Quantifying Charge Effects on Fouling Layer Strength and (Ir)Removability during Cross-Flow Microfiltration
Abstract
1. Introduction
2. Materials and Methods
2.1. Microparticle Synthesis
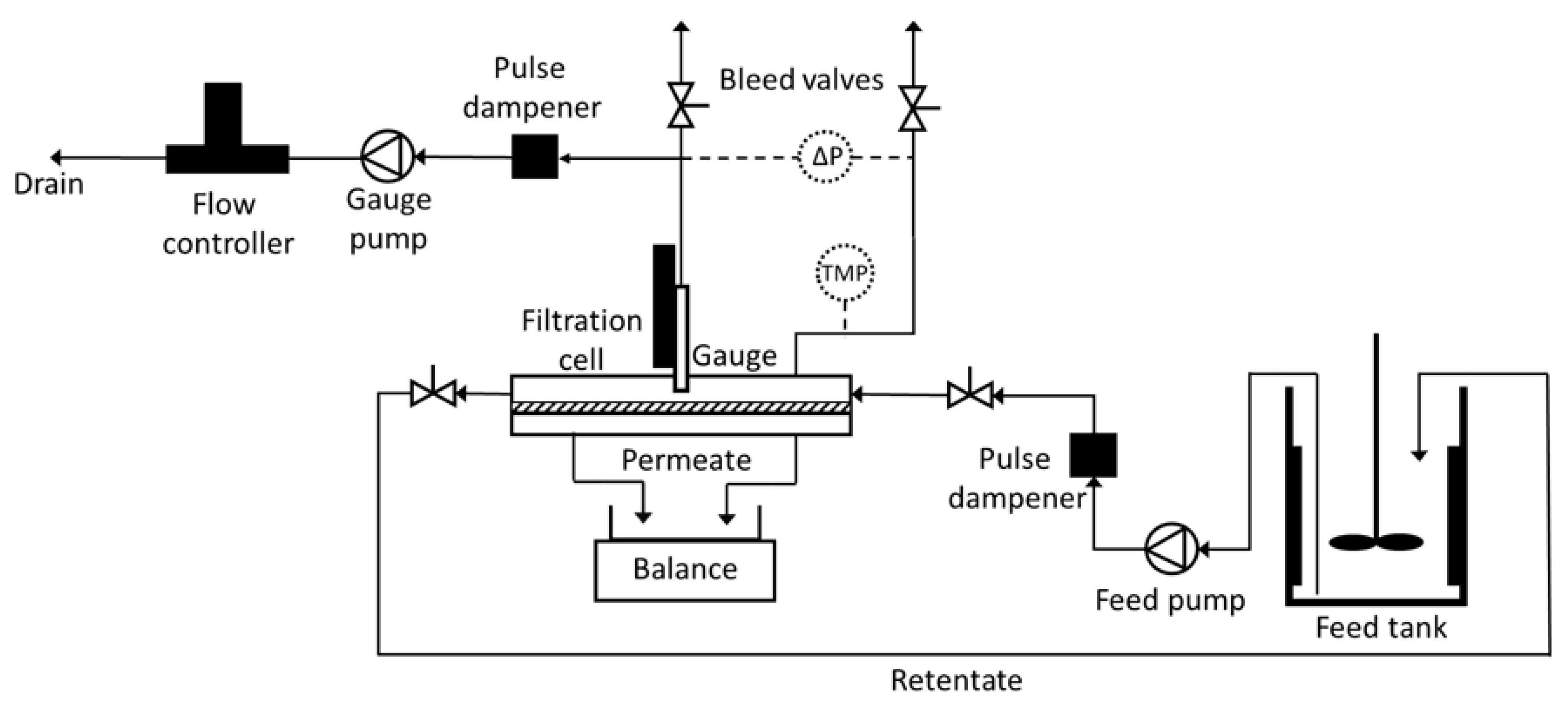
2.2. FDG Cross-Flow Filtration Rig
2.3. FDG Filtration Experiments
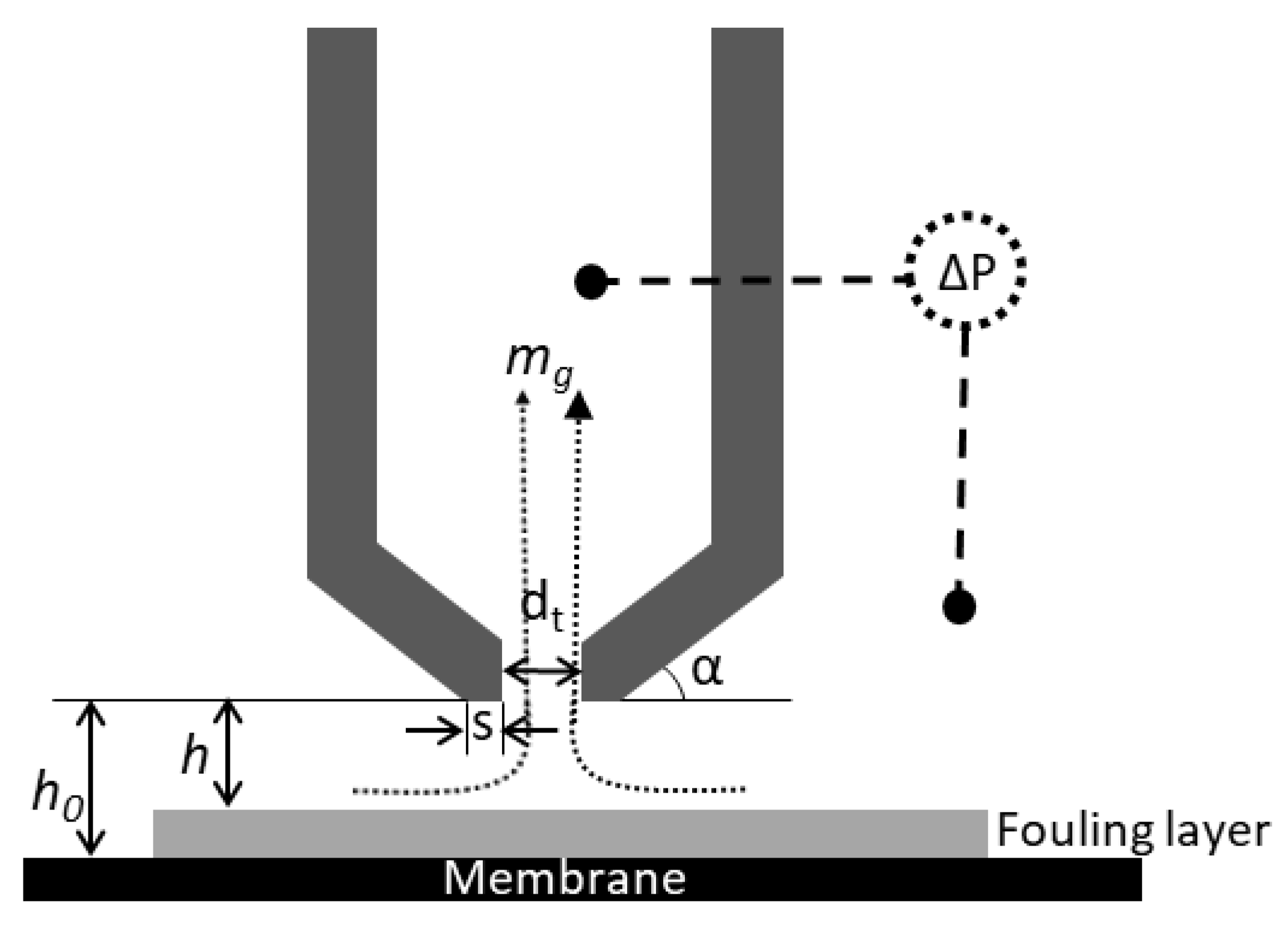
2.4. Analyses
3. Results
3.1. Characterisation of the Suspension
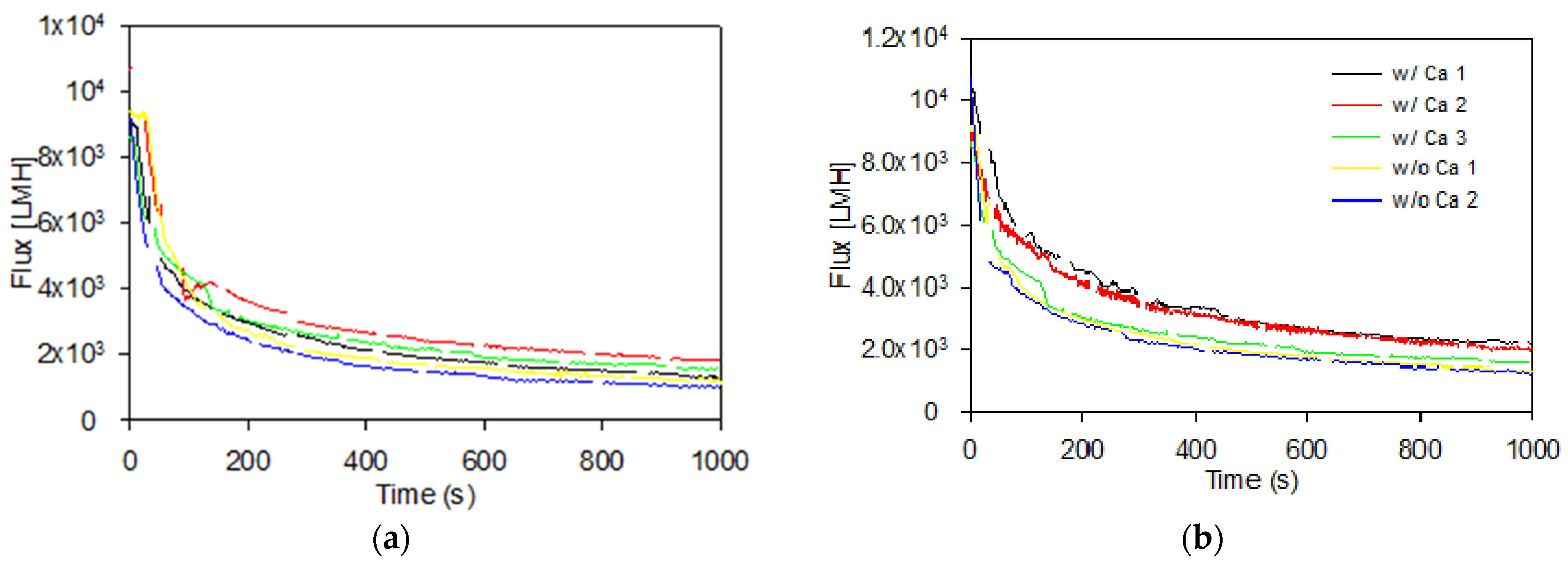
3.2. Formation of Cake Layers
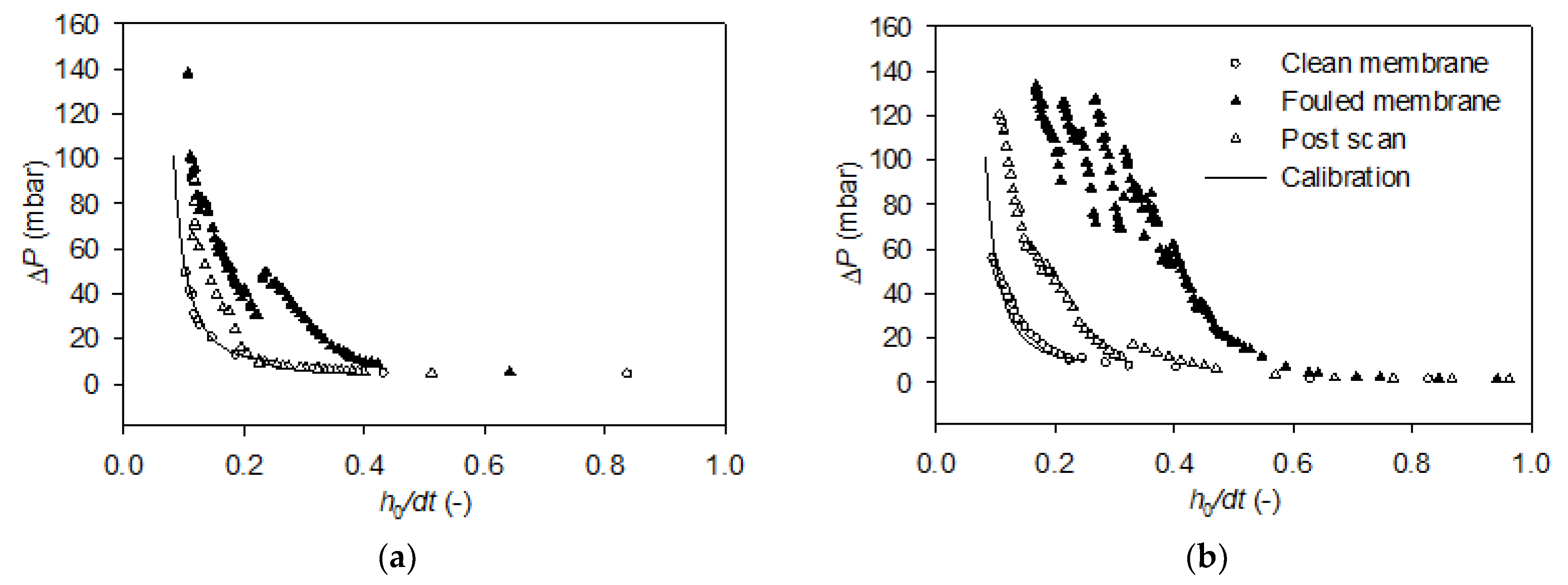
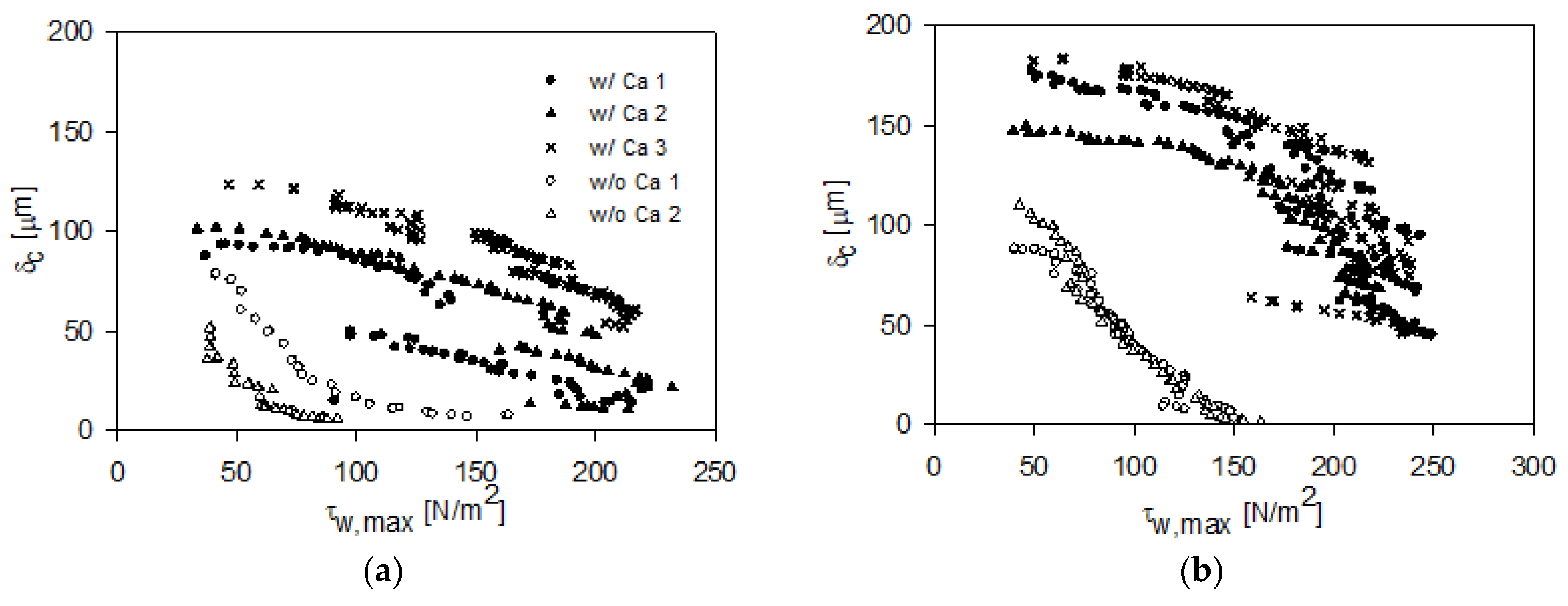
3.3. FDG Analysis of Cake Fouling Layers
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
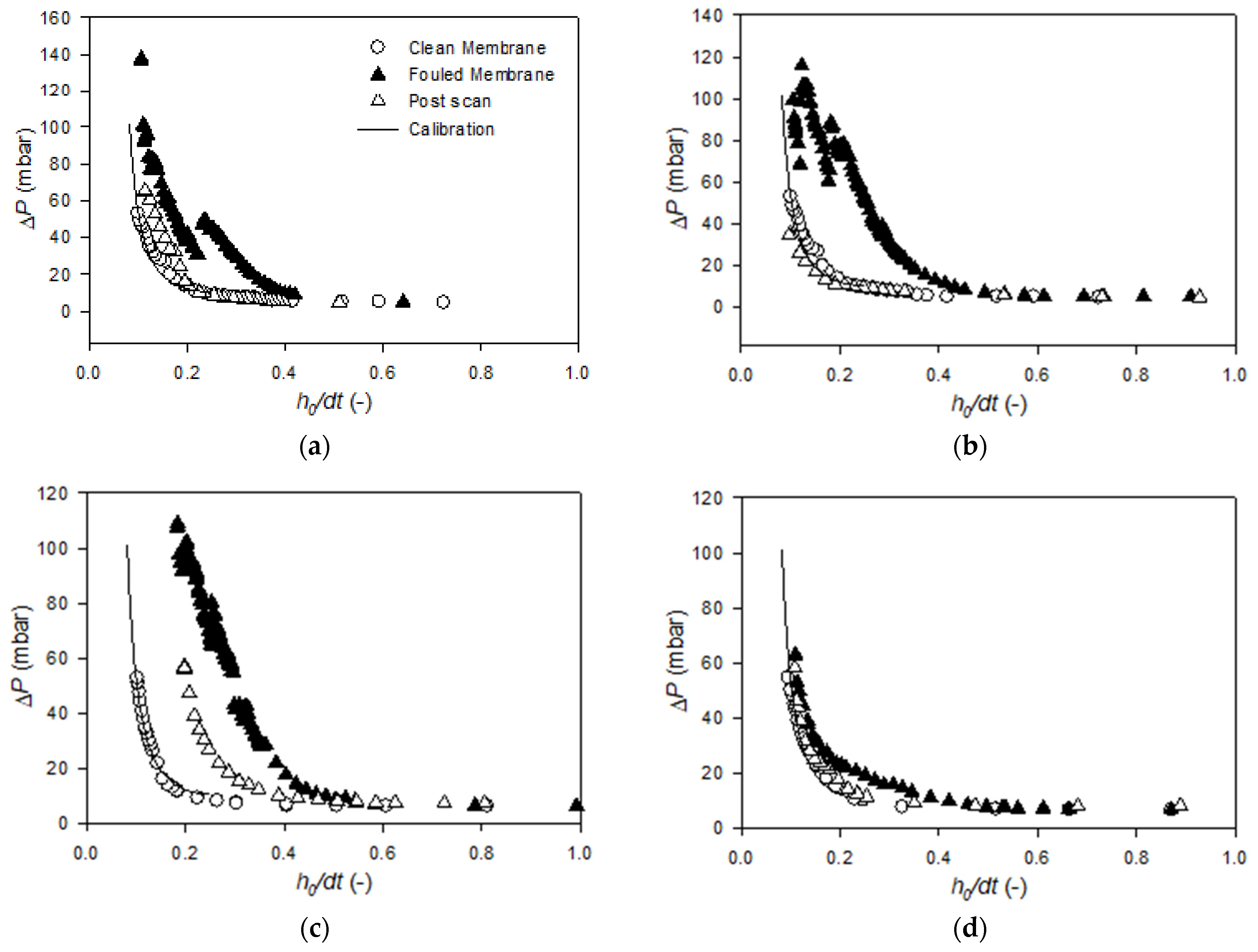
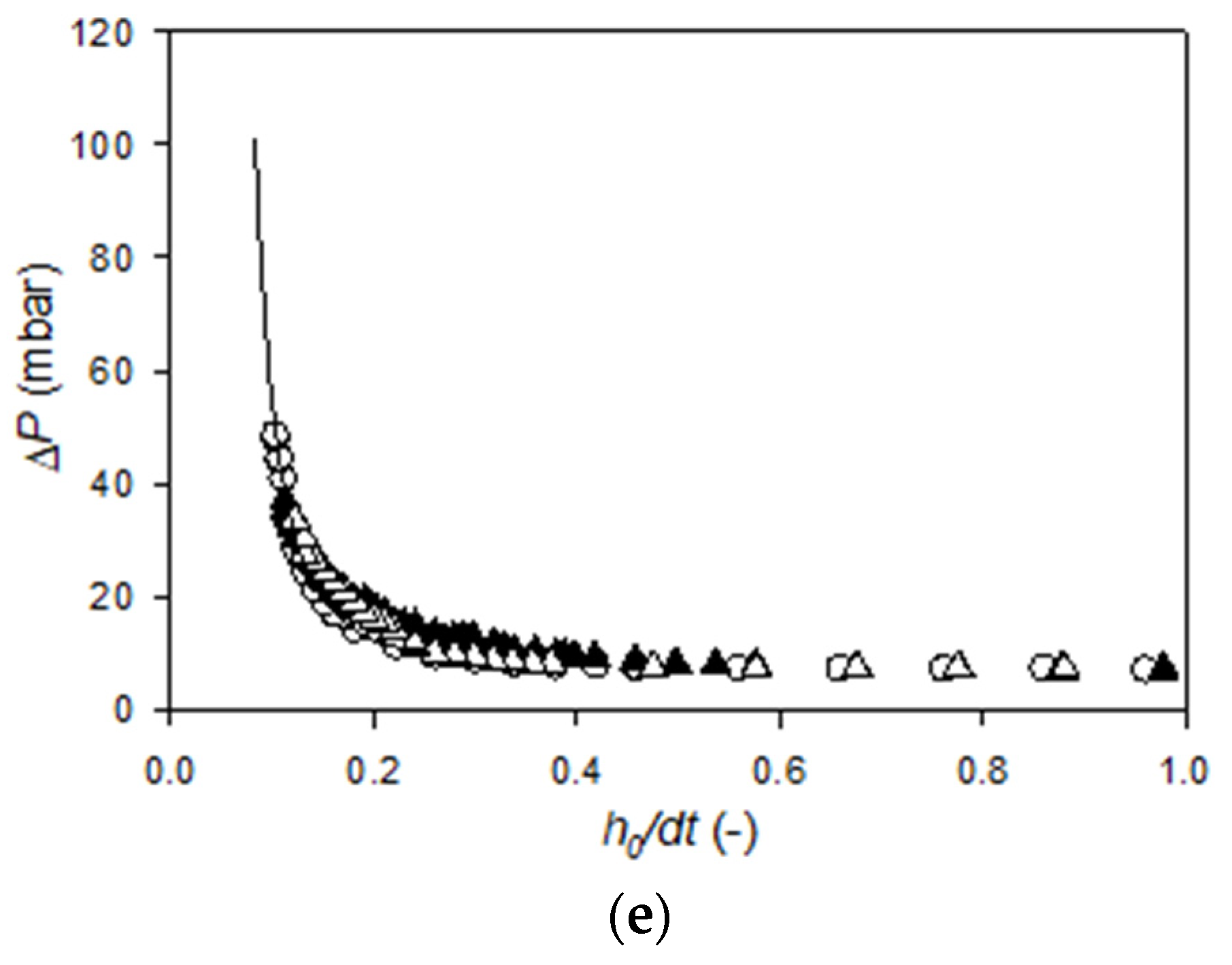
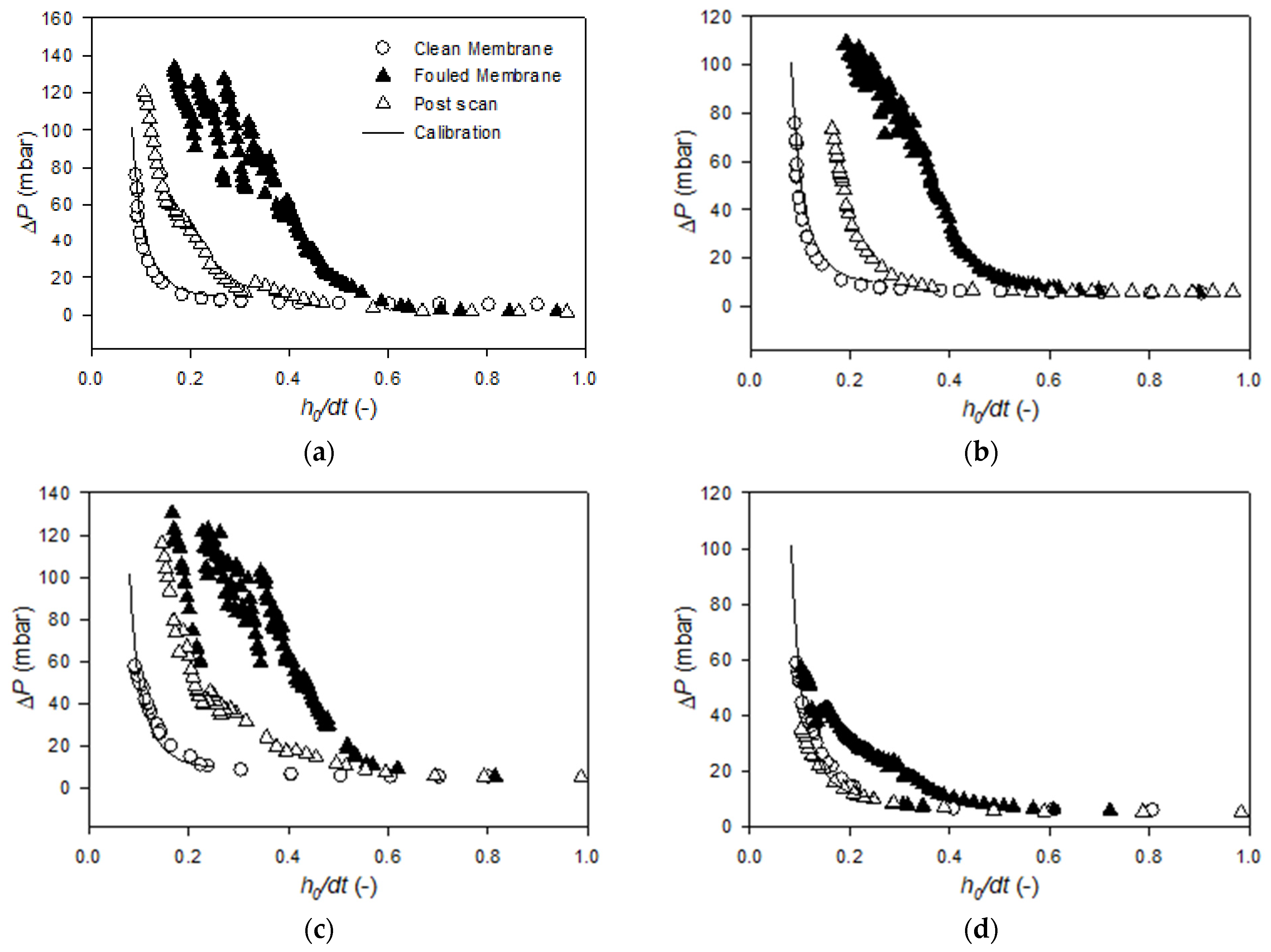
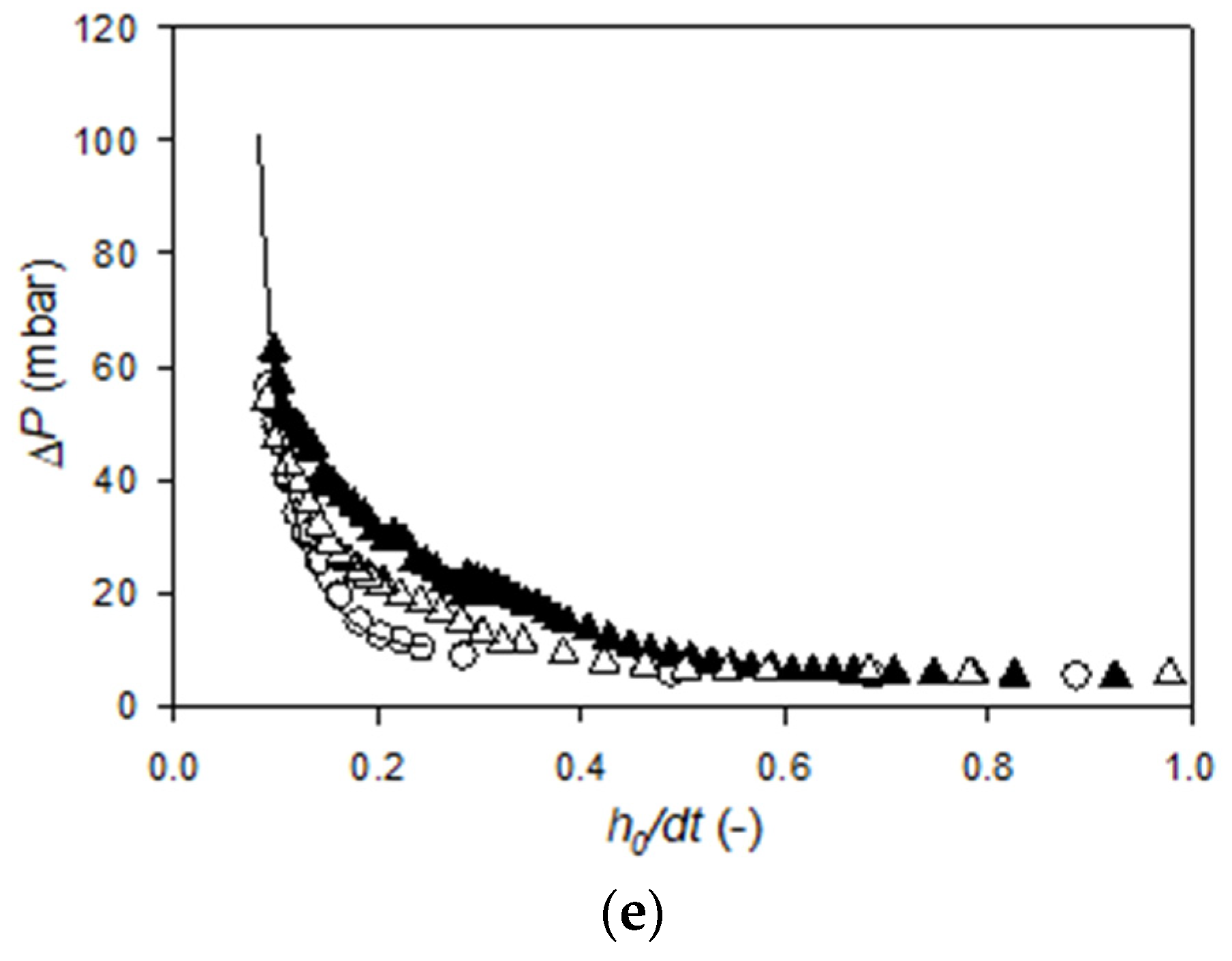
Appendix B
| Zeta Potential (mV) | Particle Diameter (µm) | |||||||
|---|---|---|---|---|---|---|---|---|
| 5 mM NaOH | 5 mM NaOH + 22.5 mM NaCl | 5 mM NaOH | 5 mM NaOH + 22.5 mM NaCl | |||||
| D10 | Median | D90 | D10 | Median | D90 | |||
| Low Charge | −30.6 ± 1.0 | −27.7 ± 0.8 | 1.27 | 1.55 | 1.97 | 1.15 | 1.39 | 1.63 |
| High Charge | −39.4 ± 0.9 | −38.7 ± 1.9 | 1.27 | 1.61 | 4.39 | 1.11 | 1.58 | 2.05 |
References
- Chen, Z.; Luo, J.; Hang, X.; Wan, Y. Physicochemical characterization of tight nanofiltration membranes for dairy wastewater treatment. J. Memb. Sci. 2018, 547, 51–63. [Google Scholar] [CrossRef]
- Hausmann, A.; Sanciolo, P.; Vasiljevic, T.; Weeks, M.; Schroën, K.; Gray, S.; Duke, M. Fouling of dairy components on hydrophobic polytetrafluoroethylene (PTFE) membranes for membrane distillation. J. Memb. Sci. 2013, 442, 149–159. [Google Scholar] [CrossRef]
- Kowalik-Klimczak, A.; Stanisławek, E. Reclamation of water from dairy wastewater using polymeric nanofiltration membranes. Desalin. Water Treat. 2018, 128, 364–371. [Google Scholar] [CrossRef]
- Werkneh, A.A.; Beyene, H.D.; Osunkunle, A.A. Recent advances in brewery wastewater treatment; approaches for water reuse and energy recovery: A review. Environ. Sustain. 2019, 2, 199–209. [Google Scholar] [CrossRef]
- Hube, S.; Eskafi, M.; Hrafnkelsdóttir, K.F.; Bjarnadóttir, B.; Bjarnadóttir, M.Á.; Axelsdóttir, S.; Wu, B. Direct membrane filtration for wastewater treatment and resource recovery: A review. Sci. Total Environ. 2020, 710, 136375. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, Y.; Feng, Z.; Rui, X.; Zhang, T.; Zhang, Z. A review on reverse osmosis and nanofiltration membranes for water purification. Polymers 2019, 11, 1252. [Google Scholar] [CrossRef]
- Guo, W.; Ngo, H.H.; Li, J. A mini-review on membrane fouling. Bioresour. Technol. 2012, 122, 27–34. [Google Scholar] [CrossRef]
- Meng, F.; Chae, S.R.; Drews, A.; Kraume, M.; Shin, H.S.; Yang, F. Recent advances in membrane bioreactors (MBRs): Membrane fouling and membrane material. Water Res. 2009, 43, 1489–1512. [Google Scholar] [CrossRef]
- Drews, A. Membrane fouling in membrane bioreactors-Characterisation, contradictions, cause and cures. J. Memb. Sci. 2010, 363, 1–28. [Google Scholar] [CrossRef]
- Huang, C.; Lin, J.; Lee, W.; Pan, J.R.; Zhao, B. Effect of coagulation mechanism on membrane permeability in coagulation-assisted microfiltration for spent filter backwash water recycling. Colloids Surf. A Physicochem. Eng. Asp. 2011, 378, 72–78. [Google Scholar] [CrossRef]
- Shi, X.; Tal, G.; Hankins, N.P.; Gitis, V. Fouling and cleaning of ultrafiltration membranes: A review. J. Water Process Eng. 2014, 1, 121–138. [Google Scholar] [CrossRef]
- Zhou, M.; Sandström, H.; Belioka, M.; Pettersson, T.; Mattsson, T. Investigation of the cohesive strength of membrane fouling layers formed during cross-flow microfiltration: The effects of pH adjustment on the properties and fouling characteristics of microcrystalline cellulose. Chem. Eng. Res. Des. 2019, 149, 52–64. [Google Scholar] [CrossRef]
- Lorenzen, S.; Ye, Y.; Chen, V.; Christensen, M.L. Direct observation of fouling phenomena during cross-flow filtration: Influence of particle surface charge. J. Memb. Sci. 2016, 510, 546–558. [Google Scholar] [CrossRef]
- Loulergue, P.; André, C.; Laux, D.; Ferrandis, J.-Y.; Guigui, C.; Cabassud, C. In-situ characterization of fouling layers: Which tool for which measurement? Desalin. Water Treat. 2011, 34, 156–162. [Google Scholar] [CrossRef]
- Lu, X.; Kujundzic, E.; Mizrahi, G.; Wang, J.; Cobry, K.; Peterson, M.; Gilron, J.; Greenberg, A.R. Ultrasonic sensor control of flow reversal in RO desalination-Part 1: Mitigation of calcium sulfate scaling. J. Memb. Sci. 2012, 419–420, 20–32. [Google Scholar] [CrossRef]
- Jørgensen, M.K.; Kujundzic, E.; Greenberg, A.R. Effect of pressure on fouling of microfiltration membranes by activated sludge. Desalin. Water Treat. 2016, 57, 6159–6171. [Google Scholar] [CrossRef]
- Ripperger, S.; Altmann, J. Crossflow microfiltration—State of the art. Sep. Purif. Technol. 2002, 26, 19–31. [Google Scholar] [CrossRef]
- Chen, V.; Li, H.; Fane, A.G. Non-invasive observation of synthetic membrane processes—A review of methods. J. Memb. Sci. 2004, 241, 23–44. [Google Scholar] [CrossRef]
- Kujundzic, E.; Greenberg, A.R.; Fong, R.; Hernandez, M. Monitoring protein fouling on polymeric membranes using ultrasonic frequency-domain reflectometry. Membranes 2011, 1, 195–216. [Google Scholar] [CrossRef]
- Lewis, W.J.T.; Chew, Y.M.J.; Bird, M.R. The application of fluid dynamic gauging in characterising cake deposition during the cross-flow microfiltration of a yeast suspension. J. Memb. Sci. 2012, 405–406, 113–122. [Google Scholar] [CrossRef][Green Version]
- Mattsson, T.; Lewis, W.J.T.; Chew, Y.M.J.; Bird, M.R. In situ investigation of soft cake fouling layers using fluid dynamic gauging. Food Bioprod. Process. 2015, 93, 205–210. [Google Scholar] [CrossRef]
- Mattsson, T.; Lewis, W.J.T.; Chew, Y.M.J.; Bird, M.R. The use of fluid dynamic gauging in investigating the thickness and cohesive strength of cake fouling layers formed during cross-flow microfiltration. Sep. Purif. Technol. 2018, 198, 25–30. [Google Scholar] [CrossRef]
- Zhou, M.; Mattsson, T. Effect of crossflow regime on the deposit and cohesive strength of membrane surface fouling. Food Bioprod. Process. 2019, 115, 185–193. [Google Scholar] [CrossRef]
- Lister, V.Y.; Lucas, C.; Gordon, P.W.; Chew, Y.M.J.; Wilson, D.I. Pressure mode fluid dynamic gauging for studying cake build-up in cross-flow microfiltration. J. Memb. Sci. 2011, 366, 304–313. [Google Scholar] [CrossRef]
- Peck, O.P.W.; Chew, Y.M.J.; Bird, M.R.; Bolhuis, A. Application of fluid dynamic gauging in the characterization and removal of biofouling deposits. Heat Transf. Eng. 2015, 36, 685–694. [Google Scholar] [CrossRef]
- Lewis, W.J.T.; Agg, A.; Clarke, A.; Mattsson, T.; Chew, Y.M.J.; Bird, M.R. Development of an automated, advanced fluid dynamic gauge for cake fouling studies in cross-flow filtrations. Sens. Actuators A. Phys. 2016, 238, 282–296. [Google Scholar] [CrossRef]
- Jones, S.A.; Chew, Y.M.J.; Wilson, D.I.; Bird, M.R. Fluid dynamic gauging of microfiltration membranes fouled with sugar beet molasses. J. Food Eng. 2012, 108, 22–29. [Google Scholar] [CrossRef]
- Arandia, K.; Balyan, U.; Mattsson, T. Investigation of membrane fouling using fluid dynamic gauging during cross-flow filtration of wood extracts. In Proceedings of ICOM2020; ICOM2020: London, UK, 2020. [Google Scholar]
- Li, S.; Heijman, S.G.J.; Verberk, J.Q.J.C.; Le, P.; Lu, J.; Kemperman, A.J.B.; Amy, G.L.; Dijk, J.C. Van Fouling control mechanisms of demineralized water backwash: Reduction of charge screening and calcium bridging effects. Water Res. 2011, 45, 6289–6300. [Google Scholar] [CrossRef]
- Lewis, W.J.T. Advanced Studies of Membrane Fouling Advanced Studies of Membrane Fouling: Investigation of Cake Fouling Using Fluid Dynamic Gauging; University of Bath: Bath, UK, 2014. [Google Scholar]

| Zeta Potential (mV) | Particle Diameter (µm) | |||||||
|---|---|---|---|---|---|---|---|---|
| 5 mM NaOH | 5 mM NaOH + 7.5 mM CaCl2 | 5 mM NaOH | 5 mM NaOH + 7.5 mM CaCl2 | |||||
| D10 | Median | D90 | D10 | Median | D90 | |||
| Low Charge | −30.6 ± 1.0 | −3.7 ± 0.5 | 1.27 | 1.55 | 1.97 | 1.45 | 3.22 | 8.76 |
| High Charge | −39.4 ± 0.9 | −5.6 ± 0.6 | 1.27 | 1.61 | 4.39 | 1.45 | 4.43 | 10.17 |
| Variable | LC w/ Ca | LC w/o Ca | HC w/ Ca | HC w/o Ca |
|---|---|---|---|---|
| Specific cake mass (g/m2) | 157 ± 15 | 141 ± 49 | 153 ± 29 | 137 ± 23 |
| Relative flux decline | 81.2 ± 2.2% | 88.6 ± 0.6% | 80.0 ± 1.8% | 87.2 ± 2.3% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jørgensen, M.K.; Mattsson, T. Quantifying Charge Effects on Fouling Layer Strength and (Ir)Removability during Cross-Flow Microfiltration. Membranes 2021, 11, 28. https://doi.org/10.3390/membranes11010028
Jørgensen MK, Mattsson T. Quantifying Charge Effects on Fouling Layer Strength and (Ir)Removability during Cross-Flow Microfiltration. Membranes. 2021; 11(1):28. https://doi.org/10.3390/membranes11010028
Chicago/Turabian StyleJørgensen, Mads Koustrup, and Tuve Mattsson. 2021. "Quantifying Charge Effects on Fouling Layer Strength and (Ir)Removability during Cross-Flow Microfiltration" Membranes 11, no. 1: 28. https://doi.org/10.3390/membranes11010028
APA StyleJørgensen, M. K., & Mattsson, T. (2021). Quantifying Charge Effects on Fouling Layer Strength and (Ir)Removability during Cross-Flow Microfiltration. Membranes, 11(1), 28. https://doi.org/10.3390/membranes11010028






