Long-Term Stable 1-butyl-3-methylimidazolium Hexafluorophosphate/Ag Metal Composite Membranes for Facilitated Olefin Transport
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Separation Performance
2.3. Characterization
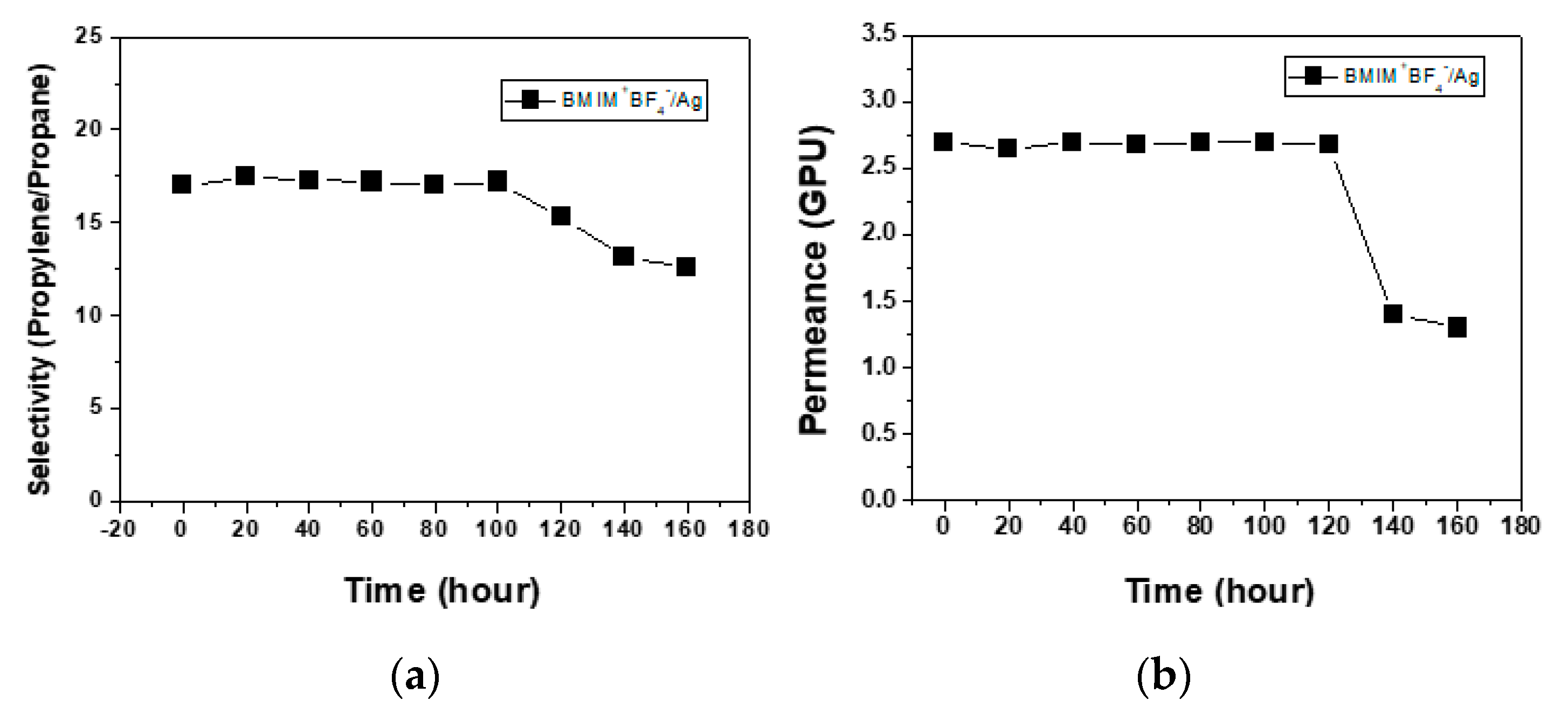
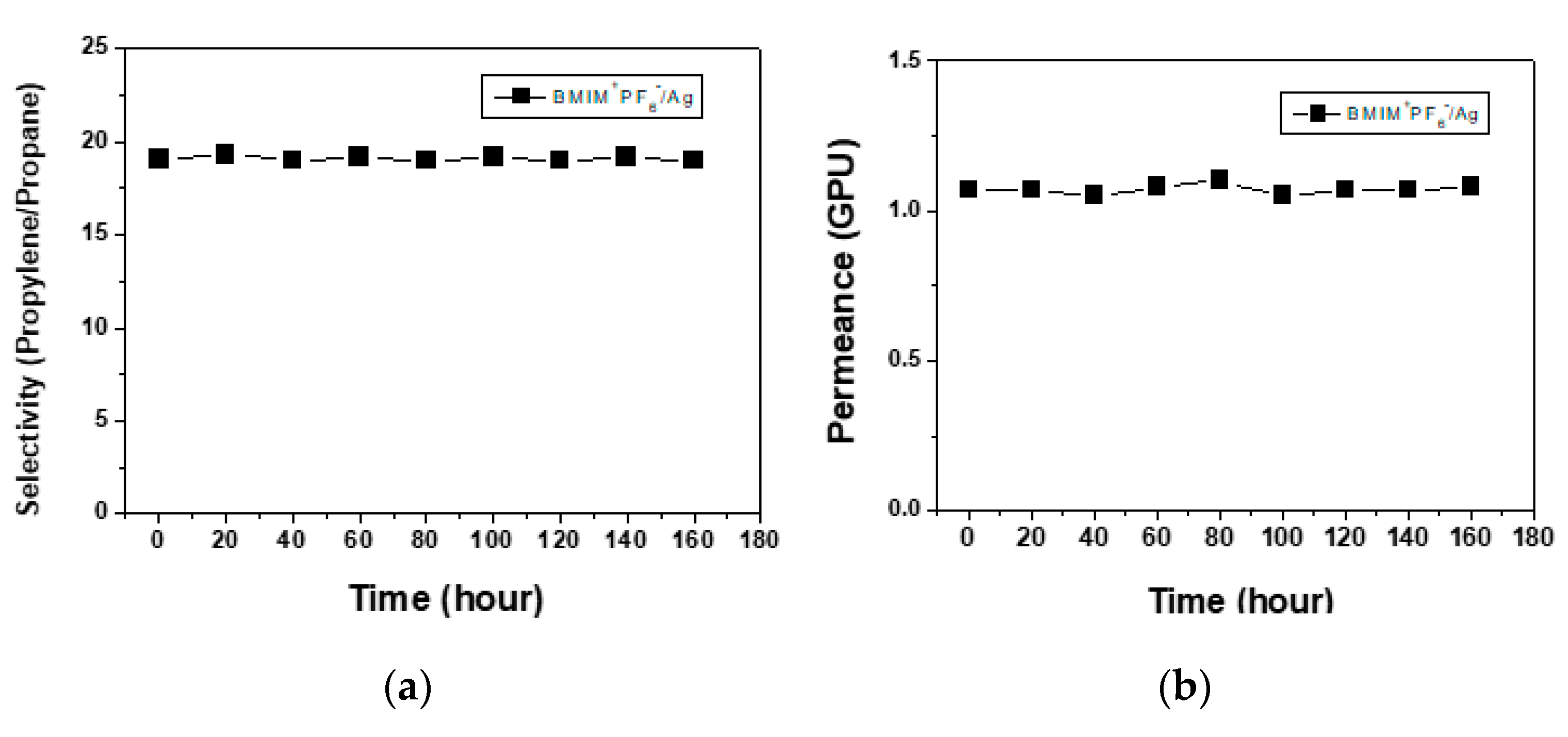
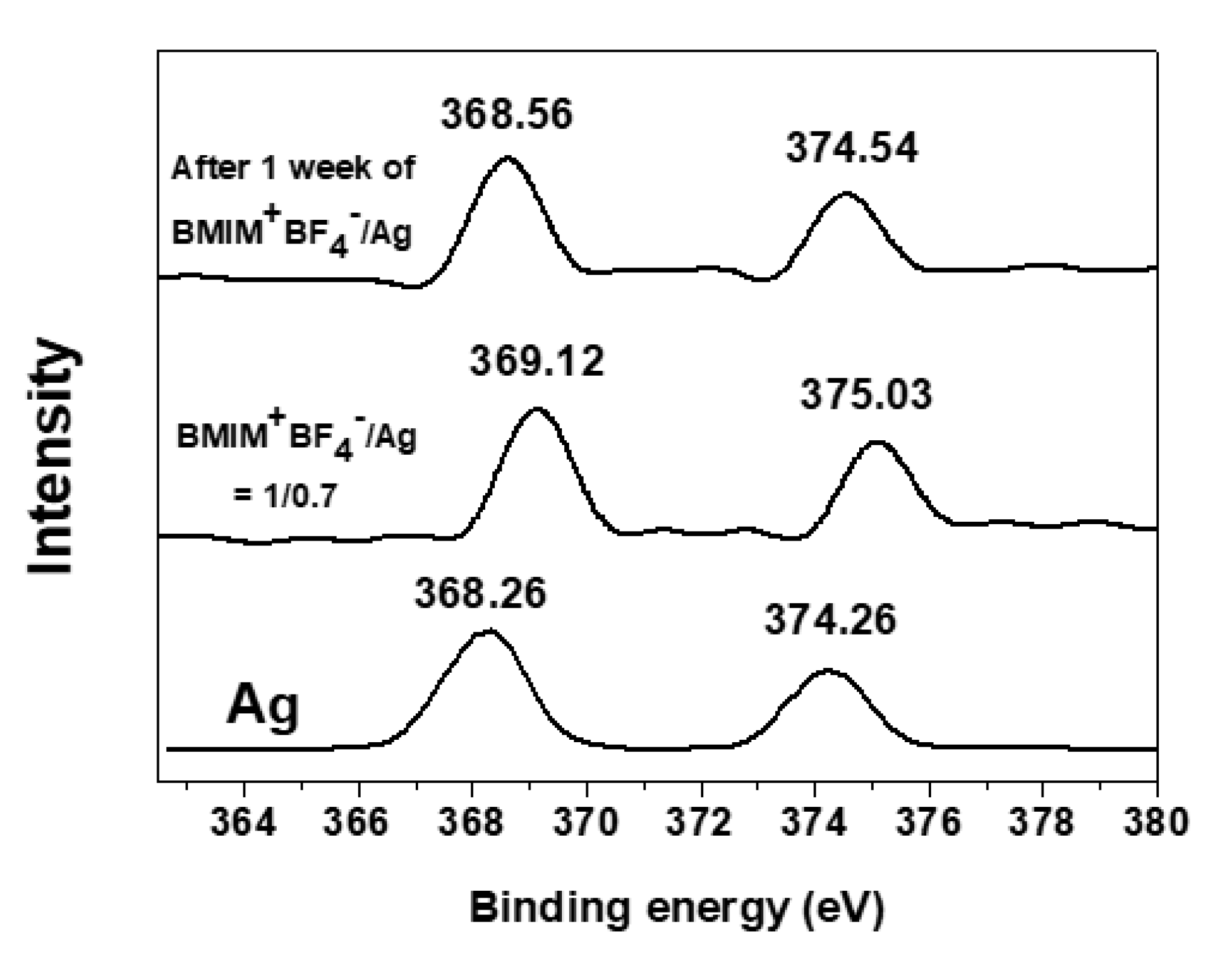
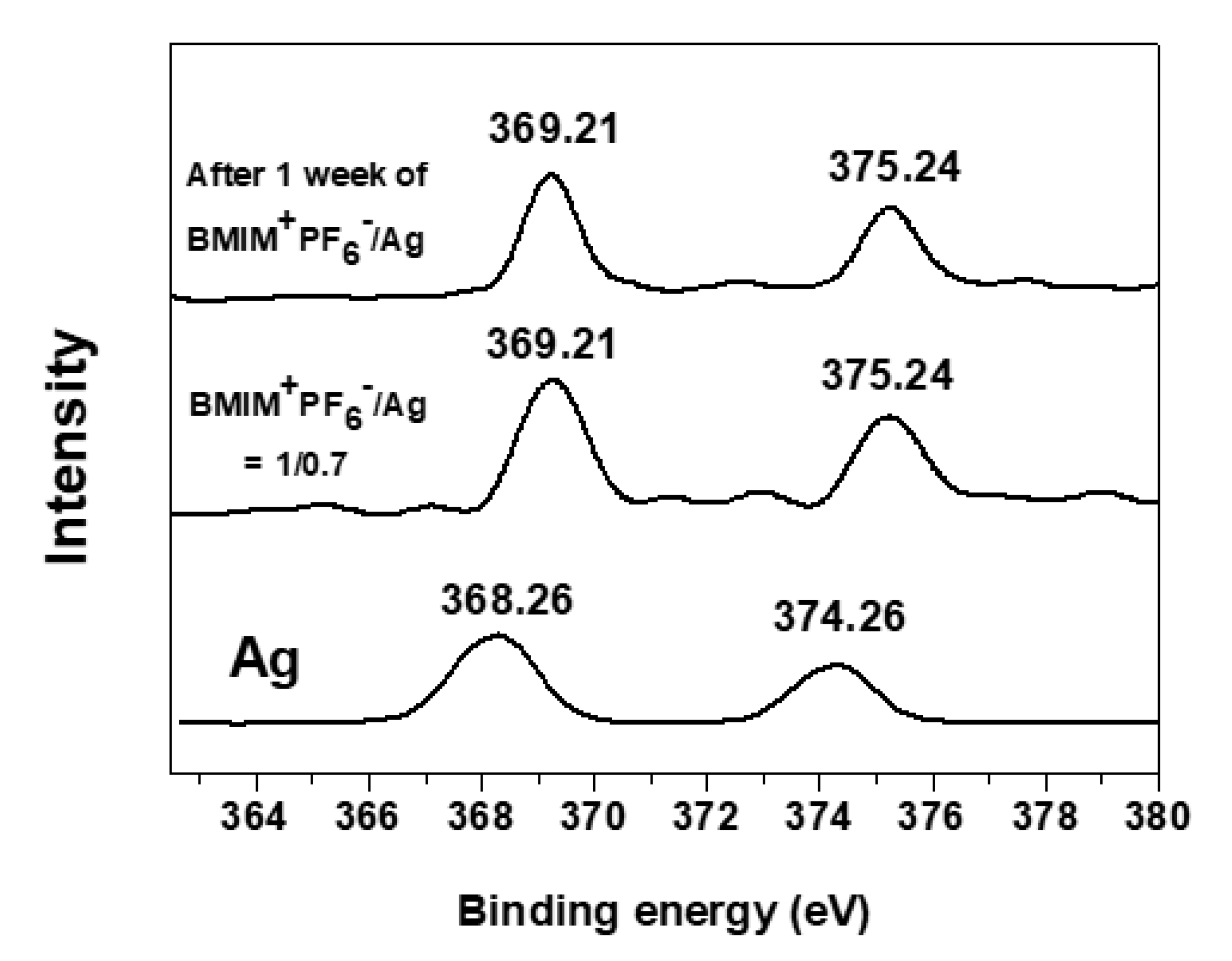
3. Results
3.1. Performance in Separation of Propylene/Propane Mixtures
3.2. Change of Binding Energies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sunderrajan, S.; Freeman, B.D.; Hall, C.K.; Pinnau, I. Propane and propylene sorption in solid polymer electrolytes based on poly(ethylene oxide) and silver salts. J. Membr. Sci. 2001, 182, 1. [Google Scholar] [CrossRef]
- Hamid, M.R.A.; Jeong, H.-K. Recent advances on mixed-matrix membranes for gas separation: Opportunities and engineering challenges. Korean J. Chem. Eng. 2018, 35, 1577. [Google Scholar] [CrossRef]
- Kim, S.Y.; Cho, Y.; Kang, S.W. Preparation and characterization of PEBAX-5513/AgBF4/BMIMBF4 membranes for olefin/paraffin separation. Polymers 2020, 12, 1550. [Google Scholar] [CrossRef] [PubMed]
- Zarca, R.; Ortiz, A.; Gorri, D.; Ortiz, I. A practical approach to fixed-site-carrier facilitated transport modeling for the separation of propylene/propane mixtures through silver containing polymeric membranes. Sep. Purif. Tech. 2017, 180, 82. [Google Scholar] [CrossRef]
- Kim, S.Y.; Cho, Y.; Kang, S.W. Correlation between functional group and separation performance in PEBAX/Ag salt/Al salt complexes for olefin separation. Polymers 2020, 12, 667. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.W.; Kang, S.W. Effect of functional group ratio in PEBAX copolymer on separation performance for facilitated olefin transport membranes. Sci. Rep. 2019, 9, 11454–11459. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.; Kang, S.W. Hybrid effect of Ag ions and polarized Ag nanoparticles in poly(ethylene oxide)/AgBF4/ionic liquid composites for long-term stable membranes. Polym. Compos. 2019, 40, 2745. [Google Scholar] [CrossRef]
- Lee, J.H.; Sudhager, P.; Cho, Y.; Kang, S.W. Effect of temperature on separation performance in ionic liquid/Ag nanocomposite membranes for olefin/paraffin mixtures. J. Ind. Eng. Chem. 2019, 74, 103. [Google Scholar] [CrossRef]
- Jeon, H.; Kang, S.W. Poly(ethylene oxide)/Ag ions and nanoparticles/1-hexyl-3-methylimidazolium tetrafluoroborate composite membranes with long-term stability for olefin/paraffin separation. RSC Adv. 2019, 9, 4771. [Google Scholar] [CrossRef]
- Wang, K.; Stiefel, E.I. Toward Separation and Purification of Olefins Using Dithiolene Complexes: An electrochemical Approach. Science 2001, 291, 106. [Google Scholar] [CrossRef] [PubMed]
- McKenna, M.; Wright, L.L.; Miller, D.J.; Tanner, L.; Haltiwanger, R.C.; DuBois, M.R. Synthesis of inequivalently bridged cyclopentadienyl dimers of molybdenum and a comparison of their reactivities with unsaturated molecules and with hydrogen. J. Am. Chem. Soc. 1983, 105, 5329. [Google Scholar] [CrossRef]
- Chae, I.S.; Kang, S.W.; Park, J.Y.; Lee, Y.G.; Lee, J.H.; Won, J.; Kang, Y.S. Surface energy level tuning of silver nanoparticle for facilitated olefin transport. Angew. Chem. Intern. Edit. 2011, 123, 3038–3041. [Google Scholar] [CrossRef]
- Hong, G.H.; Song, D.; Chae, I.S.; Oh, J.H.; Kang, S.W. Highly permeable poly(ethylene oxide) with silver nanoparticles for facilitated olefin transport. RSC Adv. 2014, 4, 4905. [Google Scholar] [CrossRef]
- Kim, M.; Kang, S.W. PEBAX-1657/Ag nanoparticles/7,7,8,8-tetracyanoquinodimethane complex for highly permeable composite membranes with long-term stability. Sci. Rep. 2019, 9, 4266. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.W.; Char, K.; Kang, Y.S. Formation of Partially Positively Charged Surface of Silver Nanoparticles by Ionic Liquid and Application for Facilitated Olefin Transport Membrane for Olefin/Parffin Separation. Chem. Mater. 2008, 20, 1308–1311. [Google Scholar] [CrossRef]



| Mixed-Gas Selectivity | Permeance (GPU) | |
|---|---|---|
| BMIM+BF4−/Ag | 17 | 2.7 |
| BMIM+PF6−/Ag | 19 | 1.1 |
| BMIM+BF4− | BMIM+PF6− | |
|---|---|---|
| Chemical structure | 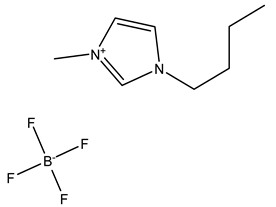 | 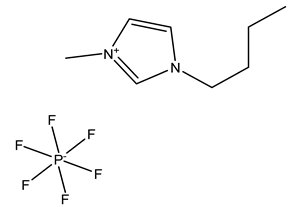 |
| Melting point | −80 °C | −8 °C |
| Solubility in water | soluble | insoluble |
| Viscosity (Pa · s) | 0.0325 | 0.05 |
| Henry’s constants (mol·kg−1·kPa−1) of C3H6 (298 K) | 5.7·10−4 | - |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, S.W. Long-Term Stable 1-butyl-3-methylimidazolium Hexafluorophosphate/Ag Metal Composite Membranes for Facilitated Olefin Transport. Membranes 2020, 10, 191. https://doi.org/10.3390/membranes10080191
Kang SW. Long-Term Stable 1-butyl-3-methylimidazolium Hexafluorophosphate/Ag Metal Composite Membranes for Facilitated Olefin Transport. Membranes. 2020; 10(8):191. https://doi.org/10.3390/membranes10080191
Chicago/Turabian StyleKang, Sang Wook. 2020. "Long-Term Stable 1-butyl-3-methylimidazolium Hexafluorophosphate/Ag Metal Composite Membranes for Facilitated Olefin Transport" Membranes 10, no. 8: 191. https://doi.org/10.3390/membranes10080191
APA StyleKang, S. W. (2020). Long-Term Stable 1-butyl-3-methylimidazolium Hexafluorophosphate/Ag Metal Composite Membranes for Facilitated Olefin Transport. Membranes, 10(8), 191. https://doi.org/10.3390/membranes10080191




