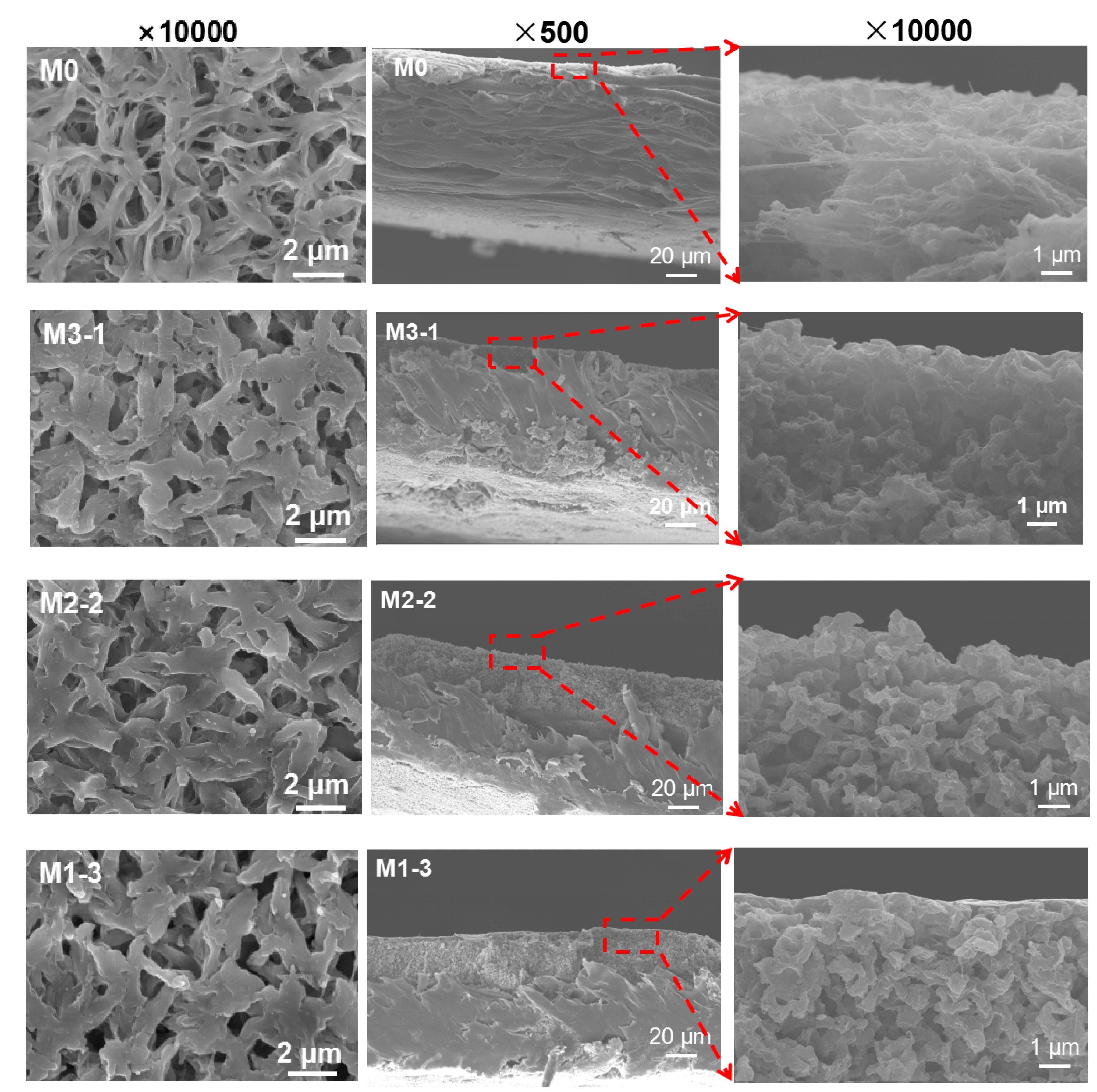
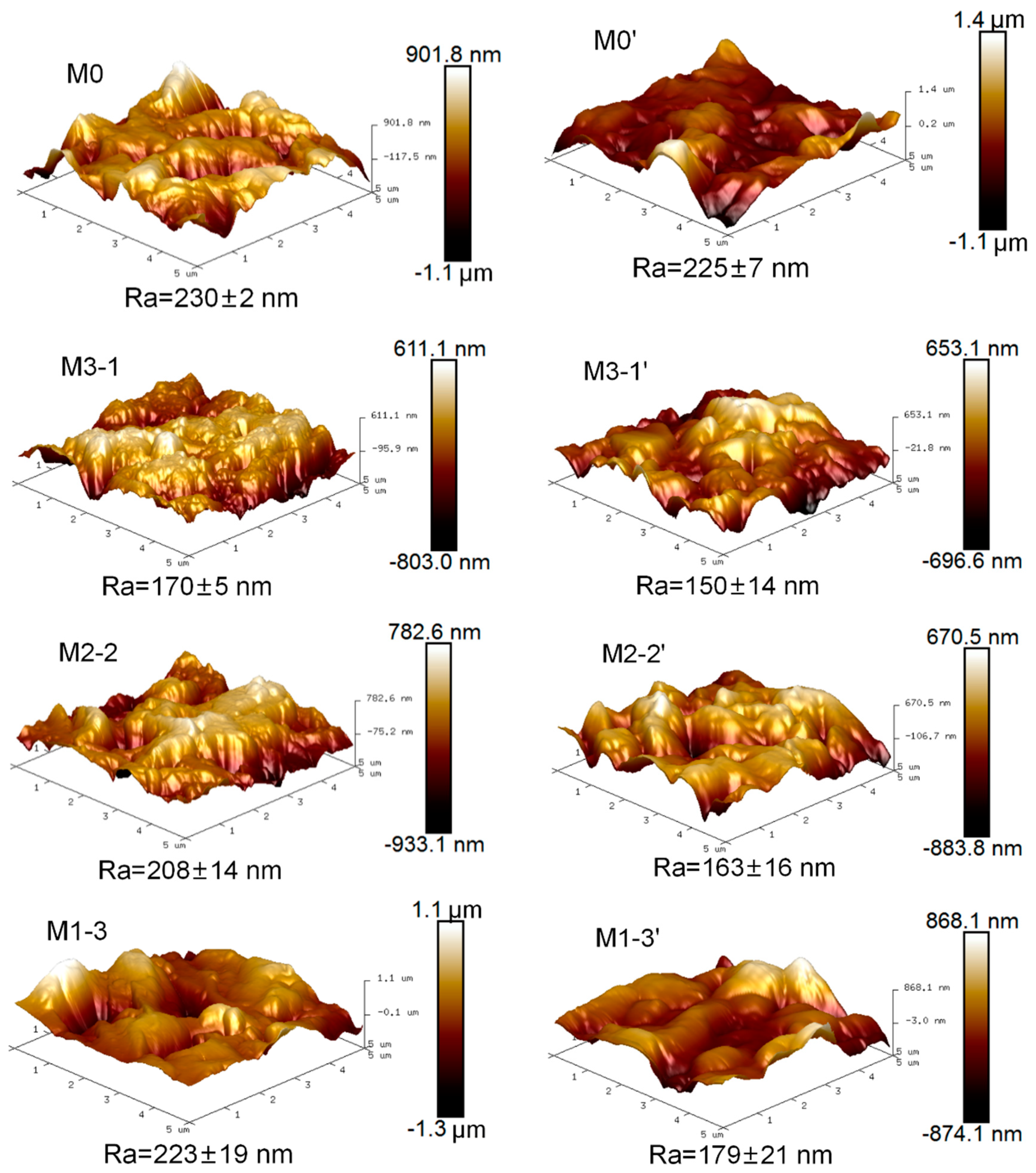

| Sample | Relative Element Content (at%) | |||
|---|---|---|---|---|
| C | N | O | F | |
| M0 | 57.54 | 1.79 | 3.92 | 36.75 |
| M3-1 | 73.26 | 4.08 | 21.15 | 1.51 |
| M2-2 | 72.57 | 6.56 | 20.09 | 0.78 |
| M1-3 | 73.51 | 8.70 | 17.58 | 0.21 |
| Foulant | Hydrogel Material | Support | Preparation Method | One Cycle Filtration (min) | FRR (%) | Rt (%) | Rir (%) | CA (°) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| BSA | P(MAAc-co-MAAm) | PVDF | UV-initiated radical graft polymerization | 120 | 96.7 | 10.7 | 3.3 | 36.6 | this work |
| TiO2, PVA | PVDF | chemically binding | 120 | 86.4 | 48.0 | 13.6 | 24.0 | [5] | |
| VSA, METMAC | PES | UV photoinitiation | 60 | 85.0 | 30.0 | 15.0 | 62.0 | [21] | |
| APT | PVDF | blending | 60 | 78.9 | - | 21.1 | 63.0 | [42] | |
| HEA | PVDF | γ ray radiation | 2 | 75.0 | - | 25.0 | 59.3 | [42] | |
| CBMA | PVDF | physisorbed free radical polymerization grafting | 60 | 92.0 | 44.0 | 8.0 | 70.2 | [29] | |
| PEGMA | PVDF | radical grafting | 60 | 90.8 | 55.5 | 9.2 | 60.0 | [37] | |
| HA | P(MAAc-co-MAAm) | PVDF | UV-initiated radical graft polymerization | 120 | 92.8 | 24.9 | 7.2 | 36.6 | this work |
| TMC, SA | PVDF | crosslinking | 60 | 91.0 | 35.0 | 8.7 | 36.0 | [39] | |
| sulfonated polyaniline | PVDF | grafting | 60 | 95.0 | 65.0 | 5.3 | 29.0 | [42] | |
| PANI, MWCNT | PVDF | blending | 120 | 85.0 | 79.0 | 15.0 | 54.8 | [42] | |
| PEG, PD | PVDF | coating | 60 | 89.1 | - | 10.9 | 61.5 | [42] | |
| PVA, Glutaraldehyde | PES | coating | 150 | 89.0 | - | 11.0 | 24.0 | [42] | |
| SA | P(MAAc-co-MAAm) | PVDF | UV-initiated radical graft polymerization | 120 | 90.8 | 30.8 | 9.2 | 36.6 | this work |
| VSA, METMAC | PES | UV photoinitiation | 60 | 86.0 | - | 14.0 | 62.0 | [21] | |
| HEA | PVDF | γ ray radiation | 2 | 92.0 | - | 11.5 | 59.3 | [43] | |
| DMAPS | PVDF | UV-initiated radical graft polymerization | 30 | 41.8 | 88.6 | 58.2 | 28.0 | [22] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, W.; Li, G.; Zhai, G.; Xie, Y.; Sun, M.; Théato, P.; Zhao, Y.; Chen, L. Facile Fabrication of Multi-Hydrogen Bond Self-Assembly Poly(MAAc-co-MAAm) Hydrogel Modified PVDF Ultrafiltration Membrane to Enhance Anti-Fouling Property. Membranes 2021, 11, 761. https://doi.org/10.3390/membranes11100761
Fu W, Li G, Zhai G, Xie Y, Sun M, Théato P, Zhao Y, Chen L. Facile Fabrication of Multi-Hydrogen Bond Self-Assembly Poly(MAAc-co-MAAm) Hydrogel Modified PVDF Ultrafiltration Membrane to Enhance Anti-Fouling Property. Membranes. 2021; 11(10):761. https://doi.org/10.3390/membranes11100761
Chicago/Turabian StyleFu, Weigui, Guoxia Li, Gaowei Zhai, Yunji Xie, Meixiu Sun, Patrick Théato, Yiping Zhao, and Li Chen. 2021. "Facile Fabrication of Multi-Hydrogen Bond Self-Assembly Poly(MAAc-co-MAAm) Hydrogel Modified PVDF Ultrafiltration Membrane to Enhance Anti-Fouling Property" Membranes 11, no. 10: 761. https://doi.org/10.3390/membranes11100761
APA StyleFu, W., Li, G., Zhai, G., Xie, Y., Sun, M., Théato, P., Zhao, Y., & Chen, L. (2021). Facile Fabrication of Multi-Hydrogen Bond Self-Assembly Poly(MAAc-co-MAAm) Hydrogel Modified PVDF Ultrafiltration Membrane to Enhance Anti-Fouling Property. Membranes, 11(10), 761. https://doi.org/10.3390/membranes11100761







