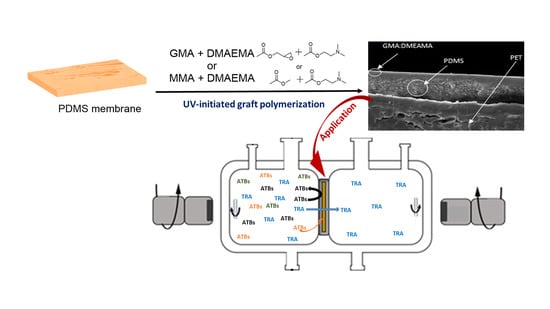
Glycidyl and Methyl Methacrylate UV-Grafted PDMS Membrane Modification toward Tramadol Membrane Selectivity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
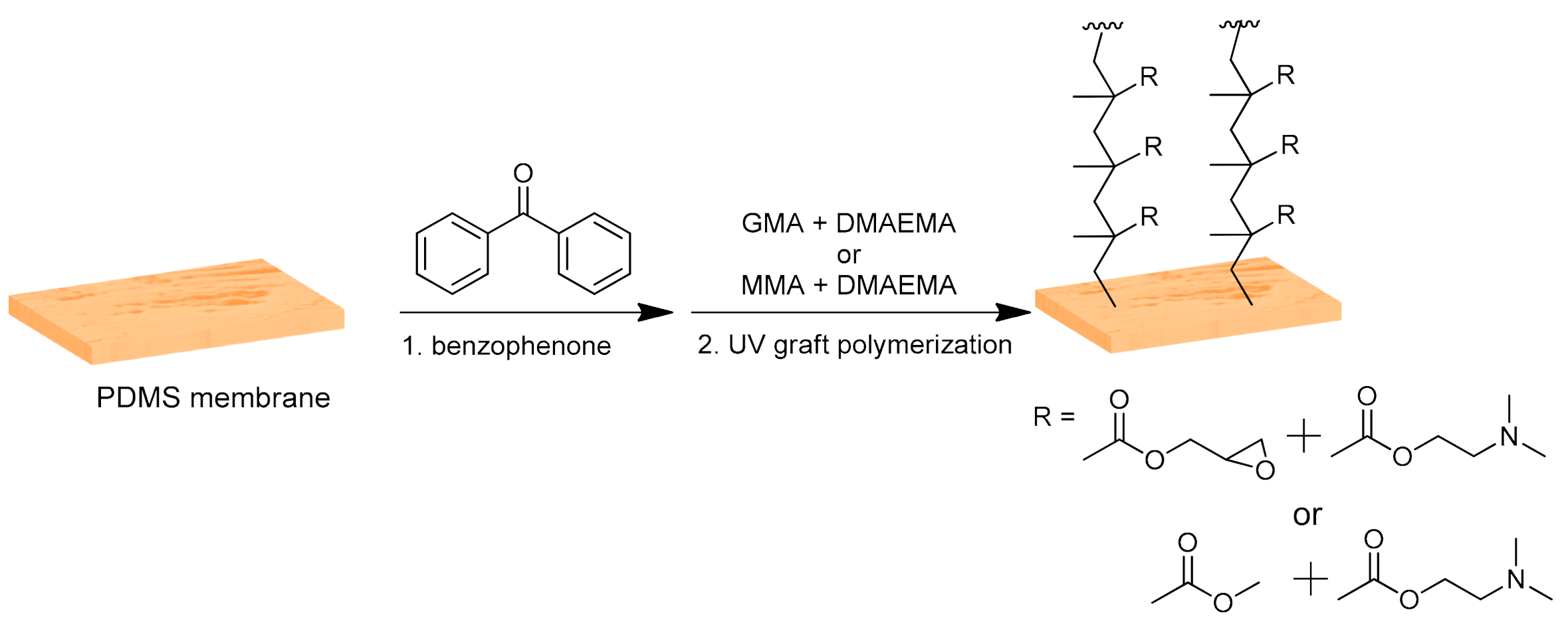
2.2. Membranes Modification
2.3. Characterization Methods
2.3.1. HPLC-UV Analysis
2.3.2. Contact Angle Measurements
2.3.3. SEM Imaging and EDX Analysis
2.3.4. FTIR Spectra
2.3.5. Specific Surface Characterization
2.4. Preparation of Model Solutions
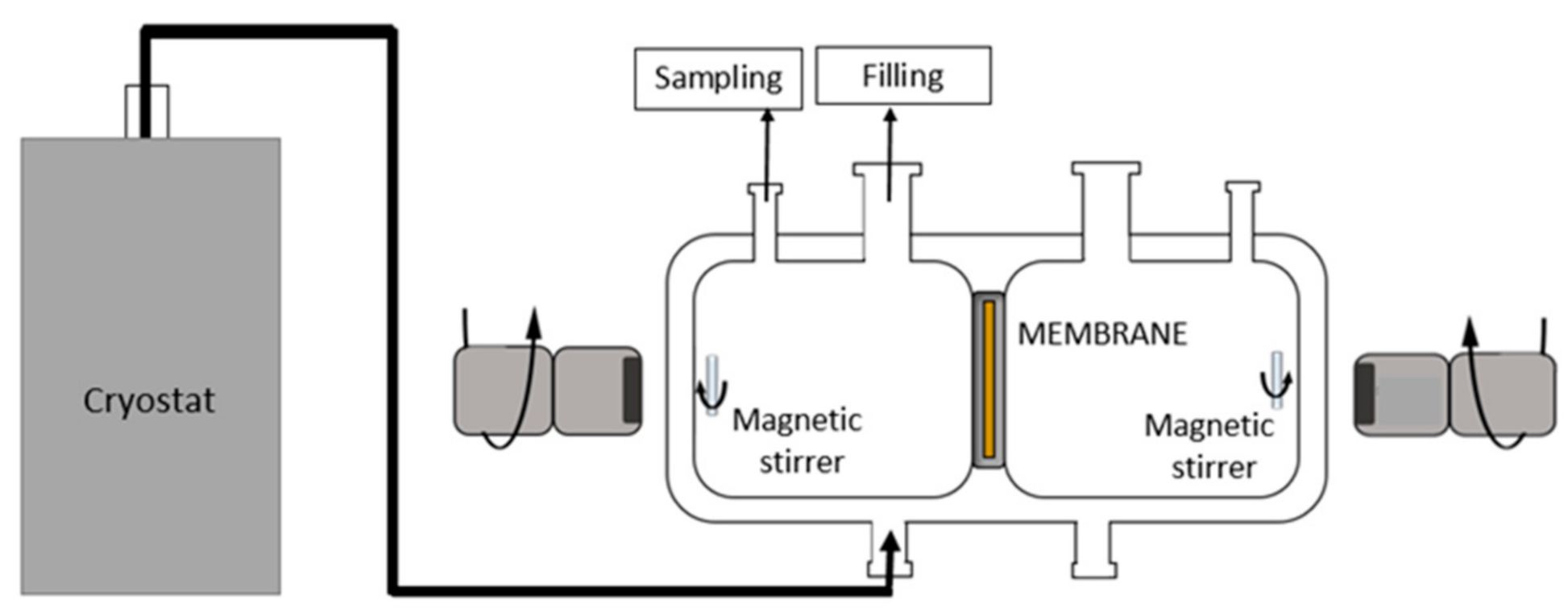
2.5. Pertraction Process
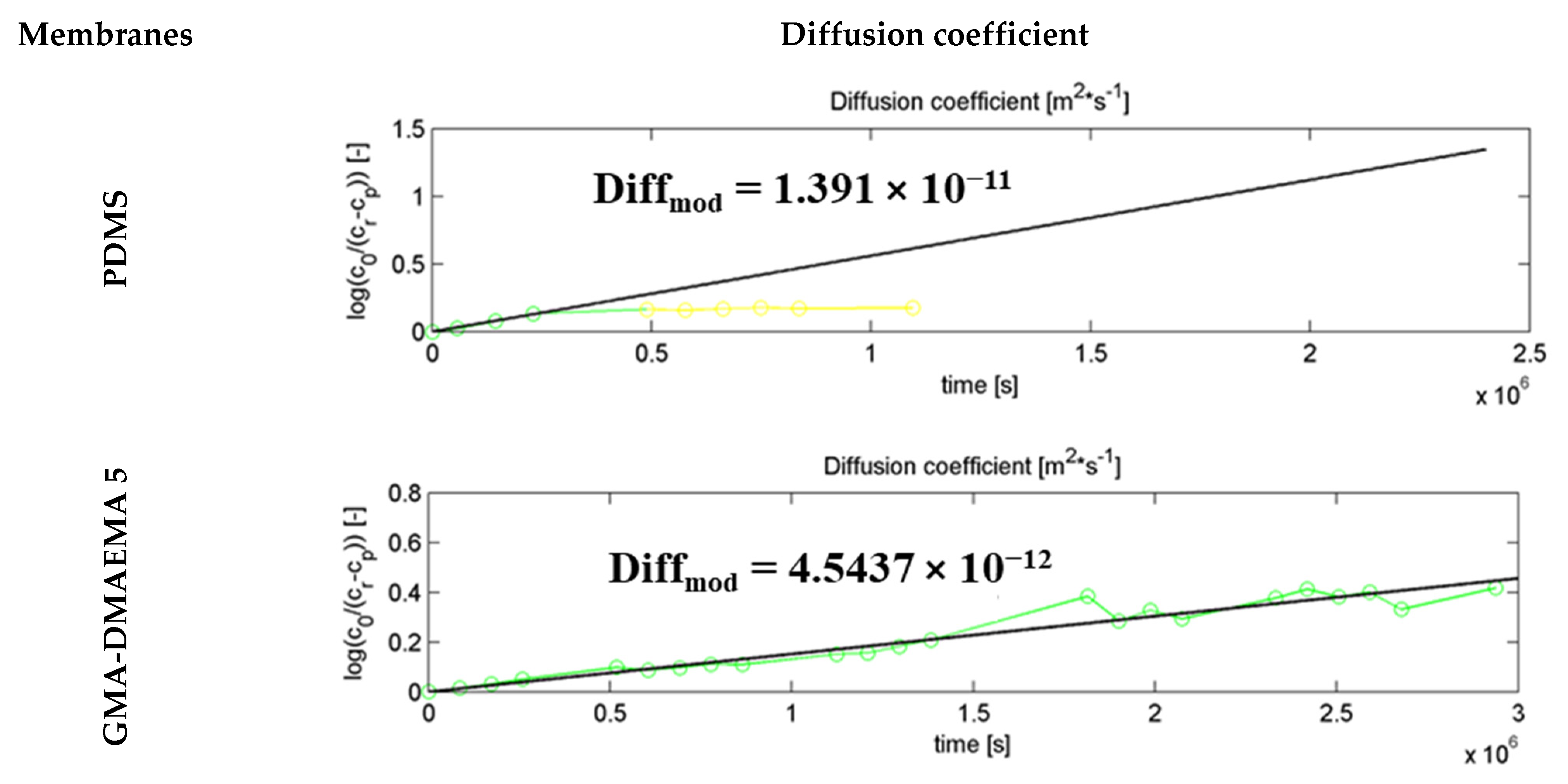
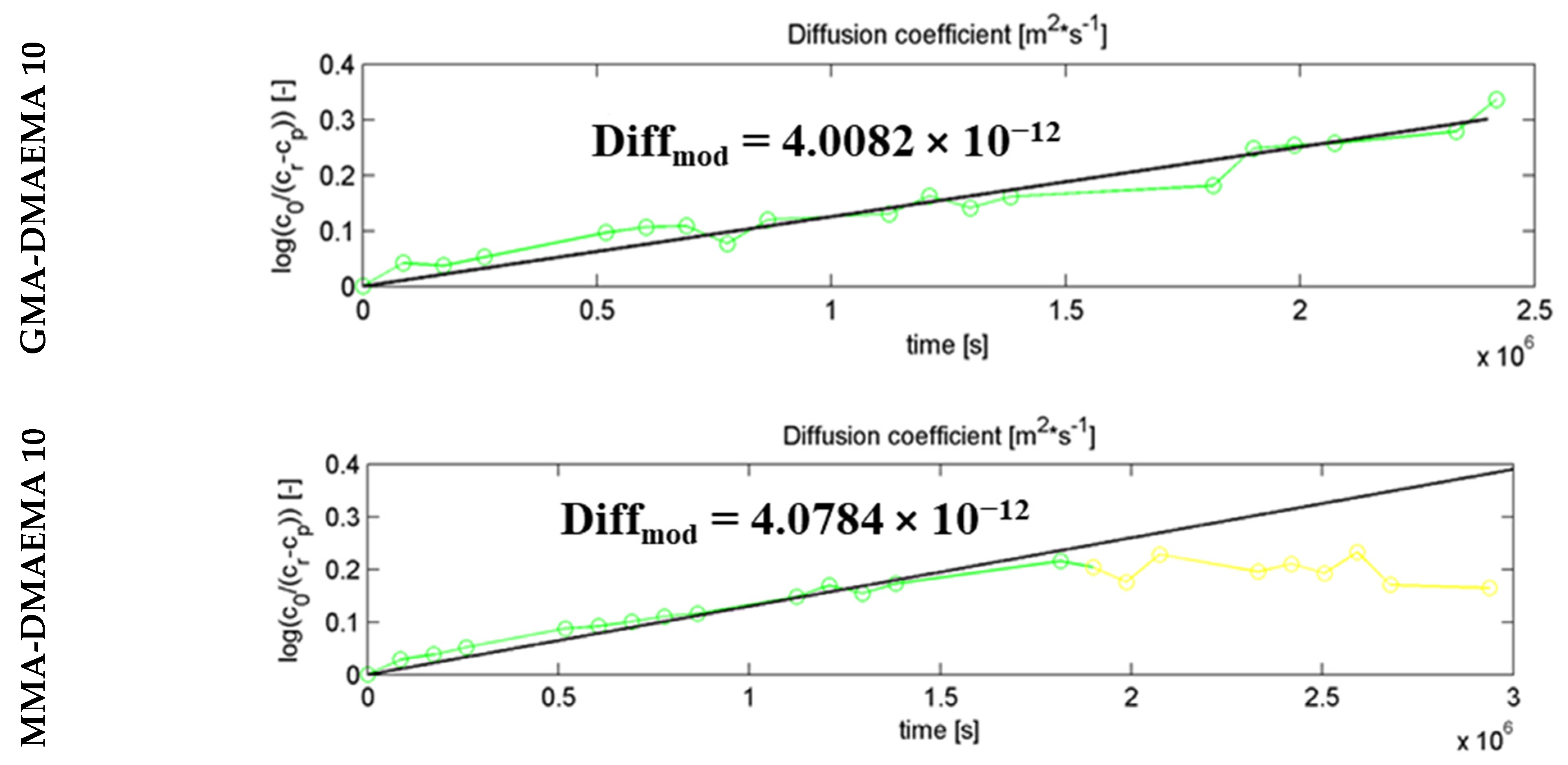
2.6. Diffusion Coefficient Calculation
3. Results
3.1. Membrane Characterization
3.1.1. Contact Angle
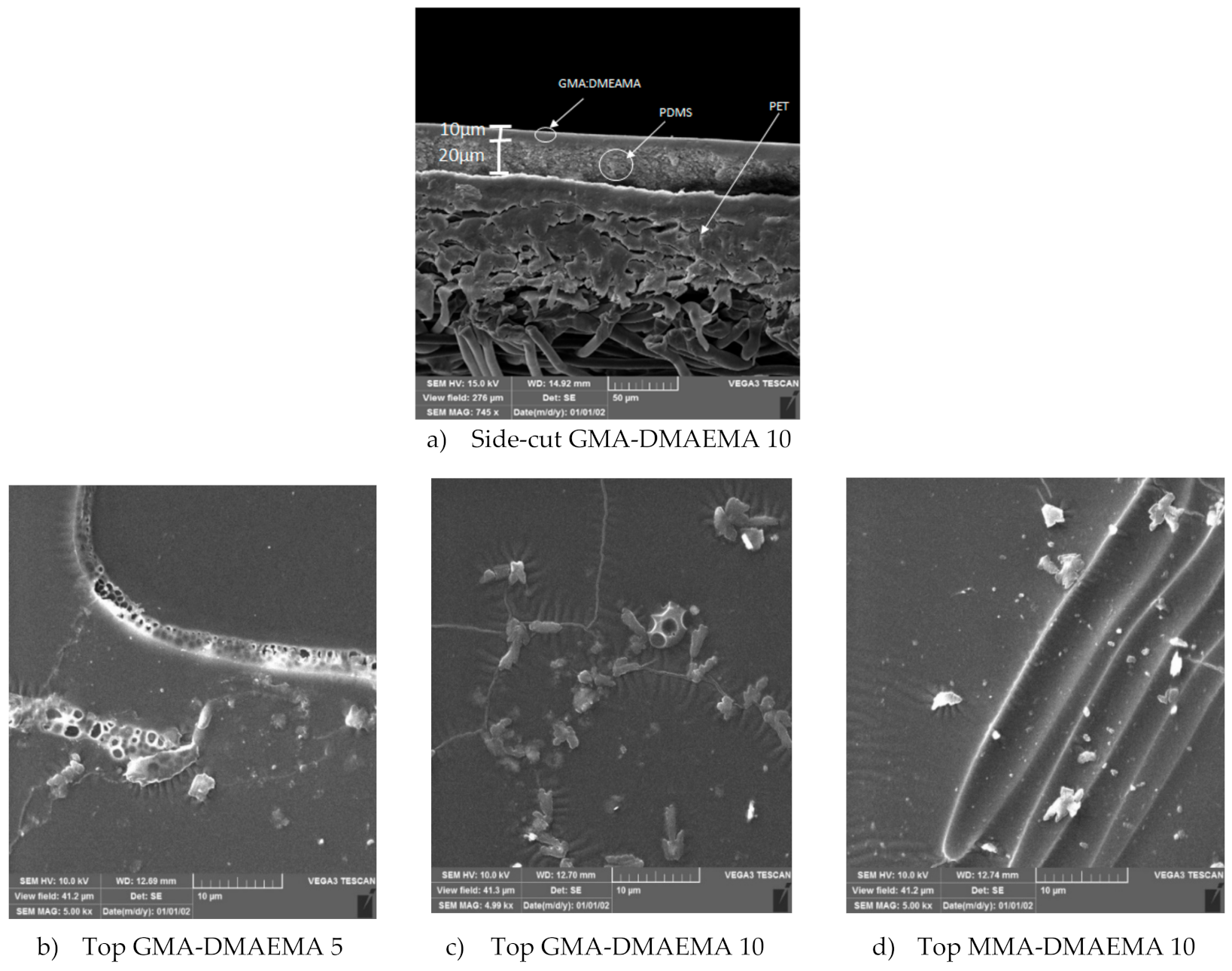
3.1.2. Scanning Electron Microscopy
3.1.3. Fourier-Transform Infrared Spectroscopy
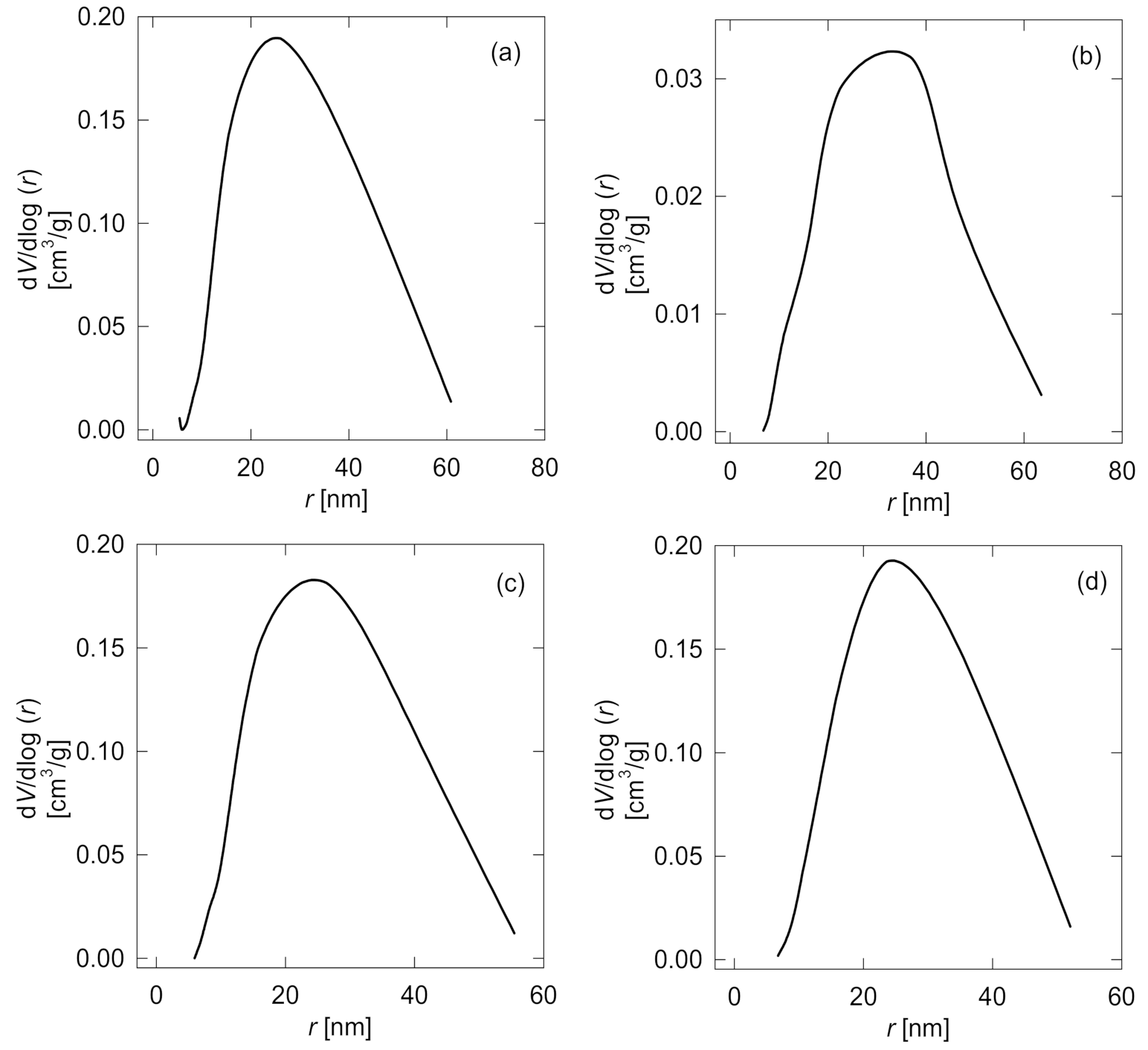
3.1.4. Specific Surface Characterization
3.1.5. Energy-Dispersive X-ray Spectroscopy
3.2. Pertractions
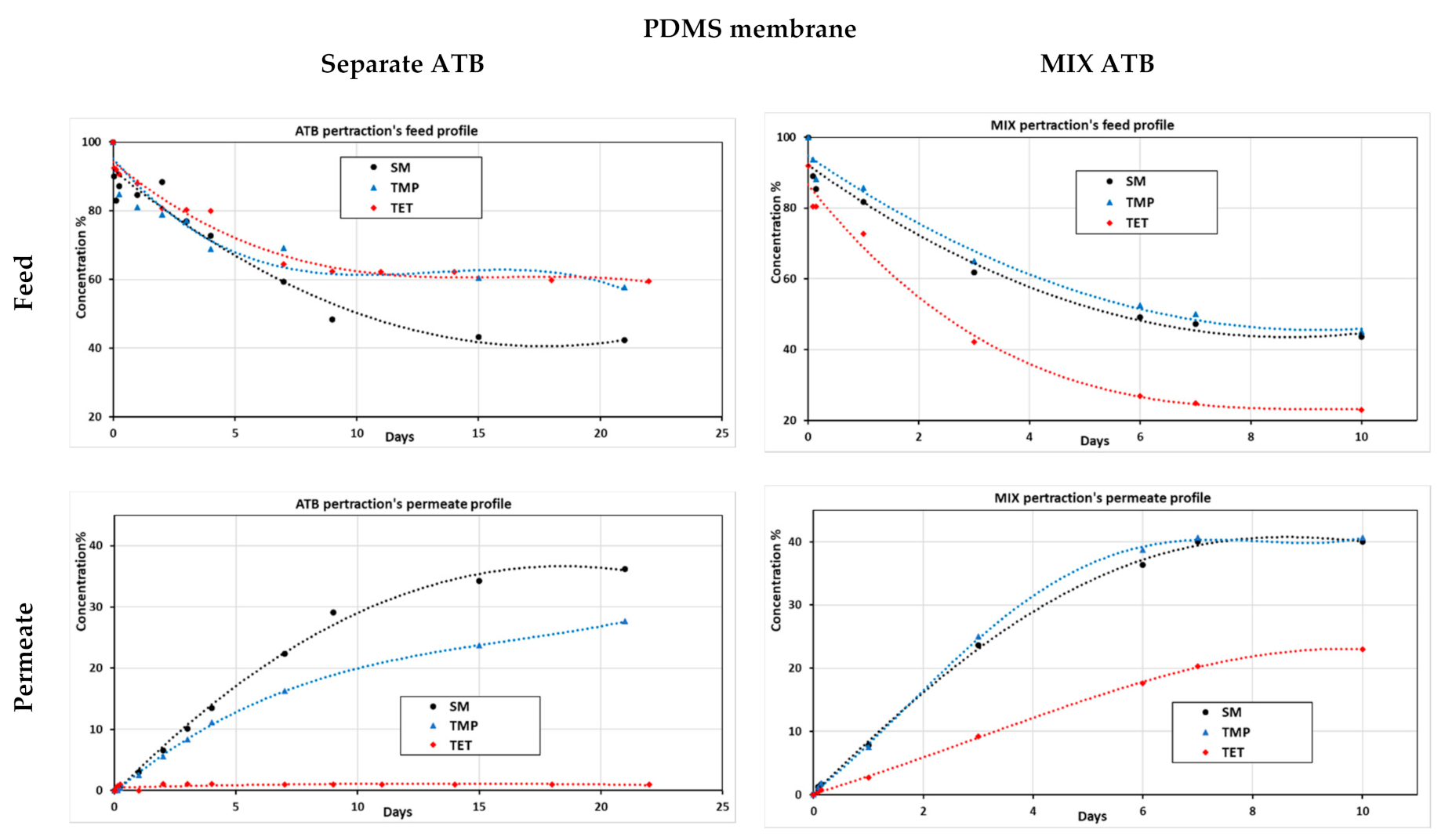
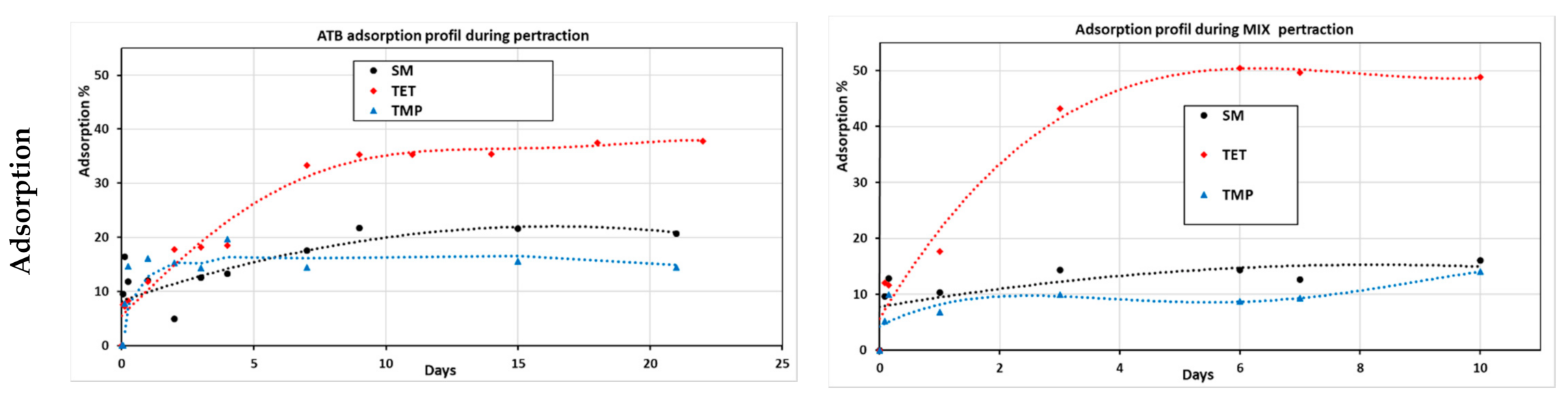
3.2.1. Mixed and Separated Antibiotic Pertraction
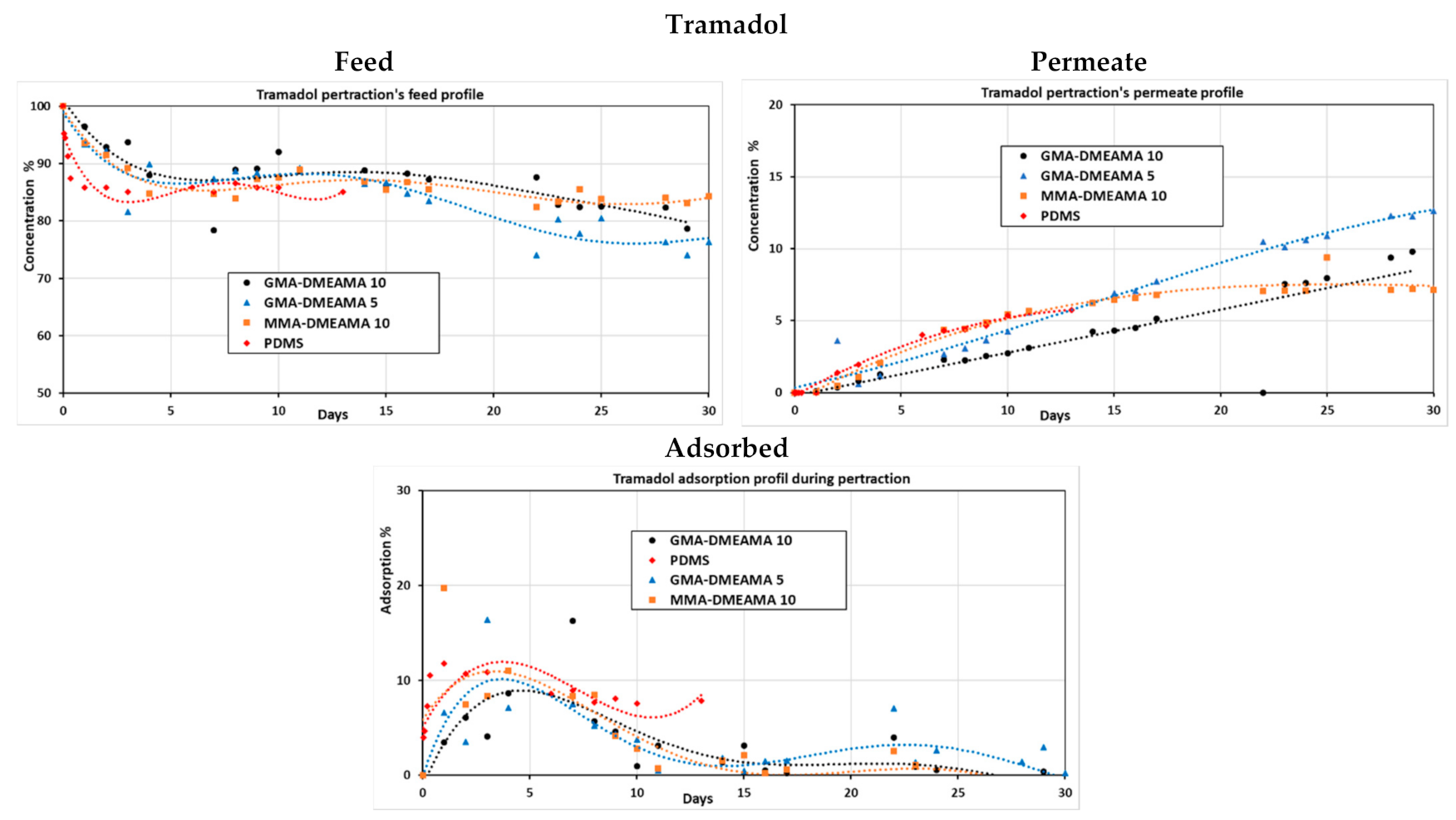
3.2.2. Tramadol Pertraction
3.2.3. Tramadol and Mixed Antibiotic Pertraction
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Diaz-Sosa, V.R.; Tapia-Salazar, M.; Wanner, J.; Cardenas-Chavez, D.L. Monitoring and Ecotoxicity Assessment of Emerging Contaminants in Wastewater Discharge in the City of Prague (Czech Republic). Water 2020, 12, 1079. [Google Scholar] [CrossRef]
- Hanna, N.; Sun, P.; Sun, Q.; Li, X.; Yang, X.; Ji, X.; Zou, H.; Ottoson, J.; Nilsson, L.E.; Berglund, B.; et al. Presence of antibiotic residues in various environmental compartments of Shandong province in eastern China: Its potential for resistance development and ecological and human risk. Environ. Int. 2018, 114, 131–142. [Google Scholar] [CrossRef]
- Kondor, A.C.; Jakab, G.; Vancsik, A.; Filep, T.; Szeberenyi, J.; Szabo, L.; Maasz, G.; Ferincz, A.; Dobosy, P.; Szalai, Z. Occurrence of pharmaceuticals in the Danube and drinking water wells: Efficiency of riverbank filtration. Environ. Pollut. 2020, 265, 114893. [Google Scholar] [CrossRef] [PubMed]
- Conde-Cid, M.; Núñez-Delgado, A.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Fernández-Calviño, D.; Arias-Estévez, M. Tetracycline and Sulfonamide Antibiotics in Soils: Presence, Fate and Environmental Risks. Processes 2020, 8, 1479. [Google Scholar] [CrossRef]
- Rodriguez-Mozaz, S.; Vaz-Moreira, I.; Della Giustina, S.V.; Llorca, M.; Barceló, D.; Schubert, S.; Berendonk, T.U.; Michael-Kordatou, I.; Fatta-Kassinos, D.; Martinez, J.L.; et al. Antibiotic residues in final effluents of European wastewater treatment plants and their impact on the aquatic environment. Environ. Int. 2020, 140, 105733. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulou, Μ.; Thoma, A.; Konstantinou, F.; Vlastos, D.; Hela, D. Assessing the human risk and the environmental fate of pharmaceutical Tramadol. Sci. Total Environ. 2020, 710, 135396. [Google Scholar] [CrossRef]
- Baek, I.H.; Kim, Y.; Baik, S.; Kim, J. Investigation of the Synergistic Toxicity of Binary Mixtures of Pesticides and Pharmaceuticals on Aliivibrio fischeri in Major River Basins in South Korea. Int. J. Environ. Res. Public Health 2019, 16, 208. [Google Scholar] [CrossRef] [Green Version]
- Zbair, M.; Ainassaari, K.; Drif, A.; Ojala, S.; Bottlinger, M.; Pirila, M.; Keiski, R.L.; Bensitel, M.; Brahmi, R. Toward new benchmark adsorbents: Preparation and characterization of activated carbon from argan nut shell for bisphenol A removal. Environ. Sci. Pollut. Res. Int. 2018, 25, 1869–1882. [Google Scholar] [CrossRef] [Green Version]
- Zbair, M.; Ainassaari, K.; El Assal, Z.; Ojala, S.; El Ouahedy, N.; Keiski, R.L.; Bensitel, M.; Brahmi, R. Steam activation of waste biomass: Highly microporous carbon, optimization of bisphenol A, and diuron adsorption by response surface methodology. Environ. Sci. Pollut. Res. Int. 2018, 25, 35657–35671. [Google Scholar] [CrossRef] [Green Version]
- Kyzas, G.Z.; Kostoglou, M. Green Adsorbents for Wastewaters: A Critical Review. Materials 2014, 7, 333–364. [Google Scholar] [CrossRef] [PubMed]
- Sassi, H.; Lafaye, G.; Ben Amor, H.; Gannouni, A.; Jeday, M.R.; Barbier, J. Wastewater treatment by catalytic wet air oxidation process over Al-Fe pillared clays synthesized using microwave irradiation. Front. Environ. Sci. Eng. 2017, 12, 2. [Google Scholar] [CrossRef]
- Kárászová, M.; Bourassi, M.; Gaálová, J. Membrane Removal of Emerging Contaminants from Water: Which Kind of Membranes Should We Use? Membranes 2020, 10, 305. [Google Scholar] [CrossRef]
- Rashed, A.O.; Merenda, A.; Kondo, T.; Lima, M.; Razal, J.; Kong, L.; Huynh, C.; Dumée, L.F. Carbon nanotube membranes—Strategies and challenges towards scalable manufacturing and practical separation applications. Sep. Purif. Technol. 2021, 257, 117929. [Google Scholar] [CrossRef]
- Doan, H.N.; Phong Vo, P.; Hayashi, K.; Kinashi, K.; Sakai, W.; Tsutsumi, N. Recycled PET as a PDMS-Functionalized electrospun fibrous membrane for oil-water separation. J. Environ. Chem. Eng. 2020, 8, 103921. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, T.; Zhao, J.; Lu, L.; Muhammad, Y.; Lan, P.; He, R.; Zou, Y.; Tong, Z. Synthesis and application of PDMS/OP-POSS membrane for the pervaporative recovery of n-butyl acetate and ethyl acetate from aqueous media. J. Membr. Sci. 2019, 591, 117324. [Google Scholar] [CrossRef]
- Gaálová, J.; Michel, M.; Bourassi, M.; Ladewig, B.P.; Kasal, P.; Jindřich, J.; Izák, P. Nafion membranes modified by cationic cyclodextrin derivatives for enantioselective separation. Sep. Purif. Technol. 2021, 266, 118538. [Google Scholar] [CrossRef]
- Bourassi, A.; Martín, E.; Bourre, M.; Fíla, V.; Gaálová, J. Separation of Diethyl Phthalate From Water by Pervaporation. WSEAS Trans. Environ. Dev. 2021, 17, 81–88. [Google Scholar] [CrossRef]
- Mohammadi, T.; Aroujalian, A.; Bakhshi, A. Pervaporation of dilute alcoholic mixtures using PDMS membrane. Chem. Eng. Sci. 2005, 60, 1875–1880. [Google Scholar] [CrossRef]
- Upadhyaya, L.; Qian, X.; Ranil Wickramasinghe, S. Chemical modification of membrane surface—Overview. Curr. Opin. Chem. Eng. 2018, 20, 13–18. [Google Scholar] [CrossRef]
- Wang, H.; Lu, X.; Lu, X.; Wang, Z.; Ma, J.; Wang, P. Improved surface hydrophilicity and antifouling property of polysulfone ultrafiltration membrane with poly(ethylene glycol) methyl ether methacrylate grafted graphene oxide nanofillers. Appl. Surf. Sci. 2017, 425, 603–613. [Google Scholar] [CrossRef]
- Regev, C.; Belfer, S.; Holenberg, M.; Fainstein, R.; Parola, A.H.; Kasher, R. Fabrication of poly(ethylene glycol) particles with a micro-spherical morphology on polymeric fibers and its application in high flux water filtration. Sep. Purif. Technol. 2019, 210, 729–736. [Google Scholar] [CrossRef]
- Raza, W.; Lee, J.; Raza, N.; Luo, Y.; Kim, K.-H.; Yang, J. Removal of phenolic compounds from industrial waste water based on membrane-based technologies. J. Ind. Eng. Chem. 2019, 71, 1–18. [Google Scholar] [CrossRef]
- Herzberg, M.; Sweity, A.; Fau-Brami, M.; Brami, M.; Fau-Kaufman, Y.; Kaufman, Y.; Fau-Freger, V.; Freger, V.; Fau-Oron, G.; Oron, G.; et al. Surface properties and reduced biofouling of graft-copolymers that possess oppositely charged groups. Biomacromolecules 2011, 12, 1169–1177. [Google Scholar] [CrossRef]
- Eshet, I.; Freger, V.; Kasher, R.; Herzberg, M.; Lei, J.; Ulbricht, M. Chemical and physical factors in design of antibiofouling polymer coatings. Biomacromolecules 2011, 12, 2681–2685. [Google Scholar] [CrossRef] [PubMed]
- Gaálová, J.; Yalcinkaya, F.; Cuřínová, P.; Kohout, M.; Yalcinkaya, B.; Koštejn, M.; Jirsák, J.; Stibor, I.; Bara, J.E.; Van der Bruggen, B.; et al. Separation of racemic compound by nanofibrous composite membranes with chiral selector. J. Membr. Sci. 2020, 596, 117728. [Google Scholar] [CrossRef]
- Nassar, A.; Ahmed, N.; Haseeb, M.; Abdel-Rahman, A.; Nasser, R. Article open access synthesis and evaluation of terpolymers as viscosity index improvers and pour point depressants. Pet. Coal 2017, 59, 442–451. [Google Scholar]
- Stawski, D.; Nowak, A. Thermal properties of poly(N,N-dimethylaminoethyl methacrylate). PLoS ONE 2019, 14, e0217441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, J.-J.; Wahid, F.; Zhang, Q.; Tao, Y.-C.; Zhong, C.; Chu, L.-Q. Facile Incorporation of Silver Nanoparticles into Quaternized Poly(2-(Dimethylamino)Ethyl Methacrylate) Brushes as Bifunctional Antibacterial Coatings. Macromol. Mater. Eng. 2017, 302, 1700069. [Google Scholar] [CrossRef]
- Johnson, L.M.; Gao, L.; Shields Iv, C.W.; Smith, M.; Efimenko, K.; Cushing, K.; Genzer, J.; López, G.P. Elastomeric microparticles for acoustic mediated bioseparations. J. Nanobiotechnol. 2013, 11, 22. [Google Scholar] [CrossRef] [Green Version]
- Dalhatu, S. Ultrasonic assisted synthesis and characterization of chitosan graf with 2- (dimethylaminoethylmethacrylate) (dmaema) Cs-g-pdmaema. J. Phys. Sci. 2021, 3, 1–10. [Google Scholar]
- Shtreimer Kandiyote, N.; Avisdris, T.; Arnusch, C.J.; Kasher, A.-O. Grafted Polymer Coatings Enhance Fouling Inhibition by an Antimicrobial Peptide on Reverse Osmosis Membranes. Langmuir 2019, 35, 1935–1943. [Google Scholar] [CrossRef]
- Dinari, A.; Abdollahi, M.; Sadeghizadeh, M. Design and fabrication of dual responsive lignin-based nanogel via “grafting from” atom transfer radical polymerization for curcumin loading and release. Sci. Rep. 2021, 11, 1962. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Wang, C.; Cheng, X.; Zhang, Y.; Li, W.; Wang, J.; Tian, Z.; Chu, W.; Korshin, G.V.; Guo, H. Interactions between the antibiotic tetracycline and humic acid: Examination of the binding sites, and effects of complexation on the oxidation of tetracycline. Water Res. 2021, 202, 117379. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; Rong, H.; Zhao, M.; Luo, H.; Chu, Z.; Wang, R. Interaction between tetracycline and microorganisms during wastewater treatment: A review. Sci. Total Environ. 2021, 757, 143981. [Google Scholar] [CrossRef] [PubMed]
- Gaálová, J.; Bourassi, M.; Soukup, K.; Trávníčková, T.; Bouša, D.; Sundararajan, S.; Losada, O.; Kasher, R.; Friess, K.; Sofer, Z. Modified Single-Walled Carbon Nanotube Membranes for the Elimination of Antibiotics from Water. Membranes 2021, 11, 720. [Google Scholar] [CrossRef]
- Kujawski, W.; Ostrowska-Gumkowska, B. Preparation and Properties of Organophilic Membranes for Pervaporation of Water-Organics Mixtures. Sep. Sci. Technol. 2003, 38, 3669–3687. [Google Scholar] [CrossRef]
- Cocchi, G.; De Angelis, M.G.; Doghieri, F. Solubility and diffusivity of liquids for food and pharmaceutical applications in crosslinked polydimethylsiloxane (PDMS) films: II. Experimental data on mixtures. J. Membr. Sci. 2015, 492, 612–619. [Google Scholar] [CrossRef]
- Abu Shawish, H.M.; Saadeh, S.M.; Al-Dalou, A.R.; Ghalwa, N.A.; Assi, A.A.A. Optimization of tramadol–PVC membrane electrodes using miscellaneous plasticizers and ion-pair complexes. Mater. Sci. Eng. C 2011, 31, 300–306. [Google Scholar] [CrossRef]
- Jahromi, Z.; Mirzaei, E.; Savardashtaki, A.; Afzali, M.; Afzali, Z. A rapid and selective electrochemical sensor based on electrospun carbon nanofibers for tramadol detection. Microchem. J. 2020, 157, 104942. [Google Scholar] [CrossRef]


| Solutions | Separate ATB | MIX ATBs | TRA | ATBs + TRA | ||
|---|---|---|---|---|---|---|
| Pollutants | SM | TMP | TET | |||
| SM (mg/L) | 200 | - | - | 100 | - | 40 |
| TMP (mg/L) | - | 200 | - | 100 | - | 40 |
| TET (mg/L) | - | - | 200 | 100 | - | 40 |
| TRA (mg/L) | - | - | - | - | 500 | 300 |
| Membranes | Contact Angle (Degrees) |
|---|---|
| PDMS | 50° |
| MMA-DMAEMA 10 | 97° |
| GMA-DMAEMA 10 | 57° |
| GMA-DMAEMA 5 | 63° |
| Sample | SBET (m2/g) | Smeso (m2/g) | Vtot (mm3liq/g) |
|---|---|---|---|
| PDMS | 12 | 12 | 86 |
| GMA-DMAEMA 10 | 1.8 | 1.6 | 12 |
| GMA-DMAEMA 5 | 14 | 11 | 67 |
| MMA-DMAEMA 10 | 10 | 10 | 67 |
| EDX Analysis. | GMA-DMAEMA 10 | MMA-DMAEMA 10 | GMA-DMAEMA 5 | PDMS [14] |
|---|---|---|---|---|
| Carbon (%) | 71.46 | 67.75 | 60.65 | 47 |
| Nitrogen (%) | 4.55 | 6.09 | 28.45 | 0 |
| Oxygen (%) | 22.46 | 25.92 | 10.90 | 29 |
| Silicon (%) | 1.53 | 0.24 | 0 | 23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bourassi, M.; Pasichnyk, M.; Oesch, O.; Sundararajan, S.; Trávničková, T.; Soukup, K.; Kasher, R.; Gaálová, J. Glycidyl and Methyl Methacrylate UV-Grafted PDMS Membrane Modification toward Tramadol Membrane Selectivity. Membranes 2021, 11, 752. https://doi.org/10.3390/membranes11100752
Bourassi M, Pasichnyk M, Oesch O, Sundararajan S, Trávničková T, Soukup K, Kasher R, Gaálová J. Glycidyl and Methyl Methacrylate UV-Grafted PDMS Membrane Modification toward Tramadol Membrane Selectivity. Membranes. 2021; 11(10):752. https://doi.org/10.3390/membranes11100752
Chicago/Turabian StyleBourassi, Mahdi, Mariia Pasichnyk, Oscar Oesch, Swati Sundararajan, Tereza Trávničková, Karel Soukup, Roni Kasher, and Jana Gaálová. 2021. "Glycidyl and Methyl Methacrylate UV-Grafted PDMS Membrane Modification toward Tramadol Membrane Selectivity" Membranes 11, no. 10: 752. https://doi.org/10.3390/membranes11100752
APA StyleBourassi, M., Pasichnyk, M., Oesch, O., Sundararajan, S., Trávničková, T., Soukup, K., Kasher, R., & Gaálová, J. (2021). Glycidyl and Methyl Methacrylate UV-Grafted PDMS Membrane Modification toward Tramadol Membrane Selectivity. Membranes, 11(10), 752. https://doi.org/10.3390/membranes11100752