Ionic Liquid in Phosphoric Acid-Doped Polybenzimidazole (PA-PBI) as Electrolyte Membranes for PEM Fuel Cells: A Review
Abstract
:1. Introduction
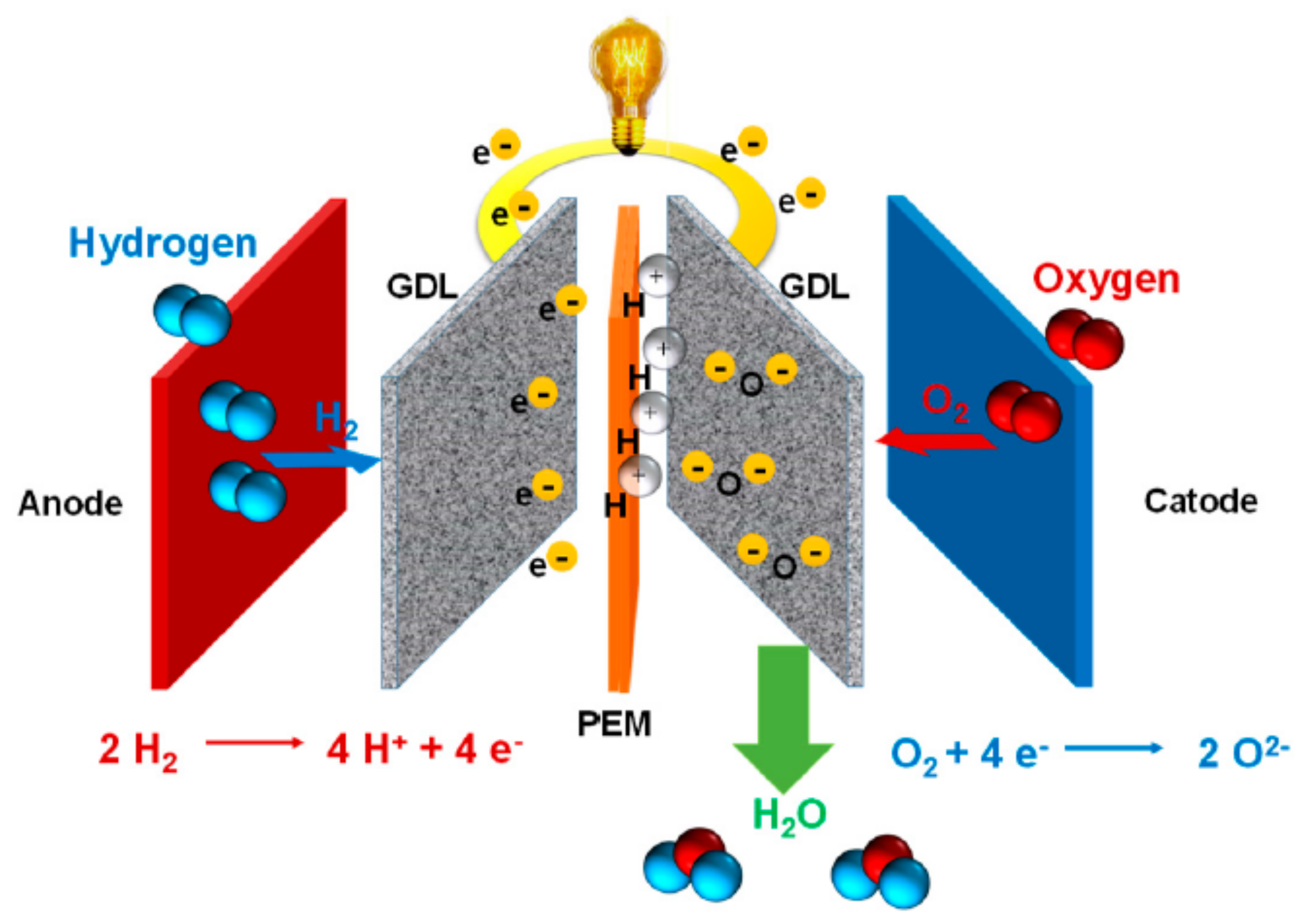
2. PBI Membrane in PEMFC
- (1)
- Proven stability in terms of electrochemical, chemical and thermal for fuel cell operations;
- (2)
- Higher mechanical tensile strength and sturdiness under heavy loads;
- (3)
- Higher gas separation capacity;
- (4)
- Good electrical insulation;
- (5)
- Cost-effective.
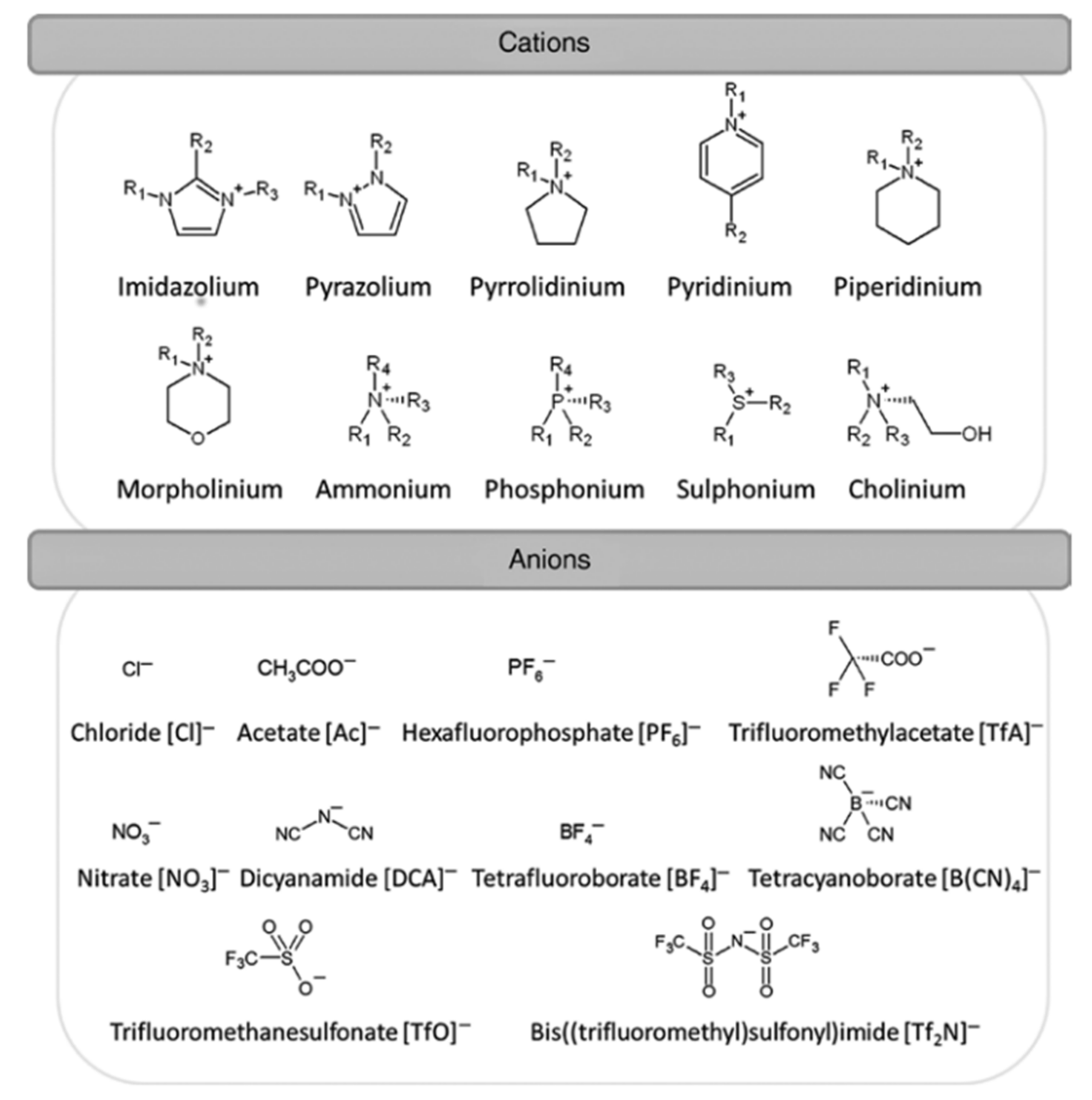
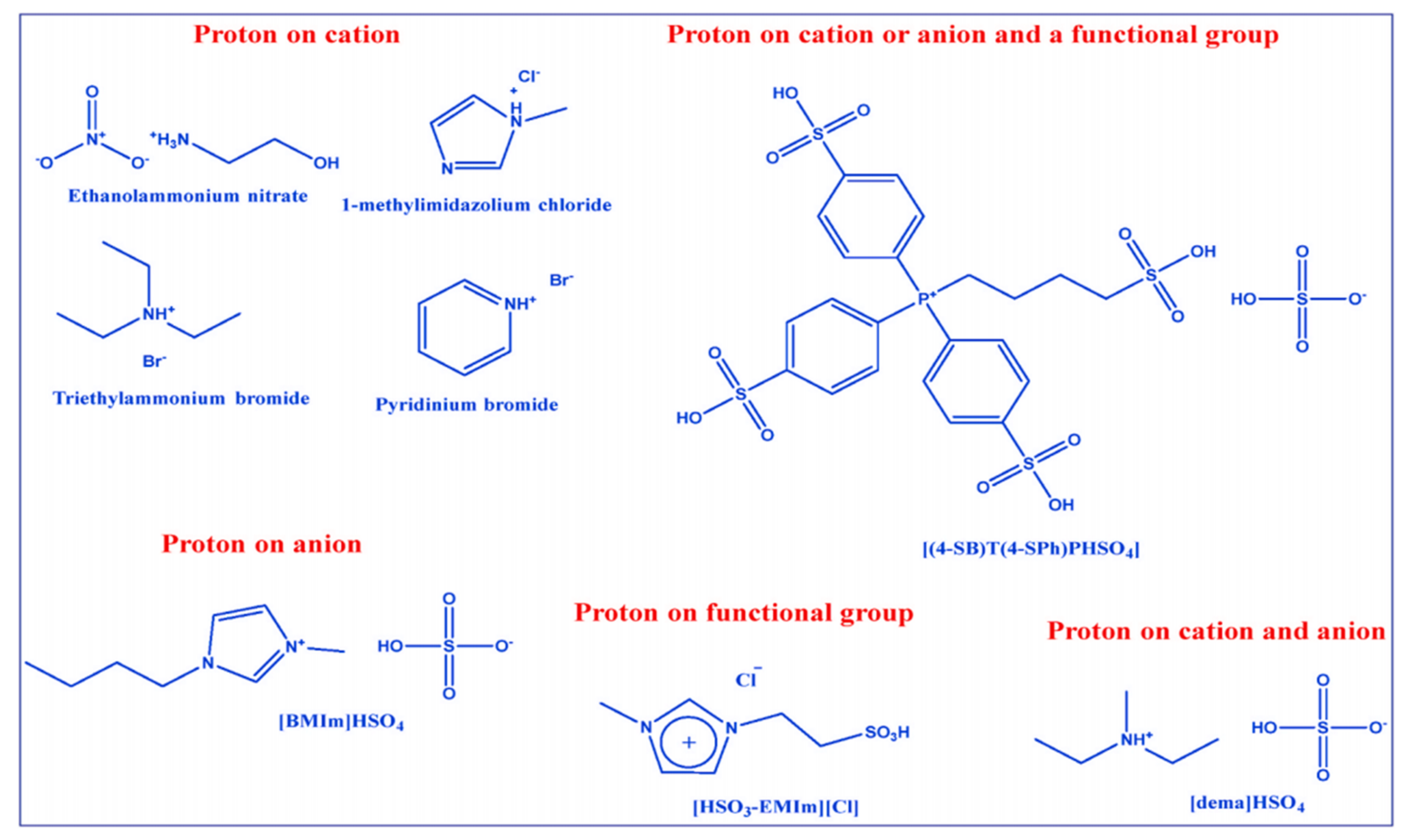
3. Ionic Liquids
4. Ionic Liquids in PBI Membranes
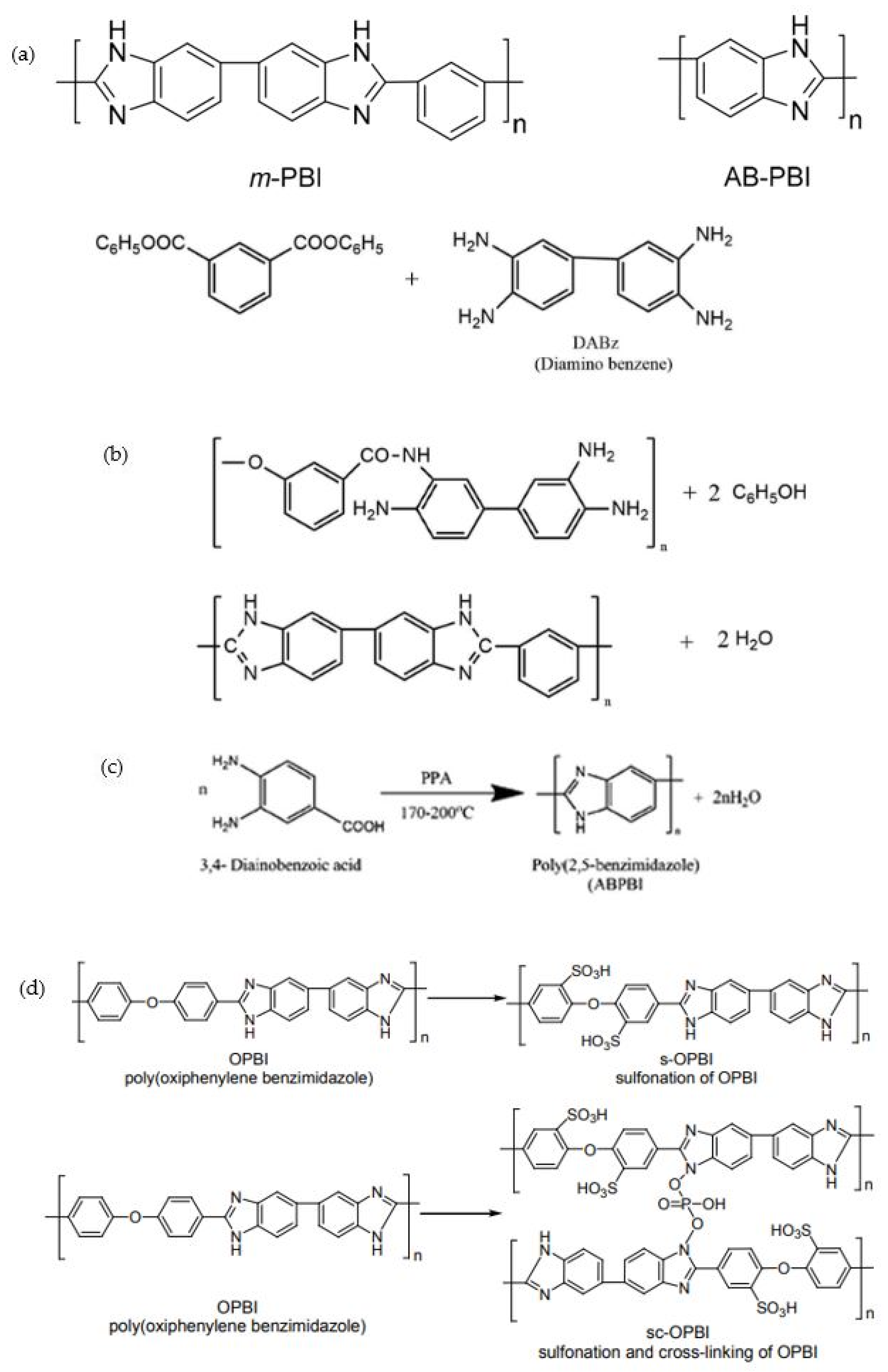
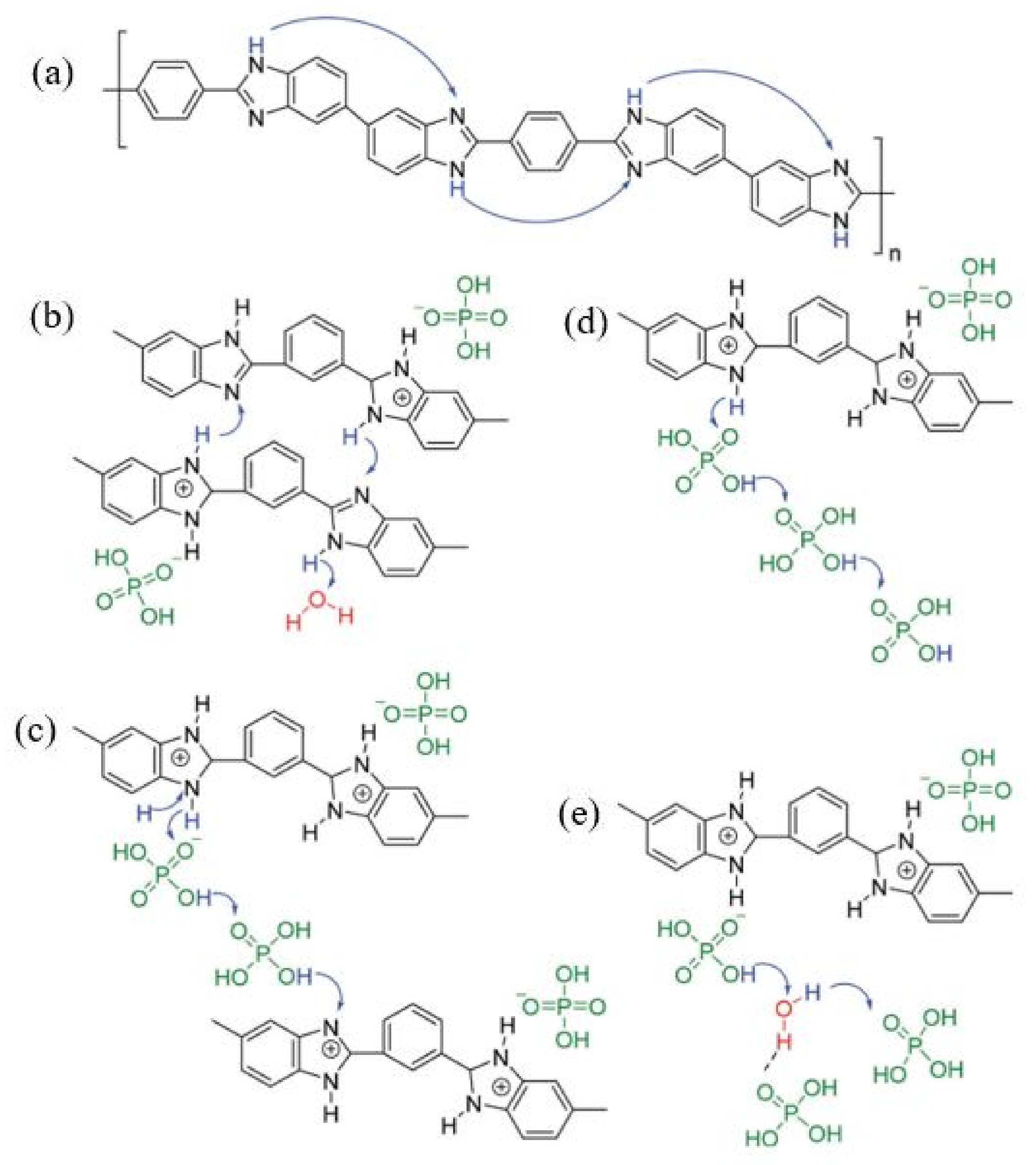
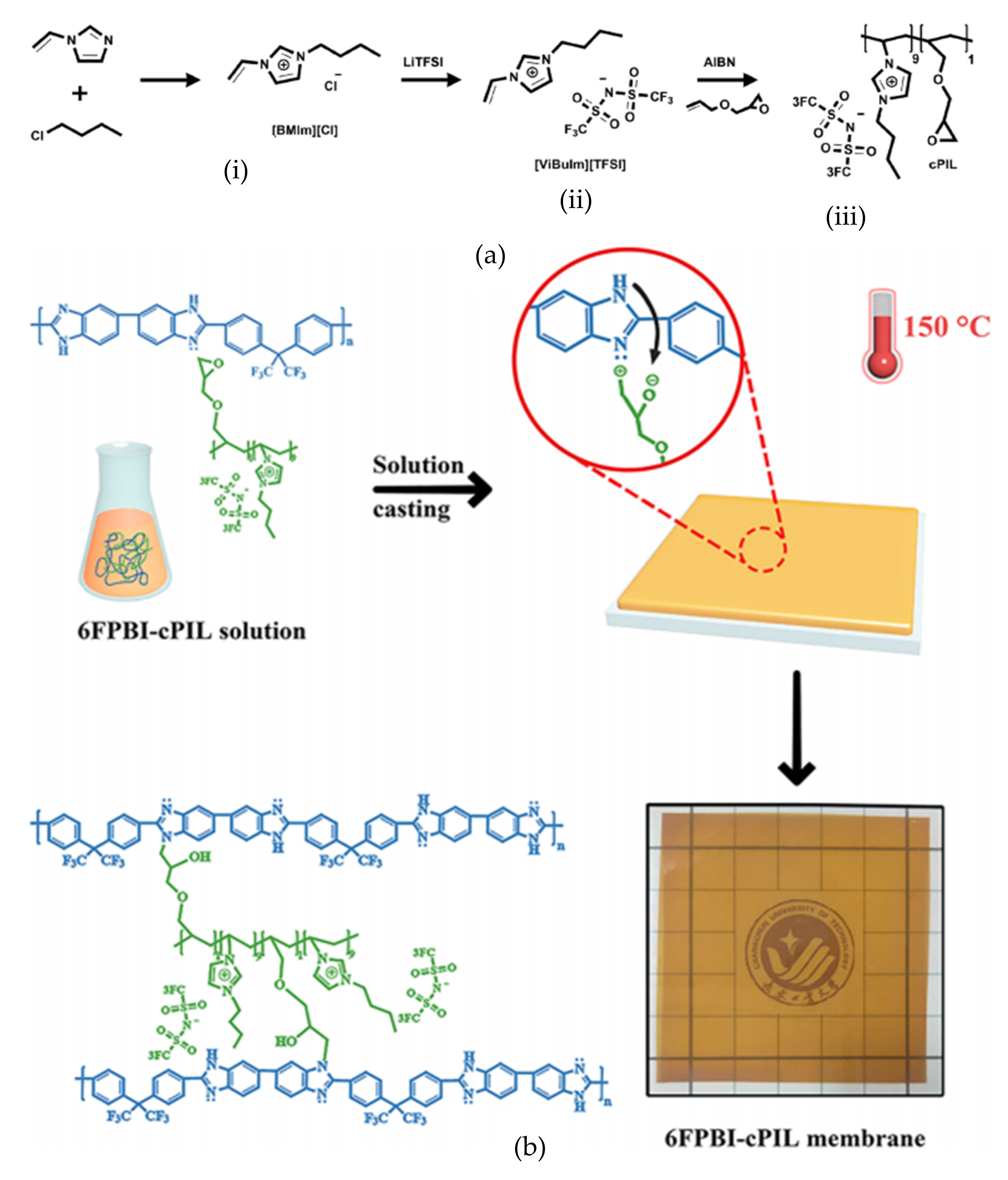
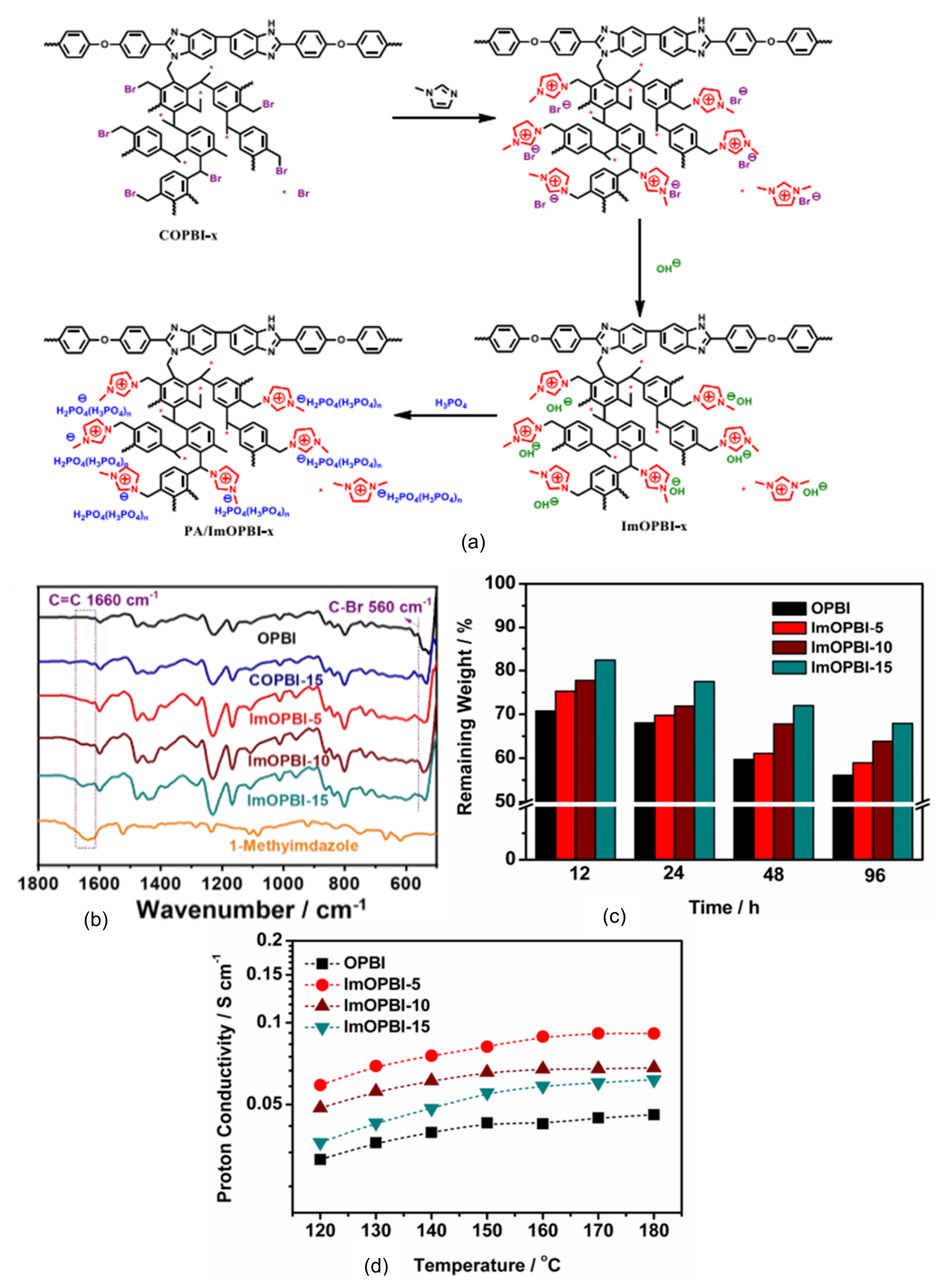
4.1. Synthesis
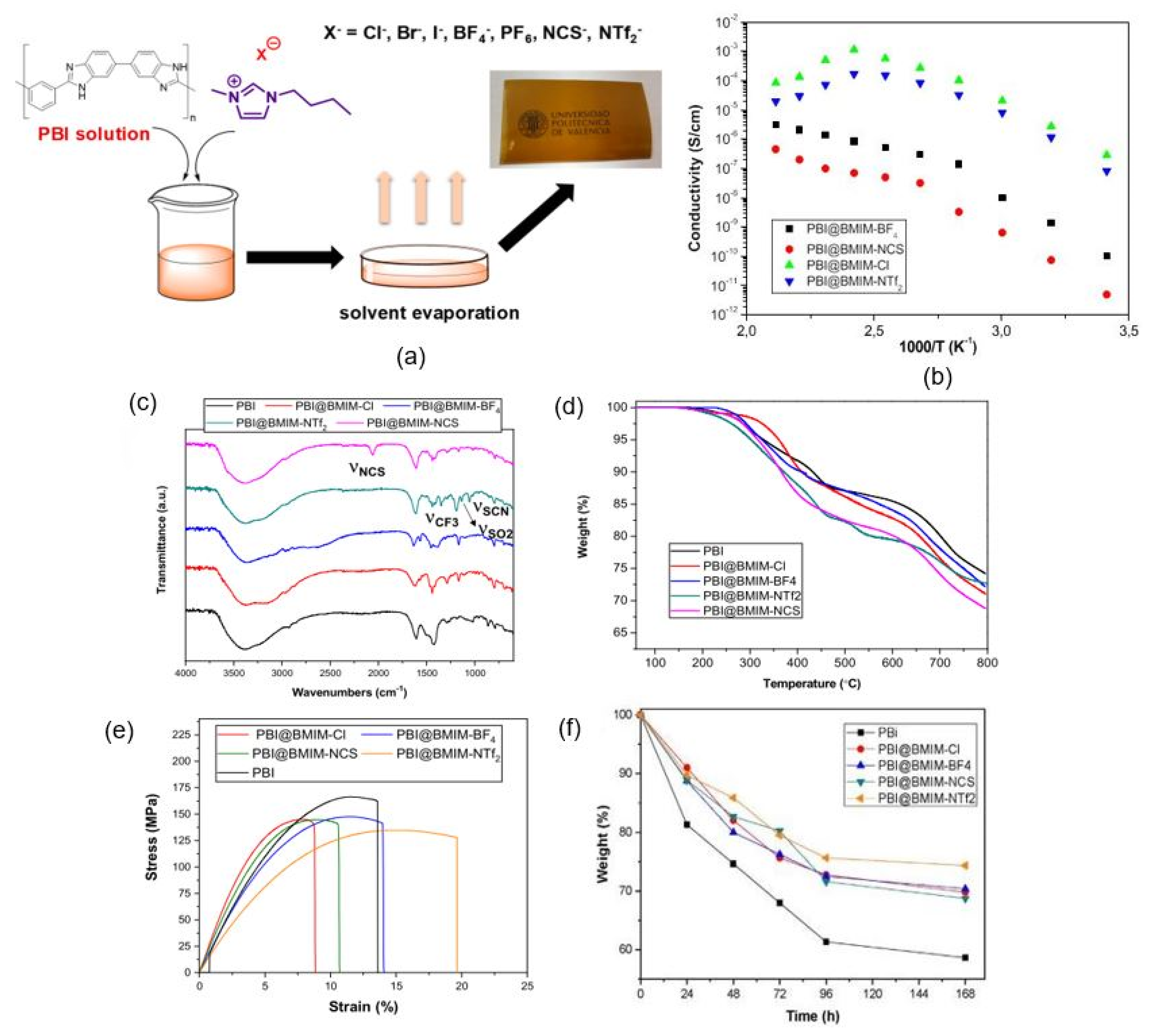
4.2. Effect of Ionic Liquids on PA/PBI Membrane Performance
5. Future Prospects
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ye, Y.S.; Rick, J.; Hwang, B.J. Water soluble polymers as proton exchange membranes for fuel cells. Polymer 2012, 4, 913–963. [Google Scholar] [CrossRef] [Green Version]
- Mohammad, N.; Mohamad, A.B.; Kadhum, A.A.H.; Loh, K.S. A review on synthesis and characterization of solid acid materials for fuel cell applications. J. Power Sources 2016, 322, 77–92. [Google Scholar] [CrossRef]
- Özdemir, Y.; Ozkan, N.; Devrim, Y. Fabrication and characterization of cross-linked polybenzimidazole based membranes for high temperature PEM fuel cells. Electrochim. Acta 2017, 245, 1–13. [Google Scholar] [CrossRef]
- Yusoff, Y.N.; Loh, K.S.; Wong, W.Y.; Daud, W.R.W.; Lee, T.K. Sulfonated graphene oxide as an inorganic filler in promoting the properties of a polybenzimidazole membrane as a high temperature proton exchange membrane. Int. J. Hydrogen Energy 2020, 45, 27510–27526. [Google Scholar] [CrossRef]
- Ying, Y.; Kamarudin, S.; Masdar, M. Silica-related membranes in fuel cell applications: An overview. Int. J. Hydrogen Energy 2018, 43, 16068–16084. [Google Scholar] [CrossRef]
- Yusoff, Y.; Samad, S.; Loh, K.S.; Lee, T.K. Structural and morphological study of sulfonated graphene oxide prepared with different precursors. J. Kejuruter. 2018, 2, 65–71. [Google Scholar]
- Mohammad, N.; Mohamad, A.B.; Kadhum, A.A.H.; Loh, K.S. Effect of silica on the thermal behaviour and ionic conductivity of mixed salt solid acid composites. J. Alloys Compd. 2017, 690, 896–902. [Google Scholar] [CrossRef]
- Escorihuela, J.; Olvera-Mancilla, J.; Alexandrova, L.; del Castillo, L.F.; Compañ, V. Recent progress in the development of composite membranes based on polybenzimidazole for high temperature proton exchange membrane (PEM) fuel cell applications. Polymers 2020, 12, 1861. [Google Scholar] [CrossRef]
- Husaini, T.; Malaysia, U.K.; Sulong, A.B.; Muhammad, S.; Utara, U.S. Effect of temperature on the mechanical performance of highly conductive composites for HT-PEMFC application. J. Kejuruter. 2018, 1, 25–30. [Google Scholar] [CrossRef]
- Goh, J.; Rahim, A.A.; Masdar, M.; Shyuan, L. Enhanced performance of polymer electrolyte membranes via modification with ionic liquids for fuel cell applications. Membranes 2021, 11, 395. [Google Scholar] [CrossRef]
- Wang, G.; Yu, Y.; Liu, H.; Gong, C.; Wen, S.; Wang, X.; Tu, Z. Progress on design and development of polymer electrolyte membrane fuel cell systems for vehicle applications: A review. Fuel Process. Technol. 2018, 179, 203–228. [Google Scholar] [CrossRef]
- Kim, D.J.; Choi, D.H.; Park, C.H.; Nam, S.Y. Characterization of the sulfonated PEEK/sulfonated nanoparticles composite membrane for the fuel cell application. Int. J. Hydrogen Energy 2016, 41, 5793–5802. [Google Scholar] [CrossRef]
- Krastev, V.; Falcucci, G.; Jannelli, E.; Minutillo, M.; Cozzolino, R. 3D CFD modeling and experimental characterization of HT PEM fuel cells at different anode gas compositions. Int. J. Hydrogen Energy 2014, 39, 21663–21672. [Google Scholar] [CrossRef]
- Asensio, J.A.; Sanchez, E.; Gomez-Romero, P. Proton-conducting membranes based on benzimidazole polymers for high-temperature PEM fuel cells. A chemical quest. Chem. Soc. Rev. 2010, 39, 3210–3239. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Shen, P.K. Recent development of polymer electrolyte membranes for fuel cells. Chem. Rev. 2012, 112, 2780–2832. [Google Scholar] [CrossRef]
- Ribeirinha, P.; Alves, I.; Vázquez, F.V.; Schuller, G.; Boaventura, M.; Mendes, A. Heat integration of methanol steam reformer with a high-temperature polymeric electrolyte membrane fuel cell. Energy 2017, 120, 468–477. [Google Scholar] [CrossRef]
- Savage, J.; Voth, G.A. Persistent subdiffusive proton transport in perfluorosulfonic acid membranes. J. Phys. Chem. Lett. 2014, 5, 3037–3042. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Kuila, T.; Nguyen, T.X.H.; Kim, N.H.; Lau, K.-T.; Lee, J.H. Polymer membranes for high temperature proton exchange membrane fuel cell: Recent advances and challenges. Prog. Polym. Sci. 2011, 36, 813–843. [Google Scholar] [CrossRef]
- Alberti, G.; Narducci, R.; di Vona, M.L.; Giancola, S. More on Nafion conductivity decay at temperatures higher than 80 °C: Preparation and first characterization of in-plane oriented layered morphologies. Ind. Eng. Chem. Res. 2013, 52, 10418–10424. [Google Scholar] [CrossRef]
- Subianto, S. Recent advances in polybenzimidazole/phosphoric acid membranes for high-temperature fuel cells. Polym. Int. 2014, 63, 1134–1144. [Google Scholar] [CrossRef]
- Li, Q.; He, R.; Jensen, J.O.; Bjerrum, N.J. PBI-based polymer membranes for high temperature fuel cells—Preparation, characterization and fuel cell demonstration. Fuel Cells 2004, 4, 147–159. [Google Scholar] [CrossRef]
- Colomban, P. Proton Conductors: Solids, Membranes, and Gels: Materials and Devices; Cambridge University Press: Cambridge, UK, 1992. [Google Scholar]
- Baozhong, X.; Savadogo, O. The effect of acid doping on the conductivity of polybenzimidazole (PBI). J. New Mater. Electrochem. Syst. 1999, 2, 95–101. [Google Scholar]
- Baozhong, X.; Savadogo, O. Hydrogen/oxygen polymer electrolyte membrane fuel cells (PEMFCs) based on alkaline-doped polybenzimidazole (PBI). Electrochem. Commun. 2000, 2, 697–702. [Google Scholar]
- Ma, Y.-L.; Wainright, J.S.; Litt, M.H.; Savinell, R.F. Conductivity of PBI membranes for high-temperature polymer electrolyte fuel cells. J. Electrochem. Soc. 2004, 151, A8–A16. [Google Scholar] [CrossRef]
- Jones, J.H.; Rozière, J. Recent advances in the functionalization of polybenzimidazole and polyetherketone for fuel cell applications. J. Membr. Sci. 2001, 185, 41–58. [Google Scholar] [CrossRef]
- Gilois, B.; Goujon, F.; Fleury, A.; Soldera, A.; Ghoufi, A. Water nano-diffusion through the Nafion fuel cell membrane. J. Membr. Sci. 2020, 602, 117958. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, L.; Yang, H.; Kong, L.; Ran, F. Alkali-tolerant polymeric gel electrolyte membrane based on cross-linked carboxylated chitosan for supercapacitors. J. Membr. Sci. 2021, 629, 119083. [Google Scholar] [CrossRef]
- De Haro, J.C.; Tatsi, E.; Fagiolari, L.; Bonomo, M.; Barolo, C.; Turri, S.; Bella, F.; Griffini, G. Lignin-based polymer electrolyte membranes for sustainable aqueous dye-sensitized solar cells. ACS Sustain. Chem. Eng. 2021, 9, 8550–8560. [Google Scholar] [CrossRef]
- Abdel-Wahed, M.S.; El-Kalliny, A.S.; Badawy, M.I.; Attia, M.S.; Gad-Allah, T.A. Core double-shell MnFe2O4@rGO@TiO2 superparamagnetic photocatalyst for wastewater treatment under solar light. Chem. Eng. J. 2020, 382, 122936. [Google Scholar] [CrossRef]
- Amici, J.; Torchio, C.; Versaci, D.; Dessantis, D.; Marchisio, A.; Caldera, F.; Bella, F.; Francia, C.; Bodoardo, S. Nanosponge-based composite gel polymer electrolyte for safer Li-O2 batteries. Polymers 2021, 13, 1625. [Google Scholar] [CrossRef]
- Radzir, N.N.M.; Abu Hanifah, S.; Ahmad, A.; Hassan, N.H.; Bella, F. Effect of lithium bis (trifluoromethylsulfonyl) imide salt-doped UV-cured glycidyl methacrylate. J. Solid State Electrochem. 2015, 19, 3079–3085. [Google Scholar] [CrossRef]
- Wang, Z.; Dong, K.; Wang, D.; Luo, S.; Liu, Y.; Yi, T.; Wang, Q.; Zhang, Y.; Hao, A. In situ construction of multibuffer structure 3D CoSn@SnOx/CoOx@C anode material for ultralong life lithium storage. Energy Technol. 2020, 8, 1–8. [Google Scholar]
- Devanathan, R. Recent developments in proton exchange membranes for fuel cells. Energy Environ. Sci. 2008, 1, 101–109. [Google Scholar] [CrossRef]
- Wainright, J.S.; Wang, J.-T.; Weng, D.; Savinell, R.F.; Litt, M. Acid-doped polybenzimidazoles: A new polymer electrolyte. J. Electrochem. Soc. 1995, 142, L121–L123. [Google Scholar] [CrossRef]
- Vogel, H.; Marvel, C.S. Polybenzimidazoles, new thermally stable polymers. J. Polym. Sci. 1961, 50, 511–539. [Google Scholar] [CrossRef]
- Das, A.; Ghosh, P.; Ganguly, S.; Banerjee, D.; Kargupta, K. Salt-leaching technique for the synthesis of porous poly (2, 5-benzimidazole) (ABPBI) membranes for fuel cell application. J. Appl. Polym. Sci. 2018, 135, 45773. [Google Scholar] [CrossRef]
- Borjigin, H.; Stevens, K.A.; Liu, R.; Moon, J.D.; Shaver, A.T.; Swinnea, S.; Freeman, B.D.; Riffle, J.S.; McGrath, J.E. Synthesis and characterization of polybenzimidazoles derived from tetraaminodiphenylsulfone for high temperature gas separation membranes. Polymer 2015, 71, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Hwang, K.; Kim, J.-H.; Kim, S.-Y.; Byun, H. Preparation of polybenzimidazole-based membranes and their potential applications in the fuel cell system. Energies 2014, 7, 1721–1732. [Google Scholar] [CrossRef]
- Zhu, L.; Swihart, M.T.; Lin, H. Unprecedented size-sieving ability in poly-benzimidazole doped with polyprotic acid for membrane H2/CO2 separation. Energy Environ. Sci. 2018, 11, 94–100. [Google Scholar] [CrossRef]
- Asensio, J.A.; Borrós, S.; Gomez-Romero, P. Proton-conducting polymers based on benzimidazoles and sulfonated benzimidazoles. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 3703–3710. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Pingitore, A.T.; Xie, W.; Yang, Z.; Perry, M.L.; Benicewicz, B.C. Sulfonated PBI gel membranes for redox flow batteries. J. Electrochem. Soc. 2019, 166, A1449–A1455. [Google Scholar] [CrossRef]
- Kumar, V.V.; Kumar, C.R.; Suresh, A.; Jayalakshmi, S.; Mudali, U.K.; Sivaraman, N. Evaluation of polybenzimidazole-based polymers for the removal of uranium, thorium and palladium from aqueous medium. R. Soc. Open Sci. 2018, 5, 171701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penchev, H.; Zaharieva, K.; Milenova, K.; Ublekov, F.; Dimova, S.; Budurova, D.; Staneva, M.; Stambolova, I.; Sinigersky, V.; Blaskov, V. Novel meta- and AB-polybenzimidazole/zinc oxide polymer hybrid nanomaterials for photocatalytic degradation of organic dyes. Mater. Lett. 2018, 230, 187–190. [Google Scholar] [CrossRef]
- Chen, D.; Yan, C.; Li, X.; Liu, L.; Wu, D.; Li, X. A highly stable PBI solvent resistant nanofiltration membrane prepared by versatile and simple cross-linking process. Sep. Purif. Technol. 2019, 224, 15–22. [Google Scholar] [CrossRef]
- Huang, F.; Pingitore, A.T.; Benecewicz, B.C. Electrochemical hydrogen separation from reformate using high temperature polybenzimidadole (PBI) membranes: The role of chemistry. ACS Sustain. Chem. Eng. 2020, 8, 6234–6242. [Google Scholar] [CrossRef]
- Montes Luna, A.D.J.; López, N.C.F.; de León, G.C.; Camacho, O.P.; Miranda, C.Y.Y.; Mercado, Y.A.P. PBI/clinoptilolite mixed-matrix membranes for binary (N2/CH4) and ternary (CO2/N2/CH4) mixed gas separation. J. Appl. Polym. Sci. 2020, 138, 50155. [Google Scholar] [CrossRef]
- Kim, D.Y.; Ryu, S.; Kim, H.-J.; Ham, H.C.; Sohn, H.; Yoon, S.P.; Han, J.; Lim, T.-H.; Kim, J.Y.; Lee, S.W.; et al. Highly selective asymmetric polybenzimidazole-4, 4′-(hexafluoroisopropylidene) bis (benzoic acid) hollow fiber membranes for hydrogen separation. Sep. Purif. Technol. 2020, 257, 117954. [Google Scholar] [CrossRef]
- Huang, F.; Pingitore, A.T.; Benicewicz, B.C. High polymer content m/p-polybenzimidazole copolymer membranes for electrochemical hydrogen separation under differential pressures. J. Electrochem. Soc. 2020, 167, 063504. [Google Scholar] [CrossRef]
- Mekhilef, S.; Saidur, R.; Safari, A. Comparative study of different fuel cell technologies. Renew. Sustain. Energy Rev. 2012, 16, 981–989. [Google Scholar] [CrossRef]
- Zeis, R. Materials and characterization techniques for high-temperature polymer electrolyte membrane fuel cells. Beilstein J. Nanotechnol. 2015, 6, 68–83. [Google Scholar] [CrossRef] [Green Version]
- Lobato, J.; Cañnizares, P.; Rodrigo, M.A.; Linares, J.J.; Pinar, F.J. Study of the influence of amount of PGI-H3PO4 in the catalytic layer of a high temperature PEMFC. Int. J. Hydrogen Energy 2010, 35, 1347–1355. [Google Scholar] [CrossRef]
- Samms, S.R.; Wasmus, S.; Savinell, R.F. Thermal stability of proton conducting acid doped polybenzimidazole in simulated fuel cell environments. J. Electrochem. Soc. 1996, 143, 1225–1232. [Google Scholar] [CrossRef]
- Melchior, J.-P.; Majer, G.; Kreuer, K.-D. Why do proton conducting polybenzimidazole phosphoric acid membranes perform well in high-temperature PEM fuel cells? Phys. Chem. Chem. Phys. 2016, 19, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Araya, S.S.; Zhou, F.; Liso, V.; Sahlin, S.L.; Vang, J.R.; Thomas, S.; Gao, X.; Jeppesen, C.; Kær, S.K. A comprehensive review of PBI-based high temperature PEM fuel cells. Int. J. Hydrogen Energy 2016, 41, 21310–21344. [Google Scholar] [CrossRef]
- Hu, Y.; Jiang, Y.; Jensen, J.O.; Cleemann, L.N.; Li, Q. Catalyst evaluation for oxygen reduction reaction in concentrated phosphoric acid at elevated temperatures. J. Power Sources 2018, 375, 77–81. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.; Xiao, L.; Benicewicz, B. Durability studies of PBI-based high temperature PEMFCs. Fuel Cells 2008, 8, 165–174. [Google Scholar] [CrossRef]
- Eguizábal, A.; Lemus, J.; Pina, M. On the incorporation of protic ionic liquids imbibed in large pore zeolites to polybenzimidazole membranes for high temperature proton exchange membrane fuel cells. J. Power Sources 2013, 222, 483–492. [Google Scholar] [CrossRef] [Green Version]
- Van de Ven, E.; Chairuna, A.; Merle, G.; Benito, S.P.; Borneman, Z.; Nijmeijer, K. Ionic liquid doped polybenzimidazole membranes for high temperature Proton Exchange Membrane fuel cell applications. J. Power Sources 2013, 222, 202–209. [Google Scholar] [CrossRef]
- Heo, P.; Kajiyama, N.; Kobayashi, K.; Nagao, M.; Sano, M.; Hibino, T. Proton conduction in Sn0.95Al0.05P2O7–PBI–PTFE composite membrane. Electrochem. Solid-State Lett. 2008, 11, B91–B95. [Google Scholar] [CrossRef]
- Oh, S.-Y.; Yoshida, T.; Kawamura, G.; Muto, H.; Sakai, M.; Matsuda, A. Inorganic-organic composite electrolytes consisting of polybenzimidazole and Cs-subtituted heteropoly acids and their application for medium temperature fuel cells. J. Mater. Chem. 2010, 20, 6359–6366. [Google Scholar] [CrossRef]
- Ghosh, S.; Maity, S.; Jana, T. Polybenzimidazole/silica nanocomposites: Organic-inorganic hybrid membranes for PEM fuel cell. J. Mater. Chem. 2011, 21, 14897–14906. [Google Scholar] [CrossRef]
- Singha, S.; Jana, T. Influence of interfacial interactions on the properties of polybenzamidazole/clay nanocomposite electrolyte membrane. Polymer 2016, 98, 20–31. [Google Scholar] [CrossRef]
- Fuentes, I.; Andrio Balado, A.; Garcia Bernabe, A.; Escorihuela Fuentes, J.; Viñas, C.; Teixidor, F.; Compañ Moreno, V. Structural and dielectric properties of cobaltacarborane composite polybenzimidazole membranes as solid polymer electrolytes at high temperature. Phys. Chem. Chem. Phys. 2018, 20, 10173–10185. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wu, X.; Wang, X.; Mamlouk, M.; Scott, K. Composite membranes of polybenzimidazole and caesium-salts-of-heteropolyacids for intermediate temperature fuel cells. J. Mater. Chem. 2011, 21, 6014–6019. [Google Scholar] [CrossRef]
- Escorihuela, J.; Narducci, R.; Compañ, V.; Costantino, F. Proton conductivity of composite polyelectrolyte membranes with metal-organic frameworks for fuel cell applications. Adv. Mater. Interfaces 2018, 6, 1801146. [Google Scholar] [CrossRef]
- Yang, J.; Liu, C.; Gao, L.; Wang, J.; Xu, Y.; He, R. Novel composite membranes of triazole modified graphene oxide and polybenzimidazole for high temperature polymer electrolyte membrane fuel cell applications. RSC Adv. 2015, 5, 101049–101054. [Google Scholar] [CrossRef]
- Kim, J.; Kim, K.; Ko, T.; Han, J.; Lee, J.-C. Polybenzimidazole composite membranes containing imidazole functionalized grapheme oxide showing high proton conductivity and improved physicochemical properties. Int. J. Hydrogen Energy 2021, 46, 12254–12262. [Google Scholar] [CrossRef]
- Compañ, V.; Escorihuela, J.; Olvera, J.; Garcia-Bernabe, A.; Andrio, A. Influence of the anion on diffusivity and mobility of ionic liquids composite polybenzimidazol membranes. Electrochim. Acta 2020, 354, 136666. [Google Scholar] [CrossRef]
- Baik, K.D.; Seo, I.S. Metallic bipolar plate with a multi-hole structure in the rib regions for polymer electrolyte membrane fuel cells. Applied Energy 2018, 212, 333–339. [Google Scholar] [CrossRef]
- Niu, B.; Luo, S.; Lu, C.; Yi, W.; Liang, J.; Guo, S.; Wang, D.; Zeng, F.; Duan, S.; Liu, Y.; et al. Polybenzimidazole and ionic liquid composite membranes for high temperature polymer electrolyte fuel cells. Solid State Ionics 2021, 361, 115569. [Google Scholar] [CrossRef]
- Muthuraja, P.; Prakash, S.; Shanmugam, V.; Radhakrsihnan, S.; Manisankar, P. Novel perovskite structured calcium titanate-PBI composite membranes for high-temperature PEM fuel cells: Synthesis and characterizations. Int. J. Hydrogen Energy 2018, 43, 4763–4772. [Google Scholar] [CrossRef]
- Guo, H.; Li, Z.; Sun, P.; Pei, H.; Zhang, L.; Cui, W.; Yin, X.; Hui, H. Enhancing proton conductivity and durability of cross-linked PBI-based high-temperature PEM: Effectively doping a novel cerium triphosphonic-isocyanurate. J. Electrochem. Soc. 2021, 168, 024510. [Google Scholar] [CrossRef]
- Krishnan, N.N.; Lee, S.; Ghorpade, R.V.; Konovalova, A.; Jang, J.H.; Kim, H.-J.; Hang, J.; Henkensmeier, D.; Han, H. Polybenzimidazole (PBI-OO) based composite membranes using sulfophenylated TiO2 as both filler and cross-linker, and their use in the HT-PEM fuel cell. J. Membr. Sci. 2018, 560, 11–20. [Google Scholar] [CrossRef]
- Abouzari-Lotf, E.; Zakeri, M.; Nasef, M.M.; Miyake, M.; Mozarmnia, P.; Bazilah, N.A. Highly durable polybenzimidazole composite membranes with phosphonated grapheme oxide for high temperature polymer electrolyte membrane fuel cells. J. Power Sources 2019, 412, 238–245. [Google Scholar] [CrossRef]
- Budak, Y.; Devrim, Y. Micro-cogeneration application of a high-temperature PEM fuel cell stack operated with polybenzimidazole based membranes. Int. J. Hydrogen Energy 2020, 45, 35198–35207. [Google Scholar] [CrossRef]
- Eren, E.O.; Ozkan, N.; Devrim, Y. Polybenzimidazole-modified carbon nanotubes as a support material for platinum-based high-temperature proton exchange membrane fuel cell electrocatalysts. Int. J. Hydrogen Energy 2020, 46, 29556–29567. [Google Scholar] [CrossRef]
- Escorihuela, J.; Sahuquillo, O.; Garcia-Bernabé, A.; Giménez, E.; Compañ, V. Phosphoric acid doped polybenzimidazole (PBI)/zeolitic imidazole framework composite membranes with significantly enhanced proton conductivity under low humidity conditions. Nanomaterials 2018, 8, 775. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Liu, X.; Yang, F.; Zhou, L.L.; Yin, B.; Wang, P.; Wang, L. Achieving high power density and excellent durability for high temperature proton exchange membrane fuel cells based on cross-linked branched polybenzimidazole and metal organic frameworks. J. Membr. Sci. 2021, 630, 119288. [Google Scholar] [CrossRef]
- Zhu, X.; Zeng, Y.; Zhang, Z.; Yang, Y.; Zhai, Y.; Wang, H.; Liu, L.; Hu, J.; Li, L. A new composite of grapheme and molecularly imprinted polymer based on ionic liquids as fuctional monomer and cross-linker for electrochemical sensing 6-benzylaminopurine. Biosens. Bioelectron. 2018, 108, 38–45. [Google Scholar] [CrossRef]
- Wang, L.; Ni, J.; Liu, D.; Gong, C. Effects of branching structures on the properties of phosphoric acid-doped polybenzimidazole as a membrane material for high-temperature proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2018, 43, 16694–16703. [Google Scholar] [CrossRef]
- Tian, X.; Wang, S.; Li, J.; Liu, F.; Wang, X.; Chen, H.; Ni, H.; Wang, Z. Composite membranes based on polybenzimidazole and ionic liquid functional Si–O–Si network for HT-PEMFC applications. Int. J. Hydrogen Energy 2017, 42, 21913–21921. [Google Scholar] [CrossRef]
- Earle, M.J.; Seddon, K.R. Ionic liquids. green solvents for the future. Pure Appl. Chem. 2000, 72, 1391–1398. [Google Scholar] [CrossRef] [Green Version]
- Plechkova, N.V.; Seddon, K.R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008, 37, 123–150. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.-S.; Rick, J.; Hwang, B.-J. Ionic liquid polymer electrolytes. J. Mater. Chem. A 2013, 1, 2719–2749. [Google Scholar] [CrossRef]
- Correia, D.M.; Fernandes, L.; Martins, P.; García-Astrain, C.; Costa, C.M.; Reguera, J.; Lanceros-Méndez, S. Ionic liquid–polymer composites: A new platform for multifunctional applications. Adv. Funct. Mater. 2020, 30, 1909736. [Google Scholar] [CrossRef]
- Ohno, H. Ionic liquids. In Fuctional Organic Liquids; Nakanishi, T., Ed.; Wiley-VCH Verlag GmbH&Co.: Weinheim, Germany, 2019; pp. 235–250. [Google Scholar]
- Łuczak, J.; Paszkiewicz-Gawron, M.; Krukowska, A.; Malankowska, A.; Zaleska-Medynska, A. Ionic liquids for nano- and microstructures preparation. Part 1: Properties and multifunctional role. Adv. Colloid Interface Sci. 2016, 230, 13–28. [Google Scholar] [CrossRef]
- Yasuda, T.; Watanabe, M. Protic ionic liquids: Fuel cell applications. MRS Bull. 2013, 38, 560–566. [Google Scholar] [CrossRef]
- Mai, N.L.; Ahn, K.; Koo, Y.-M. Methods for recovery of ionic liquids—A review. Process Biochem. 2014, 49, 872–881. [Google Scholar] [CrossRef]
- Esperança, J.; Lop, J.N.C.; Tariq, M.; Santos, L.; Magee, J.; Rebelo, L.P. Volatility of aprotic ionic liquids—A review. J. Chem. Eng. Data 2010, 55, 3–12. [Google Scholar] [CrossRef]
- Grøssereid, I.; Lethesh, K.C.; Venkatraman, V.; Fiksdahl, A. New dual functionalized zwitterions and ionic liquids; Synthesis and cellulose dissolution studies. J. Mol. Liq. 2019, 292, 111353. [Google Scholar] [CrossRef]
- Armand, M.; Endres, F.; MacFarlane, D.; Ohno, H.; Scrosati, B. Ionic-liquid materials for the electrochemical challenges of the future. Nat. Mater. 2009, 8, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Rynkowska, E.; Fatyeyeva, K.; Kujawski, W. Application of polymer-based membranes containing ionic liquids in membrane separation processes: A critical review. Rev. Chem. Eng. 2018, 34, 341–363. [Google Scholar] [CrossRef]
- Vekariya, R.L. A review of ionic liquids: Applications towards catalytic organic transformations. J. Mol. Liq. 2017, 227, 44–60. [Google Scholar] [CrossRef]
- Wilkes, J.S.; Levisky, J.A.; Wilson, R.A.; Hussey, C.L. Dialkylimidazolium chloroaluminate melts: A new class of room-temperature ionic liquids for electrochemistry, spectroscopy and synthesis. Inorg. Chem. 1982, 21, 1263–1264. [Google Scholar] [CrossRef]
- Wilkes, J.S.; Zaworotko, M. Air and water stable 1-ethyl-3-methylimidazolium based ionic liquids. J. Chem. Soc. Chem. Commun. 1992, 13, 965–967. [Google Scholar] [CrossRef]
- Davis, J.H., Jr. Task-specific ionic liquids. Chem. Lett. 2004, 33, 1072–1077. [Google Scholar] [CrossRef]
- Iqbal, B.; Muhammad, N.; Jamal, A.; Ahmad, P.; Khan, Z.U.H.; Rahim, A.; Khan, A.S.; Gonfa, G.; Iqbal, J.; Rehman, I.U. An application of ionic liquid for preparation of homogeneous collagen and alginate hydrogels for skin dressing. J. Mol. Liq. 2017, 243, 720–725. [Google Scholar] [CrossRef]
- Chatterjee, K.; Pathak, A.D.; Lakma, A.; Sharma, C.S.; Sahu, K.K.; Singh, A.K. Synthesis, characterization and application of a non-flammable dicationic ionic liquid in lithium-ion battery as electrolyte additive. Sci. Rep. 2020, 10, 9606. [Google Scholar] [CrossRef]
- Song, M.-H.; Pham, T.P.T.; Yun, Y.-S. Ionic liquid-assisted cellulose coating of chitosan hydrogel beads and their application as drug carriers. Sci. Rep. 2020, 10, 13905. [Google Scholar] [CrossRef]
- Kamble, B.B.; Ajalkar, B.D.; Tawade, A.K.; Sharma, K.K.; Mali, S.S.; Hong, C.K.; Bathula, C.; Kadam, A.N.; Tayade, S.N. Ionic liquid assisted synthesis of h-MoO3 hollow microrods and their application for electrochemical sensing of Imidacloprid pesticide in vegetables. J. Mol. Liq. 2021, 324, 115119. [Google Scholar] [CrossRef]
- Sorkhi, S.E.S.; Hashemi, M.M.; Ezabadi, A. Introduction of a novel dicationic Brönsted acidic ionic liquid based on pyrazine and its application in the synthesis of xanthenediones and 3, 4-dihydropyrimidin-2 (1 H)-ones under solvent-free conditions. Res. Chem. Intermed. 2020, 46, 2229–2246. [Google Scholar] [CrossRef]
- Ferreira, T.A.; Flores-Aguilar, J.F.; Santos, E.M.; Rodriguez, J.A.; Ibarra, I.S. New poly (ionic liquid) based fiber for determination of oxytetracycline in milk samples by application of SPME-CE technique. Molecules 2019, 24, 430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zhao, D.; Chen, G.; Liu, S.; Ji, N.; Ding, H.; Fu, J. Preparation of phosphotungstic acid based poly (ionic liquid) and its application to esterification of palmitic acid. Renew. Energy 2019, 133, 317–324. [Google Scholar] [CrossRef]
- Pillai, P.; Mandal, A. A comprehensive micro scale study of poly-ionic liquid for application in enhanced oil recovery: Synthesis, characterization and evaluation of physicochemical properties. J. Mol. Liq. 2020, 302, 112553. [Google Scholar] [CrossRef]
- Barik, B.; Kumar, A.; Nayak, P.S.; Achary, L.S.K.; Rout, L.; Dash, P. Ionic liquid assisted mesoporous silica-graphene oxide nancomposite synthesis and its application for removal of heavy metal ions from water. Mater. Chem. Phys. 2020, 239, 122028. [Google Scholar] [CrossRef]
- Skorikova, G.; Rauber, D.; Aili, D.; Martin, S.; Li, Q.; Henkensmeier, D.; Hempelmann, R. Protic ionic liquids immobilized in phosphoric acid-doped polybenzimidazole matrix enable polymer electrolyte fuel cell operation at 200 °C. J. Membr. Sci. 2020, 608, 118188. [Google Scholar] [CrossRef]
- Da Trindade, L.G.; Zanchet, L.; Martins, P.C.; Borba, K.M.; Santos, R.D.; Paiva, R.D.S.; Vermeersch, L.A.; Ticianelli, E.A.; de Souza, M.O.; Martini, E.M. The influence of ionic liquids cation on the properties of sulfonated poly (ether ether ketone)/polybenzimidazole blends applied in PEMFC. Polymer 2019, 179, 121723. [Google Scholar] [CrossRef]
- Rajabi, Z.; Javanbakht, M.; Hooshyari, K.; Badiei, A.; Adibi, M. High temperature composite membranes based on polybenzimidazole and dendrimer amine functionalized SBA-15 mesoporous silica for fuel cells. New J. Chem. 2020, 44, 5001–5018. [Google Scholar] [CrossRef]
- Shirota, H.; Mandai, T.; Fukazawa, H.; Kato, T. Comparison between dicationic and monocationic ionic liquids: Liquid density, thermal properties, surface tension, and shear viscosity. J. Chem. Eng. Data 2011, 56, 2453–2459. [Google Scholar] [CrossRef]
- Mishra, A.K.; Kuila, T.; Kim, D.-Y.; Kim, N.H.; Lee, J.H. Protic ionic liquid-functionalized mesoporous silica-based hybrid membranes for proton exchange membrane fuel cells. J. Mater. Chem. 2012, 22, 24366–24372. [Google Scholar] [CrossRef]
- Liu, F.; Wang, S.; Chen, H.; Li, J.; Wang, X.; Mao, T.; Wang, Z. The impact of poly (ionic liquid) on the phosphoric acid stability of polybenzimidazole-base HT-PEMs. Renew. Energy 2021, 163, 1692–1700. [Google Scholar] [CrossRef]
- Atabaki, F.; Yousefi, M.H.; Abdolmaleki, A.; Kalvandi, M. Poly (3, 4-ethylenedioxythiophene): Poly (styrenesulfonic acid) (PEDOT:PSS) conductivity enhancement through addition of imidazolium-ionic liquid derivatives. Polym. Technol. Eng. 2015, 54, 1009–1016. [Google Scholar] [CrossRef]
- Whitten, P.G.; Nealon, D.; Saricilar, S.Z.; Wallace, G.G. Ionic liquid solvated polymer networks for strectchable electronics. Polym. Plast. Technol. Eng. 2015, 54, 310–314. [Google Scholar] [CrossRef]
- Liu, F.; Wang, S.; Chen, H.; Li, J.; Tian, X.; Wang, X.; Mao, T.; Xu, J.; Wang, Z. Cross-linkable polymeric ionic liquid improve phosphoric acid retention and long-term conductivity stability in polybenzimidazole based PEMs. ACS Sustain. Chem. Eng. 2018, 6, 16352–16362. [Google Scholar] [CrossRef]
- Gao, C.; Hu, M.; Wang, L.; Wang, L. Synthesis and properties of phosphoric-acid-doped polybenzimidazole with hyperbranched cross-linkers decorated with imidazolium groups as high-temperature proton exchange membranes. Polymers 2020, 12, 515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Wang, S.; Liu, C.; Li, J.; Liu, F.; Tian, X.; Chen, H.; Mao, T.; Xu, J.; Wang, Z. Cage-like cross-linked membranes with excellent ionic liquid retention and elevated proton conductivity for HT-PEMFCs. Electrochim. Acta 2018, 283, 691–698. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, B.; Zhao, C.; Wang, S.; Zhang, Y.; Bu, F.; Cui, Y.; Li, X.; Na, H. Quaternized poly (ether ether ketone) s doped with phosphoric acid for high-temperature polymer electrolyte membrane fuel cells. J. Mater. Chem. A 2014, 2, 13996–14003. [Google Scholar] [CrossRef]
- Ye, H.; Huang, J.; Xu, J.J.; . Kodiweera, N.K.A.C.; Jayakody, J.R.P.; Greenbaum, S.G. New membranes based on ionic liquids for PEM fuel cells at elevated temperatures. J. Power Sources 2008, 178, 651–660. [Google Scholar] [CrossRef]
- Lin, B.; Qiao, G.; Chu, F.; Zhang, S.; Yuan, N.; Ding, J. Phosphoric acid doped hydrophobic ionic liquid-based composite membranes for anhydrous proton exchange membrane application. RSC Adv. 2017, 7, 1056–1061. [Google Scholar] [CrossRef] [Green Version]
- Mishra, A.K.; Kim, N.H.; Lee, J.H. Effects of ionic liquid-functionalized mesoporous silica on the proton conductivity of acid-doped poly (2, 5-benzimidazole) composite membranes for high-temperature fuel cells. J. Membr. Sci. 2014, 449, 136–145. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, L.; Wang, P.; Zhang, F.; Yu, S.; Shao, Z.; Yi, B. Ionic-liquid-based proton conducting membranes for anhydrous H2/Cl2 fuel-cell applications. ACS Appl. Mater. Interfaces 2014, 6, 3195–3200. [Google Scholar] [CrossRef]
- Pant, R.; Sengupta, S.; Lyulin, A.V.; Venkatnathan, A. Computational investigation of a protic ionic liquid doped poly-benzimidazole fuel cell electrolyte. J. Mol. Liq. 2020, 314, 113686. [Google Scholar] [CrossRef]
- Sen, S.; Goodwin, S.E.; Barbará, P.V.; Rance, G.A.; Wales, D.; Cameron, J.M.; Sans, V.; Mamlouk, M.; Scott, K.; Walsh, D.A. Gel-Polymer electrolytes based on poly(ionic liquid)/ionic liquid networks. ACS Appl. Polym. Mater. 2021, 3, 200–208. [Google Scholar] [CrossRef]
- Anouti, M.; Caillon-Caravanier, M.; Dridi, Y.; Galiano, H.; Lemordant, D. Synthesis and characterization of new pyrrolidinium based protic ionic liquids. good and superionic liquids. J. Phys. Chem. B 2008, 112, 13335–13343. [Google Scholar] [CrossRef] [PubMed]
- Susan, A.B.H.; Noda, A.; Mitsushima, S.; Watanabe, M. Brønsted acid–base ionic liquids and their use as new materials for anhydrous proton conductors. Chem. Commun. 2003, 938–939. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Gondosiswanto, R.; Hibbert, D.B.; Zhao, C. Critical assessment of superbase-derived protic ionic liquids as electrolytes for electrochemical applications. Electrochim. Acta 2019, 298, 413–420. [Google Scholar] [CrossRef]
- Nakamoto, H.; Watanabe, M. Brønsted acid–base ionic liquids for fuel cell electrolytes. Chem. Commun. 2007, 24, 2539–2541. [Google Scholar] [CrossRef]
- Miran, M.S.; Yasuda, T.; Susan, A.B.H.; Dokko, K.; Watanabe, M. Binary protic ionic liquid mixtures as a proton conductor: High fuel cell reaction activity and facile proton transport. J. Phys. Chem. C 2014, 118, 27631–27639. [Google Scholar] [CrossRef]
- Luo, J.; Conrad, O.; Vankelecom, I.F.J. Physicochemical properties of phosphonium-based and ammonium-based protic ionic liquids. J. Mater. Chem. 2012, 22, 20574–20579. [Google Scholar] [CrossRef] [Green Version]
- Nakamoto, H.; Noda, A.; Hayamizu, K.; Hayashi, S.; Hamaguchi, H.-O.; Watanabe, M. Proton-conducting properties of a brønsted acid−base ionic liquid and ionic melts consisting of bi s(trifluoromethanesulfonyl)imide and benzimidazole for fuel cell electrolytes. J. Phys. Chem. C 2007, 111, 1541–1548. [Google Scholar] [CrossRef]
- Yoshizawa-Fujita, M.; Byrne, N.; Forsyth, M.; Macfarlane, D.R.; Ohno, H. Proton transport properties in zwitterion blends with brønsted acids. J. Phys. Chem. B 2010, 114, 16373–16380. [Google Scholar] [CrossRef]
- Brigouleix, C.; Anouti, M.; Jacquemin, J.; Caillon-Caravanier, M.; Galiano, H.; Lemordant, D. Physicochemical characterization of morpholinium cation based protic ionic liquids used as electrolytes. J. Phys. Chem. B 2010, 114, 1757–1766. [Google Scholar] [CrossRef]
- Yoshizawa, M.; Ogihara, W.; Ohno, H. Design of new ionic liquids by neutralization of imidazole derivatives with imide-type acids. Electrochem. Solid-State Lett. 2001, 4, E25–E27. [Google Scholar] [CrossRef]
- Fernicola, A.; Panero, S.; Scrosati, B.; Tamada, M.; Ohno, H. New types of brönsted acid–base ionic liquids-based membranes for applications in PEMFCs. ChemPhysChem 2007, 8, 1103–1107. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Hu, J.; Saak, W.; Beckhaus, R.; Wittstock, G.; Vankelecom, I.F.J.; Agert, C.; Conrad, O. Protic ionic liquid and ionic melts prepared from methanesulfonic acid and 1H-1,2,4-triazole as high temperature PEMFC electrolytes. J. Mater. Chem. 2011, 21, 10426–10436. [Google Scholar] [CrossRef] [Green Version]
- Xiang, J.; Chen, R.; Wu, F.; Li, L.; Chen, S.; Zou, Q. Physicochemical properties of new amide-based protic ionic liquids and their use as materials for anhydrous proton conductors. Electrochim. Acta 2011, 56, 7503–7509. [Google Scholar] [CrossRef]
- Lalia, B.S.; Sekhon, S. Polymer electrolytes containing ionic liquids with acidic counteranion (DMRImH2PO4, R = ethyl, butyl and octyl). Chem. Phys. Lett. 2006, 425, 294–300. [Google Scholar] [CrossRef]
- Che, Q.; Sun, B.; He, R. Preparation and characterization of new anhydrous, conducting membranes based on composites of ionic liquid trifluoroacetic propylamine and polymers of sulfonated poly (ether ether) ketone or polyvinylidenefluoride. Electrochim. Acta 2008, 53, 4428–4434. [Google Scholar] [CrossRef]
- Langevin, D.; Nguyen, Q.T.; Marais, S.; Karademir, S.; Sanchez, J.-Y.; Iojoiu, C.; Martinez, M.; Mercier, R.; Judeinstein, P.; Chappey, C. High-temperature ionic-conducting material: Advanced structure and improved performance. J. Phys. Chem. C 2013, 117, 15552–15561. [Google Scholar] [CrossRef]
- Gao, J.; Liu, J.; Li, W.L.B. Proton exchange membrane fuel cell working at elevated temperature with ionic liquid as electrolyte. Int. J. Electrochem. Sci. 2011, 6, 6115–6122. [Google Scholar]
- Rogalsky, S.; Bardeau, J.-F.; Makhno, S.; Babkina, N.; Tarasyuk, O.; Cherniavska, T.; Orlovska, I.; Kozyrovska, N.; Brovko, O. New proton conducting membrane based on bacterial cellulose/polyaniline nanocomposite film impregnated with guanidinium-based ionic liquid. Polymer 2018, 142, 183–195. [Google Scholar] [CrossRef]
- Guo, J.; Wang, A.; Ji, W.; Zhang, T.; Tang, H.; Zhang, H. Protic ionic liquid-grafted polybenzimidazole as proton conducting catalyst binder for high-temperature proton exchange membrane fuel cells. Polym. Test. 2021, 96, 107066. [Google Scholar] [CrossRef]
- Koyilapu, R.; Singha, S.; Kutcherlapati, S.; Jana, T. Grafting of vinylimidazolium-type poly (ionic liquid) on silica nanoparticle through RAFT polymerization for constructing nanocomposite based PEM. Polymer 2020, 195, 122458. [Google Scholar] [CrossRef]
- Lin, J.; Korte, C. PBI-type polymers and acidic proton conducting ionic liquids—Conductivity and molecular interactions. Fuel Cells 2020, 20, 461–468. [Google Scholar] [CrossRef]
- Nag, A.; Ali, M.A.; Singh, A.; Vedarajan, R.; Matsumi, N.; Kaneko, T. N-boronated polybenzimidazole for composite electrolyte design of highly ion conducting pseudo solid-state ion gel electrolytes with a high Li-transference number. J. Mater. Chem. A 2019, 7, 4459–4468. [Google Scholar] [CrossRef]
- Liu, F.; Wang, S.; Li, J.; Tian, X.; Wang, X.; Chen, H.; Wang, Z. Polybenzimidazole/ionic-liquid-functional silica composite membranes with improved proton conductivity for high temperature proton exchange membrane fuel cells. J. Membr. Sci. 2017, 541, 492–499. [Google Scholar] [CrossRef]
- Kallem, P.; Eguizabal, A.; Mallada, R.; Pina, M.P. Constructing straight polyionic liquid microchannels for fast anhydrous proton transport. ACS Appl. Mater. Interfaces 2016, 8, 35377–35389. [Google Scholar] [CrossRef]
- Hooshyari, K.; Javanbakht, M.; Adibi, M. Novel composite membranes based on dicationic ionic liquid and polybenzimidazole mixtures as strategy for enhancing thermal and electrochemical properties of proton exchange membrane fuel cells applications at high temperature. Int. J. Hydrogen Energy 2016, 41, 10870–10883. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, S.P. Prospects of fuel cell technologies. Natl. Sci. Rev. 2017, 4, 163–166. [Google Scholar] [CrossRef]
- Raja, R.R.S.; Rashmi, W.; Khalid, W.; Wong, W.Y.; Priyanka, J. Recent progress in the development of aromatic polymer-based proton exchange membranes for fuel cell applications. Polymers 2020, 12, 1061. [Google Scholar]




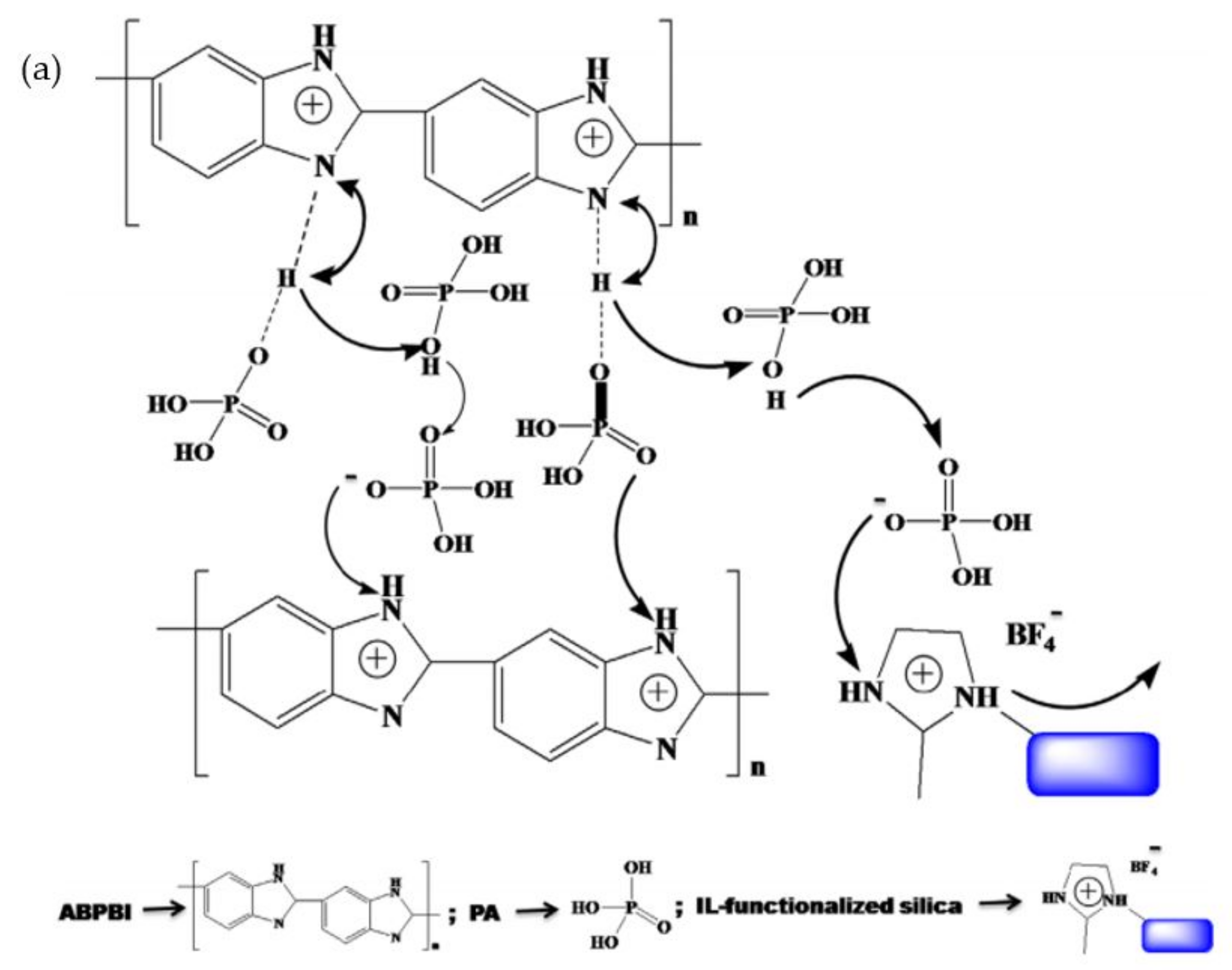
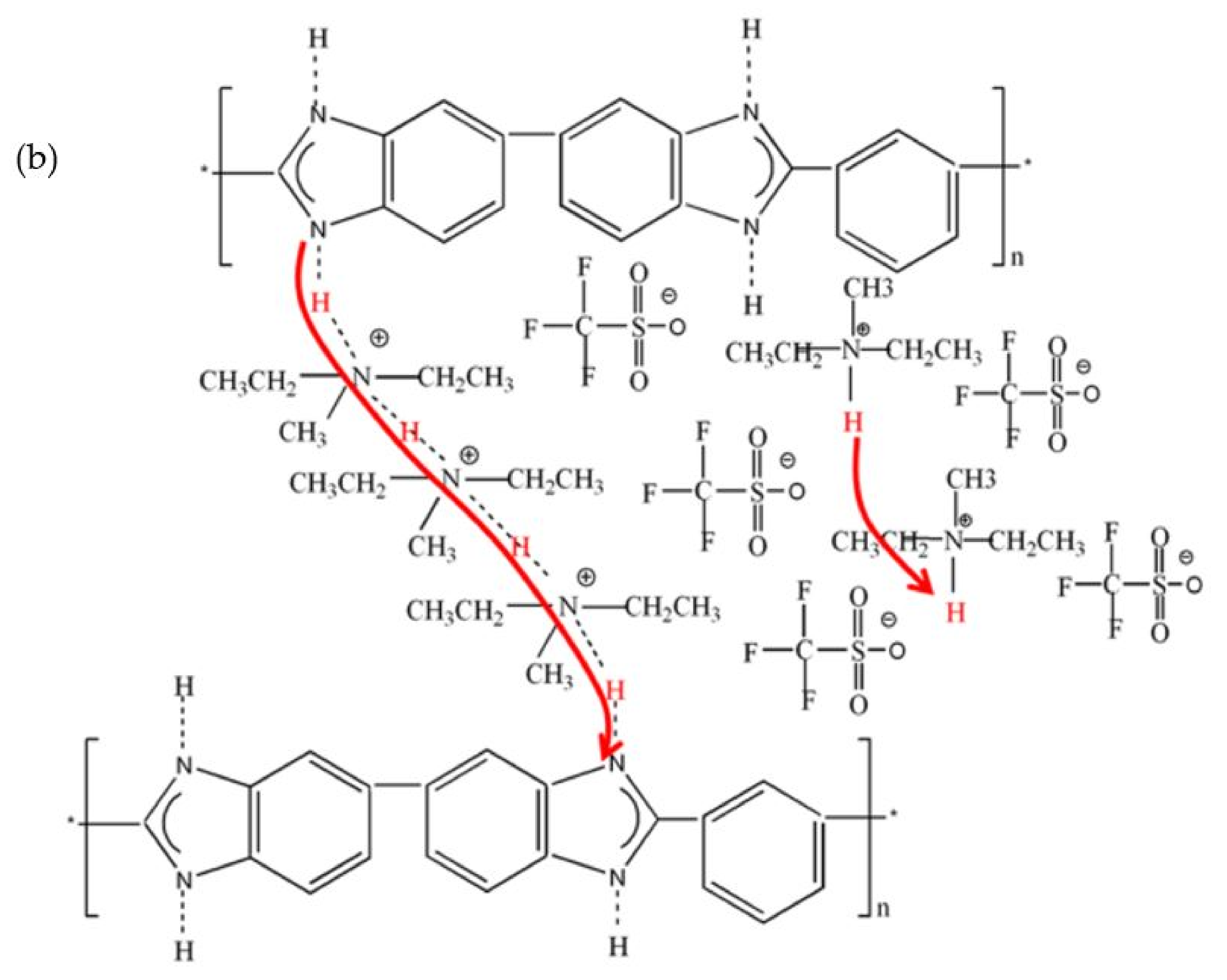

| PBI Polymer | Monomer | Method | Application | Remarks | Ref. |
|---|---|---|---|---|---|
| Sulfonated polybenzimidazole | 3,3′,4,4′-tetraaminobiphenyl (TAB) | Polymerization | Redox flow batteries | Sulfonated polybenzimidazole (s-PBI) gel membrane showed high conductivity (>240 mS/cm) and low degradation during in-cell testing | [42] |
| m and p-PBI | 3,3′-diaminobenzidine | Polymerization | Heavy metal absorbent | p-PBI polymer indicated better performance compared to m-PBI | [43] |
| m and AB-PBI/ZnO | 3,4,-diaminobenzoic | m-PBI/ZnO—doping ZnO in DMAc solution with m-PBI-based powder AB-PBI/ZnO—in situ polymerization of 3,4-diaminobenzoic with zinc nitrate. | Photocatalytic degradation of organic dyes | m-PBI/ZnO performed better as a photocatalyst compared to AB-PBI/ZnO | [44] |
| PBI | 3,3′-diaminobenzidine | Cross-linked polymerization of 4,4-dicarboxydiphenyl ether with 3,3′-diaminobenzidine in phosphorus pentaoxide/methanesulfonic acid | Nanofiltration membranes | The cross-linked PBI membranes exhibited more than 99% retention of Rose Bengal (RB) dye in DMF solvent | [45] |
| m and p-PBI | 3,3′,4,4′-tetraaminobiphenyl (TAB) | Polymerization of TAB with polyphosphoric acid | Electrochemical hydrogen separation | PBI membranes were used as polymer electrolytes for the EHS device Prepared PBI membranes operate the EHS device even in high CO, producing 99.6% purity of hydrogen products with very high power efficiencies (>72%) | [46] |
| PBI-mixed matrix membranes | 3,3′-diaminobenzidine (DAB) | Polymerization of DAB with polyphosphoric acid, followed by a casting process to dope different percentages of zeolite | Gas separation | PBI-MMMs more favorable for separation of CH4 | [47] |
| Asymmetric PHI-HFA hollow fiber | 4,4′-(hexafluoroisopropylidene)bis(benzoic acid) | Dry-jet wet spinning | Hydrogen-selective membrane | Prepared PBI-HFA had higher permselectivities of H2/N2 and H2/CO. Higher hydrogen flow rates were recorded compared to other gas | [48] |
| m/p-PBI copolymer and p-PBI homopolymer | 3,3′,4,4′-tetraaminobiphenyl (TAB) | PPA sol-gel process | Electrochemical hydrogen separation | The proton conductivity and mechanical properties of the polymer depend on the final membrane composition m/p-PBI copolymer exhibited higher creep resistance compared to homopolymer p-PBI m/p-PBI copolymer showed long-term durability and cell recoverability | [49] |
| Filler | Outcomes | Ref. |
|---|---|---|
| CaTiO3 |
| [72] |
| Cerium triphosphonic-isocyanurate (Ce-TOPT) |
| [73] |
| Sulfophenylated TiO2 |
| [74] |
| Phosphonated graphene oxide |
| [75] |
| Graphene oxide |
| [76] |
| Imidazole grapheme oxide (ImGO) and grapheme oxide |
| [68] |
| Multiwall carbon nanotubes (MWCNTs) |
| [77] |
| Zeolitic imidazolate framework |
| [78] |
| UiO-66 metal-organic framework |
| [79] |
| Ionic Liquids | Application | Remarks | Ref. |
|---|---|---|---|
| 1-methylimidazolium | Biopolymer solvent for preparation of collagen-alginate hydrogels | Ionic liquid showed a decent potential for the preparation of collagen and alginate hydrogels | [99] |
| 1,1′-(5,14-dioxo-4,6,13,15-tetraazaoctadecane-1,18-diyl) bis(3-(sec-butyl)-1H-imidazol-3-ium) bis((trifluoromethyl)-sulfonyl) imide | Electrolyte additive in lithium-ion battery | A novel dicationic room temperature ionic liquid showed a remarkable potential to subsitute conventional organic carbonate electrolyte mixture Prepared ionic liquid was safer to use at high operation temperature with no degradation, enhanced battery life, good cycling performance and Coulombic efficiency with better discharge capacities | [100] |
| 1-ethyl-3-methylimidazolium acetate | Solvent | Ionic liquid was employed as a solvent to dissolve chitosan before coating the surface of the chitosan hydrogel beads Simple but effective method for cellulose coating compared to other organic solvents | [101] |
| 1-butyl-3-methylimidazolium bromide | Co-solvent for preparation of h-MoO3 | Ionic liquid is significant for the development of hollow rod-shaped morphology h-MoO3 | [102] |
| [SO3H-Pyrazine-SO3H] Cl | Catalyst | Ionic liquid was prepared accordingly to apply as a catalyst for preparation of xanthenediones and 3,4-dihydropyrimidin-2(1H)-ones under solvent-free conditions Several advantages were achieved, including simplicity in preparing and handling the catalyst genenrality, easy workup procedure, high yields, short reaction times, catalyst can be reused and solvent-free conditions | [103] |
| 1-allyl-3-methylimidazolium chloride | Adsorbent for determination of oxytetracycline in milk sample | A simple, effective, sensitive and environmentally friendly method for determination of oxytetracycline in milk sample via SPME-CE | [104] |
| 1-(4-sulfonate)-butyl-3-vinylimidazolium | Catalyst for esterification pre-treatment | A task-specific zwitterion monomer was synthesized for production of polyzwitterion support for phosphotungstic acid grafting Phosphotungstic acid was able to immobilize in the polymer support through chemical effects, and catalytic performance is superior due to reusability of the catalyst | [105] |
| 1-hexadecyl-3-vinylimidazolium bromide | Chemical agent for oil recovery | Synthesized polyionic liquid (PIL) showed good salt tolerance behavior, thermal stability and wettability alteration ability Core flooding with PIL enhanced >30% of oil recovery after water flooding | [106] |
| 1-butyl-3-methylimidazolium chloride | Green solvent and porogen | Ionic liquid assisted the development of π-π stacking and Van Der Waals interaction toward agglomeration of the grapheme oxide sheet Ionic liquid medium also acted as a porogen to create higher surface area of composite with better active site | [107] |
| Protic Ionic Liquids | Conductivity (mS/cm) | Temperature (°C) | Ref. |
|---|---|---|---|
| Pyrrolidinium nitrate | 50.1 | 25 | [126] |
| Pyrrolidinium hydrogen sulfate | 6.8 | 25 | [126] |
| Pyrrolidinium formate | 32.9 | 25 | [126] |
| Pyrrolidinium acetate | 5.9 | 25 | [126] |
| Pyrrolidinium trifluoroacetate | 16.4 | 25 | [126] |
| Pyrrolidinium octanoate | 0.8 | 25 | [126] |
| Pyrrolidinium bis(trifluoromethanesulfonyl)amide | 39.6 | 130 | [127] |
| 7-methyl-1,5,7-triazabicyclo[4.4.0]dec-5-ene bis(trifluoromethanesulfonyl)imide | 1.54 | 30 | [128] |
| Diethylmethylammonium trifluoromethanesulfonate | 43 | 120 | [129] |
| Diethylmethylammonium hydrogen sulfate | 1.10 | 30 | [130] |
| Diethylmethylammonium bis(trifluoromethanesulfonyl)amide | 7.40 | 30 | [130] |
| Trioctylammonium triflate | 0.0303 | 25 | [131] |
| Benzimidazolium bis(trifluoromethanesulfonyl)imide | 8.3 | 140 | [132] |
| 3-(1-butyl-1H-imidazol-3-ium-3-yl)propane-1- sulfonate 1,1,1-Trifluoro-N-(trifluoromethylsulfonyl) methanesulfoneamide | 1 | 100 | [133] |
| Morpholinium formate | 9.92 | 60 | [134] |
| N-methylmorpholinium formate | 16.77 | 60 | [134] |
| N-ethylmorpholinium formate | 12.17 | 60 | [134] |
| Methylimidazolium bis(trifluoromethanesulfonyl)imide | 7.23 | 25 | [135] |
| 1-methyl-pyrazole N,N- bis(trifluoromethanesulfonyl)imide | 12 | 90 | [136] |
| 1H-1,2,4-triazole/methanesulfonic acid | 149 | 200 | [137] |
| Isobutyramide trifluoromethanesulfonate | 32.6 | 150 | [138] |
| 2,3-dimethyl-1-ethylimidazolium dihydrogenphosphate | 70 | 120 | [139] |
| Trifluoroacetic propylamine | 30 | 180 | [140] |
| Triethylammonium triflate | 31 | 130 | [141] |
| 1-ethyl-3-methylimidazolium hydrogen sulfate | 16 | 85 | [142] |
| N-butylguanidinium tetrafluoroborate | 180 | 180 | [143] |
| Ionic Liquid Type | Outcomes | Conductivity | Ref. |
|---|---|---|---|
| 2-bromo-N,N-dimetylethanamine |
| - | [144] |
| Diethylmethylammonium trifluoromethanesulfonate ([dema][TfO]) 1-ethyl-3-methylimidazolium trifluoromethanesulfonate ([emim][TfO]) 1-methylimidazolium bis(trifluoronethane sulfonyl)imide ([C1Im][NTf2]) 1-(2-Hydroxyethyl)-3-methylimidazolium bis(trifluoromethane sulfonyl)imide (HOemim][ NTf2]) |
| 108.9 mS/cm at 250 °C | [71] |
| Poly(vinylimidazolium) bromide (PVImBr) |
| 0.25 S/cm at 160 °C | [145] |
| 2-sulfoethylmethylammonium triflate [2-Sema][TfO] |
| 10 mS/cm at 100 °C | [146] |
| 1-butyl-3-metylimidazolium bis(trifluoromethane sulfonyl)imide [BMIm][TFSI] |
| 8.8 × 10−3 S/cm at 55 °C | [147] |
| 1-butyl-3-methylimidazolium dihydrogen phosphate (BMI-DHPH) |
| 0.133 S/cm at 160 °C | [118] |
| 1-metylimidazole trimethoxysilan |
| 0.106 S/cm at 170 °C | [148] |
| 1-(3-trimethoxysilylpropyl)-3-methylimidazolium chloride |
| 0.061 S/cm at 180 °C | [82] |
| Poly[1-(3H-imidazolium)ethylene] bis(trifluoromethanesulfonyl)imide |
| 50 mS/cm at 200 °C | [149] |
| 1,6-di(3-methylimidazolium)hexane bis (hexafluorophosphate) 1-butyl-3-methylimidazolium hexafluorophosphate |
| 81 mS/cm at 180 °C | [150] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seng, L.K.; Masdar, M.S.; Shyuan, L.K. Ionic Liquid in Phosphoric Acid-Doped Polybenzimidazole (PA-PBI) as Electrolyte Membranes for PEM Fuel Cells: A Review. Membranes 2021, 11, 728. https://doi.org/10.3390/membranes11100728
Seng LK, Masdar MS, Shyuan LK. Ionic Liquid in Phosphoric Acid-Doped Polybenzimidazole (PA-PBI) as Electrolyte Membranes for PEM Fuel Cells: A Review. Membranes. 2021; 11(10):728. https://doi.org/10.3390/membranes11100728
Chicago/Turabian StyleSeng, Leong Kok, Mohd Shahbudin Masdar, and Loh Kee Shyuan. 2021. "Ionic Liquid in Phosphoric Acid-Doped Polybenzimidazole (PA-PBI) as Electrolyte Membranes for PEM Fuel Cells: A Review" Membranes 11, no. 10: 728. https://doi.org/10.3390/membranes11100728
APA StyleSeng, L. K., Masdar, M. S., & Shyuan, L. K. (2021). Ionic Liquid in Phosphoric Acid-Doped Polybenzimidazole (PA-PBI) as Electrolyte Membranes for PEM Fuel Cells: A Review. Membranes, 11(10), 728. https://doi.org/10.3390/membranes11100728








