A Novel Numerical Procedure to Estimate the Electric Charge in the Pore from Filtration of Single-Salt Solutions
Abstract
:1. Introduction
2. Materials and Methods
3. Numerical Modelling
3.1. Transport Model
3.2. Adsorption Models
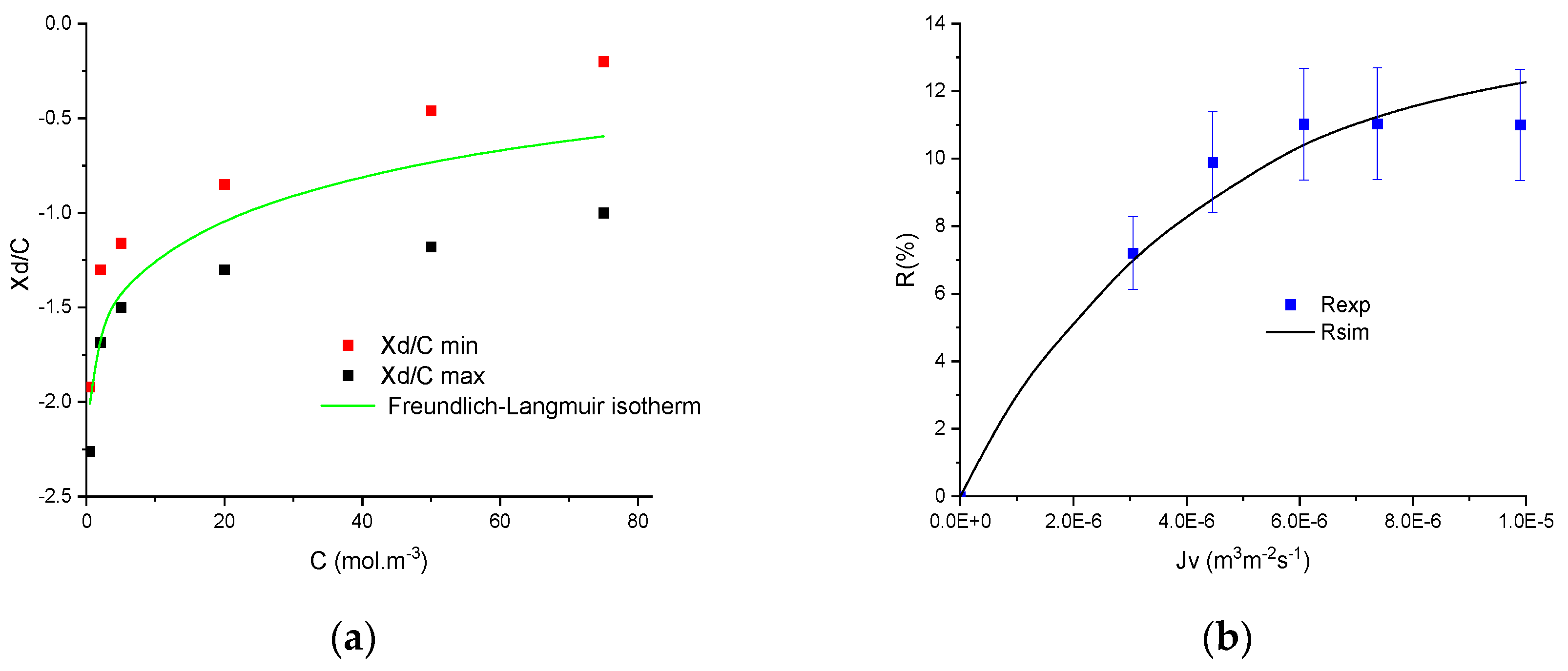
4. Results and Discussion
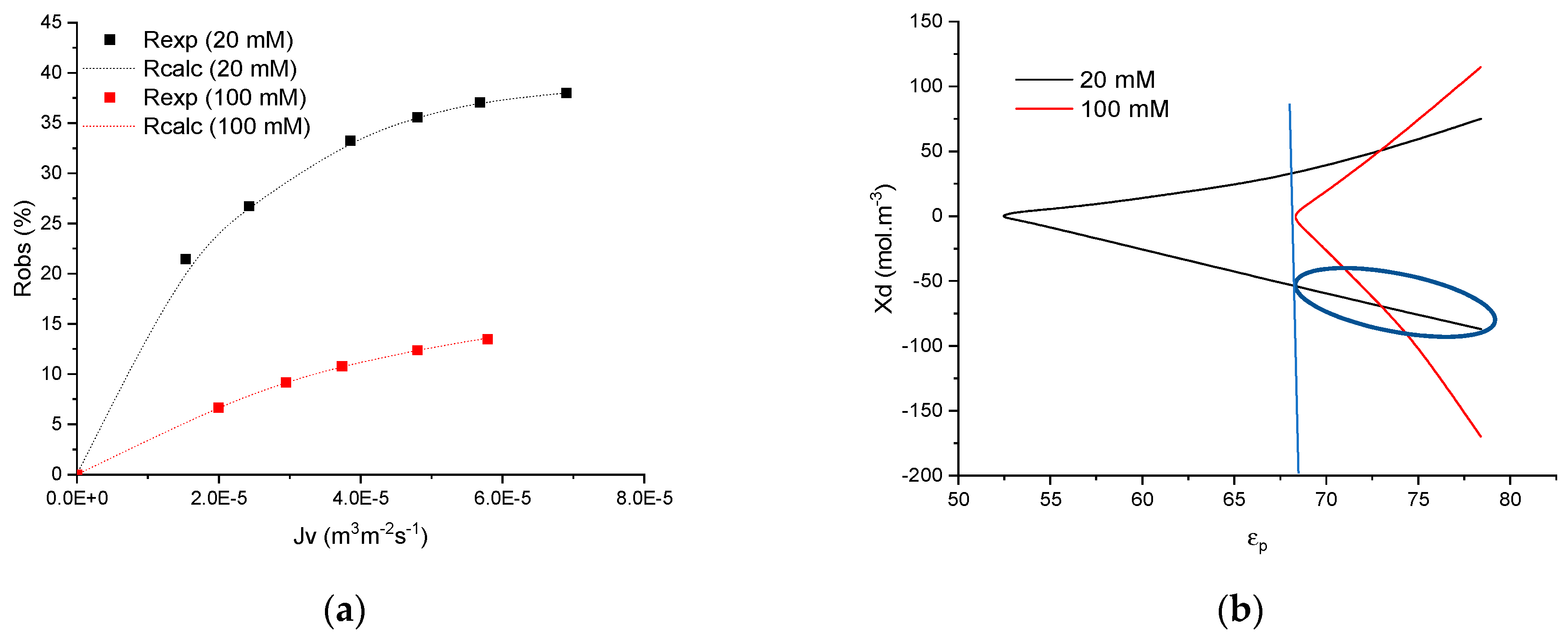
4.1. Preliminary Results
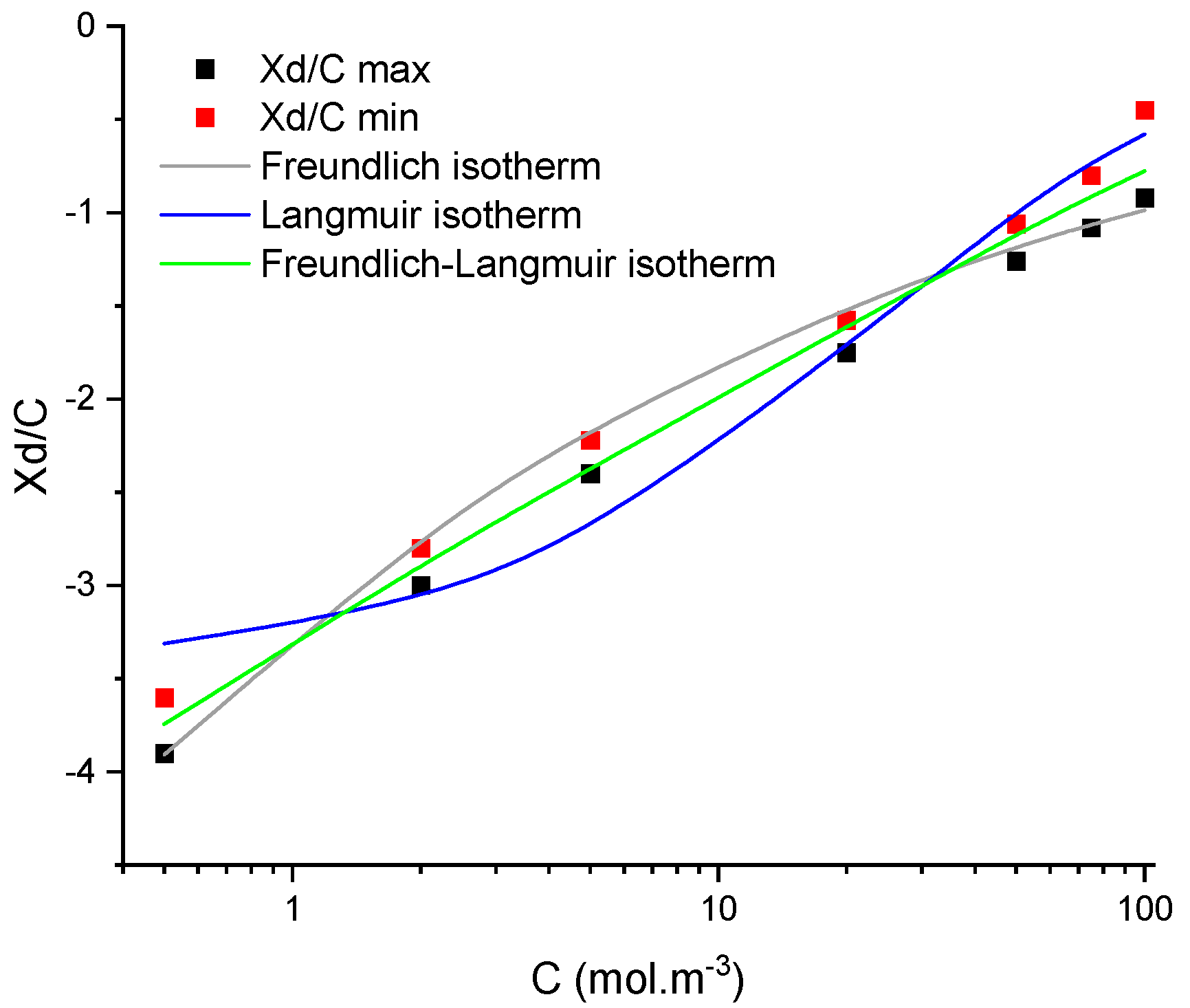
4.2. Numerical Procedure
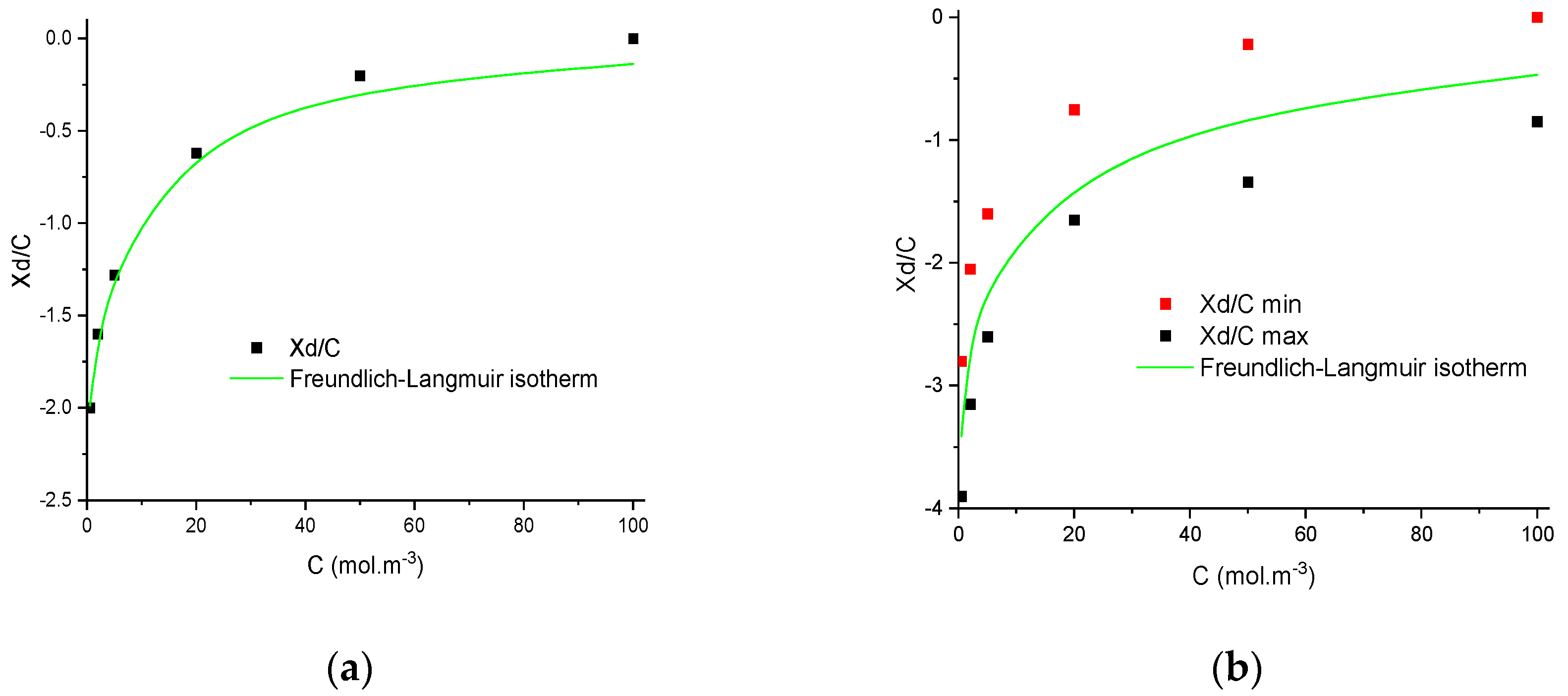
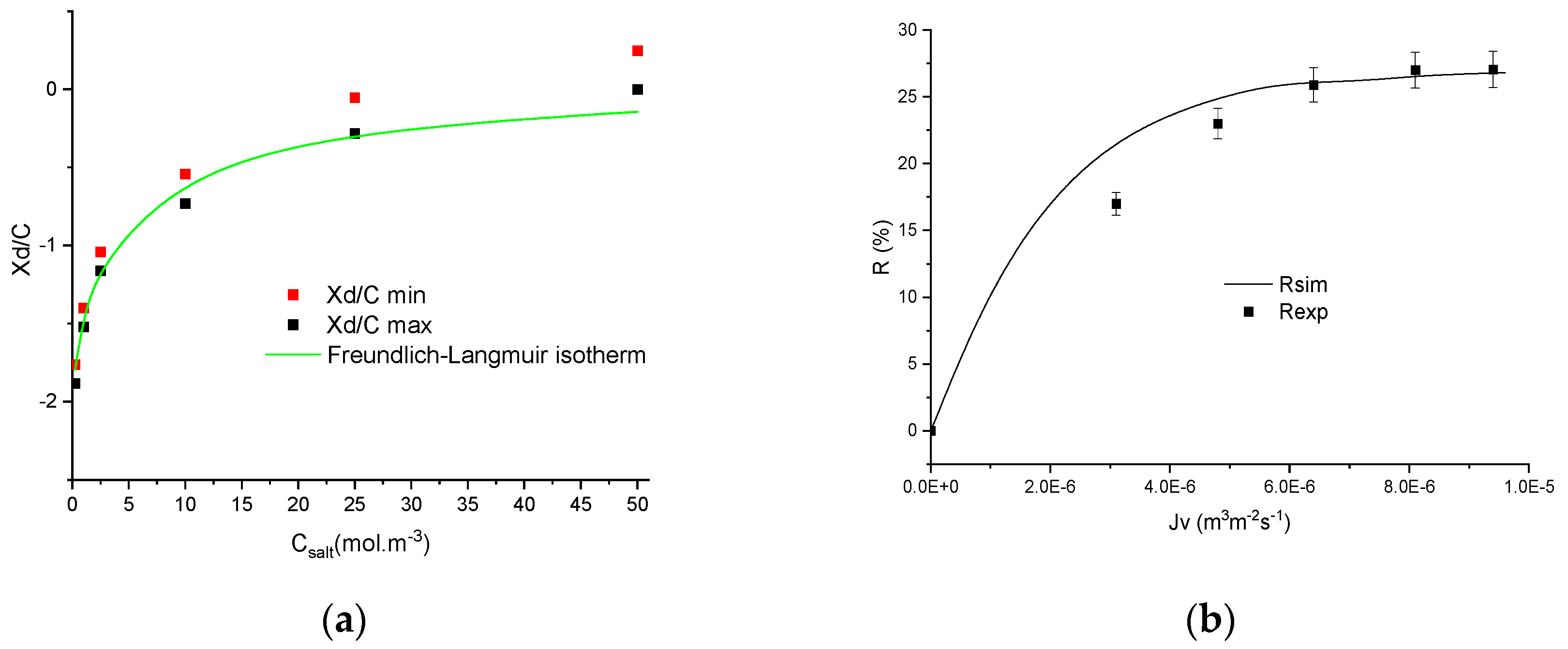
4.3. Numerical Investigation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Glossary
| ci | Concentration of ion i within the pore (mol m−3) |
| Ci,p | Permeate concentration of ion i (mol m−3) |
| Ci,f | Feed concentration of ion i (mol m−3) |
| Di,∞ | Diffusion coefficient of ion i at infinite dilution (m2 s−1) |
| e | Electronic charge (1.602 10−19 C) |
| F | Faraday constant (96487 C mol−1) |
| ji | Flux of ion i (mol m−2 s−1) |
| Jv | Permeation flux (m3 m−2 s−1) |
| Jw | Permeation flux of pure water (m3 m−2 s−1) |
| kB | Boltzmann constant (1.381 10–23 m2 kg s−2 K−1) |
| Ki,c | Ionic hindrance factor for convection (dimensionless) |
| Ki,d | Ionic hindrance factor for diffusion (dimensionless) |
| Lp | Hydraulic permeability (m3 m−2) |
| R | Gas constant (8.314 J mol−1 K−1) |
| ri,s | Stokes radius of ion i (m) |
| Ri | Observed rejection of ion i (dimensionless) |
| rp | Average pore radius (m) |
| T | Temperature (K) |
| V | Solvent velocity in the pore (m s−1) |
| x | Axial position within the pore (m) |
| Xd | Membrane effective charge density in the pore (eq m−3) |
| zi | Valence of ion i (dimensionless) |
| • | Greek letters |
| γi,p | Activity coefficient of ion i in the pore (dimensionless) |
| γi,s | Activity coefficient of ion i in the solution side of the interface (dimensionless) |
| ΔP | Applied pressure (Pa) |
| ΔWi | Dielectric exclusion energy (J) |
| ΔψD | Donnan potential (V) |
| Δπ | Osmotic pressure difference (Pa) |
| ε0 | Permittivity of free space (8.85419 × 10−12 F m−1) |
| εb | Bulk dielectric constant (dimensionless) |
| εp | Pore dielectric constant (dimensionless) |
| μ | Dynamic viscosity (Pa s) |
| φi | Steric partition coefficient (dimensionless) |
| ψ | Electrical potential within the pore (V) |
References
- Fridman-Bishop, N.; Nir, O.; Lahav, O.; Freger, V. Predicting the Rejection of Major Seawater Ions by Spiral-Wound Nanofiltration Membranes. Environ. Sci. Technol. 2015, 49, 8631–8638. [Google Scholar] [CrossRef] [PubMed]
- Fernández de Labastida, M.; Yaroshchuk, A. Nanofiltration of multi-ion solutions: Quantitative control of concentration polarization and interpretation by solution-diffusion-electro-migration model. Membranes 2021, 11, 272. [Google Scholar] [CrossRef] [PubMed]
- Diaz, P.A.B.; de Araujo Kronemberger, F.; Habert, A.C. Predictive study of succinic acid transport via nanofiltration membranes using a Maxwell–Stefan approach. J. Chem. Technol. Biotechnol. 2021, 96, 785–800. [Google Scholar] [CrossRef]
- Tsuru, T.; Nakao, S.; Kimura, S. Calculation of ion rejection by extended Nernst-Planck equation with charged reverse osmosis membranes for single and mixed electrolyte solutions. J. Chem. Eng. Jpn. 1991, 24, 511–517. [Google Scholar] [CrossRef] [Green Version]
- Szymczyk, A.; Fievet, P. Investigating transport properties of nanofiltration membranes by means of a steric, electric and dielectric exclusion model. J. Membr. Sci. 2005, 252, 77–88. [Google Scholar] [CrossRef]
- Bandini, S.; Vezzani, D. Nanofiltration modeling: The role of dielectric exclusion in membrane characterization. Chem. Eng. Sci. 2003, 58, 3303–3326. [Google Scholar] [CrossRef]
- Bowen, W.R.; Mukhtar, H. Characterisation and prediction of separation performance of nanofiltration membranes. J. Membr. Sci. 1996, 112, 263–274. [Google Scholar] [CrossRef]
- Silva, V.; Geraldes, V.; Brites Alves, A.M.; Palacio, L.; Prádanos, P.; Hernández, A. Multi-ionic nanofiltration of highly concentrated salt mixtures in the seawater range. Desalination 2011, 277, 29–39. [Google Scholar] [CrossRef]
- Schaep, J.; Vandecasteele, C.; Mohammad, A.W.; Bowen, W.R. Modelling the retention of ionic components for different nanofiltration membranes. Sep. Purif. Technol. 2001, 22–23, 169. [Google Scholar] [CrossRef]
- Zerafat, M.M.; Shariati-Niassar, M.; Hashemi, S.J.; Sabbaghi, S.; Ismail, A.F.; Matsuura, T. Mathematical modeling of nanofiltration for concentrated electrolyte solutions. Desalination 2013, 320, 17–23. [Google Scholar] [CrossRef]
- Dutournié, P.; Limousy, L.; Blel, W.; Déon, S.; Fievet, P. Understanding of ion transport in a na-mordenite membrane: Use of numerical modeling to estimate surface-solute interactions in the pore. Ind. Eng. Chem. Res. 2014, 53, 8221–8227. [Google Scholar] [CrossRef]
- Bowen, W.R.; Mohammad, A.W.; Hilal, N. Characterisation of nanofiltration membranes for predictive purposes–Use of salts, uncharged solutes and atomic force microscopy. J. Membr. Sci. 1997, 126, 91–105. [Google Scholar] [CrossRef]
- Idil Mouhoumed, E.; Szymczyk, A.; Schäfer, A.; Paugam, L.; La, Y.H. Physico-chemical characterization of polyamide NF/RO membranes: Insight from streaming current measurements. J. Membr. Sci. 2014, 461, 130–138. [Google Scholar] [CrossRef] [Green Version]
- Datta, S.; Conlisk, A.T.; Kanani, D.M.; Zydney, A.L.; Fissell, W.H.; Roy, S. Characterizing the surface charge of synthetic nanomembranes by the streaming potential method. J. Colloid Interface Sci. 2010, 348, 85–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Déon, S.; Escoda, A.; Fievet, P. A transport model considering charge adsorption inside pores to describe salts rejection by nanofiltration membranes. Chem. Eng. Sci. 2011, 66, 2823–2832. [Google Scholar] [CrossRef]
- Efligenir, A.; Fievet, P.; Déon, S.; Salut, R. Characterization of the isolated active layer of a NF membrane by electrochemical impedance spectroscopy. J. Membr. Sci. 2015, 477, 172–182. [Google Scholar] [CrossRef]
- Montalvillo, M.; Silva, V.; Palacio, L.; Calvo, J.I.; Carmona, F.J.; Hernández, A.; Prádanos, P. Charge and dielectric characterization of nanofiltration membranes by impedance spectroscopy. J. Membr. Sci. 2014, 454, 163–173. [Google Scholar] [CrossRef]
- Bason, S.; Oren, Y.; Freger, V. Characterization of ion transport in thin films using electrochemical impedance spectroscopy: II: Examination of the polyamide layer of RO membranes. J. Membr. Sci. 2007, 302, 10–19. [Google Scholar] [CrossRef]
- Escoda, A.; Déon, S.; Fievet, P. Assessment of dielectric contribution in the modeling of multi-ionic transport through nanofiltration membranes. J. Membr. Sci. 2011, 378, 214–223. [Google Scholar] [CrossRef]
- Lanteri, Y.; Szymczyk, A.; Fievet, P. Influence of steric, electric, and dielectric effects on membrane potential. Langmuir 2008, 24, 7955–7962. [Google Scholar] [CrossRef]
- Dutournié, P.; Déon, S.; Limousy, L. Understanding the separation of anion mixtures by TiO2 membranes: Numerical investigation and effect of alkaline treatment on physicochemical properties. Chem. Eng. J. 2019, 363, 365–373. [Google Scholar] [CrossRef]
- Déon, S.; Dutournié, P.; Limousy, L.; Bourseau, P. Transport of salt mixtures through nanofiltration membranes: Numerical identification of electric and dielectric contributions. Sep. Purif. Technol. 2009, 69, 225–233. [Google Scholar] [CrossRef]
- Déon, S.; Escoda, A.; Fievet, P.; Dutournié, P.; Bourseau, P. How to use a multi-ionic transport model to fully predict rejection of mineral salts by nanofiltration membranes. Chem. Eng. J. 2012, 189–190, 24–31. [Google Scholar] [CrossRef]
- Said, A.; Limousy, L.; Nouali, H.; Michelin, L.; Halawani, J.; Toufaily, J.; Hamieh, T.; Dutournié, P.; Daou, T.J. Synthesis of mono- and bi-layer MFI zeolite films on macroporous alumina tubular supports: Application to nanofiltration. J. Cryst. Growth 2015, 428, 71–79. [Google Scholar] [CrossRef]
- Said, A.; Daou, T.J.; Limousy, L.; Bikai, J.; Halwani, J.; Toufaily, J.; Hamieh, T.; Dutournié, P. Surface energy modification of a Na-mordenite thin layer treated by an alkaline solution. Mater. Express 2015, 5, 451–456. [Google Scholar] [CrossRef]
- Bowen, W.R.; Welfoot, J.S. Modelling the performance of membrane nanofiltration-critical assessment and model development. Chem. Eng. Sci. 2002, 57, 1121–1137. [Google Scholar] [CrossRef]
- Bowen, W.R.; Mohammad, A.W. Diafiltration by nanofiltration: Prediction and optimization. AIChE J. 1998, 44, 1799–1812. [Google Scholar] [CrossRef]
- Ferry, J.D. Statistical evaluation of sieve constants in ultrafiltration. J. Gen. Physiol. 1935, 20, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Born, M. Volumen and hydratationswärme der ionen. Z. Physik. Chem. 1920, 1, 45–48. [Google Scholar] [CrossRef]
- Vezzani, D.; Bandini, S. Donnan equilibrium and dielectric exclusion for characterization of nanofiltration membranes. Desalination 2002, 149, 477–483. [Google Scholar] [CrossRef]
- Yaroshchuk, A.E. Dielectric exclusion of ions from membranes. Adv. Colloid Interface Sci. 2000, 85, 193–230. [Google Scholar] [CrossRef]
- Déon, S.; Dutournié, P.; Bourseau, P. Modeling nanofiltration with Nernst-Planck approach and polarization layer. AIChE J. 2007, 53, 1952–1969. [Google Scholar] [CrossRef]
- Petkovska, M. Discrimination between adsorption isotherm models based on nonlinear frequency response results. Adsorption 2014, 20, 385–395. [Google Scholar] [CrossRef]
- Dutournié, P.; Limousy, L.; Zouaoui, N.; Mahzoul, H.; Chevereau, E. Facilitated transport of monovalent salt mixture through ultrafiltration Na-Mordenite membrane: Numerical investigations of electric and dielectric contributions. Desalination 2011, 280, 397–402. [Google Scholar] [CrossRef]
- Dutournié, P.; Limousy, L.; Anquetil, J.; Déon, S. Modification of the selectivity properties of tubular ceramic membranes after alkaline treatment. Membranes 2017, 7, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
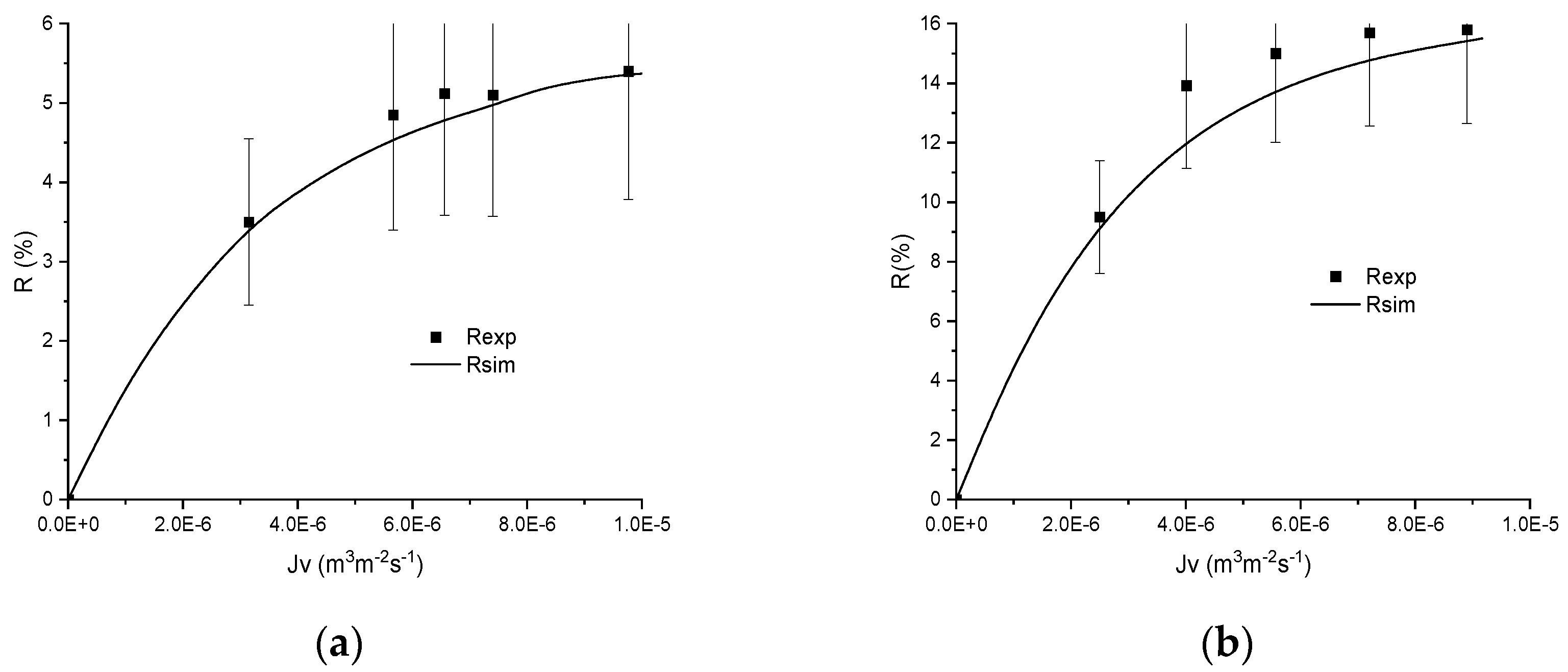
| Membranes | TiO2 | Na-Mordenite | MFI–MFI |
|---|---|---|---|
| Lp × 1014 (m3 m−2) | 4.8 | 1.3 | 0.8 |
| RVB12 max (%) | 62 | 3 | 36 |
| rp (nm) | 1.45 | 8.5 | 2.15 |
| Membranes | Salts | Qmax | KLF | n |
|---|---|---|---|---|
| TiO2 | NaCl | 3.37 | 1.20 | 0.022 |
| NaI | 2.13 | 1.10 | 0.151 | |
| Na2SO4 | 1.12 | 1.05 | 0.057 | |
| Na-MOR | NaCl | 2.01 | 1.29 | −0.010 |
| NaF | 3.19 | 1.15 | 0.051 | |
| MFI/MFI | NaI | 2.13 | 1.00 | 0.102 |
| NaF | 1.26 | 1.10 | 0.050 | |
| Na2SO4 | 1.87 | 0.99 | 0.274 |
| Membranes | Salts | εp min | εp max | Xd/Cf min | Xd/Cf max |
|---|---|---|---|---|---|
| TiO2 | NaCl | 77.6 | 78.4 | −3.8 | 0 |
| NaI | 78.4 | 78.4 | −2.0 | 0 | |
| Na2SO4 | 77.8 | 78.4 | −1.0 | +0.36 | |
| Na-MOR | NaCl | 77.6 | 78.4 | −2.3 | 0 |
| NaF | 73.3 | 78.4 | −3.9 | 0 | |
| MFI/MFI | NaI | 77.6 | 78.4 | −2.3 | 0 |
| NaF | 77.7 | 78.4 | −1.3 | 0 | |
| Na2SO4 | 77.9 | 78.4 | −1.9 | +0.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dutournié, P.; Daou, T.J.; Déon, S. A Novel Numerical Procedure to Estimate the Electric Charge in the Pore from Filtration of Single-Salt Solutions. Membranes 2021, 11, 726. https://doi.org/10.3390/membranes11100726
Dutournié P, Daou TJ, Déon S. A Novel Numerical Procedure to Estimate the Electric Charge in the Pore from Filtration of Single-Salt Solutions. Membranes. 2021; 11(10):726. https://doi.org/10.3390/membranes11100726
Chicago/Turabian StyleDutournié, Patrick, T. Jean Daou, and Sébastien Déon. 2021. "A Novel Numerical Procedure to Estimate the Electric Charge in the Pore from Filtration of Single-Salt Solutions" Membranes 11, no. 10: 726. https://doi.org/10.3390/membranes11100726
APA StyleDutournié, P., Daou, T. J., & Déon, S. (2021). A Novel Numerical Procedure to Estimate the Electric Charge in the Pore from Filtration of Single-Salt Solutions. Membranes, 11(10), 726. https://doi.org/10.3390/membranes11100726








