Immobilisation and Release of Radical Scavengers on Nanoclays for Chemical Reinforcement of Proton Exchange Membranes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CeO2 Embedded and Amino Functionalised HNT and Their Characterisation
2.2.1. HNT Pre-Treatment
2.2.2. Preparation of CeO2@HNT
2.2.3. Surface Functionalisation of CeO2@HNT
2.2.4. Physico-Chemical Characterisation of HNT
2.3. Membrane Preparation and Characterisation
2.3.1. Fenton Reaction Protocol
2.3.2. Membrane Characterisation
3. Results and Discussion
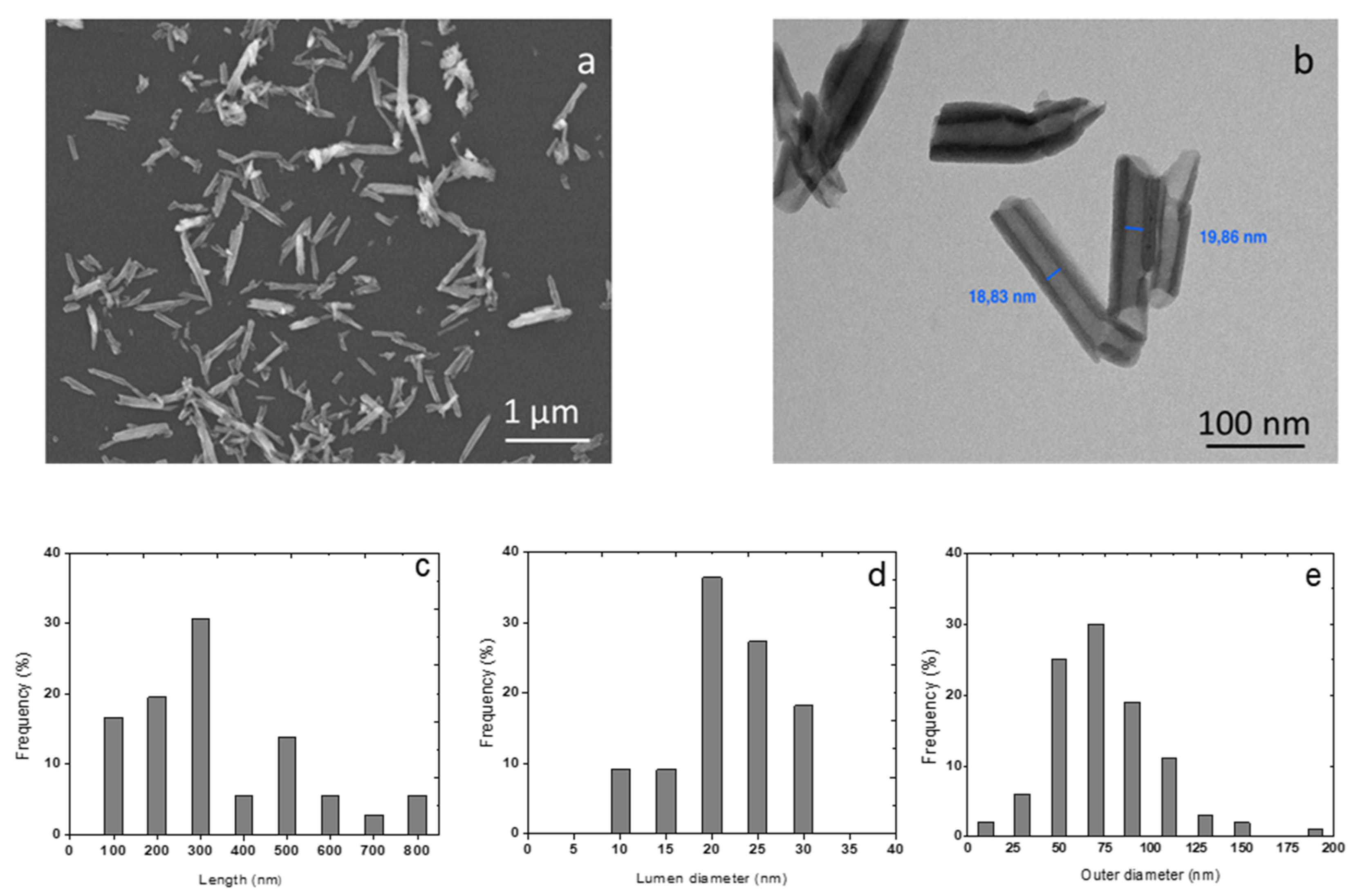
3.1. Characterisation of HNTs
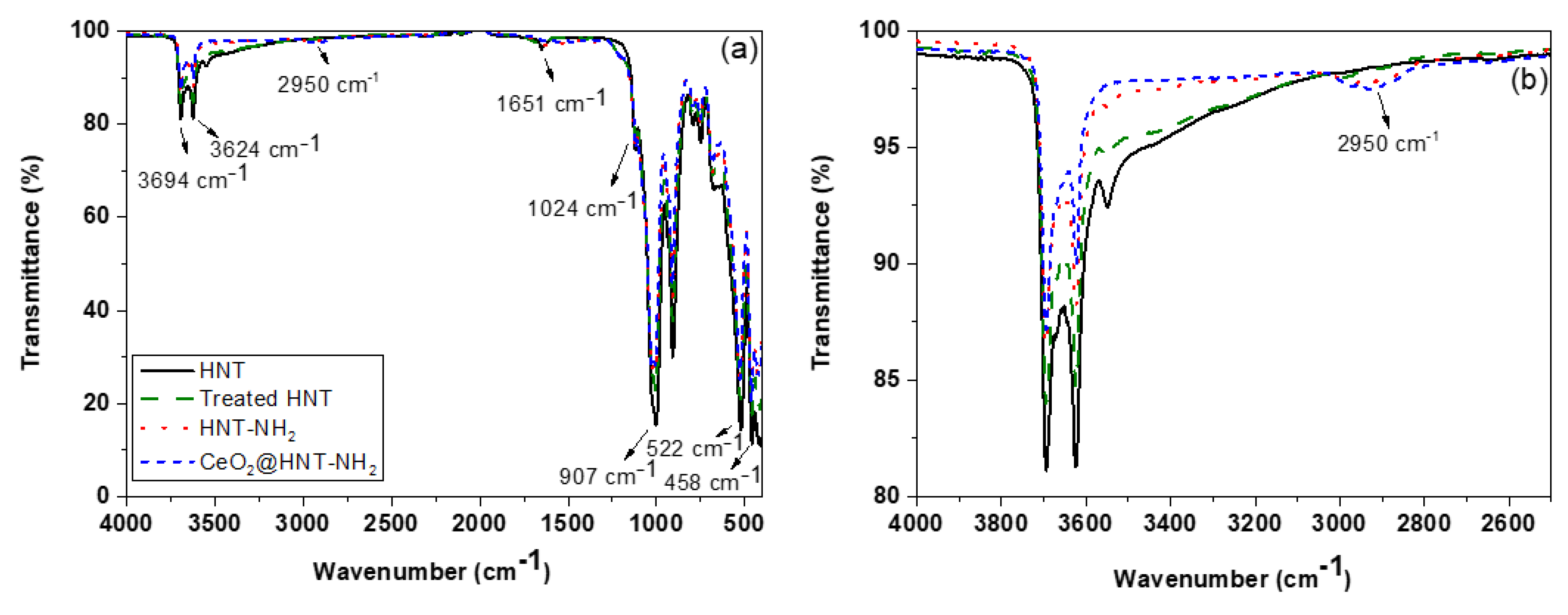
3.2. Preparation and Characterisation of CeO2@HNT
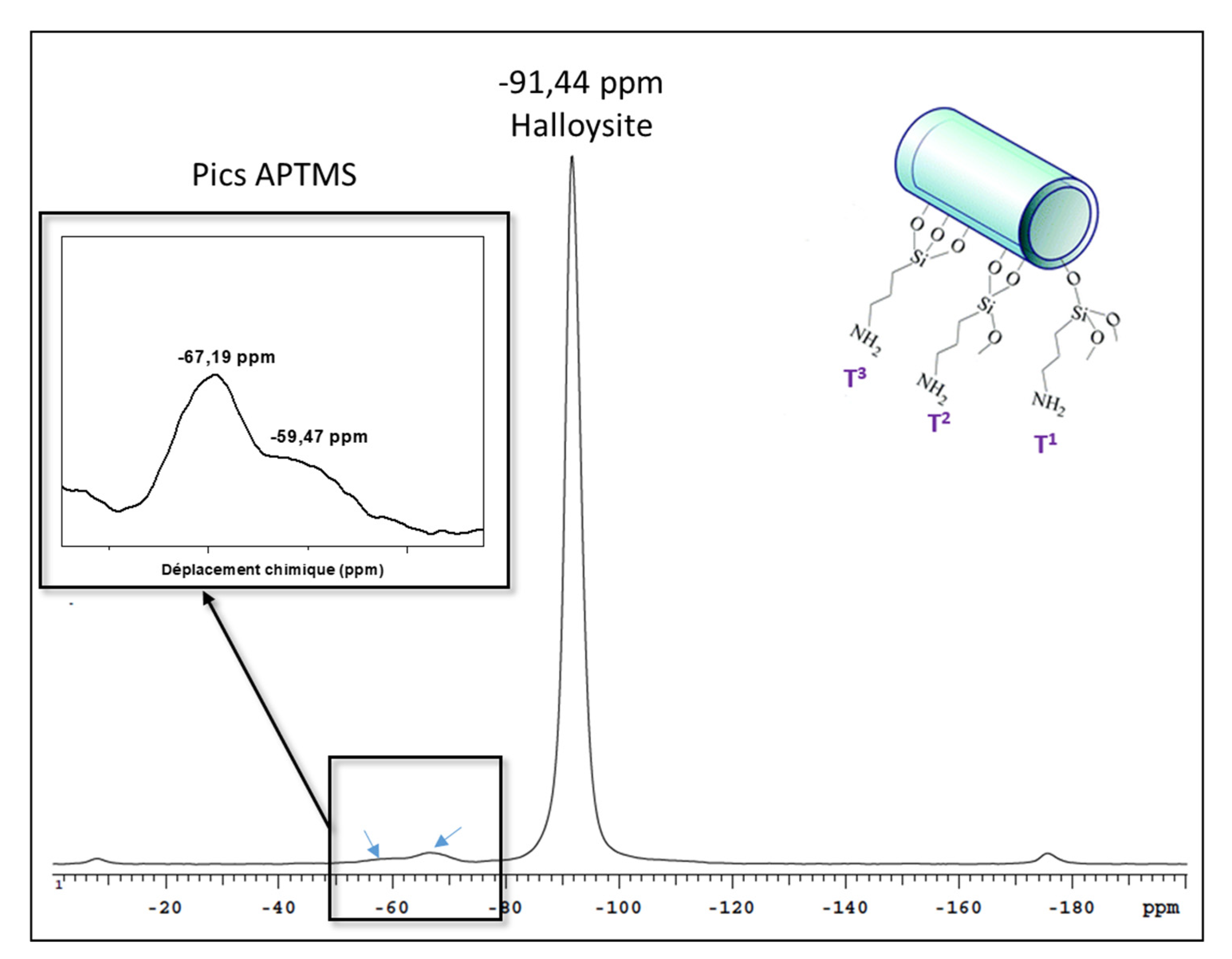
3.3. Preparation and Characterisation of CeO2@HNT-NH2
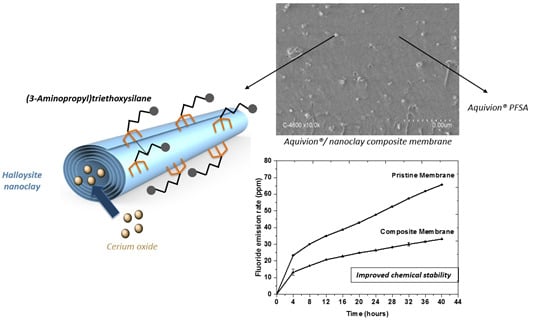
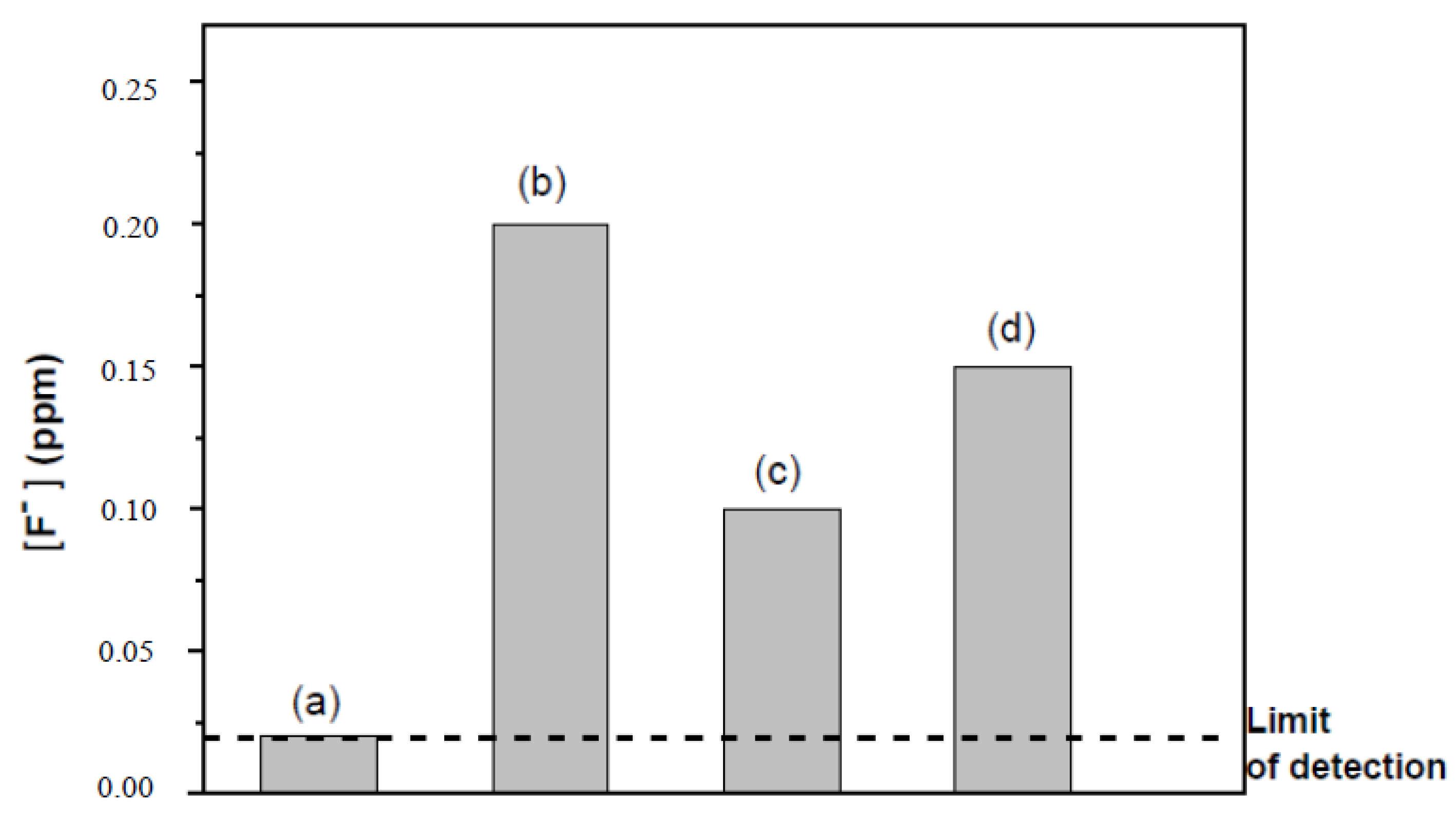
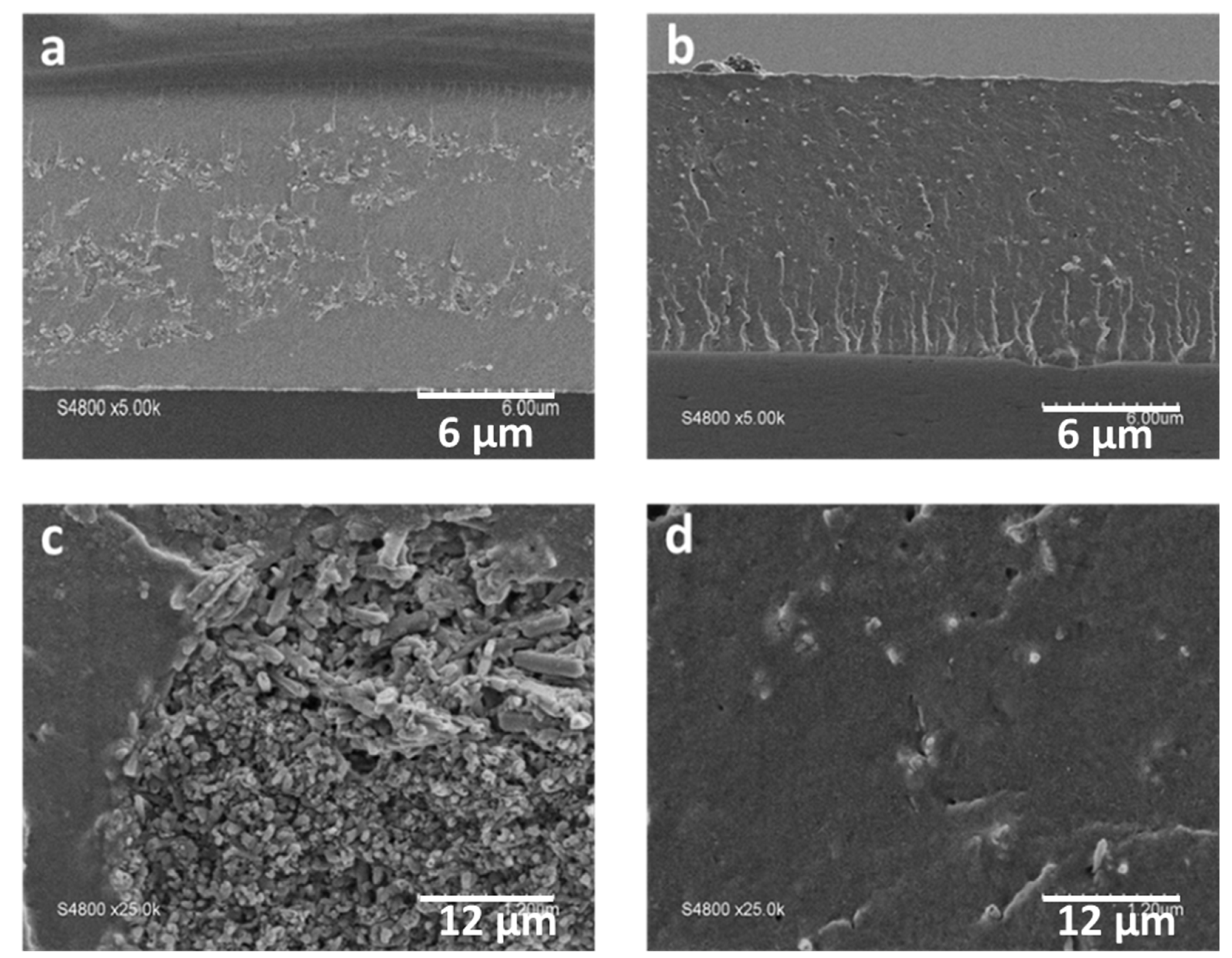
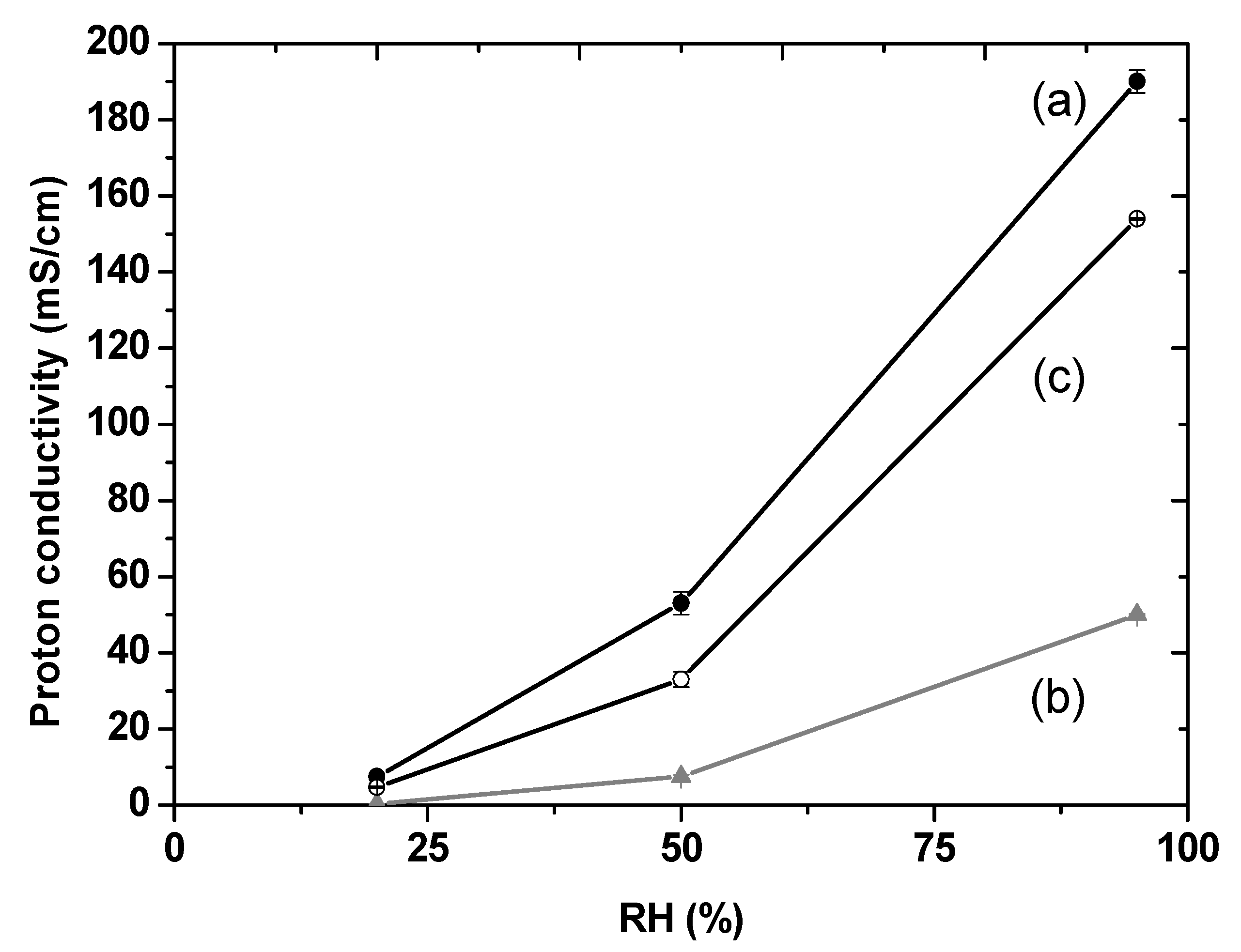
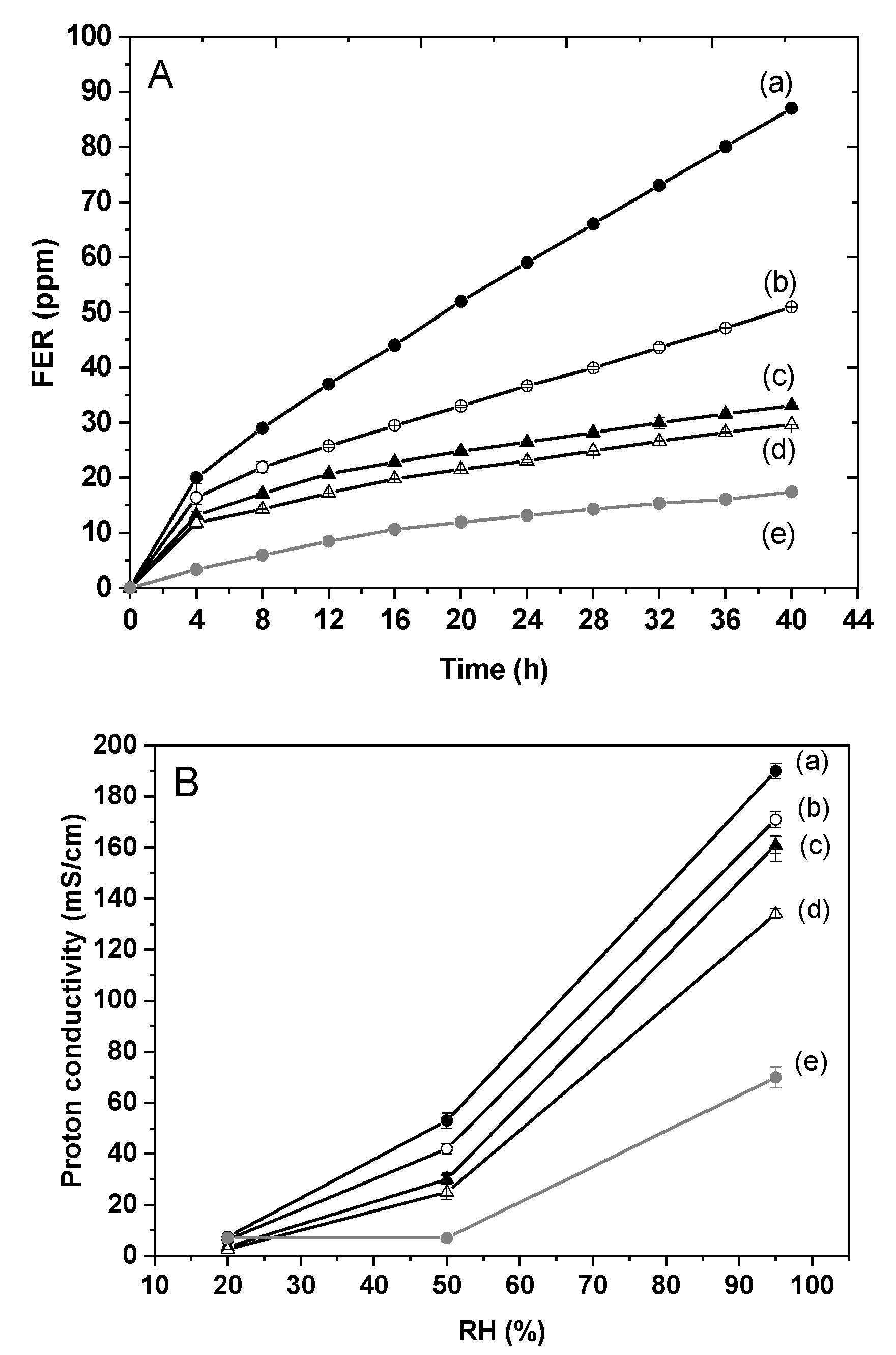
3.4. Composite Membrane Characterisation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Bruijn, F.A.; Dam, V.A.T.; Janssen, G.J.M. Review: Durability and degradation issues of PEM fuel cell components. Fuel Cells 2008, 8, 3–22. [Google Scholar] [CrossRef]
- Kusoglu, A.; Weber, A.Z. New insights into perfluorinated Sulfonic-Acid Ionomers. Chem. Rev. 2017, 117, 987–1104. [Google Scholar] [CrossRef] [PubMed]
- Borup, R.; Meyers, J.; Pivovar, B.; Kim, Y.S.; Mukundan, R.; Garland, N.; Myers, D.; Wilson, M.; Garzon, F.; Wood, D.; et al. Scientific aspects of polymer electrolyte fuel cell durability and degradation. Chem. Rev. 2007, 107, 3904–3951. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.energy.gov/eere/fuelcells/doe-technical-targets-fuel-cell-systems-and-stacks-transportation-applications (accessed on 15 June 2020).
- Rodgers, M.P.; Bonville, L.J.; Kunz, H.R.; Slattery, D.K.; Fenton, J.M. Fuel cell perfluorinated sulfonic acid membrane degradation correlating accelerated stress testing and lifetime. Chem. Rev. 2012, 112, 6075–6103. [Google Scholar] [CrossRef] [PubMed]
- Zatoń, M.; Rozière, J.; Jones, D.J. Current understanding of chemical degradation mechanisms of perfluorosulfonic acid membranes and their mitigation strategies: A review. Sustain. Energy Fuels 2017, 1, 409–438. [Google Scholar] [CrossRef]
- Subianto, S.; Pica, M.; Casciola, M.; Cojocaru, P.; Merlo, L.; Hards, G.; Jones, D.J. Physical and chemical modifi cation routes leading to improved mechanical properties of per fl uorosulfonic acid membranes for PEM fuel cells. J. Power Sources 2013, 233, 216–230. [Google Scholar] [CrossRef]
- Curtin, D.E.; Lousenberg, R.D.; Henry, T.J.; Tangeman, P.C.; Tisack, M.E. Advanced materials for improved PEMFC performance and life. J. Power Sources 2004, 131, 41–48. [Google Scholar] [CrossRef]
- Venkatesan, S.v.; Lim, C.; Holdcroft, S.; Kjeang, E. Progression in the morphology of fuel cell membranes upon conjoint chemical and mechanical degradation. J. Electrochem. Soc. 2016, 163, F637–F643. [Google Scholar] [CrossRef]
- Mauritz, K.A.; Moore, R.B. State of understanding of Nafion. Chem. Rev. 2004, 104, 4535–4585. [Google Scholar] [CrossRef]
- Ramaswamy, N.; Hakim, N.; Mukerjee, S. Degradation mechanism study of perfluorinated proton exchange membrane under fuel cell operating conditions. Electrochim. Acta 2008, 53, 3279–3295. [Google Scholar] [CrossRef]
- Huang, X.; Solasi, R.; Zou, Y.U.E.; Feshler, M.; Reifsnider, K.; Condit, D.; Burlatsky, S.; Madden, T. Mechanical endurance of polymer electrolyte membrane and PEM fuel cell durability. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 2346–2357. [Google Scholar] [CrossRef]
- Alberti, G.; Narducci, R.; Di Vona, M.L.; Giancola, S. Annealing of nafion 1100 in the presence of an annealing agent: A powerful method for increasing ionomer working temperature in PEMFCs. Fuel Cells 2013, 13, 42–47. [Google Scholar] [CrossRef]
- Giancola, S.; Arciniegas, R.A.B.; Fahs, A.; Chailan, J.-F.; Di Vona, M.L.; Knauth, P.; Narducci, R. Study of Annealed Aquivion® Ionomers with the INCA Method. Membranes 2019, 9, 134. [Google Scholar] [CrossRef] [PubMed]
- Zatoń, M.; Cavaliere, S.; Jones, D.J.; Rozière, J. Design of Heterogeneities and Interfaces with Nanofibers in Fuel Cell Membranes BT—Handbook of Nanofibers. In Handbook of Nanofibers; Barhoum, A., Bechelany, M., Makhlouf, A., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–37. [Google Scholar]
- Bahar, B.; Hobson, A.R.; Kolde, J.A.; Zuckerbrod, D.; Bahar, B.; Hobson, A.R.; Kolde, J.A.; Zuckerbrod, D. Ultra-Thin Integral Composite Membrane. U.S. Patent No. 5,547,551, 20 August 1996. [Google Scholar]
- Xiao, P.; Li, J.S.; Tang, H.L.; Wang, Z.; Pan, M. Physically stable and high performance Aquivion/ePTFE composite membrane for high temperature fuel cell application. J. Memb. Sci. 2013, 442, 65–71. [Google Scholar] [CrossRef]
- Sood, R.; Cavaliere, S.; Jones, D.J.; Rozière, J. Electrospun nanofibre composite polymer electrolyte fuel cell and electrolysis membranes. Nano Energy 2016, 26, 729–745. [Google Scholar] [CrossRef]
- Subianto, S.; Donnadio, A.; Cavaliere, S.; Pica, M.; Casciola, M.; Jones, D.J.; Rozière, J. Reactive coaxial electrospinning of ZrP/ZrO2 nanofibres. J. Mater. Chem. A 2014, 2, 13359–13365. [Google Scholar] [CrossRef]
- Choi, J.; Lee, K.; Wycisk, R.; Pintauro, P.N.; Mather, P.T. Nanofiber composite membranes with low equivalent weight perfluorosulfonic acid polymers. J. Mater. Chem. 2010, 20, 6282–6290. [Google Scholar] [CrossRef]
- Cele, N.P.; Sinha Ray, S.; Pillai, S.K.; Ndwandwe, M.; Nonjola, S.; Sikhwivhilu, L.; Mathe, M.K. Carbon nanotubes based nafion compositemembranes for fuel cell applications. Fuel Cells 2010, 10, 64–71. [Google Scholar]
- Jérôme, R.; Thomassin, J.-M.; Kollar, J.; Caldarella, G.; Germain, A.; Detrembleur, C. Beneficial effect of carbon nanotubes on the performances of Nafion membranes in fuel cell applications. J. Memb. Sci. 2007, 303, 252–257. [Google Scholar]
- Donnadio, A.; Pica, M.; Carbone, A.; Gatto, I.; Posati, T.; Mariangeloni, G.; Casciola, M. Double filler reinforced ionomers: A new approach to the design of composite membranes for fuel cell applications. J. Mater. Chem. A 2015, 3, 23530–23538. [Google Scholar] [CrossRef]
- Guo, C.; Zhou, L.; Lv, J. Effects of expandable graphite and modified ammonium polyphosphate on the flame-retardant and mechanical properties of wood flour-polypropylene composites. Polym. Polym. Compos. 2013, 21, 449–456. [Google Scholar] [CrossRef]
- Di Noto, V.; Gliubizzi, R.; Negro, E.; Pace, G. Effect of SiO2 on relaxation phenomena and mechanism of ion conductivity of [Nafion/(SiO2)x] composite membranes. J. Phys. Chem. B 2006, 110, 24972–24986. [Google Scholar] [CrossRef] [PubMed]
- Thayumanasundaram, S.; Piga, M.; Lavina, S.; Negro, E.; Jeyapandian, M.; Ghassemzadeh, L.; Müller, K.; Di Noto, V. Hybrid inorganic–organic proton conducting membranes based on Nafion, SiO2 and triethylammonium trifluoromethanesulfonate ionic liquid. Electrochim. Acta 2010, 55, 1355–1365. [Google Scholar] [CrossRef]
- Burgaz, E.; Lian, H.; Alonso, R.H.; Estevez, L.; Kelarakis, A.; Giannelis, E.P. Nafion-clay hybrids with a network structure. Polymer (Guildf) 2009, 50, 2384–2392. [Google Scholar] [CrossRef]
- Herrera Alonso, R.; Estevez, L.; Lian, H.; Kelarakis, A.; Giannelis, E.P. Nafion-clay nanocomposite membranes: Morphology and properties. Polymer (Guildf) 2009, 50, 2402–2410. [Google Scholar] [CrossRef][Green Version]
- Beauger, C.; Lainé, G.; Burr, A.; Taguet, A.; Otazaghine, B.; Rigacci, A. Nafion®-sepiolite composite membranes for improved proton exchange membrane fuel cell performance. J. Memb. Sci. 2013, 430, 167–179. [Google Scholar] [CrossRef]
- Nicotera, I.; Enotiadis, A.; Angjeli, K.; Coppola, L.; Ranieri, G.A.; Gournis, D. Effective improvement of water-retention in nanocomposite membranes using novel organo-modified clays as fillers for high temperature PEMFCs. J. Phys. Chem. B 2011, 115, 9087–9097. [Google Scholar] [CrossRef]
- Zatoń, M.; Rozière, J.; Jones, D.J. Mitigation of PFSA membrane chemical degradation using composite cerium oxide–PFSA nanofibres. J. Mater. Chem. A 2017, 5, 5390–5401. [Google Scholar] [CrossRef]
- Yuan, X.Z.; Li, H.; Zhang, S.; Martin, J.; Wang, H. A review of polymer electrolyte membrane fuel cell durability test protocols. J. Power Sources 2011, 196, 9107–9116. [Google Scholar] [CrossRef]
- Gubler, L.; Dockheer, S.M.; Koppenol, W.H. Radical (HO●, H● and HOO●) formation and ionomer degradation in polymer electrolyte fuel cells. J. Electrochem. Soc. 2011, 158, B755. [Google Scholar] [CrossRef]
- Mittal, V.O.; Kunz, H.R.; Fenton, J.M. Membrane degradation mechanisms in PEMFCs. J. Electrochem. Soc. 2007, 154, B652. [Google Scholar] [CrossRef]
- Peron, J.; Nedellec, Y.; Jones, D.; Rozière, J. The effect of dissolution, migration and precipitation of platinum in Nafion®-based membrane electrode assemblies during fuel cell operation at high potential. J. Power Sources 2008, 185, 1209–1217. [Google Scholar] [CrossRef]
- Healy, J.; Hayden, C.; Xie, T.; Olson, K.; Waldo, R.; Brundage, M.; Gasteiger, H.; Abbott, J. Aspects of the chemical degradation of PFSA ionomers used in PEM fuel cells. Fuel Cells 2005, 5, 302–308. [Google Scholar] [CrossRef]
- Gubler, L.; Koppenol, W.H. Kinetic simulation of the chemical stabilization mechanism in fuel cell membranes using cerium and manganese redox couples. J. Electrochem. Soc. 2011, 159, B211–B218. [Google Scholar] [CrossRef]
- Endoh, E. Development of highly durable PFSA membrane and MEA for PEMFC under high temperature and low humidity conditions. ECS Trans. 2008, 16, 1229–1240. [Google Scholar] [CrossRef]
- Pearman, B.P.; Mohajeri, N.; Brooker, R.P.; Rodgers, M.P.; Slattery, D.K.; Hampton, M.D.; Cullen, D.a.; Seal, S. The degradation mitigation effect of cerium oxide in polymer electrolyte membranes in extended fuel cell durability tests. J. Power Sources 2013, 225, 75–83. [Google Scholar] [CrossRef]
- Danilczuk, M.; Perkowski, A.J.; Schlick, S. Ranking the stability of perfluorinated membranes used in fuel cells to attack by hydroxyl radicals and the effect of Ce(III): A competitive kinetics approach based on spin trapping ESR. Macromolecules 2010, 43, 3352–3358. [Google Scholar] [CrossRef]
- Schlick, S.; Danilczuk, M.; Drews, A.R.; Kukreja, R.S. Scavenging of Hydroxyl Radicals by Ceria Nanoparticles: Effect of Particle Size and Concentration. J. Phys. Chem. C 2016, 120, 6885–6890. [Google Scholar] [CrossRef]
- Lei, M.; Yang, T.Z.; Wang, W.J.; Huang, K.; Zhang, Y.C.; Zhang, R.; Jiao, R.Z.; Fu, X.L.; Yang, H.J.; Wang, Y.G.; et al. One-dimensional manganese oxide nanostructures as radical scavenger to improve membrane electrolyte assembly durability of proton exchange membrane fuel cells. J. Power Sources 2013, 230, 96–100. [Google Scholar] [CrossRef]
- Trogadas, P.; Parrondo, J.; Ramani, V. CeO2 surface oxygen vacancy concentration governs in situ free radical scavenging efficacy in polymer electrolytes. ACS Appl. Mater. Interfaces 2012, 4, 5098–5102. [Google Scholar] [CrossRef]
- Baker, A.M.; Mukundan, R.; Spernjak, D.; Judge, E.J.; Advani, S.G.; Prasad, A.K.; Borup, R.L. Cerium migration during PEM fuel cell accelerated stress testing. J. Electrochem. Soc. 2016, 163, 1023–1031. [Google Scholar] [CrossRef]
- Stewart, S.M.; Spernjak, D.; Borup, R.; Datye, A.; Garzon, F. Cerium migration through hydrogen fuel cells during accelerated stress testing. ECS Electrochem. Lett. 2014, 3, F19–F22. [Google Scholar] [CrossRef]
- Zatoń, M.; Prélot, B.; Donzel, N.; Rozière, J.; Jones, D.J. Migration of Ce and Mn ions in PEMFC and its impact on PFSA membrane degradation. J. Electrochem. Soc. 2018, 165, F3281–F3289. [Google Scholar] [CrossRef]
- D’Urso, C.; Oldani, C.; Baglio, V.; Merlo, L.; Aricò, A.S. Towards fuel cell membranes with improved lifetime: Aquivion® Perfluorosulfonic Acid membranes containing immobilized radical scavengers. J. Power Sources 2014, 272, 753–758. [Google Scholar] [CrossRef]
- D’Urso, C.; Oldani, C.; Baglio, V.; Merlo, L.; Aricò, A.S. Immobilized transition metal-based radical scavengers and their effect on durability of Aquivion® perfluorosulfonic acid membranes. J. Power Sources 2016, 301, 317–325. [Google Scholar] [CrossRef]
- Park, Y.; Kim, D. Chemical stability enhancement of Nafion membrane by impregnation of a novel organic ·OH radical scavenger, 3,4-dihydroxy-cinnamic acid. J. Memb. Sci. 2018, 566, 1–7. [Google Scholar] [CrossRef]
- Jones, D.J.; Rozière, J. Advances in the development of inorganic–organic membranes for fuel cell applications. In Fuel Cells I—Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2008; Volume 215, pp. 219–264. [Google Scholar]
- Chua, S.; Fang, R.; Sun, Z.; Wu, M.; Gu, Z.; Wang, Y.; Hart, J.N.; Sharma, N.; Li, F.; Wang, D.W. Hybrid solid polymer electrolytes with two-dimensional inorganic nanofillers. Chem. A Eur. J. 2018, 24, 18180–18203. [Google Scholar] [CrossRef] [PubMed]
- Veerabadran, N.G.; Price, R.; Lvov, Y.M. Clays nanotubes for encapsulation and sustaines release of drugs. Nano 2007, 02, 115–120. [Google Scholar] [CrossRef]
- Yuan, P.; Tan, D.; Annabi-Bergaya, F. Properties and applications of halloysite nanotubes: Recent research advances and future prospects. Appl. Clay Sci. 2015, 112–113, 75–93. [Google Scholar] [CrossRef]
- Kausar, A. Review on Polymer/Halloysite Nanotube nanocofmposite. Polym. Plast. Technol. Eng. 2018, 57, 548–564. [Google Scholar] [CrossRef]
- Joussein, E.; Petit, S.; Churchman, J.; Theng, B.; Righi, D.; Delvaux, B. Halloysite clay minerals—A review. Clay Miner. 2005, 40, 383–426. [Google Scholar] [CrossRef]
- Gaaz, T.; Sulong, A.; Kadhum, A.; Al-Amiery, A.; Nassir, M.; Jaaz, A. The impact of halloysite on the thermo-mechanical properties of polymer composites. Molecules 2017, 22, 838. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, A.K.; Tarwal, N.L.; Shinde, P.S.; Kadam, P.M.; Patil, R.S.; Barman, S.R.; Patil, P.S. Effective utilization of spray pyrolyzed CeO2 as optically passive counter electrode for enhancing optical modulation of WO3. Solid State Ionics 2009, 180, 1324–1331. [Google Scholar] [CrossRef]
- Pasbakhsh, P.; Churchman, G.J.; Keeling, J.L. Characterisation of properties of various halloysites relevant to their use as nanotubes and microfibre fillers. Appl. Clay Sci. 2013, 74, 47–57. [Google Scholar] [CrossRef]
- Zargarian, S.S.; Haddadi-Asl, V.; Hematpour, H. Carboxylic acid functionalization of halloysite nanotubes for sustained release of diphenhydramine hydrochloride. J. Nanoparticle Res. 2015, 17, 218. [Google Scholar] [CrossRef]
- Falcón, J.M.; Sawczen, T.; Aoki, I.V. Dodecylamine-Loaded halloysite nanocontainers for active anticorrosion coatings. Front. Mater. 2015, 2, 1–13. [Google Scholar] [CrossRef]
- Lun, H.; Ouyang, J.; Yang, H. Natural halloysite nanotubes modified as an aspirin carrier. RSC Adv. 2014, 4, 44197–44202. [Google Scholar] [CrossRef]
- Bauluz Lázaro, B. Halloysite and kaolinite: Two clay minerals with geological and technological importance. Rev. la Acad. Ciencias Exactas Físicas Químicas y Nat. Zaragoza 2015, 70, 7–38. [Google Scholar]
- Churchman, G.J.; Lowe, D.J. Alteration, formation, and occurrence of minerals in soils introduction: The role of mineralogy in soil science. In Handbook of Soil Science; CRC Press: Boca Raton, FL, USA, 2012; Volume 1, pp. 33–48. [Google Scholar]
- Saklar, S.; Yorukoglu, A. Effects of acid leaching on halloysite. Physicochem. Probl. Miner. Process. 2015, 51, 83–94. [Google Scholar]
- Ambikadevi, V.R.; Lalithambika, M. Effect of organic acids on ferric iron removal from iron-stained kaolinite. Appl. Clay Sci. 2000, 16, 133–145. [Google Scholar] [CrossRef]
- Bediako, E.G.; Nyankson, E.; Dodoo-Arhin, D.; Agyei-Tuffour, B.; Łukowiec, D.; Tomiczek, B.; Yaya, A.; Efavi, J.K. Modified halloysite nanoclay as a vehicle for sustained drug delivery. Heliyon 2018, 4, e00689. [Google Scholar] [CrossRef] [PubMed]
- Abdullayev, E.; Price, R.; Shchukin, D.; Lvov, Y. Halloysite tubes as nanocontainers for anticorrosion coating with benzotriazole. ACS Appl. Mater. Interfaces 2009, 1, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
- Shu, Z.; Zhang, Y.; Ouyang, J.; Yang, H. Characterization and synergetic antibacterial properties of ZnO and CeO2 supported by halloysite. Appl. Surf. Sci. 2017, 420, 833–838. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Yuan, P.; Southon, P.D.; Liu, Z.; Kepert, C.J. Organosilane functionalization of halloysite nanotubes for enhanced loading and controlled release. Nanotechnology 2012, 23, 375705. [Google Scholar] [CrossRef]
- Yuan, P.; Southon, P.D.; Liu, Z.; Green, M.E.R.; Hook, J.M.; Antill, S.J.; Kepert, C.J. Functionalization of Halloysite Clay Nanotubes by Grafting with γ-Aminopropyltriethoxysilane. J. Phys. Chem. C 2008, 112, 15742–15751. [Google Scholar] [CrossRef]
- Joo, Y.; Sim, J.H.; Jeon, Y.; Lee, S.U.; Sohn, D. Opening and blocking the inner-pores of halloysite. Chem. Commun. 2013, 49, 4519–4521. [Google Scholar] [CrossRef]
- Giancola, S.; Zatoń, M.; Reyes-Carmona, Á.; Dupont, M.; Donnadio, A.; Cavaliere, S.; Rozière, J.; Jones, D.J. Composite short side chain PFSA membranes for PEM water electrolysis. J. Memb. Sci. 2019, 570–571, 69–76. [Google Scholar] [CrossRef]
- Baker, A.M.; Babu, S.K.; Mukundan, R.; Advani, S.G.; Prasad, A.K.; Spernjak, D.; Borup, R.L. Cerium ion mobility and diffusivity rates in perfluorosulfonic acid membranes measured via hydrogen pump operation. J. Electrochem. Soc. 2017, 164, F1272–F1278. [Google Scholar] [CrossRef]
- Park, J.; Kim, D. Effect of cerium/18-crown-6-ether coordination complex OH quencher on the properties of sulfonated poly(ether ether ketone) fuel cell electrolyte membranes. J. Memb. Sci. 2014, 469, 238–244. [Google Scholar] [CrossRef]
| Material | Al (wt %) | Si (wt %) | O (wt %) | N (wt %) | Fe (wt %) | Ce (wt %) |
|---|---|---|---|---|---|---|
| HNT | 21.85 | 22.00 | 55.15 | - | 0.34 | - |
| treated HNT | 18.39 | 21.36 | 56.57 | - | 0.23 | - |
| CeO2@HNT | 18.39 | 21.36 | 56.57 | - | 0.23 | 8.0 |
| CeO2@HNT-NH2 | 11.21 | 16.21 | 72.40 | 1.30 | 0.23 | 8.0 |
| Membrane | Young Modulus (N/mm²) | Breaking Strength (N mm²) | Yield Stress (N mm²) |
|---|---|---|---|
| Aquivion® 830 EW | 238 ± 7 | 14 ± 0.7 | 10 ± 0.3 |
| Aquivion® + 4 % CeO2@HNT-NH2 | 237 ± 5 | 11 ± 1.3 | 9 ± 0.6 |
| Aquivion® + 10 % CeO2@HNT-NH2 | 326 ± 9 | 15 ± 1.5 | 12 ± 0.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akrout, A.; Delrue, A.; Zatoń, M.; Duquet, F.; Spanu, F.; Taillades-Jacquin, M.; Cavaliere, S.; Jones, D.; Rozière, J. Immobilisation and Release of Radical Scavengers on Nanoclays for Chemical Reinforcement of Proton Exchange Membranes. Membranes 2020, 10, 208. https://doi.org/10.3390/membranes10090208
Akrout A, Delrue A, Zatoń M, Duquet F, Spanu F, Taillades-Jacquin M, Cavaliere S, Jones D, Rozière J. Immobilisation and Release of Radical Scavengers on Nanoclays for Chemical Reinforcement of Proton Exchange Membranes. Membranes. 2020; 10(9):208. https://doi.org/10.3390/membranes10090208
Chicago/Turabian StyleAkrout, Alia, Aude Delrue, Marta Zatoń, Fanny Duquet, Francesco Spanu, Mélanie Taillades-Jacquin, Sara Cavaliere, Deborah Jones, and Jacques Rozière. 2020. "Immobilisation and Release of Radical Scavengers on Nanoclays for Chemical Reinforcement of Proton Exchange Membranes" Membranes 10, no. 9: 208. https://doi.org/10.3390/membranes10090208
APA StyleAkrout, A., Delrue, A., Zatoń, M., Duquet, F., Spanu, F., Taillades-Jacquin, M., Cavaliere, S., Jones, D., & Rozière, J. (2020). Immobilisation and Release of Radical Scavengers on Nanoclays for Chemical Reinforcement of Proton Exchange Membranes. Membranes, 10(9), 208. https://doi.org/10.3390/membranes10090208