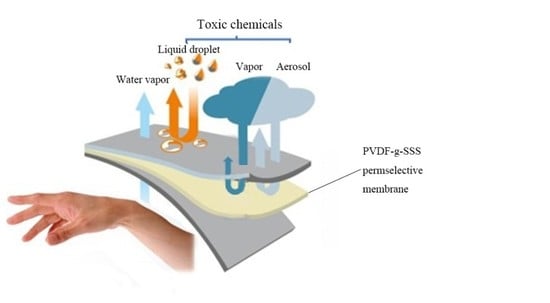
Preparation and Chemical Protective Clothing Application of PVDF Based Sodium Sulfonate Membrane
Abstract
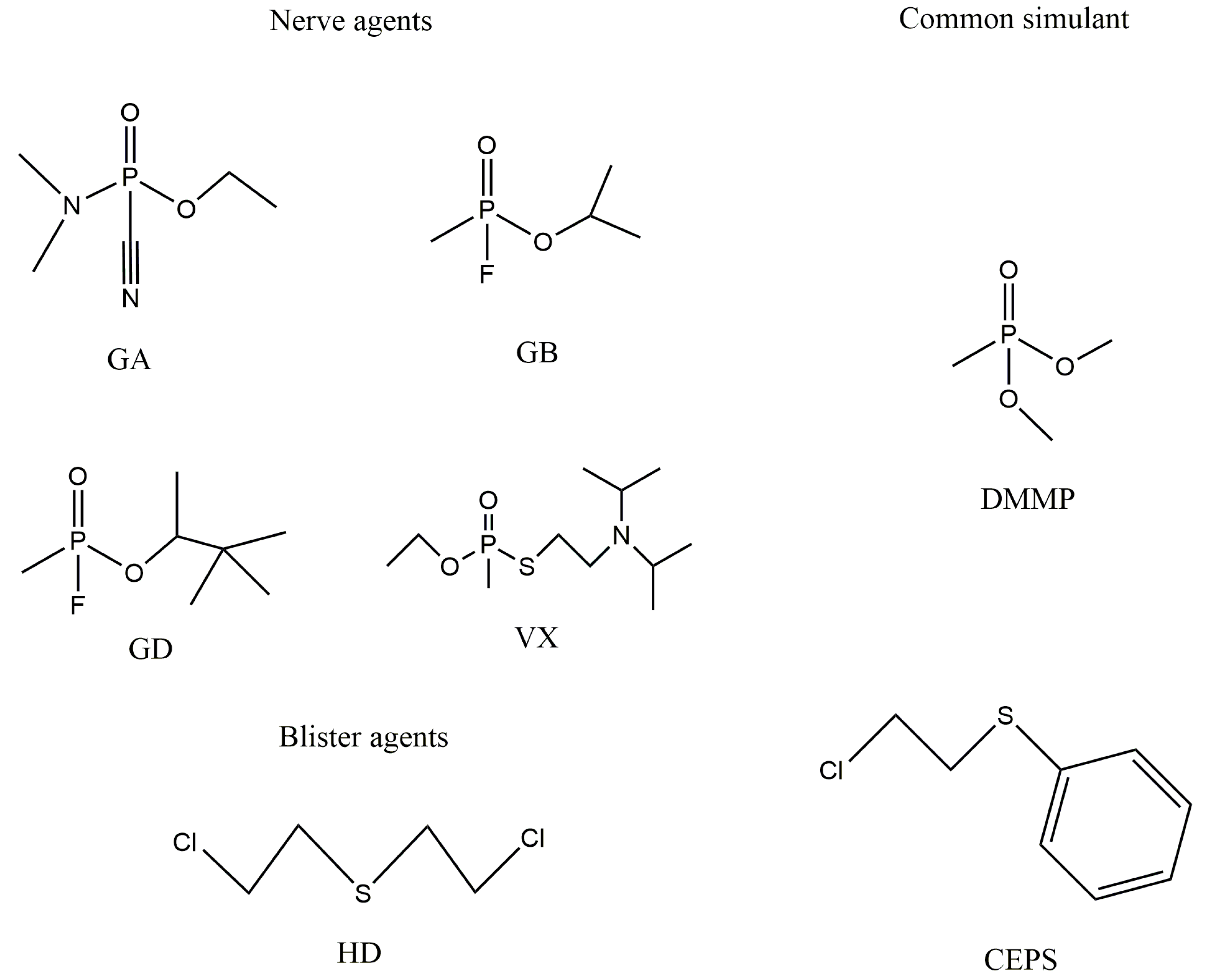
1. Introduction
2. Materials and Methods
2.1. Materials
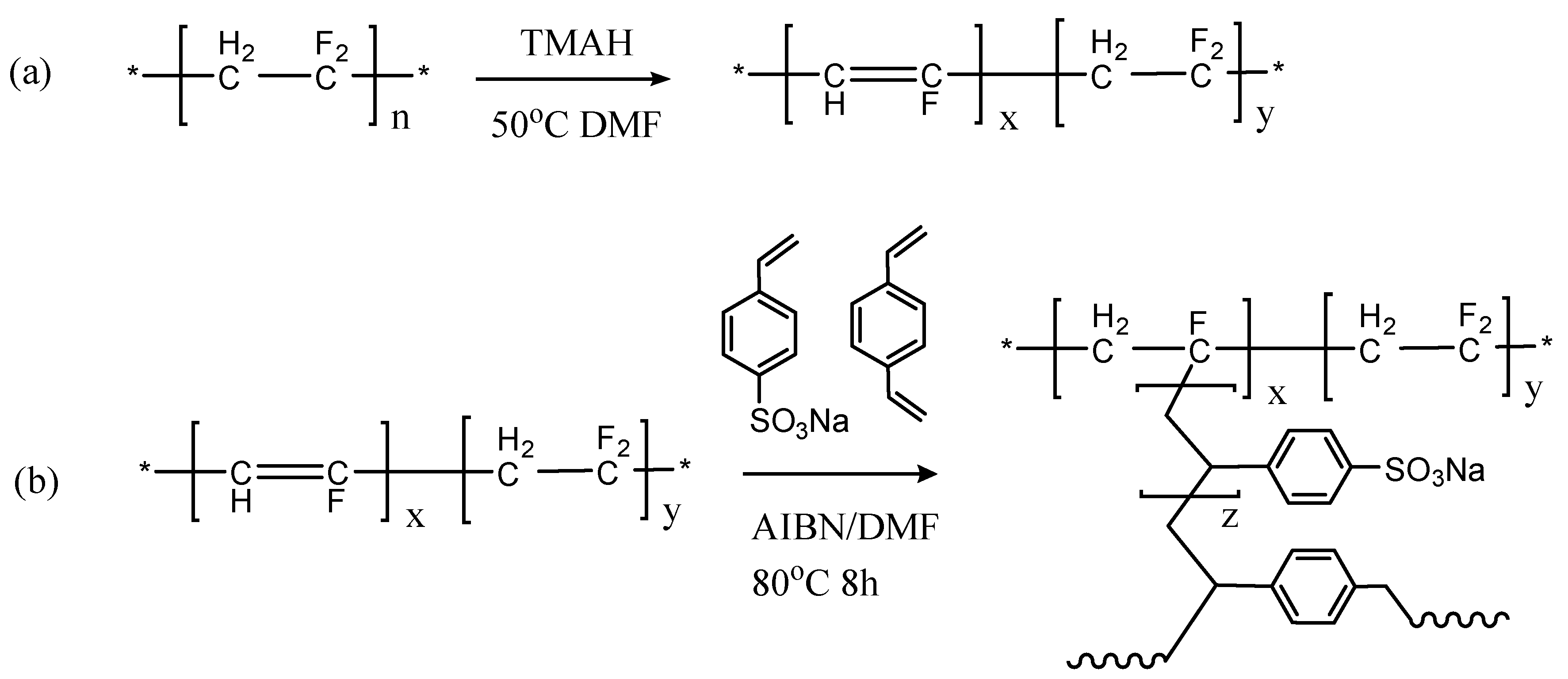
2.2. Membranes Preparation
2.3. Infrared Spectrum Analysis
2.4. Morphology Analysis
2.5. Water Uptake (WU), Ion-Exchange Capacity (IEC), and Linear Swelling Ratio (LSR)
2.6. Water Contact Angle (CA)
2.7. Evaluation of Mechanical Properties of Membranes
2.8. Evaluation of Permeations and Selectivities
3. Results
3.1. Synthesis and Characterization of PVDF-g-SSS
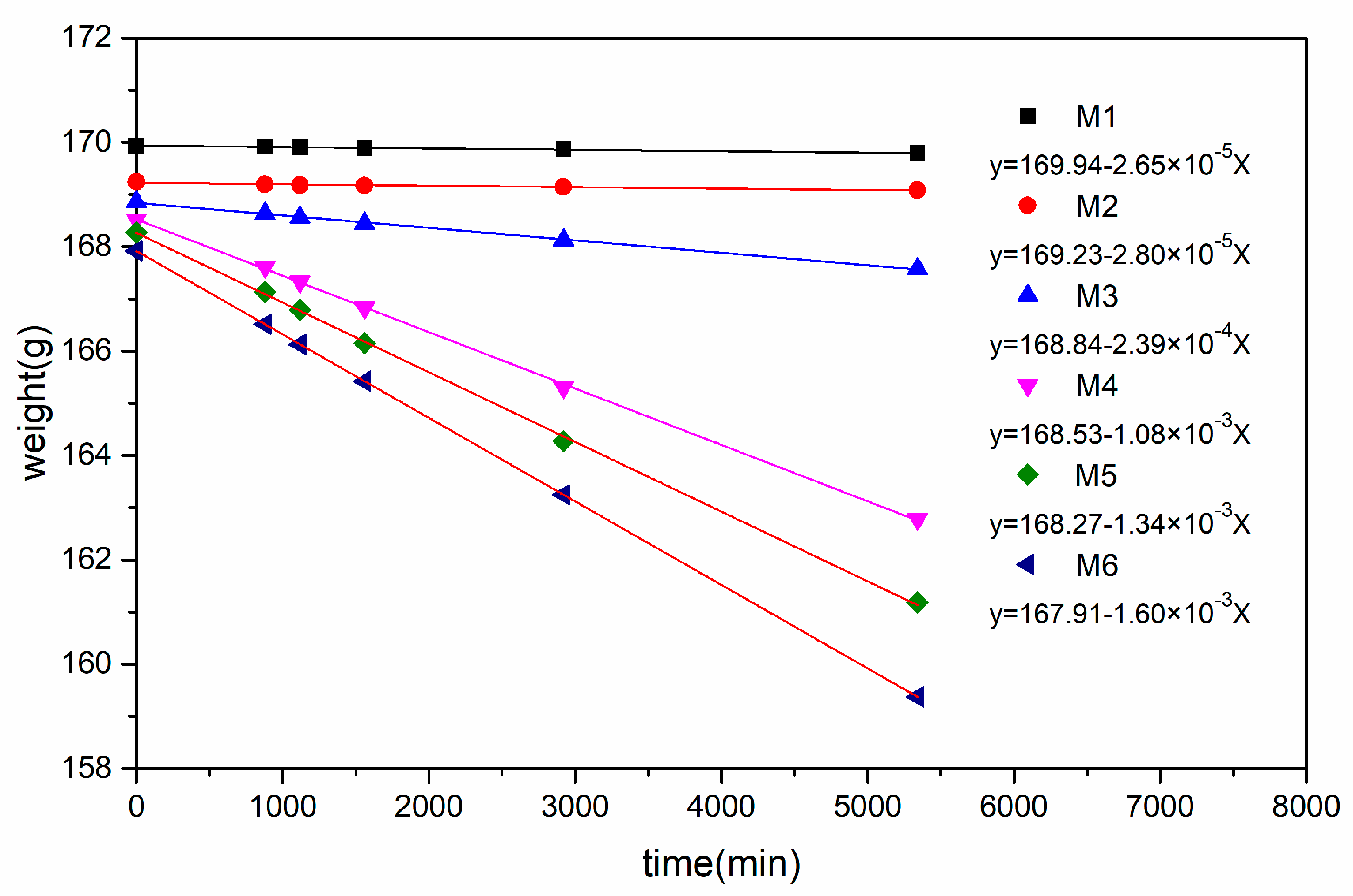
3.2. IEC, WU, and LSR
3.3. Water Contact Angle
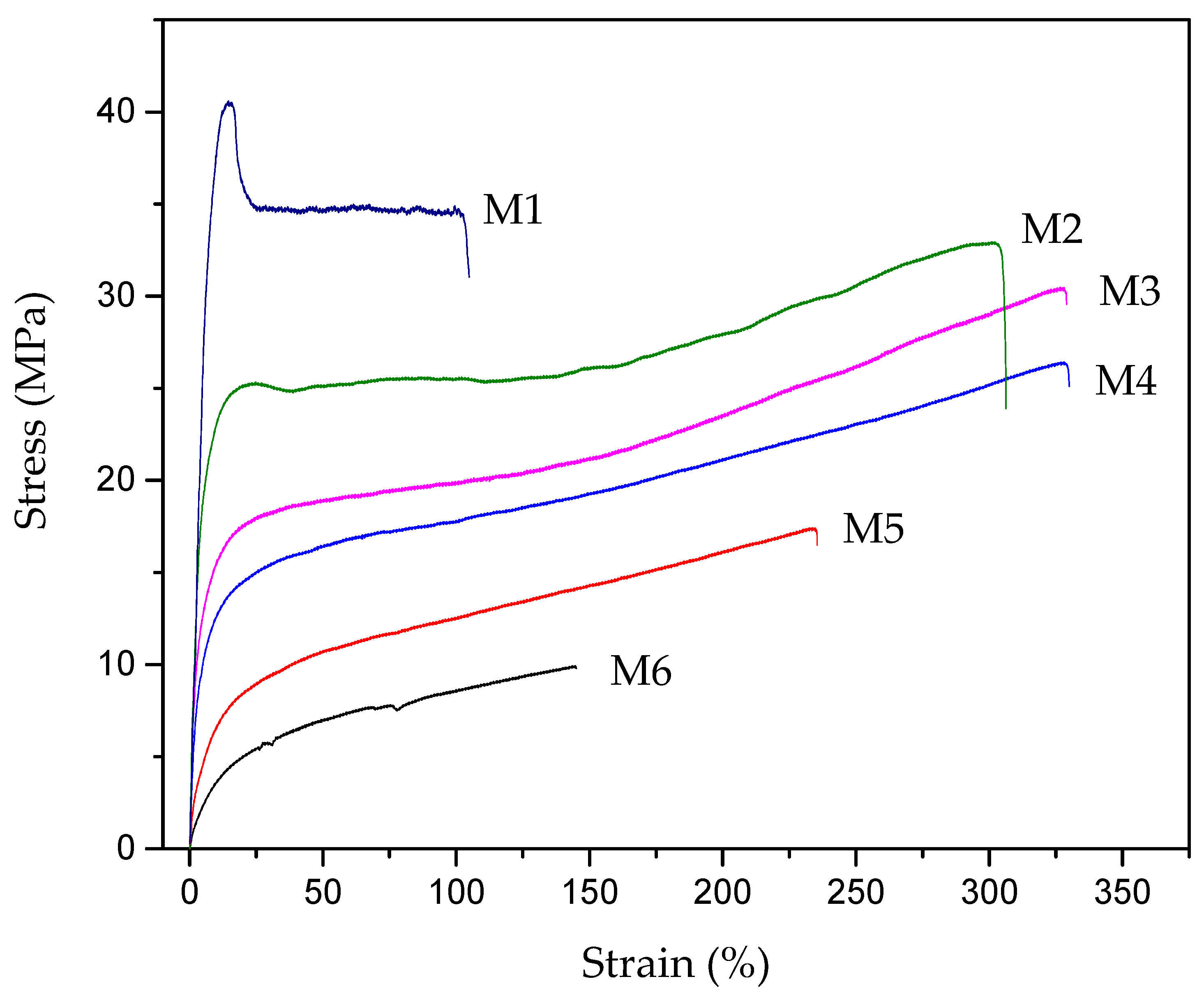
3.4. Mechanical Properties
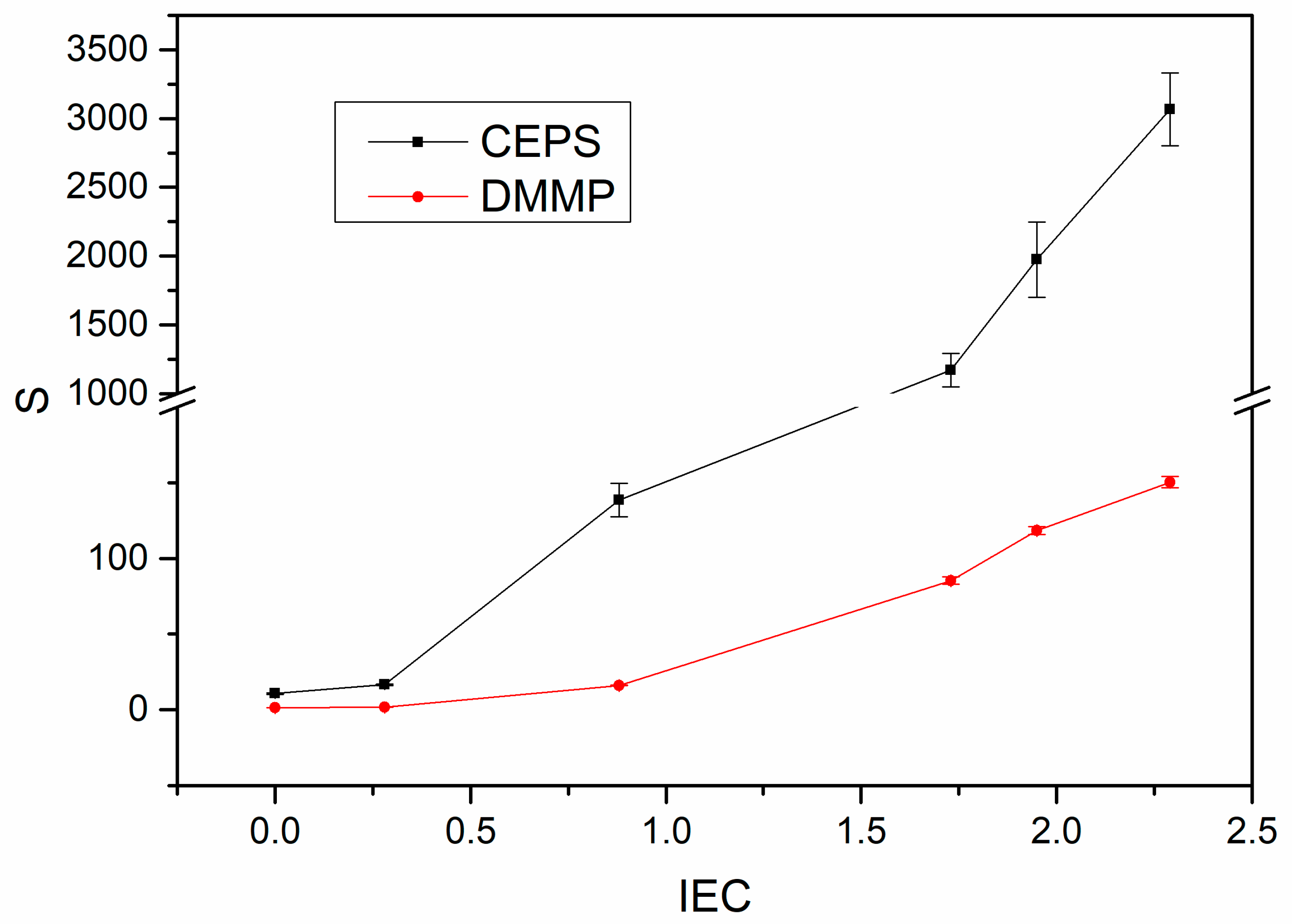
3.5. Permeations and Selectivities of Membranes
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jortani, S.A.; Snyder, J.W.; Valdes, R. The role of the clinical laboratory in managing chemical or biological terrorism. Clin. Chem. 2000, 46, 1883–1893. [Google Scholar] [CrossRef] [PubMed]
- Jouypazadeh, H.; Farrokhpour, H. DFT and TD-DFT study of the adsorption and detection of sulfur mustard chemical warfare agent by the C-24, C12Si12, Al12N12, Al12P12, Be12O12, B12N12 and Mg12O12 nanocages. J. Mol. Struct. 2018, 1164, 227–238. [Google Scholar] [CrossRef]
- Dubey, V.; Rao, N.; Maiti, S.N.; Gupta, A.K. Sorption of sulfur mustard and its oxygen analog in black and nonblack-filled butyl rubber membranes. J. Appl. Polym. Sci. 1998, 69, 503–511. [Google Scholar] [CrossRef]
- Almquist, C.B.; Hwang, S.T. The permeation of organophosphorus compounds in silicone rubber membranes. J. Membr. Sci. 1999, 153, 57–69. [Google Scholar] [CrossRef]
- Rivin, D.; Lindsay, R.S.; Shuely, W.J.; Rodriguez, A. Liquid permeation through nonporous barrier materials. J. Membr. Sci. 2005, 246, 39–47. [Google Scholar] [CrossRef]
- Lu, X.; Nguyen, V.; Zeng, X.; Elliott, B.J.; Gin, D.L. Selective rejection of a water-soluble nerve agent stimulant using a nanoporous lyotropic liquid crystal-butyl rubber vapor barrier material: Evidence for a molecular size-discrimination mechanism. J. Membr. Sci. 2008, 318, 397–404. [Google Scholar] [CrossRef]
- Schreuder-Gibson, H.L.; Truong, Q.; Walker, J.E.; Owens, J.R.; Wander, J.D.; Jones, W.E. Chemical and biological protection and detection in fabrics for protective clothing. Mrs Bull. 2003, 28, 574–578. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, Y.; Zhang, Q.; Wang, M.; Wang, Y.; Ji, T.; Gao, Q. Preparation and properties of spherical activated carbon-based inner materials for chemical protective clothing. China Text. Lead. 2018, 2, 64–67. [Google Scholar] [CrossRef]
- Ramaseshan, R.; Sundarrajan, S.; Liu, Y.; Barhate, R.S.; Lala, N.L.; Ramakrishna, S. Functionalized polymer nanofibre membranes for protection from chemical warfare stimulants. Nanotechnology 2006, 17, 2947–2953. [Google Scholar] [CrossRef]
- Rivin, D.; Meermeier, G.; Schneider, N.S.; Vishnyakov, A.; Neimark, A.V. Simultaneous transport of water and organic molecules through polyelectrolyte membranes. J. Phys. Chem. B 2004, 108, 8900–8909. [Google Scholar] [CrossRef]
- Schneider, N.S.; Rivin, D. Interaction of dimethyl methylphosphonate with Nafion in acid and cation modifications. Polymer 2004, 45, 6309–6320. [Google Scholar] [CrossRef]
- Schneider, N.S.; Rivin, D. Solvent transport in hydrocarbon and perfluorocarbon ionomers. Polymer 2006, 47, 3119–3131. [Google Scholar] [CrossRef]
- Schneider, N.S.; Rivin, D. Steady state analysis of water vapor transport in ionomers. Polymer 2010, 51, 671–678. [Google Scholar] [CrossRef]
- Jung, K.H.; Pourdeyhimi, B.; Zhang, X.W. Chemical protection performance of polystyrene sulfonic acid-filled polypropylene nonwoven membranes. J. Membr. Sci. 2010, 362, 137–142. [Google Scholar] [CrossRef]
- Vishnyakov, A.; Neimark, A.V. Molecular dynamics simulation of nanoscale distribution and mobility of water and dimethylmethylphosphonate in sulfonated polystyrene. J. Phys. Chem. B 2008, 112, 14905–14910. [Google Scholar] [CrossRef]
- Ruiz-Colon, E.; Perez-Perez, M.; Suleiman, D. Synthesis and characterization of novel phosphonated and sulfonated poly(styrene-isobutylene-styrene) for fuel cell and protective clothing applications. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 1424–1435. [Google Scholar] [CrossRef]
- Ruiz-Colon, E.; Perez-Perez, M.; Suleiman, D. Influence of carboxylated and phosphonated single-walled carbon nanotubes on the transport properties of sulfonated poly(styrene-isobutylene-styrene) membranes. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 2475–2495. [Google Scholar] [CrossRef]
- Ruiz-Colon, E.; Perez-Perez, M.; Suleiman, D. Transport properties of blended sulfonated poly(styrene-isobutylene-styrene) and isopropyl phosphate membranes. J. Appl. Polym. Sci. 2019, 136, 47009. [Google Scholar] [CrossRef]
- Suleiman, D.; Carreras, G.; Soto, Y. Effect of block composition, size and functionality of poly(styrene-isobutylene-styrene) copolymers. J. Appl. Polym. Sci. 2013, 128, 2297–2306. [Google Scholar] [CrossRef]
- Nafion 117. Available online: https://www.fuelcellstore.com/nafion-117?search=nafion%20117 (accessed on 23 June 2020).
- Barlow, C.Y.; Morgan, D.C. Polymer film packaging for food: An environmental assessment. Resour. Conserv. Recycl. 2013, 78, 74–80. [Google Scholar] [CrossRef]
- Cai, J.; Lv, X.; Xing, Y.; Zhao, X. Carbon dioxide adsorption on poly(vinylidene chloride)-based carbons with ultrahigh microporosities prepared by facile carbonization. Mater. Lett. 2014, 114, 37–39. [Google Scholar] [CrossRef]
- Cha, B.J.; Yang, J.M. Preparation of poly(vinylidene fluoride) hollow fiber membranes for microfiltration using modified TIPS process. J. Membr. Sci. 2007, 291, 191–198. [Google Scholar] [CrossRef]
- Fontananova, E.; Jansen, J.C.; Cristiano, A.; Curcio, E.; Drioli, E. Effect of additives in the casting solution on the formation of PVDF membranes. Desalination 2006, 192, 190–197. [Google Scholar] [CrossRef]
- Gregorio, R.; Ueno, E.M. Effect of crystalline phase, orientation and temperature on the dielectric properties of poly (vinylidene fluoride) (PVDF). J. Mater. Sci. 1999, 34, 4489–4500. [Google Scholar] [CrossRef]
- Nguyen, L.; Mighri, F.; Deyrail, Y.; Elkoun, S. Conductive materials for proton exchange membrane fuel cell bipolar plates made from PVDF, PET and Co-continuous PVDF/PET filled with carbon additives. Fuel Cells 2010, 10, 938–948. [Google Scholar] [CrossRef]
- Prakash, G.K.S.; Smart, M.C.; Wang, Q.J.; Atti, A.; Pleynet, V.; Yang, B.; McGrath, K.; Olah, G.A.; Narayanan, S.R.; Chun, W.; et al. High efficiency direct methanol fuel cell based on poly(styrenesulfonic) acid (PSSA)-poly(vinylidene fluoride) (PVDF) composite membranes. J. Fluor. Chem. 2004, 125, 1217–1230. [Google Scholar] [CrossRef]
- Nasef, M.M.; Saidi, H.; Dahlan, K.Z.M. Single-step radiation induced grafting for preparation of proton exchange membranes for fuel cell. J. Membr. Sci. 2009, 339, 115–119. [Google Scholar] [CrossRef]
- Golcuk, S.; Muftuoglu, A.E.; Celik, S.U.; Bozkurt, A. Synthesis and characterization of polymer electrolyte membranes based on PVDF and styrene via photoinduced grafting. J. Polym. Res. 2013, 20, 144. [Google Scholar] [CrossRef]
- Sharma, P.P.; Yadav, V.; Gahlot, S.; Lebedeva, O.V.; Chesnokova, A.N.; Srivastava, D.N.; Raskulova, T.V.; Kulshrestha, V. Acid resistant PVDF-co-HFP based copolymer proton exchange membrane for electro-chemical application. J. Membr. Sci. 2019, 573, 485–492. [Google Scholar] [CrossRef]
- Kim, Y.; Shin, S.-H.; Chang, I.S.; Moon, S.-H. Characterization of uncharged and sulfonated porous poly(vinylidene fluoride) membranes and their performance in microbial fuel cells. J. Membr. Sci. 2014, 463, 205–214. [Google Scholar] [CrossRef]
- Li, C.; Song, Y.; Wang, X.; Zhang, Q. Synthesis, characterization and application of S-TiO2/PVDF-g-PSSA composite membrane for improved performance in MFCs. Fuel 2020, 264, 116847. [Google Scholar] [CrossRef]
- Li, C.; Wang, L.; Wang, X.; Kong, M.; Zhang, Q.; Li, G. Synthesis of PVDF-g-PSSA proton exchange membrane by ozone-induced graft copolymerization and its application in microbial fuel cells. J. Membr. Sci. 2017, 527, 35–42. [Google Scholar] [CrossRef]
- Kim, Y.W.; Lee, D.K.; Lee, K.J.; Kim, J.H. Single-step synthesis of proton conducting poly(vinylidene fluoride) (PVDF) graft copolymer electrolytes. Eur. Polym. J. 2008, 44, 932–939. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, L.; Li, H. Research advances in selectively permeable materials of skin protection. J. Funct. Polym. 2020, 3, 226–244. [Google Scholar] [CrossRef]
- Wu, B.; Lin, X.; Ge, L.; Wu, L.; Xu, T. A novel route for preparing highly proton conductive membrane materials with metal-organic frameworks. Chem. Commun. 2013, 49, 143–145. [Google Scholar] [CrossRef]
- Al Munsur, A.Z.; Hossain, I.; Nam, S.Y.; Chae, J.E.; Kim, T.-H. Quaternary ammonium-functionalized hexyl bis(quaternary ammonium)-mediated partially crosslinked SEBSs as highly conductive and stable anion exchange membranes. Int. J. Hydrog. Energy 2020, 45, 15658–15671. [Google Scholar] [CrossRef]
- Bai, T.-T.; Cong, M.-Y.; Jia, Y.-X.; Ma, K.-K.; Wang, M. Preparation of self-crosslinking anion exchange membrane with acid block performance from side-chain type polysulfone. J. Membr. Sci. 2020, 599, 117831. [Google Scholar] [CrossRef]
- Kozmai, A.E.; Nikonenko, V.V.; Zyryanova, S.; Pismenskaya, N.D.; Dammak, L.; Baklouti, L. Modelling of anion-exchange membrane transport properties with taking into account the change in exchange capacity and swelling when varying bathing solution concentration and pH. J. Membr. Sci. 2019, 590, 117291. [Google Scholar] [CrossRef]
- Haubold, H.G.; Vad, T.; Jungbluth, H.; Hiller, P. Nano structure of NAFION: A SAXS study. Electrochim. Acta 2001, 46, 1559–1563. [Google Scholar] [CrossRef]
- Cao, L.; He, X.; Jiang, Z.; Li, X.; Li, Y.; Ren, Y.; Yang, L.; Wu, H. Channel-facilitated molecule and ion transport across polymer composite membranes. Chem. Soc. Rev. 2017, 46, 6725–6745. [Google Scholar] [CrossRef]
- Wijmans, J.G.; Baker, R.W. The solution-diffusion model—A review. J. Membr. Sci. 1995, 107, 1–21. [Google Scholar] [CrossRef]
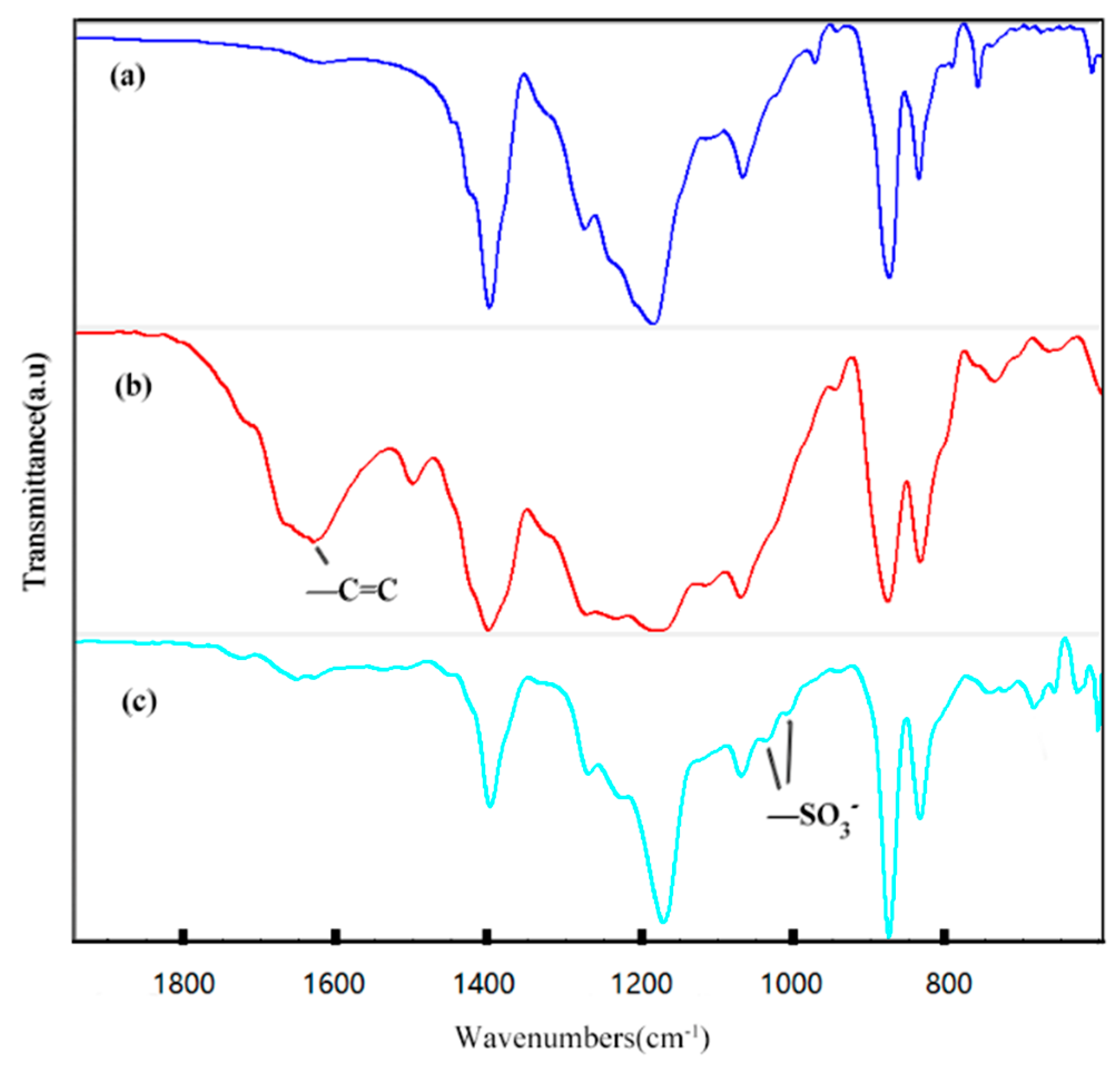


| Sample | PVDF (g) | SSS (g) | DVB (g) | AIBN (g) | DMF (mL) |
|---|---|---|---|---|---|
| M1 | 20 | 0 | 0 | 0 | 200 |
| M2 | 20 | 2.5 | 0.25 | 0.025 | 225 |
| M3 | 20 | 6 | 0.6 | 0.06 | 260 |
| M4 | 20 | 10.4 | 1.04 | 0.104 | 304 |
| M5 | 20 | 16 | 1.6 | 0.16 | 360 |
| M6 | 20 | 24 | 2.4 | 0.24 | 440 |
| Sample | IEC (mmol/g) | WU (%) | LSR (%) | CA of Water (o) |
|---|---|---|---|---|
| M1 | 0 | 1.3 | 0 | 81.9 ± 3.5 |
| M2 | 0.28 | 8.3 | 1. 7 | 70.9 ± 2.8 |
| M3 | 0.88 | 14.3 | 5.0 | 61.2 ± 2.5 |
| M4 | 1.73 | 23.1 | 8. 3 | 48.0 ± 2.6 |
| M5 | 1.95 | 53.4 | 16. 7 | 39.3 ± 2.3 |
| M6 | 2.29 | 77.4 | 28. 3 | 23.6 ± 1.9 |
| Sample | Thickness (μm) | Strength (MPa) | Elongation-at-Break (%) |
|---|---|---|---|
| M1 | 85 | 40.6 ± 2.1 | 112.0 ± 12.8 |
| M2 | 120 | 32.9 ± 2.3 | 306.3 ± 24.2 |
| M3 | 110 | 30.5 ± 2.6 | 329.0 ± 29.2 |
| M4 | 125 | 26.4 ± 1.9 | 329.9 ± 25.7 |
| M5 | 130 | 17.4 ± 2.0 | 235.5 ± 18.3 |
| M6 | 130 | 9.9 ± 1.1 | 145.1 ± 13.5 |
| Sample | VTR of Water (g m−2 day−1) | VP of Water ×10−9 (mol s−1 m−1) | VP of DMMP ×10−9 (mol s−1 m−1) | VP of CEPS × 10−9 (mol s−1 m−1) | Selectivity (Water/DMMP) | Selectivity (Water/CEPS) |
|---|---|---|---|---|---|---|
| M1 | 60 ± 3 | 3.32 ± 0.16 | 2.60 ± 0.09 | 0.31 ± 0.03 | 1.28 ± 0.03 | 10.71 ± 0.52 |
| M2 | 64 ± 4 | 4.95 ± 0.31 | 3.07 ± 0.15 | 0.30 ± 0.03 | 1.61 ± 0.04 | 16.5 ± 0.62 |
| M3 | 548 ± 15 | 38.76 ± 1.06 | 2.41 ± 0.10 | 0.28 ± 0.03 | 16.08 ± 0.16 | 138.43 ± 11.05 |
| M4 | 2476 ± 34 | 199.04 ± 2.73 | 2.33 ± 0.12 | 0.17 ± 0.02 | 85.42 ± 2.49 | 1170.82 ± 121.69 |
| M5 | 3072 ± 47 | 256.84 ± 3.93 | 2.17 ± 0.10 | 0.13 ± 0.02 | 118.35 ± 2.55 | 1975.69 ± 273.72 |
| M6 | 3668 ± 54 | 306.67 ± 4.35 | 2.04 ± 0.10 | 0.10 ± 0.01 | 150.32 ± 3.76 | 3066.70 ± 263.17 |
| Membrane | IEC (mmol/g) | Strength (MPa) | VTR of Water (g m−2 Day−1) | Selectivity (Water/DMMP) | Selectivity (Water/CEPS) | Reference |
|---|---|---|---|---|---|---|
| PSS-nonwoven | - | 9 | 4500 | 80 | 1500 | [14] |
| Nafion-117 | 0.89 | 43 | - | 66 | 340 | [14] |
| SIBS-g-PVPA | 1.8 | - | - | 2.4 | - | [16] |
| SIBS 61-CNT0.5 | 1.41 | - | - | 32 | - | [17] |
| SIBS84-IP 1 | 2.2 | - | - | 50 | - | [18] |
| SIBS-30-100k-84.4-Ba | - | - | - | 35 | - | [19] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Wang, X.; Wang, D.; Li, H.; Li, L.; Zhang, S.; Zhou, C.; Zheng, X.; Men, Q.; Zhong, J.; et al. Preparation and Chemical Protective Clothing Application of PVDF Based Sodium Sulfonate Membrane. Membranes 2020, 10, 190. https://doi.org/10.3390/membranes10080190
Zhao Y, Wang X, Wang D, Li H, Li L, Zhang S, Zhou C, Zheng X, Men Q, Zhong J, et al. Preparation and Chemical Protective Clothing Application of PVDF Based Sodium Sulfonate Membrane. Membranes. 2020; 10(8):190. https://doi.org/10.3390/membranes10080190
Chicago/Turabian StyleZhao, Yue, Xinbo Wang, Deyin Wang, Heguo Li, Lei Li, Shouxin Zhang, Chuan Zhou, Xiaohui Zheng, Quanfu Men, Jinyi Zhong, and et al. 2020. "Preparation and Chemical Protective Clothing Application of PVDF Based Sodium Sulfonate Membrane" Membranes 10, no. 8: 190. https://doi.org/10.3390/membranes10080190
APA StyleZhao, Y., Wang, X., Wang, D., Li, H., Li, L., Zhang, S., Zhou, C., Zheng, X., Men, Q., Zhong, J., & Wu, L. (2020). Preparation and Chemical Protective Clothing Application of PVDF Based Sodium Sulfonate Membrane. Membranes, 10(8), 190. https://doi.org/10.3390/membranes10080190