Condensing Effect of Cholesterol on hBest1/POPC and hBest1/SM Langmuir Monolayers
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures and hbest1 Purification
2.2. Monolayers Experiments
2.3. Brewster Angle Microscopy Studies
3. Results
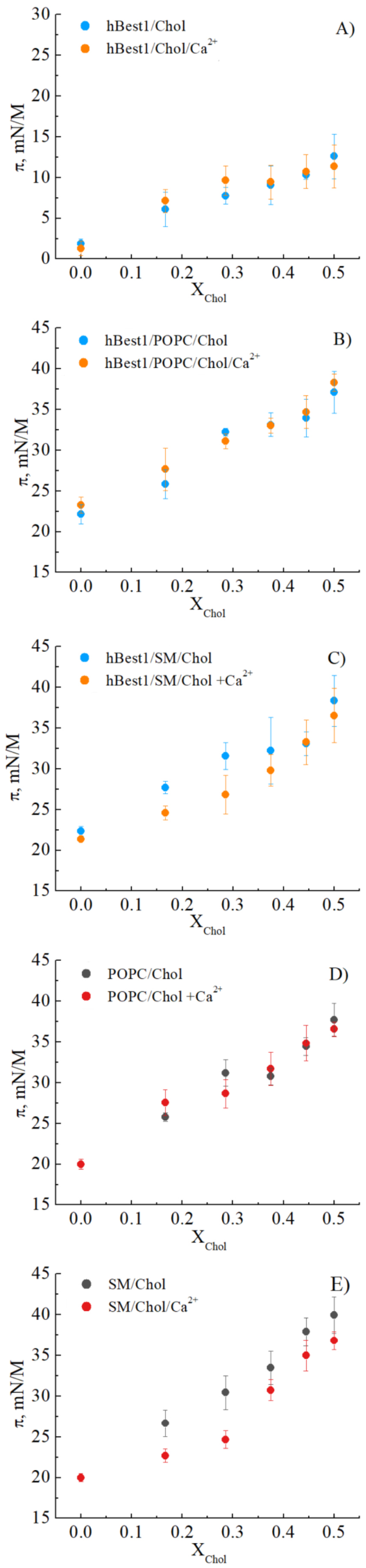
3.1. Condensing Effect of Cholesterol on hbest1, hbest1/POPC and hbest1/SM Monolayers
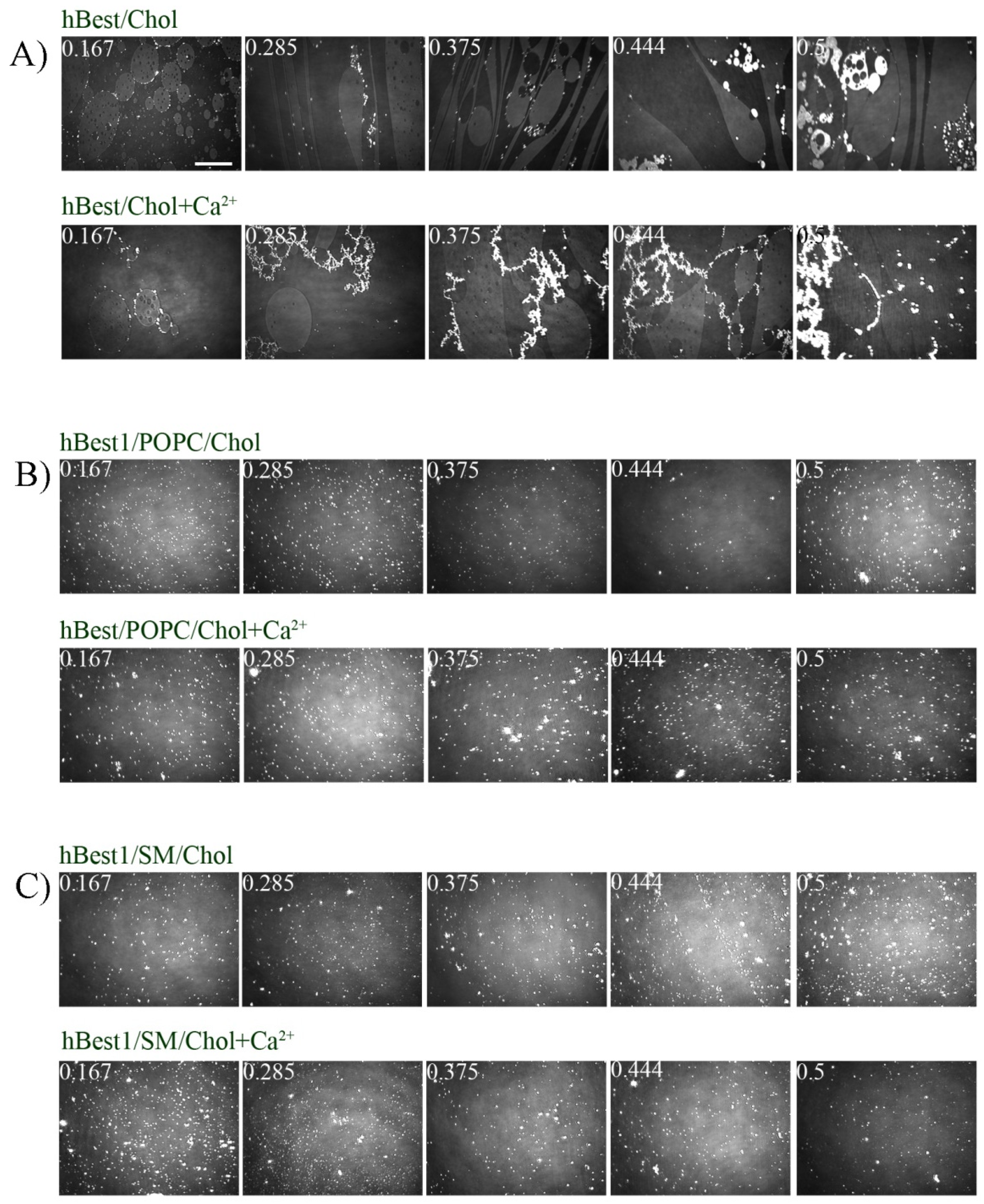
3.2. Morphology of hBest1/Chol, hBest1/POPC/Chol and hBest1/SM/Chol Monolayers
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sun, H.; Tsunenari, T.; Yau, K.-W.; Nathans, J. The vitelliform macular dystrophy protein defines a new family of chloride channels. Proc. Natl. Acad. Sci. USA 2002, 99, 4008–4013. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Yoon, B.-E.; Berglund, K.; Oh, S.-J.; Park, H.; Shin, H.-S.; Augustine, G.J.; Lee, C.J. Channel-Mediated Tonic GABA Release from Glia. Science 2010, 330, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Woo, D.H.; Han, K.-S.; Shim, J.W.; Yoon, B.-E.; Kim, E.; Bae, J.Y.; Oh, S.-J.; Hwang, E.M.; Marmorstein, A.D.; Bae, Y.C.; et al. TREK-1 and Best1 Channels Mediate Fast and Slow Glutamate Release in Astrocytes upon GPCR Activation. Cell 2012, 151, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Burgess, R.; Millar, I.D.; Leroy, B.P.; Urquhart, J.E.; Fearon, I.M.; De Baere, E.; Brown, P.D.; Robson, A.G.; Wright, G.A.; Kestelyn, P.; et al. Biallelic Mutation of BEST1 Causes a Distinct Retinopathy in Humans. Am. J. Hum. Genet. 2008, 82, 19–31. [Google Scholar] [CrossRef]
- Hartzell, H.C.; Qu, Z.; Yu, K.; Xiao, Q.; Chien, L.-T. Molecular Physiology of Bestrophins: Multifunctional Membrane Proteins Linked to Best Disease and Other Retinopathies. Physiol. Rev. 2008, 88, 639–672. [Google Scholar] [CrossRef]
- Marmorstein, A.D.; Cross, H.E.; Peachey, N.S. Functional roles of bestrophins in ocular epithelia. Prog. Retin. Eye Res. 2009, 28, 206–226. [Google Scholar] [CrossRef]
- Boon, C.J.; Klevering, B.J.; Leroy, B.P.; Hoyng, C.B.; Keunen, J.E.; Hollander, A.I.D. The spectrum of ocular phenotypes caused by mutations in the BEST1 gene. Prog. Retin. Eye Res. 2009, 28, 187–205. [Google Scholar] [CrossRef]
- Querques, G.; Zerbib, J.; Santacroce, R.; Margaglione, M.; Delphin, N.; Rozet, J.-M.; Kaplan, J.; Martinelli, D.; Noci, N.D.; Soubrane, G.; et al. Functional and clinical data of Best vitelliform macular dystrophy patients with mutations in the BEST1 gene. Mol. Vis. 2009, 15, 2960–2972. [Google Scholar]
- Mohler, C.W.; Fine, S.L. Long-term evaluation of patients with Best’s vitelliform dystrophy. Ophthalmology 1981, 88, 688–692. [Google Scholar] [CrossRef]
- Clemett, R. Vitelliform dystrophy: Long-term observations on New Zealand pedigrees. Aust. N. Z. J. Ophthalmol. 1991, 19, 221–227. [Google Scholar] [CrossRef]
- Seddon, J. Assessment of mutations in the best macular dystrophy (VMD2) gene in patients with adult-onset foveomacular vitelliform dystrophy, age-related maculopathy, and bull’s-eye maculopathy. Ophthalmology 2001, 108, 2060–2067. [Google Scholar] [CrossRef]
- Renner, A.B.; Tillack, H.; Kraus, H.; Krämer, F.; Mohr, N.; Weber, B.H.; Foerster, M.H.; Kellner, U. Late Onset is Common in Best Macular Dystrophy Associated with VMD2 Gene Mutations. Ophthalmology 2005, 112, 586–592.e2. [Google Scholar] [CrossRef] [PubMed]
- Wabbels, B.; Preising, M.N.; Kretschmann, U.; Demmler, A.; Lorenz, B. Genotype-phenotype correlation and longitudinal course in ten families with Best vitelliform macular dystrophy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2006, 244, 1453–1466. [Google Scholar] [CrossRef] [PubMed]
- Lindblom, G.; Orädd, G. Lipid lateral diffusion and membrane heterogeneity. Biochim. Biophys. Acta (BBA) Biomembr. 2009, 1788, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Bagatolli, L.A.; Ipsen, J.H.; Simonsen, A.C.; Mouritsen, O.G. An outlook on organization of lipids in membranes: Searching for a realistic connection with the organization of biological membranes. Prog. Lipid Res. 2010, 49, 378–389. [Google Scholar] [CrossRef]
- Brown, D.; London, E. Structure and Origin of Ordered Lipid Domains in Biological Membranes. J. Membr. Biol. 1998, 164, 103–114. [Google Scholar] [CrossRef]
- Somerharju, P.; Virtanen, J.A.; Cheng, K.H. Lateral organisation of membrane lipids. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 1999, 1440, 32–48. [Google Scholar] [CrossRef]
- Todeschini, A.R.; Hakomori, S.-I. Functional role of glycosphingolipids and gangliosides in control of cell adhesion, motility, and growth, through glycosynaptic microdomains. Biochim. Biophys. Acta (BBA) Gen. Subj. 2008, 1780, 421–433. [Google Scholar] [CrossRef]
- Ramstedt, B.; Slotte, J.P. Sphingolipids and the formation of sterol-enriched ordered membrane domains. Biochim. Biophys. Acta (BBA) Biomembr. 2006, 1758, 1945–1956. [Google Scholar] [CrossRef]
- Lingwood, D.; Kaiser, H.-J.; Levental, I.; Simons, K. Lipid rafts as functional heterogeneity in cell membranes. Biochem. Soc. Trans. 2009, 37, 955–960. [Google Scholar] [CrossRef]
- Ohvo-Rekilä, H. Cholesterol interactions with phospholipids in membranes. Prog. Lipid Res. 2002, 41, 66–97. [Google Scholar] [CrossRef]
- Zhang, Z.; Bhide, S.Y.; Berkowitz, M.L. Molecular Dynamics Simulations of Bilayers Containing Mixtures of Sphingomyelin with Cholesterol and Phosphatidylcholine with Cholesterol. J. Phys. Chem. B 2007, 111, 12888–12897. [Google Scholar] [CrossRef] [PubMed]
- Ipsen, J.H.; Karlström, G.; Mourtisen, O.; Wennerström, H.; Zuckermann, M. Phase equilibria in the phosphatidylcholine-cholesterol system. Biochim. Biophys. Acta (BBA) Biomembr. 1987, 905, 162–172. [Google Scholar] [CrossRef]
- Simons, K.; Ikonen, E. Functional rafts in cell membranes. Nature 1997, 387, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Bretscher, M.S.; Munro, S. Cholesterol and the Golgi apparatus. Science 1993, 261, 1280–1281. [Google Scholar] [CrossRef]
- Demel, R.; Bruckdorfer, K.; Van Deenen, L. Structural requirements of sterols for the interaction with lecithin at the air-water interface. Biochim. Biophys. Acta (BBA) Biomembr. 1972, 255, 311–320. [Google Scholar] [CrossRef]
- Demel, R.; Van Kessel, W.G.; Van Deenen, L. The properties of polyunsaturated lecithins in monolayers and liposomes and the interactions of these lecithins with cholesterol. Biochim. Biophys. Acta (BBA) Biomembr. 1972, 266, 26–40. [Google Scholar] [CrossRef]
- Yeagle, P.L. Cholesterol and related sterols: Roles in membrane structure and function. In The Membranes of Cells, 3rd ed.; Yeagle, P.L., Ed.; Academic Press: Boston, MA, USA, 2016; Chapter 9; pp. 189–218. [Google Scholar]
- Boesze-Battaglia, K.; Albert, A.D. Cholesterol modulation of photoreceptor function in bovine retinal rod outer segments. J. Biol. Chem. 1990, 265, 20727–20730. [Google Scholar] [CrossRef]
- Mladenova, K.; Moskova-Doumanova, V.; Tabashka, I.; Petrova, S.; Lalchev, Z.; Doumanov, J. Establishment and characterization of stably transfected mdck cell line, expressing hbest1 protein. Bulg. J. Agric. Sci. 2013, 19, 159–162. [Google Scholar]
- Mladenova, K.; Petrova, S.; Georgiev, G.A.; Moskova-Doumanova, V.; Lalchev, Z.; Doumanov, J. Interaction of Bestrophin-1 with 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) in surface films. Colloids Surf. B Biointerfaces 2014, 122, 432–438. [Google Scholar] [CrossRef]
- Smith, P.; Krohn, R.; Hermanson, G.; Mallia, A.; Gartner, F.; Provenzano, M.; Fujimoto, E.; Goeke, N.; Olson, B.; Klenk, D. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Mladenova, K.; Petrova, S.; Andreeva, T.D.; Moskova-Doumanova, V.; Topouzova-Hristova, T.; Kalvachev, Y.; Balashev, K.; Bhattacharya, S.S.; Chakarova, C.; Lalchev, Z.; et al. Effects of Ca2+ ions on bestrophin-1 surface films. Colloids Surf. B Biointerfaces 2017, 149, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Dupuy, A.D.; Engelman, D.M. Protein area occupancy at the center of the red blood cell membrane. Proc. Natl. Acad. Sci. USA 2008, 105, 2848–2852. [Google Scholar] [CrossRef]
- Mangiarotti, A.; Galassi, V.V.; Puentes, E.N.; Oliveira, R.G.; Del Pópolo, M.G.; Wilke, N. Hopanoids Like Sterols Form Compact but Fluid Films. Langmuir 2019. [Google Scholar] [CrossRef]
- Jurak, M. Thermodynamic Aspects of Cholesterol Effect on Properties of Phospholipid Monolayers: Langmuir and Langmuir–Blodgett Monolayer Study. J. Phys. Chem. B 2013, 117, 3496–3502. [Google Scholar] [CrossRef]
- Andreeva, T.D.; Petrova, S.; Mladenova, K.; Moskova-Doumanova, V.; Topouzova-Hristova, T.; Petseva, Y.; Mladenov, N.; Balashev, K.; Lalchev, Z.; Doumanov, J. Effects of Ca2+, Glu and GABA on hBest1 and composite hBest1/POPC surface films. Colloids Surf. B Biointerfaces 2018, 161, 192–199. [Google Scholar] [CrossRef]
- Rujoi, M.; Borchman, D.; Dupré, D.B.; Yappert, M. Interactions of Ca2+ with Sphingomyelin and Dihydrosphingomyelin. Biophys. J. 2002, 82, 3096–3104. [Google Scholar] [CrossRef]
- Feng, R.-J.; Lin, L.; Li, Y.-Y.; Liu, M.-H.; Guo, Y.; Zhang, Z. Effect of Ca2+ to Sphingomyelin Investigated by Sum Frequency Generation Vibrational Spectroscopy. Biophys. J. 2017, 112, 2173–2183. [Google Scholar] [CrossRef] [PubMed]
- Del Castillo-Santaella, T.; Maldonado-Valderrama, J.; Faraudo, J.; Martín-Molina, A. Specific Ion Effects in Cholesterol Monolayers. Materials 2016, 9, 340. [Google Scholar] [CrossRef]
- Mladenov, N.; Petrova, S.D.; Mladenova, K.; Bozhinova, D.; Moskova-Doumanova, V.; Topouzova-Hristova, T.; Videv, P.; Veleva, R.; Kostadinova, A.; Staneva, G.; et al. Miscibility of hBest1 and sphingomyelin in surface films—A prerequisite for interaction with membrane domains. Colloids Surf. B Biointerfaces 2020, 189, 110893. [Google Scholar] [CrossRef]
- Wydro, P. Sphingomyelin/phosphatidylcholine/cholesterol monolayers—Analysis of the interactions in model membranes and Brewster Angle Microscopy experiments. Colloids Surf. B Biointerfaces 2012, 93, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.F.; Caseli, L.; Conde, J.M.; Dynarowicz-Łątka, P. New look for an old molecule—Solid/solid phase transition in cholesterol monolayers. Chem. Phys. Lipids 2019, 225, 104819. [Google Scholar] [CrossRef] [PubMed]
- Fantini, J.; Barrantes, F.J. How cholesterol interacts with membrane proteins: An exploration of cholesterol-binding sites including CRAC, CARC, and tilted domains. Front. Physiol. 2013, 4, 31. [Google Scholar] [CrossRef]
- Lee, A.G. A Database of Predicted Binding Sites for Cholesterol on Membrane Proteins, Deep in the Membrane. Biophys. J. 2018, 115, 522–532. [Google Scholar] [CrossRef]
- Mouritsen, O.G.; Zuckermann, M.J. What’s so special about cholesterol? Lipids 2004, 39, 1101–1113. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, R.F.M.; Fedorov, A.; Prieto, M. Sphingomyelin/Phosphatidylcholine/Cholesterol Phase Diagram: Boundaries and Composition of Lipid Rafts. Biophys. J. 2003, 85, 2406–2416. [Google Scholar] [CrossRef]
- Veatch, S.L.; Keller, S.L. Organization in Lipid Membranes Containing Cholesterol. Phys. Rev. Lett. 2002, 89, 268101. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Videv, P.; Mladenov, N.; Andreeva, T.; Mladenova, K.; Moskova-Doumanova, V.; Nikolaev, G.; Petrova, S.D.; Doumanov, J.A. Condensing Effect of Cholesterol on hBest1/POPC and hBest1/SM Langmuir Monolayers. Membranes 2021, 11, 52. https://doi.org/10.3390/membranes11010052
Videv P, Mladenov N, Andreeva T, Mladenova K, Moskova-Doumanova V, Nikolaev G, Petrova SD, Doumanov JA. Condensing Effect of Cholesterol on hBest1/POPC and hBest1/SM Langmuir Monolayers. Membranes. 2021; 11(1):52. https://doi.org/10.3390/membranes11010052
Chicago/Turabian StyleVidev, Pavel, Nikola Mladenov, Tonya Andreeva, Kirilka Mladenova, Veselina Moskova-Doumanova, Georgi Nikolaev, Svetla D. Petrova, and Jordan A. Doumanov. 2021. "Condensing Effect of Cholesterol on hBest1/POPC and hBest1/SM Langmuir Monolayers" Membranes 11, no. 1: 52. https://doi.org/10.3390/membranes11010052
APA StyleVidev, P., Mladenov, N., Andreeva, T., Mladenova, K., Moskova-Doumanova, V., Nikolaev, G., Petrova, S. D., & Doumanov, J. A. (2021). Condensing Effect of Cholesterol on hBest1/POPC and hBest1/SM Langmuir Monolayers. Membranes, 11(1), 52. https://doi.org/10.3390/membranes11010052




