Synthesis of Cis-Cisoid or Cis-Transoid Poly(Phenyl-Acetylene)s Having One or Two Carbamate Groups as Oxygen Permeation Membrane Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Measurements
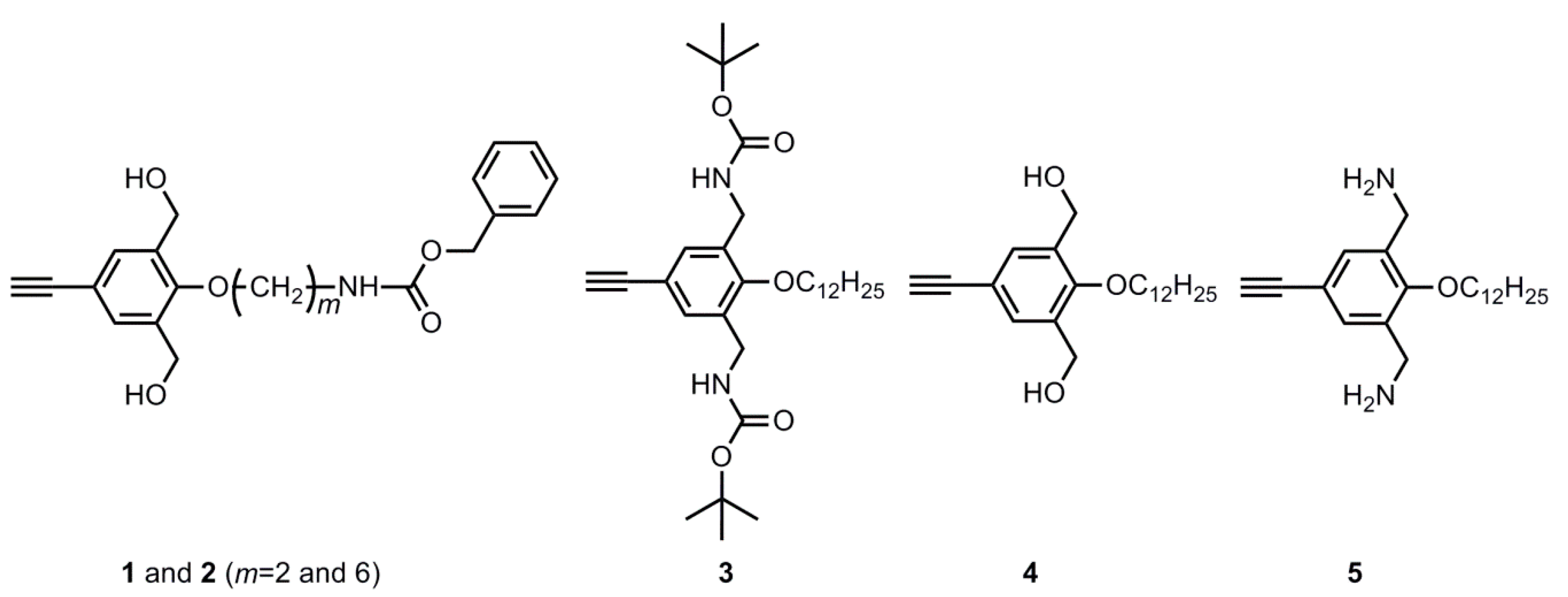
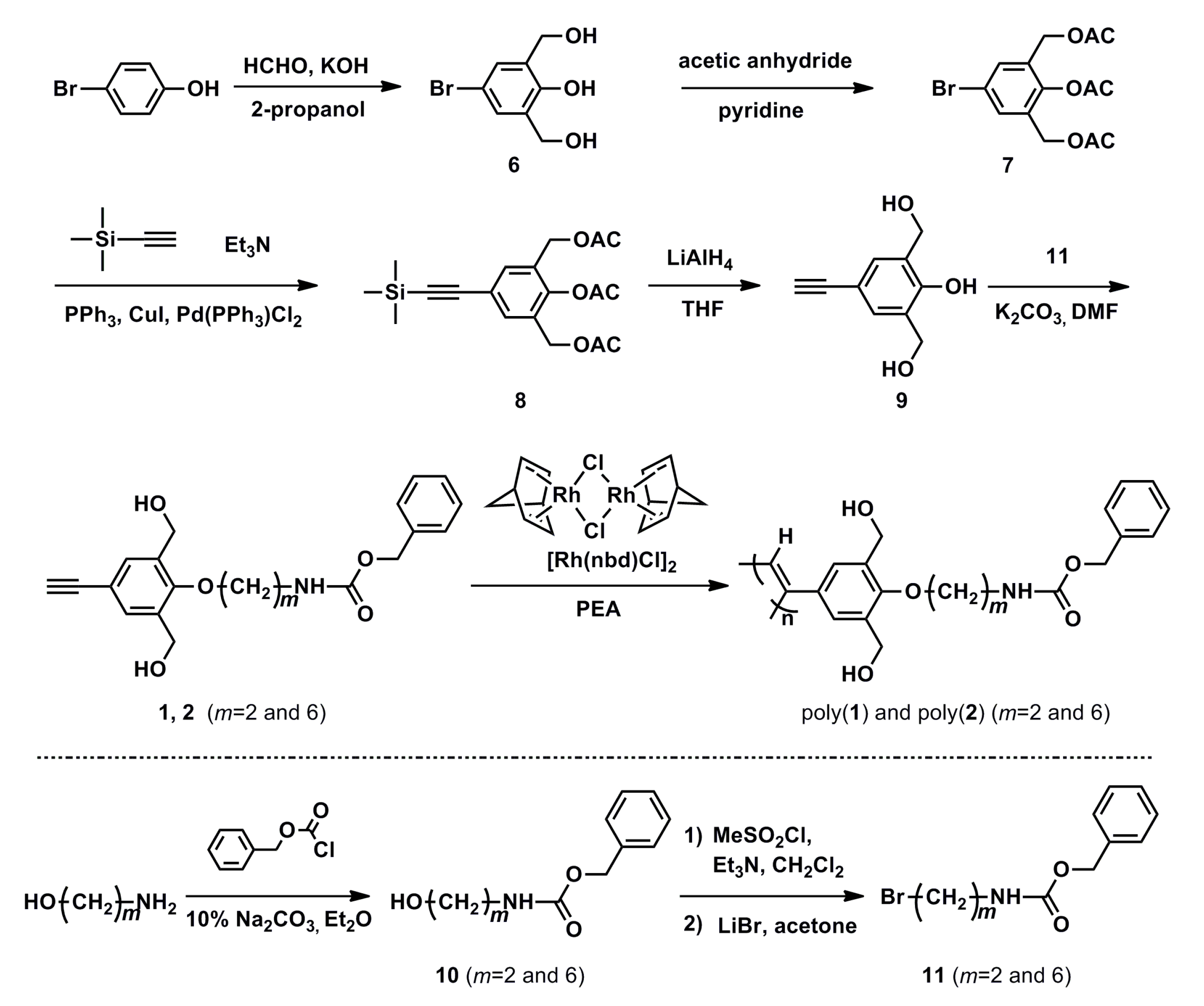
2.3. Synthesis of Monomer 1
2.3.1. N-Benzyloxycarbonyl-2-aminoethanol (10, m = 2)
2.3.2. N-Benzyloxycarbonyl-2-bromoethylamine (11, m = 2)
2.3.3. 4-(N-Benzyloxycarbonyl-2-ethylamino)benzyloxy-3,5-bis(hydroxymethyl)phenylacetylene (1, m = 2)
2.4. Synthesis of Monomer 2
2.4.1. N-(Benzyloxycarbonyl)-6-amino-1-hexanol (10, m = 6)
2.4.2. N-(Benzyloxycarbonyl)-6-bromohexylamine (11, m = 6)
2.4.3. 4-(N-Benzyloxycarbonyl-6-hexylamino)benzyloxy-3,5-bis(hydroxymethyl)phenylacetylene (2, m = 6)
2.5. Synthesis of Monomer 3
2.5.1. 4-Dodecyloxy-3,5-bis(hydroxymethyl)phenylacetylene (4)
2.5.2. 4-Dodecyloxy-3,5-bis(bromomethyl)phenylacetylene (12)
2.5.3. 4-Dodecyloxy-3,5-bis(azidomethyl)phenylacetylene (13)
2.5.4. 4-Dodecyloxy-3,5-bis(aminomethyl)phenylacetylene (5)
2.5.5. 4-Dodecyloxy-3,5-bis(tert-butoxycarbonylamino)) Phenyl Acetylene (3)
2.6. Polymerization of Monomers 1–3
- Poly(1)
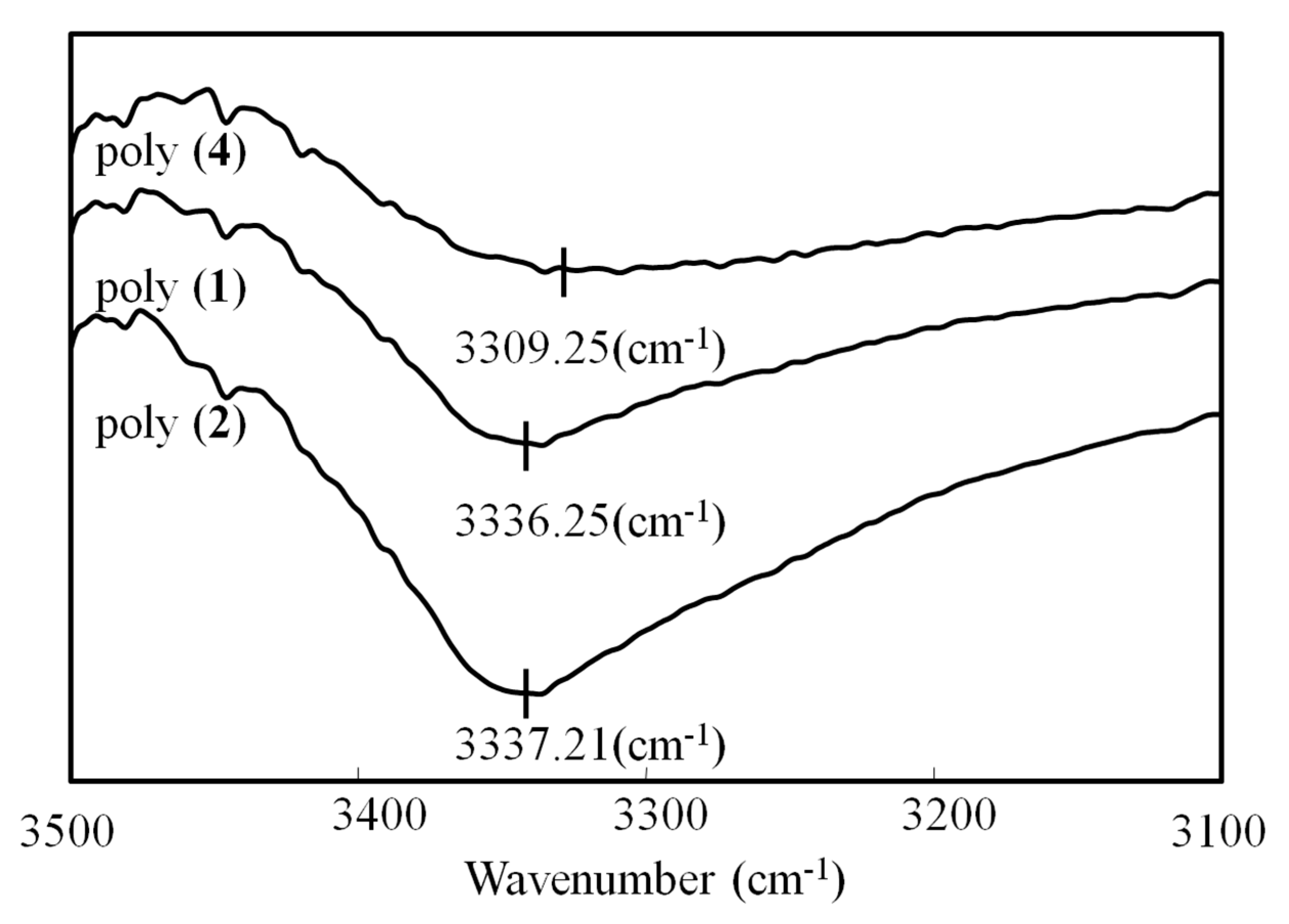
- 1 H NMR(400 MHz, dimethylsulfoxide-d6 (DMSO-d6), δ): 7.37 (br, PhH), 6.68 (br, cis proton in the main chain), 5.70 (br, CH2NHCO), 5.07–4.63 (br, PhCH2OCO, Ph(CH2OH)2), 4.28 (br, Ph(CH2OH)2). IR (cm−1, KBr): 3334 (NH, OH), 1690 (C=O), 1480 (CH), 1261 (C–O), 1096 (C–N).
- Poly(2)
- 1 H NMR(400 MHz, DMSO-d6, δ): 7.38–7.27 (br, PhH), 6.73 (br, cis proton in the main chain), 5.76 (br, CH2NHCO), 5.29–4.68 (br, PhCH2OCO, Ph(CH2OH)2), 4.33 (br, Ph(CH2OH)2), 3.04 (br, CH2CH2NH, PhOCH2CH2,), 1.46–1.30 (t, 6H, CH2(CH2)3NH). IR (cm−1, KBr): 3334 (NH, OH), 1690 (C=O), 1480 (CH), 1261 (C–O), 1096 (C–N).
- Poly(3)
- 1 H NMR(400 MHz, CDCl3, TMS, δ): 7.50 (br, PhH), 5.10 (br, Ph(CH2NHCO)2), 3.48 (br, Ph(CH2NH)2, PhOCH2CH2), 2.02 (br, PhOCH2CH2CH2), 1.66–1.54 (br, ((OCH3)3)2), 1.23–1.09 (br, CH2(CH2)9CH3), 0.86 (br, CH2CH3). IR (cm−1, KBr): 3370 (N–H), 1691 (C=O), 1480 (CH), 1168 (C–O), 1096 (C–N).
2.7. Membrane Preparation
2.8. Estimation of Polymers as Oxygen Permeation Membranes
2.8.1. Membrane Strength
2.8.2. Oxygen Permeation
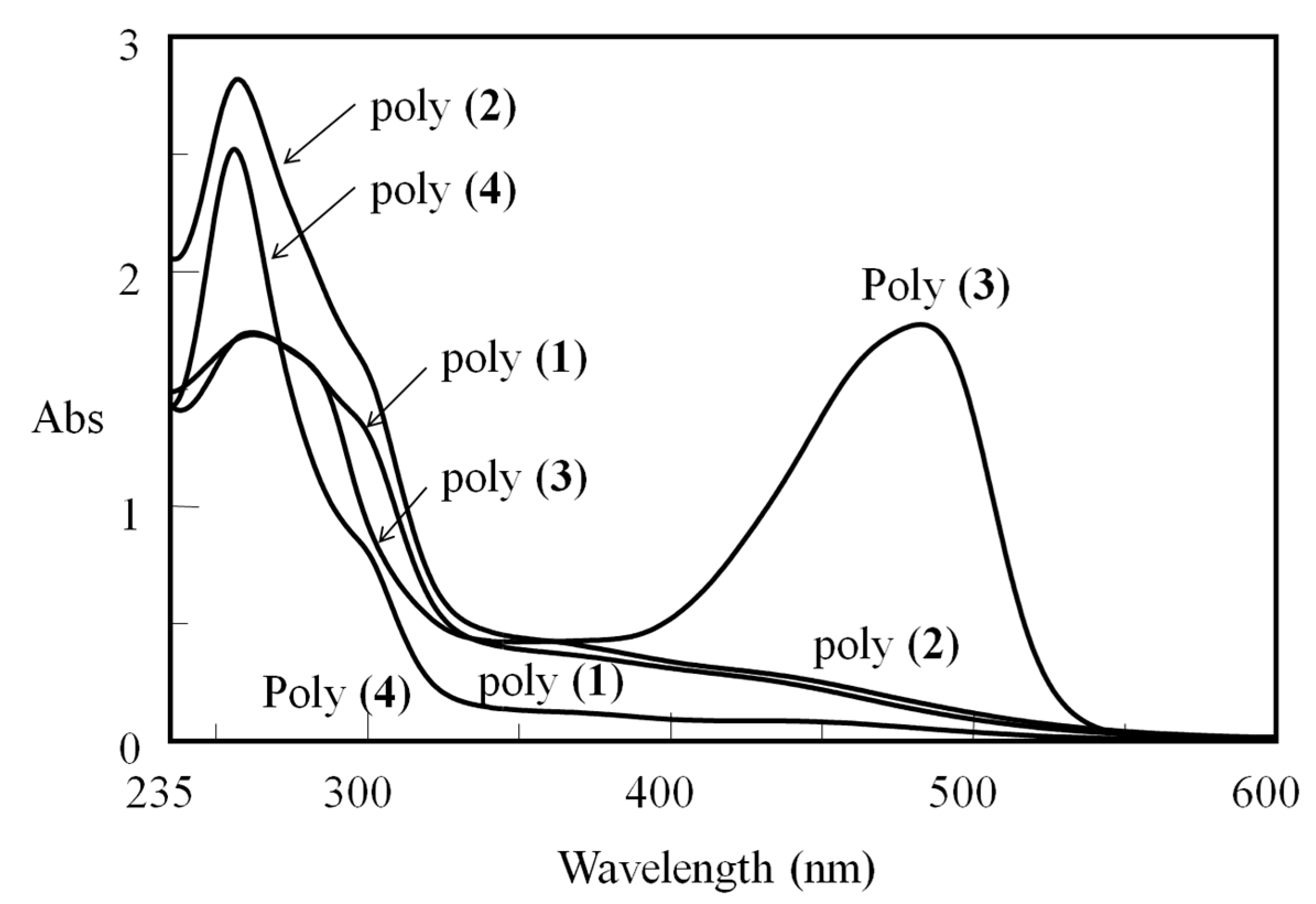
3. Results and Discussion
3.1. Synthesis of Monomers 1–3
3.2. Synthesis of poly(1)–poly(3)
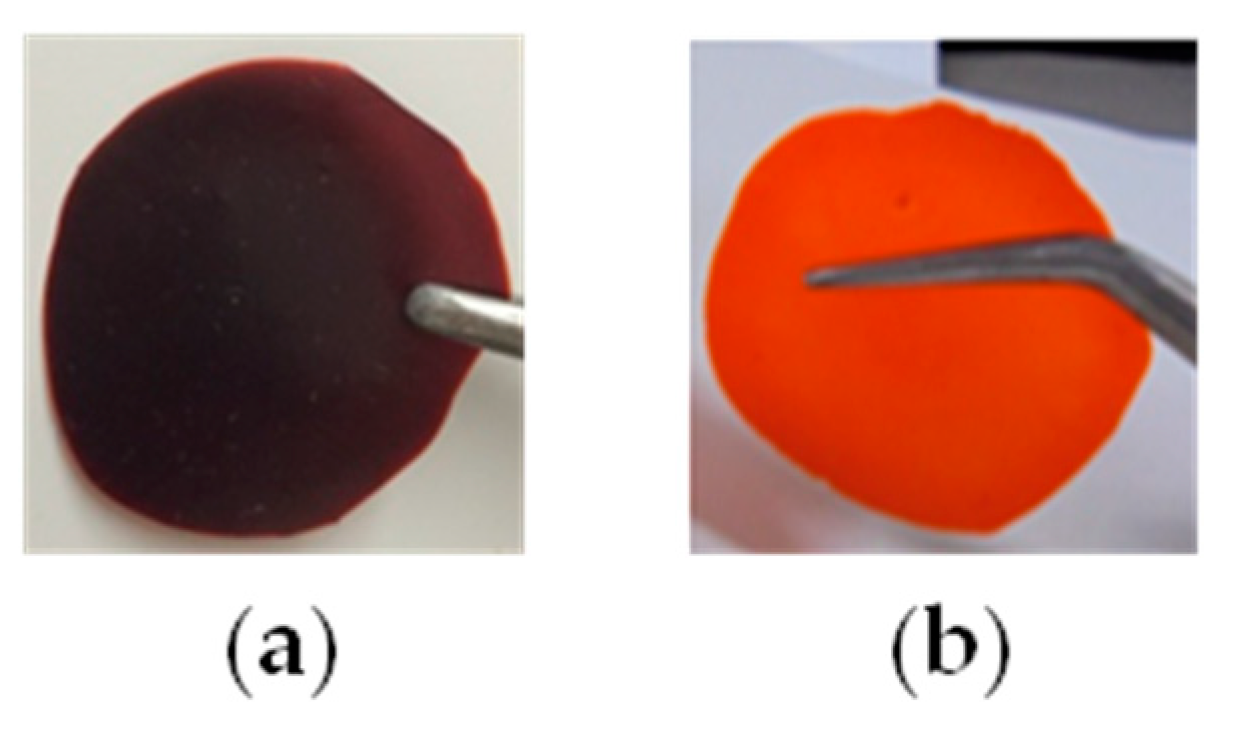
3.3. Effects of the Main Chain Conformation on the Solubility and Membrane Strengths
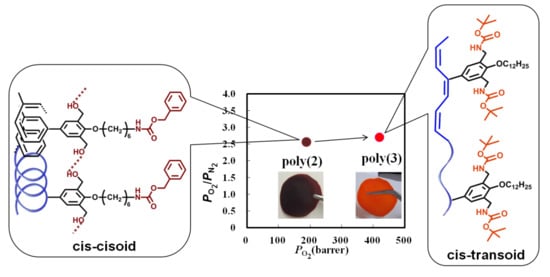
3.4. Oxygen Permeability of the Membranes from the New Polymers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Appendix A
- If these polymers made hydrogen bonds intermolecularly, it also affected their solubility largely. However, we have already reported that these polymers from the monomers having two hydroxy groups (such as 1, 2 and 4) had intramolecular hydrogen bonds because the long alkyl groups could prevent from forming intermolecular hydrogen bonds. Therefore, the effects of the intermolecular hydrogen bonds on the solubility is thought to be not large.
- Since the synthesis of these monomers needed multi-step synthesis, the total yields were not high (5.5–7.7%).
References
- Ito, T.; Shirakawa, H.; Ikeda, S. Simultaneous polymerization and formation of polyacetylene film on the surface of concentrated soluble Ziegler-type catalyst solution. J. Polym. Sci. Part A Polym. Chem. 1974, 12, 11–20. [Google Scholar] [CrossRef]
- Shirakawa, H.; Louis, E.J.; MacDiarmid, A.G.; Chiang, C.K.; Heeger, A.J. Synthesis of electrically conducting organic polymers: Halogen derivatives of polyacetylene, (CH)x. J. Chem. Soc. Chem. Commun. 1977, 578–580. [Google Scholar] [CrossRef]
- Chiang, C.K.; Fincher, C.R.; Park, Y.W.; Heeger, A.J.; Shirakawa, H.; Louis, E.J.; Gau, S.C.; MacDiarmid, A.G. Electrical conductivity in doped polyacetylene. Phys. Rev. Lett. 1977, 39, 1098–1101. [Google Scholar] [CrossRef]
- MacDiarmid, A.G. “Synthetic metals”: A novel role for organic polymers (Nobel Lecture). Angew. Chem. Int. Ed. 2001, 40, 2581–2590. [Google Scholar] [CrossRef]
- Shirakawa, H. The discovery of polyacetylene film: The dawning of an era of conducting polymers (Nobel Lecture). Angew. Chem. Int. Ed. 2001, 40, 2574–2580. [Google Scholar] [CrossRef]
- Heeger, A.J. Semiconducting and metallic polymers: The fourth generation of polymeric materials (Nobel Lecture). Angew. Chem. Int. Ed. 2001, 40, 2591–2611. [Google Scholar] [CrossRef]
- Alagi, K. Helical polyacetylene: Asymmetric polymerization in a chiral liquid-crystal field. Chem. Rev. 2009, 109, 5354–5401. [Google Scholar]
- Liu, J.; Lam, J.W.Y.; Tang, B.Z. Acetylenic polymers: Syntheses, structures, and functions. Chem. Rev. 2009, 109, 5799–5867. [Google Scholar] [CrossRef]
- Rudick, J.G.; Percec, V. Induced helical backbone conformations of self-organizable dendronized polymers. Acc. Chem. Res. 2008, 41, 1641–1652. [Google Scholar] [CrossRef]
- Percec, V.; Aqad, E.; Peterca, M.; Rudick, J.G.; Lemon, L.; Ronda, J.C.; De, B.B.; Heiney, P.A.; Meijer, E.W. Steric communication of chiral information observed in dendronized polyacetylenes. J. Am. Chem. Soc. 2006, 128, 16365–16372. [Google Scholar] [CrossRef]
- Yashima, E.; Maeda, K.; Iida, H.; Furusho, Y.; Nagai, K. Helical polymers: Synthesis, structures, and functions. Chem. Rev. 2009, 109, 6102–6211. [Google Scholar] [CrossRef] [PubMed]
- Shiotsuki, M.; Sanda, F.; Masuda, T. Polymerization of substituted acetylenes and features of the formed polymers. Polym. Chem. 2011, 2, 1044–1058. [Google Scholar] [CrossRef]
- Motoshige, R.; Mawatari, Y.; Motoshige, A.; Yoshida, Y.; Sasaki, T.; Yoshimizu, H.; Suzuki, T.; Tsujita, Y.; Tabata, M. Mutual conversion between stretched and contracted helices accompanied by a drastic change in color and spatial structure of poly(phenylacetylene) prepared with a [Rh(nbd)Cl]2-amine catalyst. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 752–759. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Aoki, T.; Kaneko, T.; Teraguchi, M.; Shi, Z.; Jia, H. Subnanoporous highly oxygen pemselective membranes from poly(conjugated hyperbranched macromonomer)s synthesized by one-pot simultaneous two-mode homopolymerization of 1,3-bis(silyl)phenylacetylene using a single Rh catalytic system: Control of their structures and pemselectivities. Macromolecules 2017, 50, 7121–7136. [Google Scholar]
- Liu, L.; Zhang, G.; Aoki, T.; Wang, Y.; Kaneko, T.; Teraguchi, M.; Zhang, C.; Dong, H. Synthesis of one-handed helical block copoly(substituted acetylene)s consisting of dynamic cis-transoidal and static cis-cisoidal block: Chiral teleinduction in helix-sense-selective polymerization using a chiral living polymer as an initiator. ACS Macro Lett. 2016, 5, 1381–1385. [Google Scholar] [CrossRef]
- Jin, Y.; Aoki, T.; Kwak, G. Control of intamolecular hydrogen bonding in a conformation-switchable helical spring polymer by solvent and temperature. Angew. Chem. Int. Ed. 2020, 59, 1837–1844. [Google Scholar] [CrossRef] [PubMed]
- Aoki, T.; Kaneko, T.; Maruyama, N.; Sumi, A.; Takahashi, M.; Sato, T.; Teraguchi, M. Helix-sense-selective polymerization of phenylacetylene having two hydroxy groups using a chiral catalytic system. J. Am. Chem. Soc. 2003, 125, 6346–6347. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Namikoshi, T.; Zang, Y.; Aoki, T.; Hadano, S.; Abe, Y.; Wasuzu, I.; Tsutsuba, T.; Teraguchi, M.; Kaneko, T. Top-down preparation method of self-supporting supramolecular polymeric membranes using highly selective photocyclicaromatization of cis-cisoid helical poly(phenylacetylene)s in membrane state. J. Am. Chem. Soc. 2013, 135, 602–605. [Google Scholar] [CrossRef]
- Teraguchi, M.; Aoki, T.; Kaneko, T.; Tanioka, D. Helix-sense-selective polymerization of achiral phenylacetylenes with two N-alkylamide groups to generate the one-handed helical polymers stabilized by intramolecular hydrogen bonds. ACS Macro Lett. 2012, 1, 1258–1261. [Google Scholar] [CrossRef]
- Sanda, F.; Endo, T. Syntheses and functions of polymers based on amino acids. Macromol. Chem. Phys. 1999, 200, 2651–2661. [Google Scholar] [CrossRef]
- Bauri, K.; Roy, S.G.; De, P. Side-chain amino-acid-derived cationic chiral polymers by controlled radical polymerization. Macromol. Chem. Phys. 2015, 217, 365–379. [Google Scholar] [CrossRef]
- Suyama, K.; Shirai, M. Photobase generators: Recent progress and application trend in polymer systems. Prog. Polym. Sci. 2009, 34, 194–209. [Google Scholar] [CrossRef]
- Guo, X.; Facchetti, A.; Marks, T.J. Imide-and amide-functionalized polymer semiconductors. Chem. Rev. 2014, 114, 8943–9021. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Sanda, F.; Masuda, T. Synthesis and properties of amino acid-based polyacetylenes. Macromolecules 2003, 36, 3932–3937. [Google Scholar] [CrossRef]
- Sogawa, H.; Shiotsuki, M.; Sanda, F. α-Propargyl amino acid-derived optically active novel substituted polyacetylenes: Synthesis, secondary structures, and responsiveness to ions. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 2008–2018. [Google Scholar] [CrossRef]
- Shirakawa, Y.; Suzuki, Y.; Terada, K.; Shiotsuki, M.; Masuda, T.; Sanda, F. Synthesis and secondary structure of poly(1-methylpropargyl-N-alkylcarbamate)s. Macromolecules 2010, 43, 5575–5581. [Google Scholar] [CrossRef]
- Liu, R.; Shiotsuki, M.; Masuda, T.; Sanda, F. Synthesis and chiroptical properties of hydroxyphenylglycine-based poly(m-phenyleneethynylene-p-phenyleneethynylene)s. Macromolecules 2009, 42, 6115–6122. [Google Scholar] [CrossRef]
- Saeed, I.; Khan, F.Z.; Shiotsuki, M.; Masuda, T. Synthesis and properties of carbamate- and amine-containing poly(phenylacetylenes). J. Polym. Sci. Part A Polym. Chem. 2009, 47, 1853–1863. [Google Scholar] [CrossRef]
- Sanda, F.; Yukawa, Y.; Masuda, T. Synthesis and properties of optically active substituted polyacetylenes having carboxyl and/or amino groups. Polymer 2004, 45, 849–854. [Google Scholar] [CrossRef]
- Zang, Y.; Aoki, T.; Teraguchi, M.; Kaneko, T.; Ma, L.; Jia, H. Synthesis and oxygen permeation of novel polymers of phenylacetylenes having two hydroxyl groups via different lengths of spacers. Polymer 2015, 56, 199–206. [Google Scholar] [CrossRef]
- Yamada, Y.K.; Okada, C.; Yoshida, K.; Umeda, Y.; Arima, S.; Sato, N.; Kai, T.; Takayanagi, H.; Harigaya, Y. Convenient synthesis of 7’ and 6’-bromo-D-tryptophan and their derivatives by enzymatic optical resolution using D-aminoacylase. Tetrahedron 2002, 58, 7851–7861. [Google Scholar] [CrossRef]
- Carrasco, M.R.; Alvarado, C.I.; Dashner, S.T.; Wong, A.J.; Wong, M.A. Synthesis of aminooxy and N-alkylaminooxy amines for use in bioconjugation. J. Org. Chem. 2010, 75, 5757–5759. [Google Scholar] [CrossRef] [PubMed]
- Nativi, C.; Francesconi, O.; Gabrielli, G.; Simone, I.D.; Turchetti, B.; Mello, T.; Mannelli, L.D.C.; Ghelardini, C.; Buzzini, P.; Roelens, S. Aminopyrrolic synthetic receptors for monosaccharides: A class of carbohydrate-binding agents endowed with antibiotic activity versus pathogenic yeasts. Chem. Eur. J. 2012, 18, 5064–5072. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Aoki, T.; Shoji, K.; Teraguchi, M.; Kaneko, T.; Ma, L.; Jia, H.; Miao, F. Synthesis and oxygen permeation of novel well-defined homopoly(phenylacetylene)s with different sizes and shapes of oligosiloxanyl side groups. J. Membrane Sci. 2018, 56, 26–38. [Google Scholar] [CrossRef]

| No. | Monomer b | Rf c | Solvent | Yield (%) d | Mw (×106) e | Mw/Mne |
|---|---|---|---|---|---|---|
| 1 | 1 | 0.20 | THF | 52.4 | 4.80 | 2.32 |
| 2 | 2 | 0.30 | THF | 38.0 | 1.40 | 6.50 |
| 3 | 3 | 0.93 | toluene | 72.4 | 2.90 | 4.31 |
| 4 | 4 | 0.80 | toluene | 43.2 | 3.10 | 5.40 |
| 5 | 5 | 0.00 | toluene | 4.60 | - f | - f |
| No. | Polymer | Solubility a | Membrane-Forming Ability b | Maximum Flexural Stress (×103) (σ/KPa) c | Color d | ||
|---|---|---|---|---|---|---|---|
| Toluene | THF | DMF | |||||
| 1 | poly(1) | − | + | + | + e | 0.968 | deep red |
| 2 | poly(2) | − | + | + | ++ e | 2.40 | deep red |
| 3 | poly(3) | ++ | ++ | − | +++ f | 53.6 | orange |
| 4 | poly(4) | + | + | − | + f | 4.29 | deep red |
| 5 | poly(5) | − | − | − | − g | − g | yellow h |
| No. | Membrane a | PO2 (Barrer) b | PO2/PN2 | DO2c | DO2/DN2 | SO2d | SO2/SN2 |
|---|---|---|---|---|---|---|---|
| 1 | poly(2) | 188 | 2.56 | 11.6 | 1.05 | 16.1 | 2.44 |
| 2 | poly(3) | 420 | 2.70 | 184 | 1.06 | 2.28 | 2.54 |
| 3 | poly(4) | 3.09 | 3.04 | 3.41 | 1.25 | 0.909 | 2.44 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zang, Y.; Lun, Y.; Teraguchi, M.; Kaneko, T.; Jia, H.; Miao, F.; Zhang, X.; Aoki, T. Synthesis of Cis-Cisoid or Cis-Transoid Poly(Phenyl-Acetylene)s Having One or Two Carbamate Groups as Oxygen Permeation Membrane Materials. Membranes 2020, 10, 199. https://doi.org/10.3390/membranes10090199
Zang Y, Lun Y, Teraguchi M, Kaneko T, Jia H, Miao F, Zhang X, Aoki T. Synthesis of Cis-Cisoid or Cis-Transoid Poly(Phenyl-Acetylene)s Having One or Two Carbamate Groups as Oxygen Permeation Membrane Materials. Membranes. 2020; 10(9):199. https://doi.org/10.3390/membranes10090199
Chicago/Turabian StyleZang, Yu, Yinghui Lun, Masahiro Teraguchi, Takashi Kaneko, Hongge Jia, Fengjuan Miao, Xunhai Zhang, and Toshiki Aoki. 2020. "Synthesis of Cis-Cisoid or Cis-Transoid Poly(Phenyl-Acetylene)s Having One or Two Carbamate Groups as Oxygen Permeation Membrane Materials" Membranes 10, no. 9: 199. https://doi.org/10.3390/membranes10090199
APA StyleZang, Y., Lun, Y., Teraguchi, M., Kaneko, T., Jia, H., Miao, F., Zhang, X., & Aoki, T. (2020). Synthesis of Cis-Cisoid or Cis-Transoid Poly(Phenyl-Acetylene)s Having One or Two Carbamate Groups as Oxygen Permeation Membrane Materials. Membranes, 10(9), 199. https://doi.org/10.3390/membranes10090199