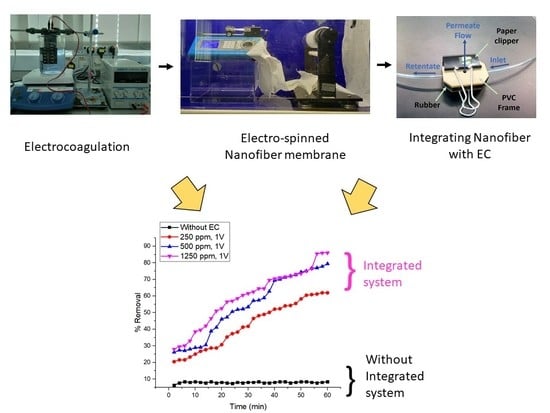
Integrated Membrane–Electrocoagulation System for Removal of Celestine Blue Dyes in Wastewater
Abstract
1. Introduction
| Anode | (1) | |
| At alkaline condition | (2) | |
| At acidic condition | (3) | |
| Cathode | (4) |
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Stock Solutions
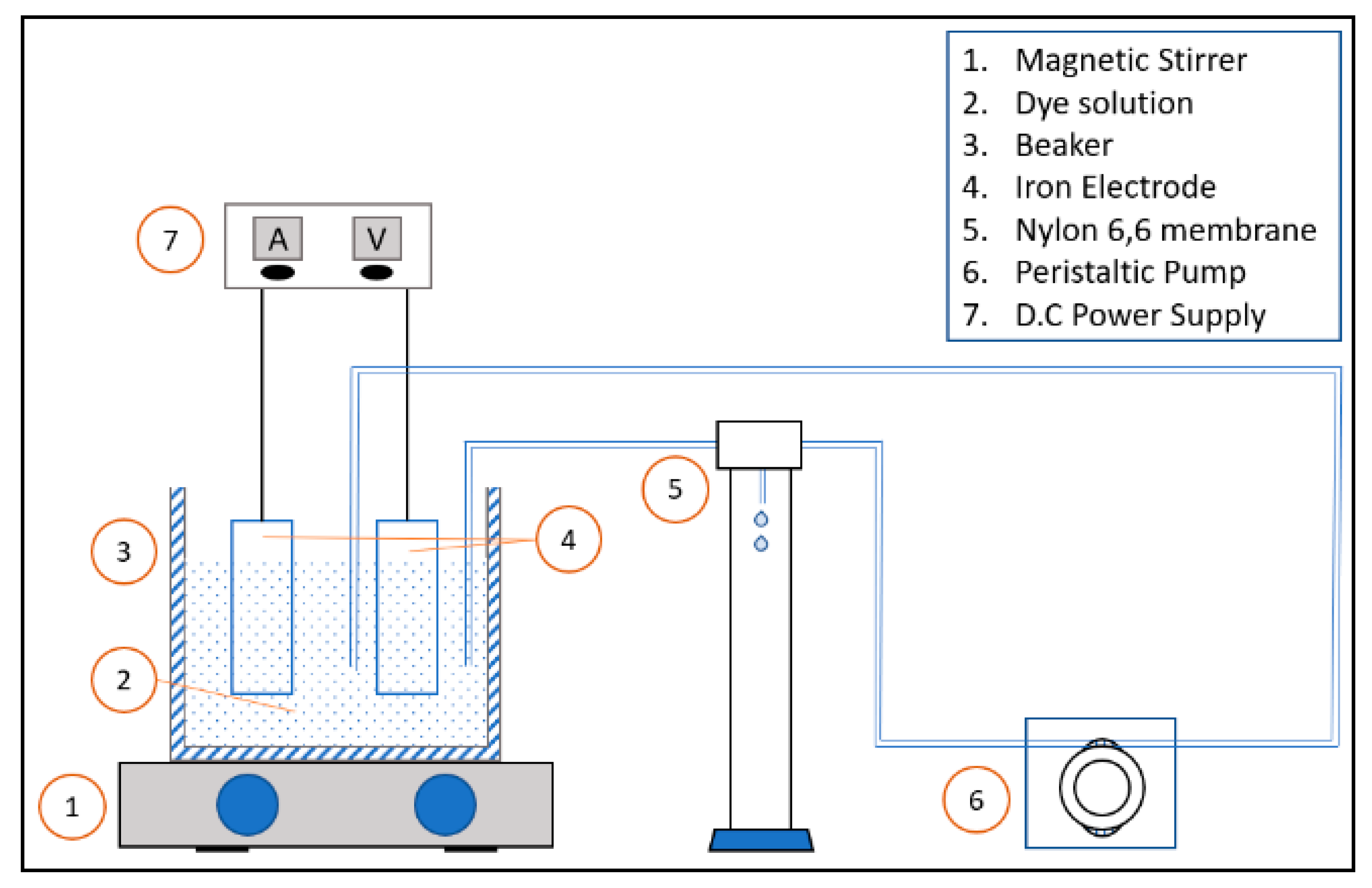
2.3. Equipment and Apparatus
2.4. Instrument Analysis
2.5. Nylon 6,6 Nanofiber Membrane Synthesis and Characterization
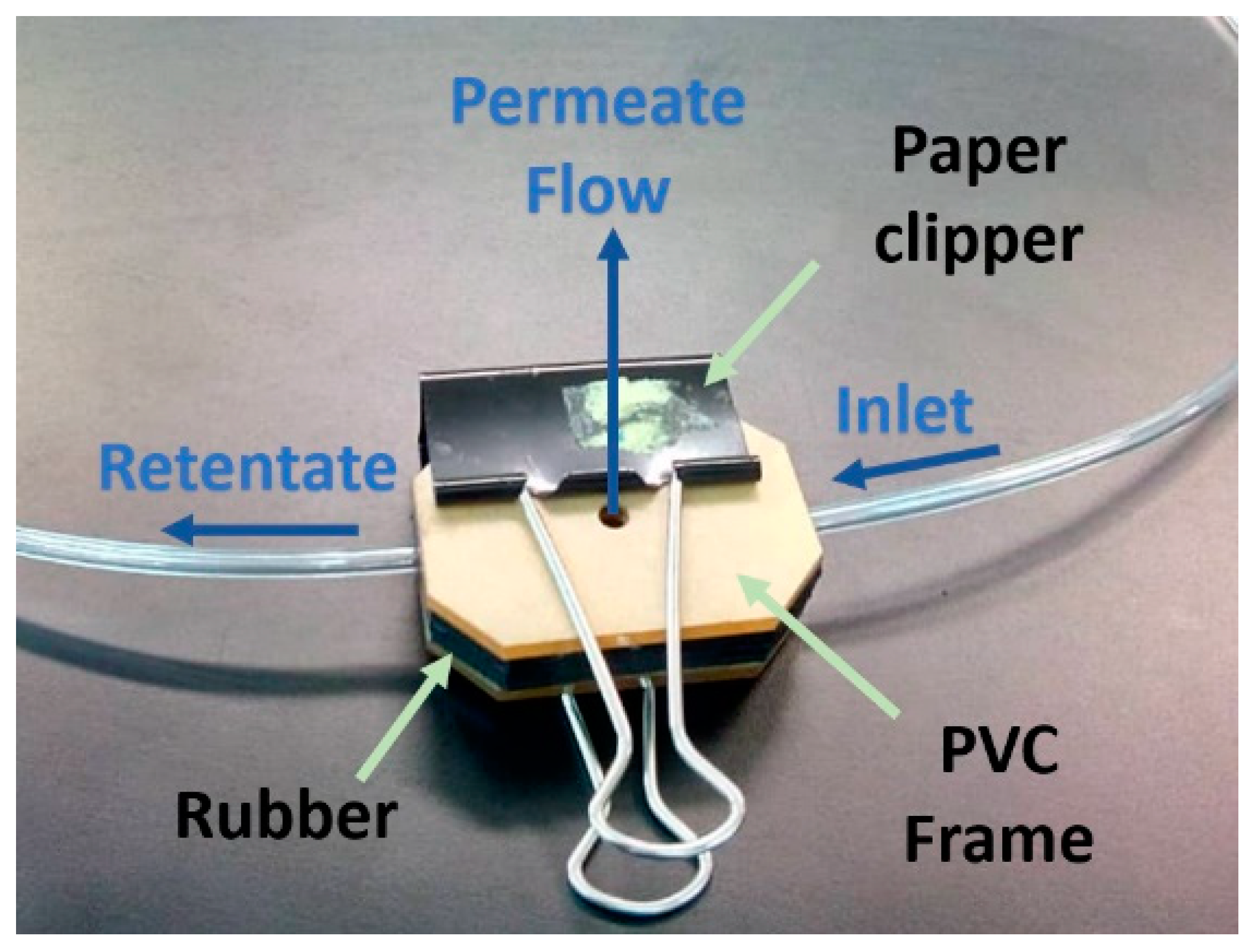
2.6. Experiment Procedure
2.7. Electrocoagulation Method
3. Results and Discussion
3.1. Characteristics of Nylon 6,6 NANOFIBER membrane
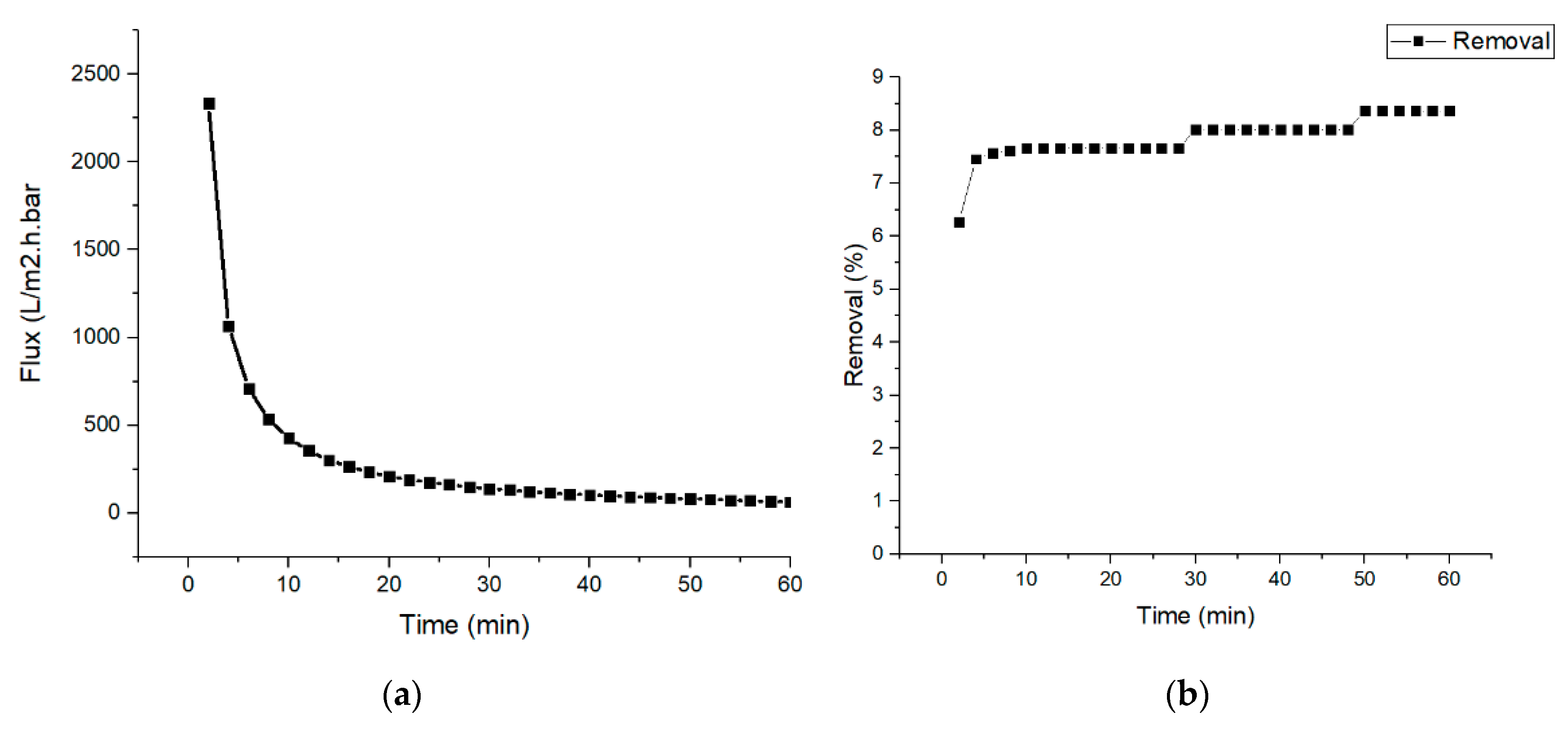
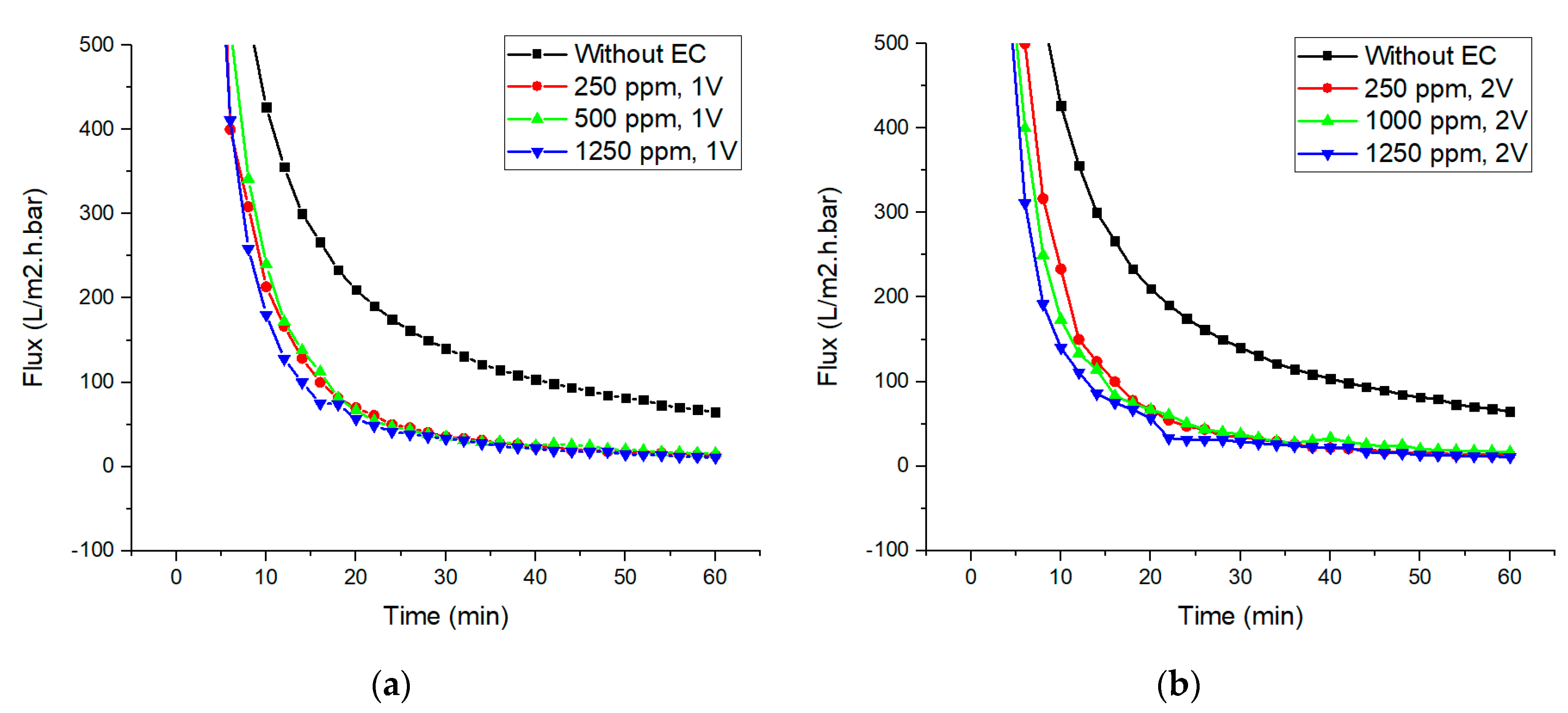
3.2. Permeability of Nylon 6,6 Nanofiber Membrane
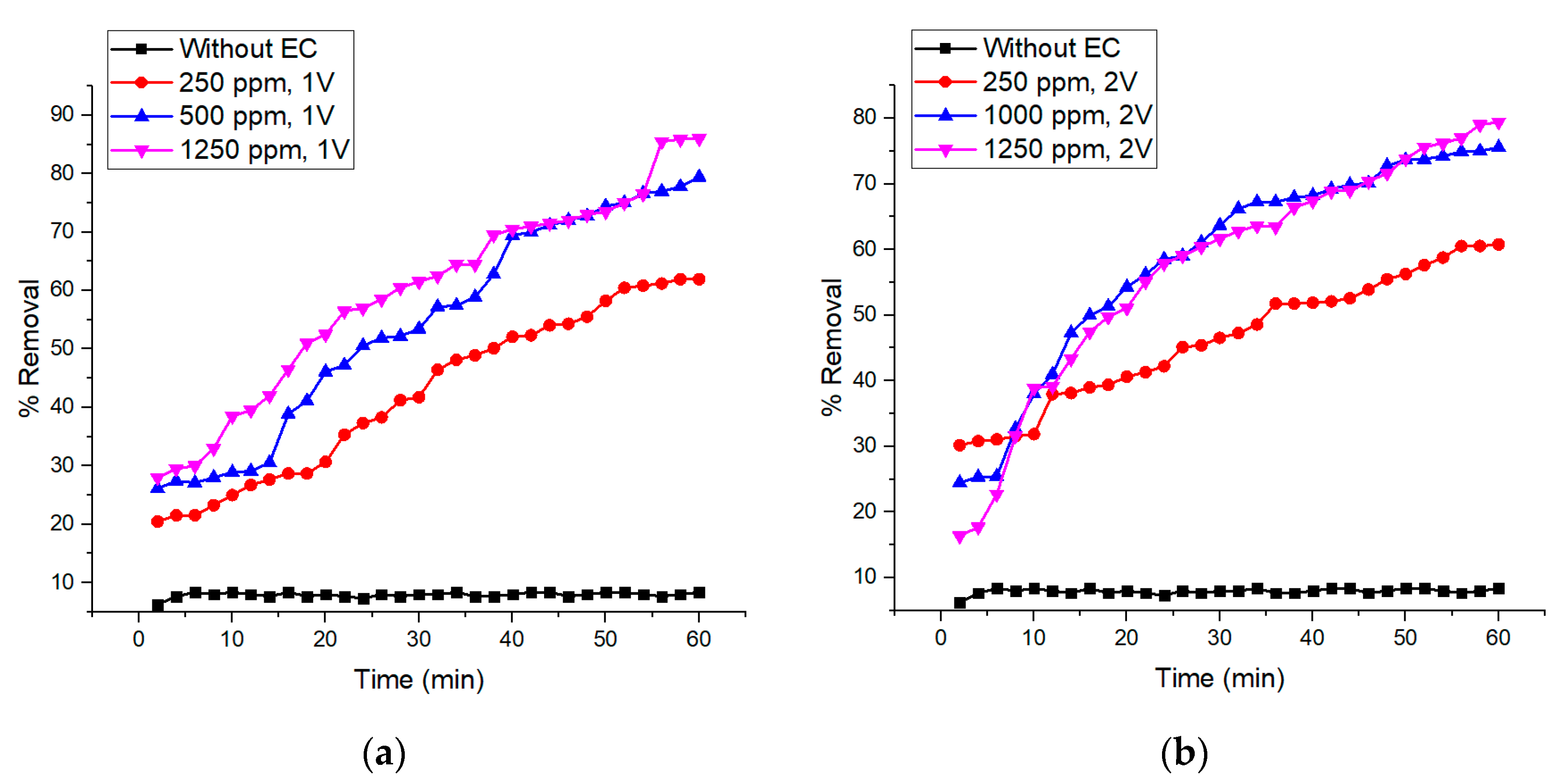
3.3. Integrated Membrane–Electrocoagulation System
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- MIDA. Textiles and Textile Products. Available online: https://www.mida.gov.my/home/textiles-and-textile-products/posts/ (accessed on 21 January 2020).
- Mouni, L.; Belkhiri, L.; Bollinger, J.-C.; Bouzaza, A.; Assadi, A.; Tirri, A.; Dahmoune, F.; Madani, K.; Remini, H. Removal of Methylene Blue from aqueous solutions by adsorption on Kaolin: Kinetic and equilibrium studies. Appl.Clay Sci. 2018, 153, 38–45. [Google Scholar] [CrossRef]
- Yuan, Y.; Ning, X.A.; Zhang, Y.; Lai, X.; Li, D.; He, Z.; Chen, X. Chlorobenzene levels, component distribution, and ambient severity in wastewater from five textile dyeing wastewater treatment plants. Ecotoxicol. Environ. Saf. 2020, 193, 110257. [Google Scholar] [CrossRef]
- Kandisa, R.V.; Saibaba Kv, N. Dye Removal by Adsorption: A Review. J. Bioremed. Biodegrad. 2016, 7. [Google Scholar] [CrossRef]
- Pearce, C. The removal of colour from textile wastewater using whole bacterial cells: A review. Dyes Pigments 2003, 58, 179–196. [Google Scholar] [CrossRef]
- El-Sikaily, A.; Khaled, A.; El Nemr, A. Textile Dyes Xenobiotic and Their Harmful Effect. In Non-Conventional Textile Waste Water Treatment; Nemr, A.E., Ed.; Nova Science Publishers: New York, NY, USA, 2012; pp. 31–64. [Google Scholar]
- Drumond Chequer, F.M.; de Oliveira, G.A.R.; Anastacio Ferraz, E.R.; Carvalho, J.; Boldrin Zanoni, M.V.; de Oliveir, D.P. Textile Dyes: Dyeing Process and Environmental Impact. In Eco-Friendly Textile Dyeing and Finishing; IntechOpen: london, UK, 2013. [Google Scholar] [CrossRef]
- Liu, J.; Liu, A.; Wang, W.; Li, R.; Zhang, W.X. Feasibility of nanoscale zero-valent iron (nZVI) for enhanced biological treatment of organic dyes. Chemosphere 2019, 237, 124470. [Google Scholar] [CrossRef] [PubMed]
- Paz, A.; Carballo, J.; Perez, M.J.; Dominguez, J.M. Biological treatment of model dyes and textile wastewaters. Chemosphere 2017, 181, 168–177. [Google Scholar] [CrossRef]
- Bhatia, D.; Sharma, N.R.; Singh, J.; Kanwar, R.S. Biological methods for textile dye removal from wastewater: A review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1836–1876. [Google Scholar] [CrossRef]
- Hao, N.; Nie, Y.; Xu, Z.; Jin, C.; Fyda, T.J.; Zhang, J.X.J. Microfluidics-enabled acceleration of Fenton oxidation for degradation of organic dyes with rod-like zero-valent iron nanoassemblies. J. Colloid. Interface Sci. 2020, 559, 254–262. [Google Scholar] [CrossRef]
- Javaid, R.; Qazi, U.Y. Catalytic Oxidation Process for the Degradation of Synthetic Dyes: An Overview. Int. J. Environ. Res. Public Health 2019, 16, 2066. [Google Scholar] [CrossRef]
- Rambabu, K.; Bharath, G.; Monash, P.; Velu, S.; Banat, F.; Naushad, M.; Arthanareeswaran, G.; Loke Show, P. Effective treatment of dye polluted wastewater using nanoporous CaCl2 modified polyethersulfone membrane. Process Saf. Environ. Prot. 2019, 124, 266–278. [Google Scholar] [CrossRef]
- Ghadhban, M.Y.; Majdi, H.S.; Rashid, K.T.; Alsalhy, Q.F.; Lakshmi, D.S.; Salih, I.K.; Figoli, A. Removal of Dye from a Leather Tanning Factory by Flat-Sheet Blend Ultrafiltration (UF) Membrane. Membranes (Basel) 2020, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, H.R.; El-Molla, S.A.; Saif, M. Improvement of physicochemical properties of Fe2O3/MgO nanomaterials by hydrothermal treatment for dye removal from industrial wastewater. Powder Technol. 2013, 249, 225–233. [Google Scholar] [CrossRef]
- Saiful Amran, S.N.B.; Wongso, V.; Abdul Halim, N.S.; Husni, M.K.; Sambudi, N.S.; Wirzal, M.D.H. Immobilized carbon-doped TiO2 in polyamide fibers for the degradation of methylene blue. J. Asian Ceram. Soc. 2019, 7, 321–330. [Google Scholar] [CrossRef]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Park, J.H.; Wang, J.J.; Xiao, R.; Tafti, N.; DeLaune, R.D.; Seo, D.C. Degradation of Orange G by Fenton-like reaction with Fe-impregnated biochar catalyst. Bioresour. Technol. 2018, 249, 368–376. [Google Scholar] [CrossRef]
- Nunez, J.; Yeber, M.; Cisternas, N.; Thibaut, R.; Medina, P.; Carrasco, C. Application of electrocoagulation for the efficient pollutants removal to reuse the treated wastewater in the dyeing process of the textile industry. J. Hazard. Mater. 2019, 371, 705–711. [Google Scholar] [CrossRef]
- Keyikoglu, R.; Can, O.T.; Aygun, A.; Tek, A. Comparison of the effects of various supporting electrolytes on the treatment of a dye solution by electrocoagulation process. Colloid Interface Sci. Commun. 2019, 33, 100210. [Google Scholar] [CrossRef]
- Ghanbari, F.; Moradi, M.; Eslami, A.; Emamjomeh, M.M. Electrocoagulation/Flotation of Textile Wastewater with Simultaneous Application of Aluminum and Iron as Anode. Environ. Processes 2014, 1, 447–457. [Google Scholar] [CrossRef]
- Casillas, H.A.M.; Cocke, D.L.; Gomes, J.A.; Morkovsky, P.R.; Parga, J.; Peterson, E.; Garcia, C. Electrochemistry Behind Electrocoagulation Using Iron Electrodes. ECS Trans. 2007, 6, 1–15. [Google Scholar] [CrossRef]
- Balasubramaniam, L.; Wirzal, M.D.H.; Putra, Z.A.; Nordin, N.A.H.M.; Bilad, M.R. Kinetic study on celestine blue dye removal using electrocoagulation method. Int. J. Technol. Res. Eng. 2018, 1, 2347–4718. [Google Scholar]
- Kabdaşlı, I.; Arslan-Alaton, I.; Ölmez-Hancı, T.; Tünay, O. Electrocoagulation applications for industrial wastewaters: A critical review. Environ. Technol. Rev. 2012, 1, 2–45. [Google Scholar] [CrossRef]
- Yadav, A.K.; Singh, L.; Mohanty, A.; Satya, S.; Sreekrishnan, T.R. Removal of various pollutants from wastewater by electrocoagulation using iron and aluminium electrode. Desalin. Water Treat. 2012, 46, 352–358. [Google Scholar] [CrossRef]
- Vlyssides, A.G.; Papaioannou, D.; Loizidoy, M.; Karlis, P.K.; Zorpas, A.A. Testing an electrochemical method for treatment of textile dye wastewater. Waste Manag. 2000, 20, 569–574. [Google Scholar] [CrossRef]
- Zeng, H.; Yu, Z.; Peng, Y.; Zhu, L. Environmentally friendly electrostatically driven self-assembled LDH/GO/PVDF composite membrane for water treatment. Appl. Clay Sci. 2019, 183. [Google Scholar] [CrossRef]
- Cheng, X.; Zhou, W.; Li, P.; Ren, Z.; Wu, D.; Luo, C.; Tang, X.; Wang, J.; Liang, H. Improving ultrafiltration membrane performance with pre-deposited carbon nanotubes/nanofibers layers for drinking water treatment. Chemosphere 2019, 234, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Weschenfelder, S.E.; Fonseca, M.J.C.; Costa, B.R.S.; Borges, C.P. Influence of the use of surfactants in the treatment of produced water by ceramic membranes. J. Water Process Eng. 2019, 32, 100955. [Google Scholar] [CrossRef]
- Permogorov, N. Membrane Technologies for Water Treatment: Removal of Toxic Trace Elements with Emphasis on Arsenic, Fluoride and Uranium. Johns. Matthey Technol. Rev. 2016, 60, 273–276. [Google Scholar] [CrossRef]
- Alqaheem, Y.; Alomair, A.; Vinoba, M.; Pérez, A. Polymeric Gas-Separation Membranes for Petroleum Refining. Int. J. Polym. Sci. 2017, 2017, 4250927. [Google Scholar] [CrossRef]
- Mohshim, D.F.; Mukhtar, H.b.; Man, Z.; Nasir, R. Latest Development on Membrane Fabrication for Natural Gas Purification: A Review. J. Eng. 2013, 2013, 101746. [Google Scholar] [CrossRef]
- Guiga, W.; Lameloise, M.-L. Membrane separation in food processing. In Green Food Processing Techniques, 1st ed.; Chemat, F., Vorobiev, E., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 245–287. [Google Scholar] [CrossRef]
- Conidi, C.; Drioli, E.; Cassano, A. Membrane-based agro-food production processes for polyphenol separation, purification and concentration. Curr. Opin. Food Sci. 2018, 23, 149–164. [Google Scholar] [CrossRef]
- Al-Husaini, I.S.; Yusoff, A.R.M.; Lau, W.-J.; Ismail, A.F.; Al-Abri, M.Z.; Wirzal, M.D.H. Iron oxide nanoparticles incorporated polyethersulfone electrospun nanofibrous membranes for effective oil removal. Chem. Eng. Res. Des. 2019, 148, 142–154. [Google Scholar] [CrossRef]
- Santos, P.G.; Scherer, C.M.; Fisch, A.G.; Rodrigues, M.A.S. Petrochemical wastewater treatment: Water recovery using membrane distillation. J. Clean. Prod. 2020, 267. [Google Scholar] [CrossRef]
- Li, B.; Cui, Y.; Japip, S.; Thong, Z.; Chung, T.-S. Graphene oxide (GO) laminar membranes for concentrating pharmaceuticals and food additives in organic solvents. Carbon 2018, 130, 503–514. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Mukherjee, D.; Deb, N.; Swarnakar, S.; Banerjee, S. Application of green synthesized ZnO nanoparticle coated ceramic ultrafiltration membrane for remediation of pharmaceutical components from synthetic water: Reusability assay of treated water on seed germination. J. Environ. Chem. Eng. 2020, 8. [Google Scholar] [CrossRef]
- Foong, C.Y.; Wirzal, M.D.H.; Bustam, M.A. A review on nanofibers membrane with amino-based ionic liquid for heavy metal removal. J. Mol. Liq. 2020, 297. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Wirzal, M.D.H.; Nordin, N.A.H.; Abd Halim, N.S. Development of Polyvinylidene fluoride (PVDF)-ZIF-8 Membrane for Wastewater Treatment. IOP Conf. Ser. Earth Environ. Sci. 2018, 140. [Google Scholar] [CrossRef]
- Azizo, A.S.; Wirzal, M.D.H.; Bilad, M.R.; Yusoff, A.R.M. Assessment of nylon 6, 6 nanofibre membrane for microalgae harvesting. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2017; Volume 1891, p. 020032. [Google Scholar]
- Aussawasathien, D.; Teerawattananon, C.; Vongachariya, A. Separation of micron to sub-micron particles from water: Electrospun nylon-6 nanofibrous membranes as pre-filters. J. Membr. Sci. 2008, 315, 11–19. [Google Scholar] [CrossRef]
- Somensi, C.A.; Simionatto, E.L.; Bertoli, S.L.; Wisniewski, A., Jr.; Radetski, C.M. Use of ozone in a pilot-scale plant for textile wastewater pre-treatment: Physico-chemical efficiency, degradation by-products identification and environmental toxicity of treated wastewater. J. Hazard. Mater. 2010, 175, 235–240. [Google Scholar] [CrossRef]
- Yalcinkaya, F. A review on advanced nanofiber technology for membrane distillation. J. Eng. Fibers Fabr. 2019, 14. [Google Scholar] [CrossRef]
- Hobbs, C.; Taylor, J.; Hong, S. Effect of surface roughness on fouling of RO and NF membranes during filtration of a high organic surficial groundwater. J. Water Supply Res. Technol.-AQUA 2006, 55, 559–570. [Google Scholar] [CrossRef]
- Abdullah, N.; Yusof, N.; Lau, W.J.; Jaafar, J.; Ismail, A.F. Recent trends of heavy metal removal from water/wastewater by membrane technologies. J. Ind. Eng. Chem. 2019, 76, 17–38. [Google Scholar] [CrossRef]
- Halim, N.S.A.; Wirzal, M.D.H.; Bilad, M.R.; Yusoff, A.R.M.; Nordin, N.A.H.M.; Putra, Z.A.; Jaafar, J. Effect of Solvent Vapor Treatment on Electrospun Nylon 6,6 Nanofiber Membrane. IOP Conf. Ser. Mater. Sci. Eng. 2018, 429. [Google Scholar] [CrossRef]
- Wei, M.-C.; Wang, K.-S.; Huang, C.-L.; Chiang, C.-W.; Chang, T.-J.; Lee, S.-S.; Chang, S.-H. Improvement of textile dye removal by electrocoagulation with low-cost steel wool cathode reactor. Chem. Eng. J. 2012, 192, 37–44. [Google Scholar] [CrossRef]
- Wirzal, M.D.H.; Yusoff, A.R.M.; Zima, J.; Barek, J. Degradation of Ampicillin and Penicillin G using Anodic Oxidation. Int. J. Electrochem. Sci. 2013, 8, 8978–8988. [Google Scholar]
- Rohadi, N. Impact of Adding Sodium Chloride to Change of Turbidity and Iron Concentration on Treatment Waste Water Using Electrocoagulation Process. J. Phys. Conf. Ser. 2019, 1364. [Google Scholar] [CrossRef]
- Arroyo, M.G.; Perez-Herranz, V.; Montanes, M.T.; Garcia-Anton, J.; Guinon, J.L. Effect of pH and chloride concentration on the removal of hexavalent chromium in a batch electrocoagulation reactor. J. Hazard. Mater. 2009, 169, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Larue, O.; Vorobiev, E. Floc size estimation in iron induced electrocoagulation and coagulation using sedimentation data. Int. J. Miner. Process. 2003, 71, 1–15. [Google Scholar] [CrossRef]
- Abdelrasoul, A.; Doan, H.; Lohi, A. Fouling in Membrane Filtration and Remediation Methods. In Mass Transfer—Advances in Sustainable Energy and Environment Oriented Numerical Modeling; Nakajima, H., Ed.; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef]
- Zhang, Y.; Ou, H.; Liu, H.; Ke, Y.; Zhang, W.; Liao, G.; Wang, D. Polyimide-based carbon nanofibers: A versatile adsorbent for highly efficient removals of chlorophenols, dyes and antibiotics. Colloids Surf. A Physicochem. Eng. Asp. 2018, 537, 92–101. [Google Scholar] [CrossRef]
- Cui, M.H.; Sangeetha, T.; Gao, L.; Wang, A.J. Efficient azo dye wastewater treatment in a hybrid anaerobic reactor with a built-in integrated bioelectrochemical system and an aerobic biofilm reactor: Evaluation of the combined forms and reflux ratio. Bioresour. Technol. 2019, 292, 122001. [Google Scholar] [CrossRef]

| Membrane | Pore Size (µm) | Porosity (%) | Surface Roughness (nm) |
|---|---|---|---|
| Nylon 6,6 nanofiber | 0.2 | 71.3 ± 2.0 | 56.5 ± 10 |
| Voltage | Removal Efficiency | Energy Usage (kWh/m3) |
|---|---|---|
| 1 V | 43.2% | 0.75 |
| 2 V | 42.0% | 1.65 |
| 10 V | 100.0% | 31.88 |
| Method | Treatment Mechanism | Influencing Variable | Type of Electrode | pH | Dye Removal | Reference |
|---|---|---|---|---|---|---|
| EC | Physicochemical | Voltage, NaCl concentration | Iron | 7 | 43.2% | [23] |
| Integrated nanofiber (NF) membrane and EC | Physicochemical and Filtration | Voltage supplied, time | Iron | 7 | 79.4% | This paper |
| Adsorptive carbon nanofibers | Adsorption | pH, concentration | - | 3–11 | 77.8% | [54] |
| Integrated bio-electrochemical and anaerobic system | Biological and bio-electrochemical | Reactor configuration and reflux ratio | Graphite | 7.27 | 97.5% | [55] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saad, M.S.; Balasubramaniam, L.; Wirzal, M.D.H.; Abd Halim, N.S.; Bilad, M.R.; Md Nordin, N.A.H.; Adi Putra, Z.; Ramli, F.N. Integrated Membrane–Electrocoagulation System for Removal of Celestine Blue Dyes in Wastewater. Membranes 2020, 10, 184. https://doi.org/10.3390/membranes10080184
Saad MS, Balasubramaniam L, Wirzal MDH, Abd Halim NS, Bilad MR, Md Nordin NAH, Adi Putra Z, Ramli FN. Integrated Membrane–Electrocoagulation System for Removal of Celestine Blue Dyes in Wastewater. Membranes. 2020; 10(8):184. https://doi.org/10.3390/membranes10080184
Chicago/Turabian StyleSaad, Muhammad Syaamil, Lila Balasubramaniam, Mohd Dzul Hakim Wirzal, Nur Syakinah Abd Halim, Muhammad Roil Bilad, Nik Abdul Hadi Md Nordin, Zulfan Adi Putra, and Fuad Nabil Ramli. 2020. "Integrated Membrane–Electrocoagulation System for Removal of Celestine Blue Dyes in Wastewater" Membranes 10, no. 8: 184. https://doi.org/10.3390/membranes10080184
APA StyleSaad, M. S., Balasubramaniam, L., Wirzal, M. D. H., Abd Halim, N. S., Bilad, M. R., Md Nordin, N. A. H., Adi Putra, Z., & Ramli, F. N. (2020). Integrated Membrane–Electrocoagulation System for Removal of Celestine Blue Dyes in Wastewater. Membranes, 10(8), 184. https://doi.org/10.3390/membranes10080184