Magnetically Aligned and Enriched Pathways of Zeolitic Imidazolate Framework 8 in Matrimid Mixed Matrix Membranes for Enhanced CO2 Permeability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Magnetic ZIF−8 Synthesis
2.3. Membrane Formation
2.3.1. Native Matrimid Membranes
2.3.2. Homogeneous m–ZIF−8/Matrimid MMMs
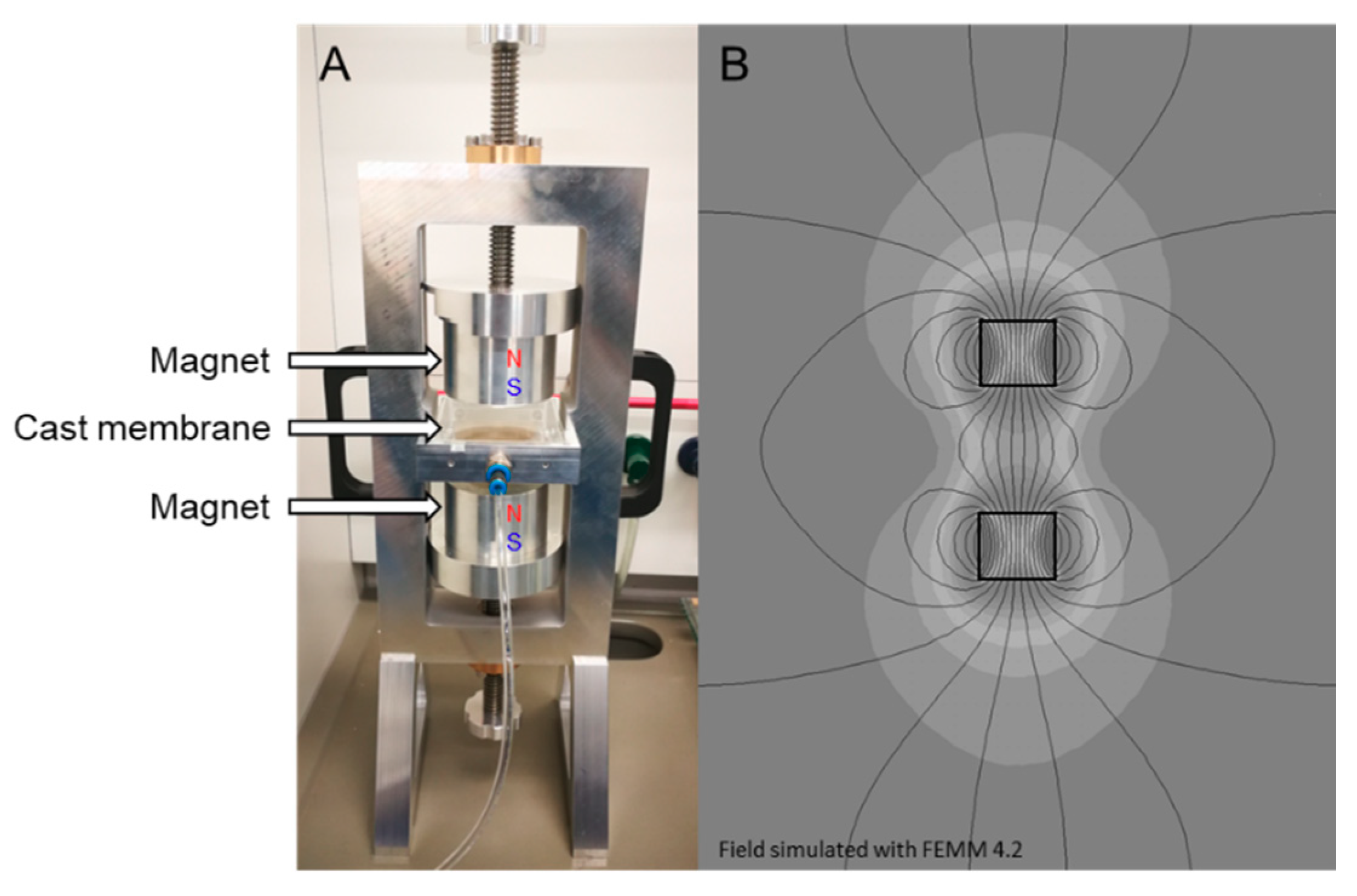
2.3.3. Magnetically Aligned m–ZIF−8/Matrimid MMMs
2.4. Characterization
3. Results and Discussion
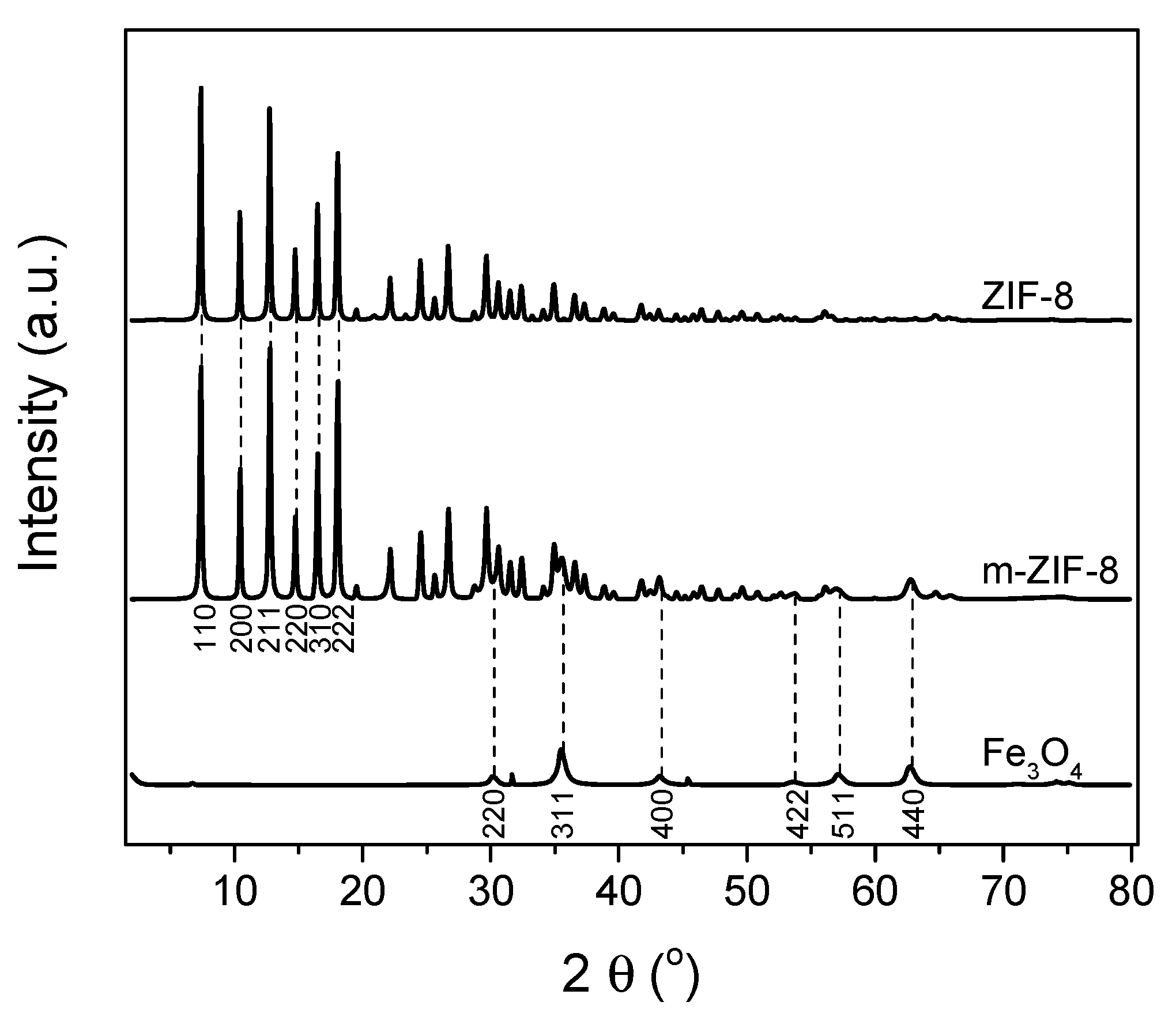
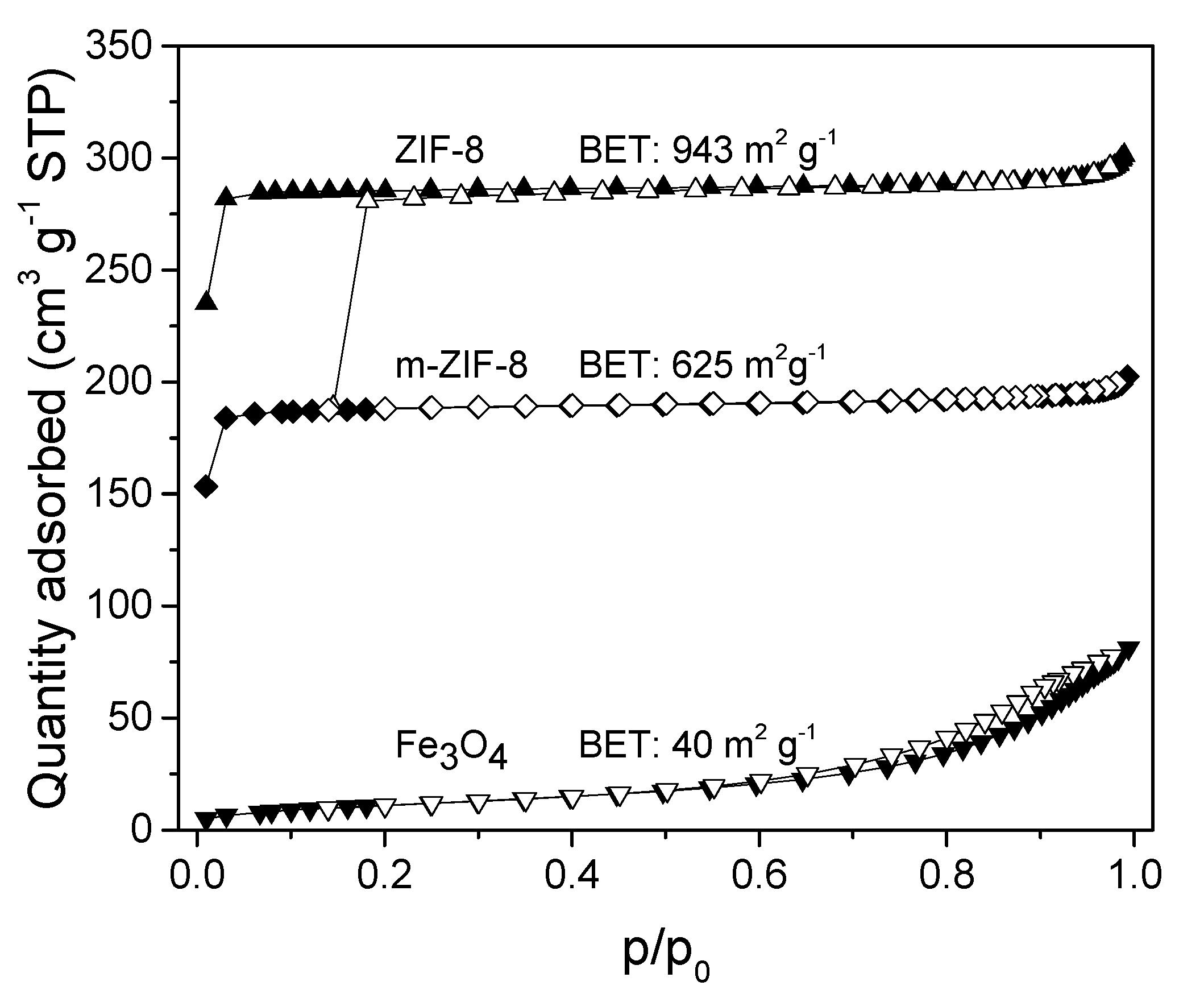
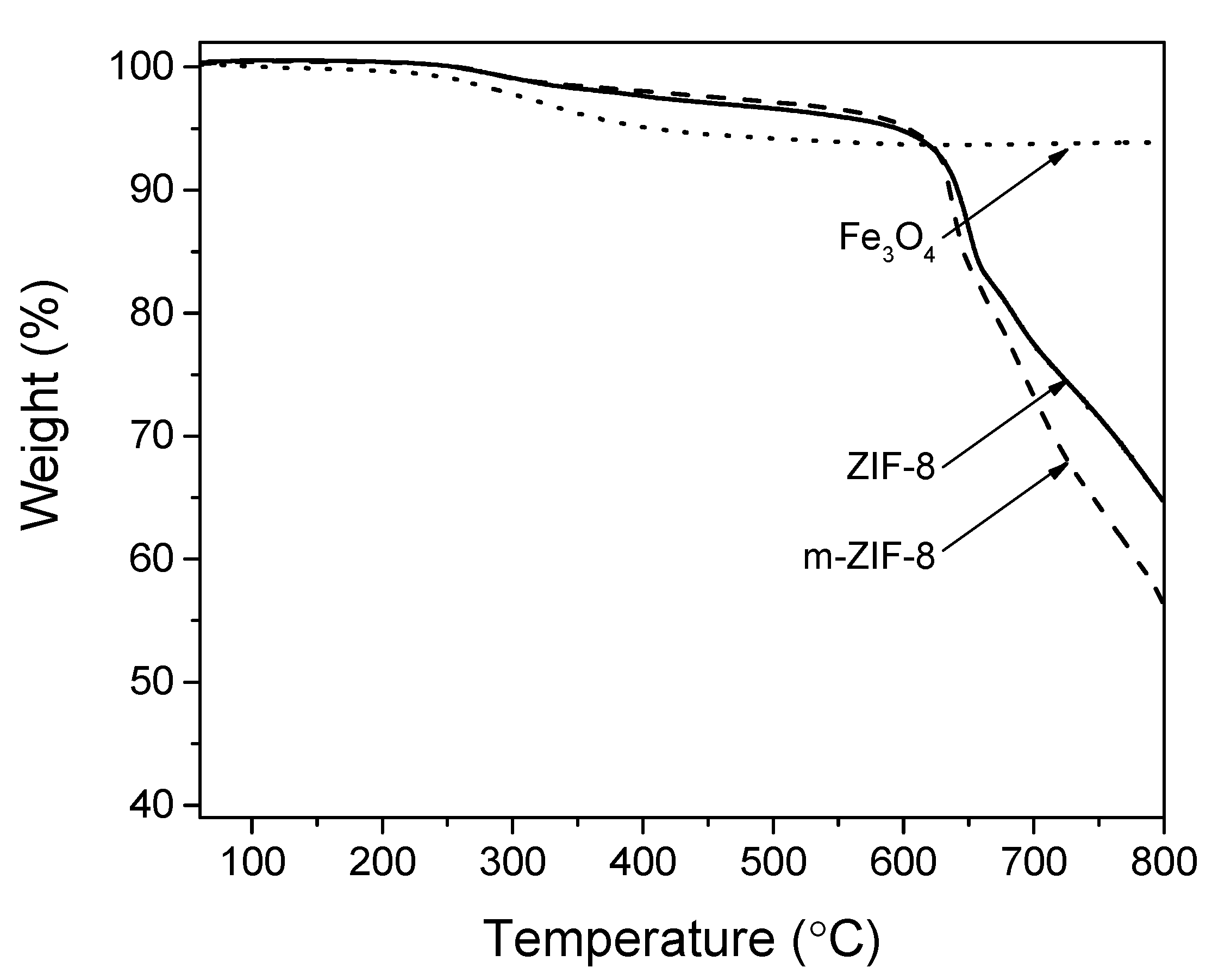
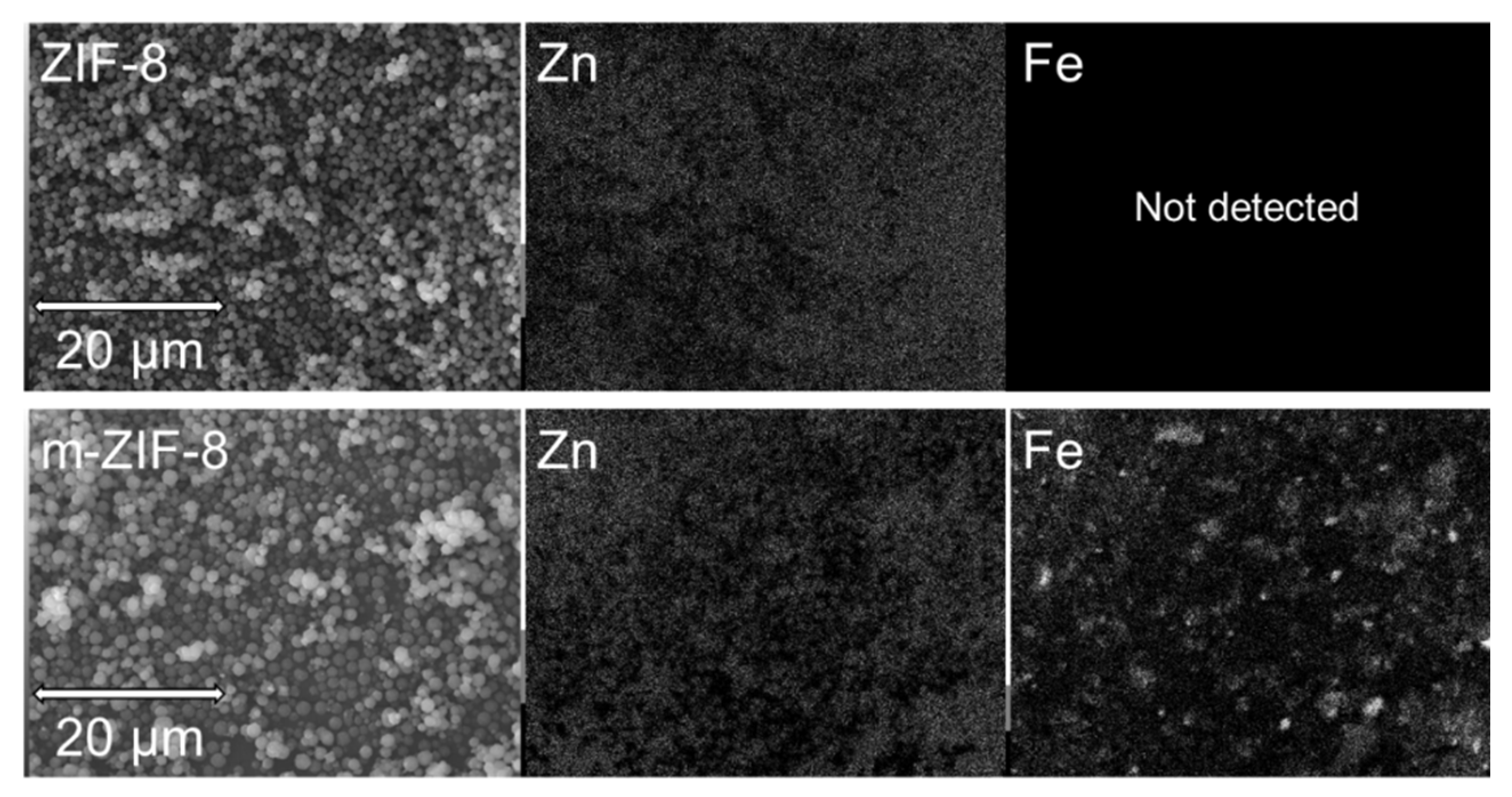
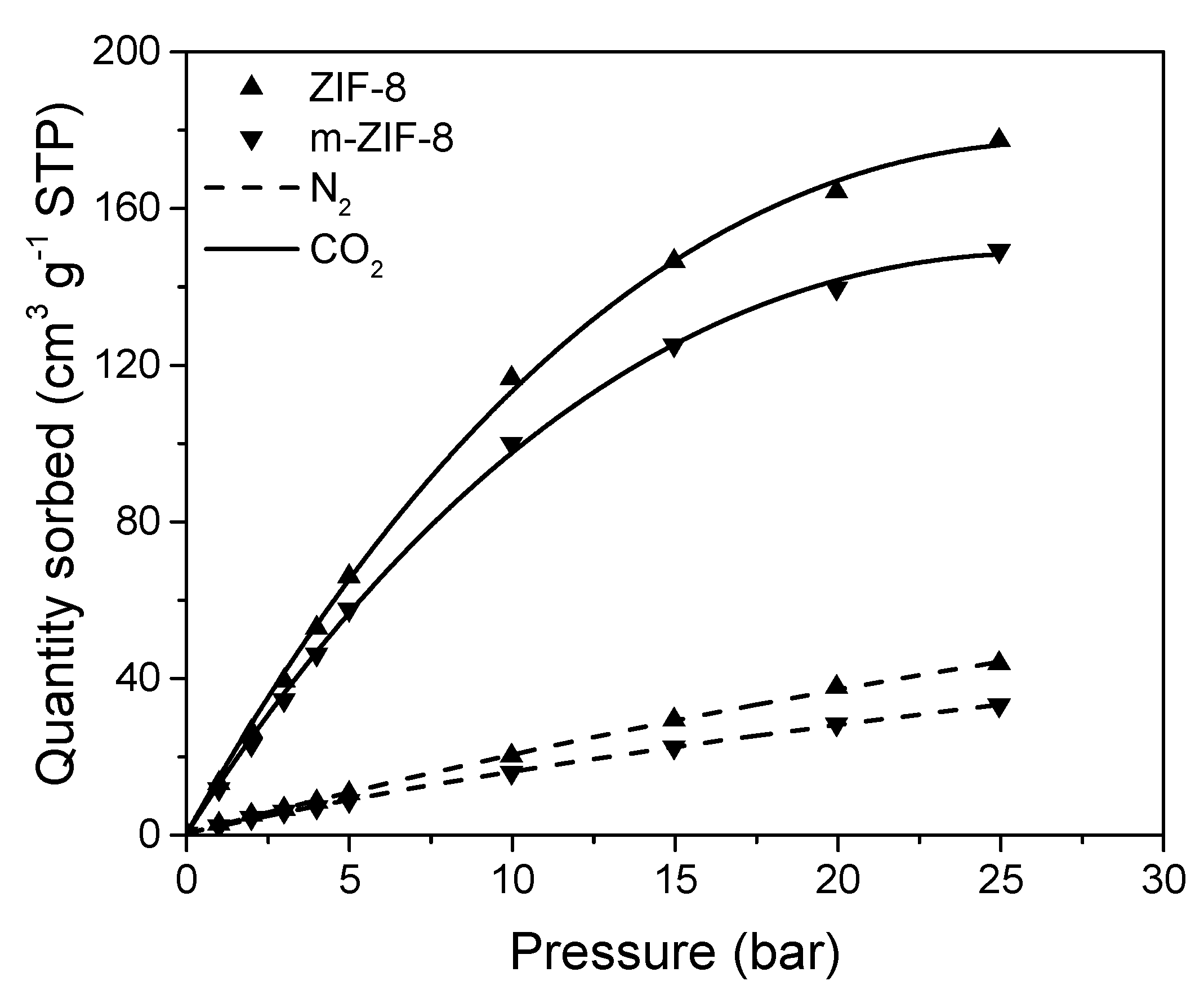
3.1. ZIF−8, m–ZIF−8 and Fe3O4 Analysis
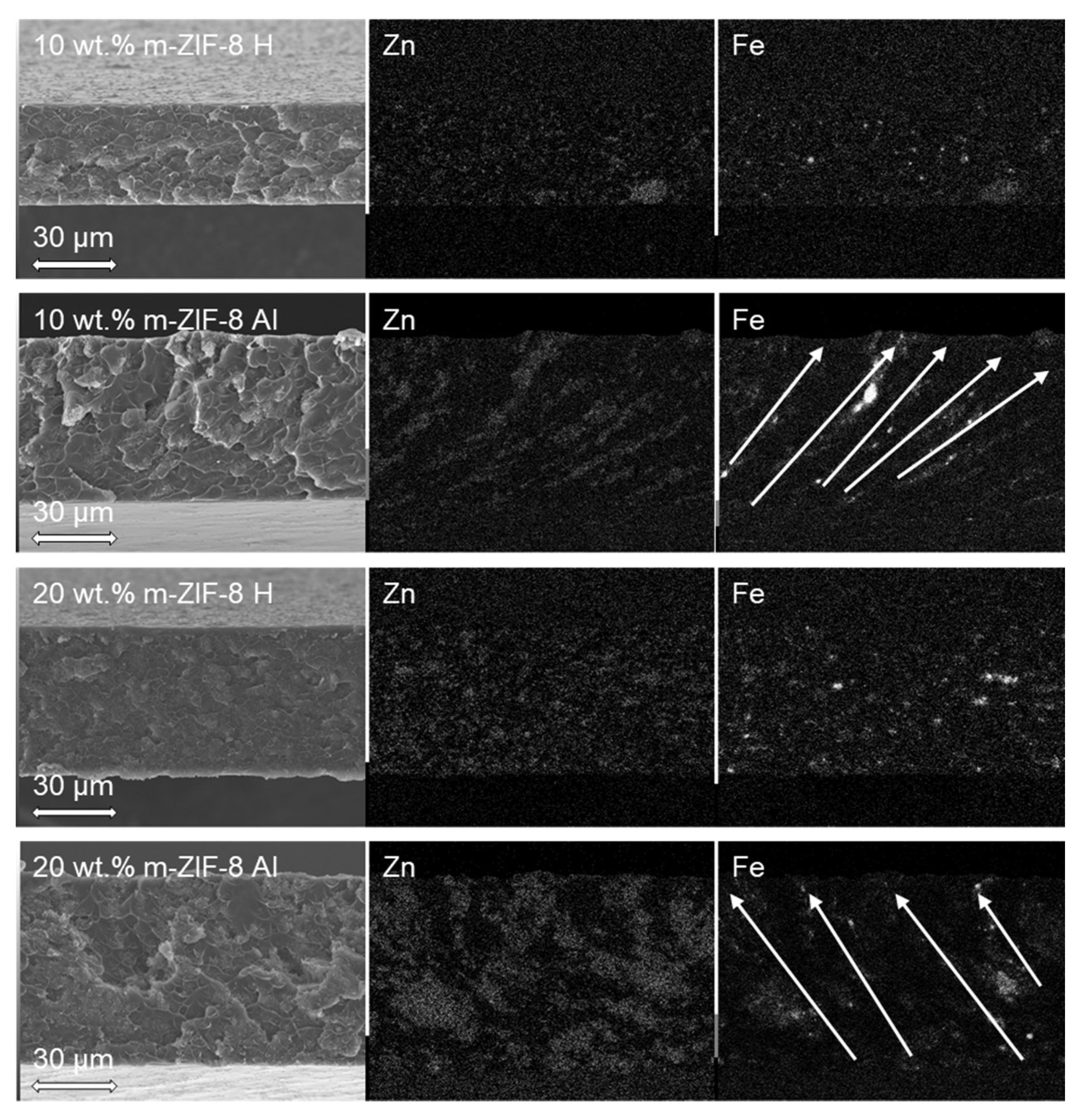
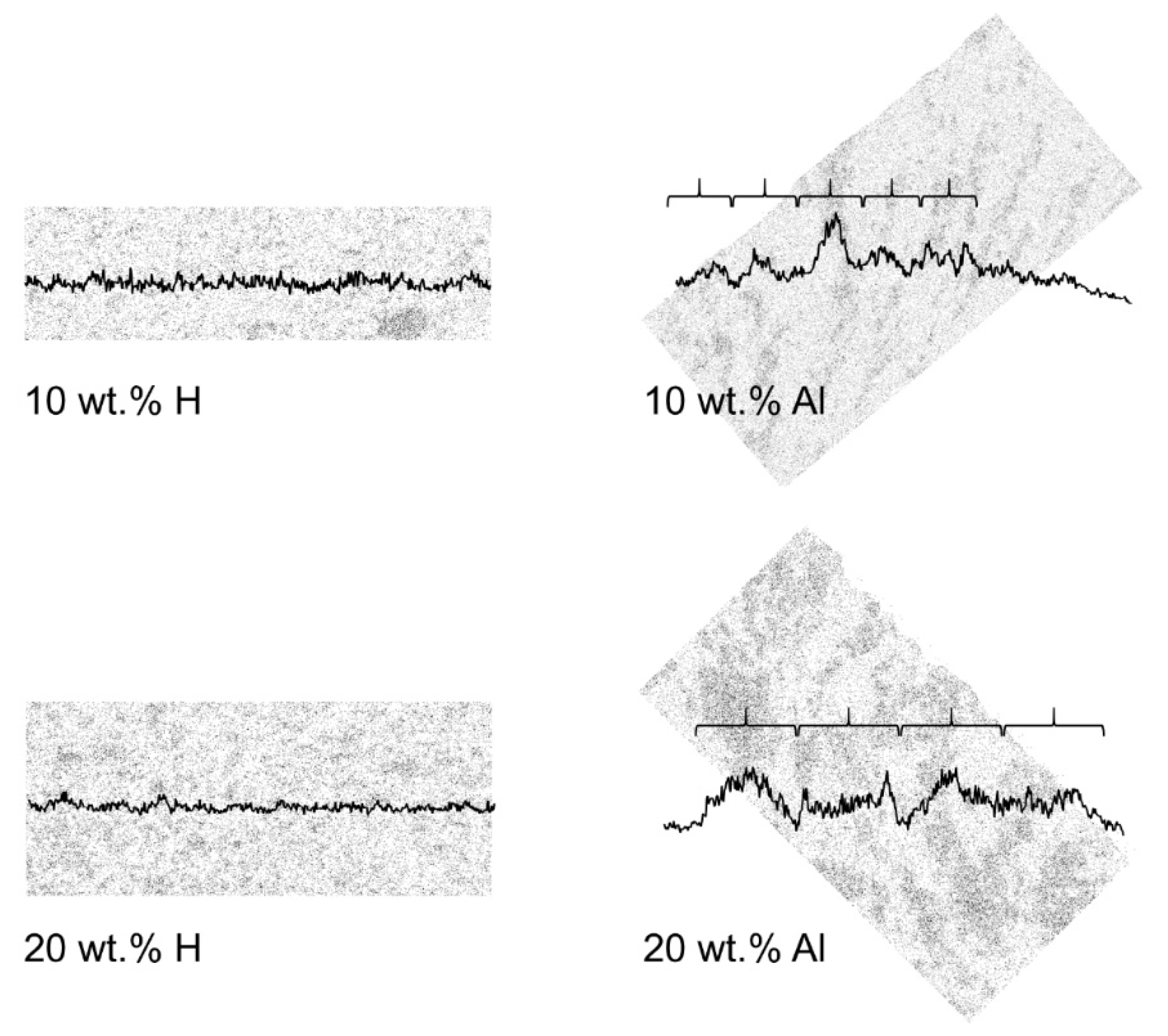
3.2. Membrane Analysis
4. Conclusions
Author Contributions
Funding

Acknowledgments
Conflicts of Interest
References
- Ismail, A.F.; Khulbe, K.C.; Matsuura, T. Gas Separation Membranes: Polymeric and Inorganic; Springer: Heidelberg, Germany, 2015; ISBN 9783319010953. [Google Scholar]
- Robeson, L.M. Correlation of separation factor versus permeability for polymeric membranes. J. Memb. Sci. 1991, 62, 165–185. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Memb. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Biswal, B.P.; Chaudhari, H.D.; Banerjee, R.; Kharul, U.K. Chemically Stable Covalent Organic Framework (COF)-Polybenzimidazole Hybrid Membranes: Enhanced Gas Separation through Pore Modulation. Chem. Eur. J. 2016, 22, 4695–4699. [Google Scholar] [CrossRef] [PubMed]
- Thür, R.; Van Velthoven, N.; Slootmaekers, S.; Didden, J.; Verbeke, R.; Smolders, S.; Dickmann, M.; Egger, W.; De Vos, D.; Vankelecom, I.F.J. Bipyridine-based UiO−67 as novel filler in mixed-matrix membranes for CO2-selective gas separation. J. Memb. Sci. 2019, 576, 78–87. [Google Scholar] [CrossRef]
- Kertik, A.; Wee, L.H.; Pfannmöller, M.; Bals, S.; Martens, J.A.; Vankelecom, I.F.J. Highly selective gas separation membrane using in situ amorphised metal-organic frameworks. Energy Environ. Sci. 2017, 10, 2342–2351. [Google Scholar] [CrossRef]
- Yuan, J.; Zhu, H.; Sun, J.; Mao, Y.; Liu, G.; Jin, W. Novel ZIF−300 Mixed-Matrix Membranes for Efficient CO2 Capture. ACS Appl. Mater. Interfaces 2017, 9, 38575–38583. [Google Scholar] [CrossRef]
- Jeazet, H.B.T.; Staudt, C.; Janiak, C. Metal–organic frameworks in mixed-matrix membranes for gas separation. Dalt. Trans. 2012, 41, 14003–14027. [Google Scholar] [CrossRef]
- Li, H.; Wang, K.; Sun, Y.; Lollar, C.T.; Li, J.; Zhou, H.C. Recent advances in gas storage and separation using metal–organic frameworks. Mater. Today 2018, 21, 108–121. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef] [Green Version]
- Yuan, S.; Feng, L.; Wang, K.; Pang, J.; Bosch, M.; Lollar, C.; Sun, Y.; Qin, J.; Yang, X.; Zhang, P.; et al. Stable Metal-Organic Frameworks: Design, Synthesis, and Applications. Adv. Mater. 2018, 30, 1–35. [Google Scholar] [CrossRef] [Green Version]
- Phan, A.; Doonan, C.J.; Uribe-Romo, F.J.; Knobler, C.B.; O’Keeffe, M.; Yaghi, O.M. Synthesis, Structure, and Carbon Dioxide Capture Properties of Zeolitic Imidazolate Frameworks. Acc. Chem. Res. 2010, 43, 58–67. [Google Scholar] [CrossRef]
- Chen, B.; Yang, Z.; Zhu, Y.; Xia, Y. Zeolitic imidazolate framework materials: Recent progress in synthesis and applications. J. Mater. Chem. A 2014, 2, 16811–16831. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, Y.; Zeng, G.; Zhao, L.; Lai, Z. Rapid synthesis of zeolitic imidazolate framework−8 (ZIF−8) nanocrystals in an aqueous system. Chem. Commun. 2011, 47, 2071–2073. [Google Scholar] [CrossRef]
- Tanaka, S.; Kida, K.; Okita, M.; Ito, Y.; Miyake, Y. Size-Controlled synthesis of zeolitic imidazolate framework−8 (ZIF−8) crystals in an aqueous system at room temperature. Chem. Lett. 2012, 41, 1337–1339. [Google Scholar] [CrossRef]
- Cravillon, J.; Nayuk, R.; Springer, S.; Feldhoff, A.; Huber, K.; Wiebcke, M. Controlling Zeolitic Imidazolate Framework Nano- and Microcrystal Formation: Insight into Crystal Growth by Time-Resolved In Situ Static Light Scattering. Chem. Mater. 2011, 23, 2130–2141. [Google Scholar] [CrossRef]
- Lee, Y.R.; Jang, M.S.; Cho, H.Y.; Kwon, H.J.; Kim, S.; Ahn, W.S. ZIF−8: A comparison of synthesis methods. Chem. Eng. J. 2015, 271, 276–280. [Google Scholar] [CrossRef]
- Bustamante, E.L.; Fernández, J.L.; Zamaro, J.M. Influence of the solvent in the synthesis of zeolitic imidazolate framework−8 (ZIF−8) nanocrystals at room temperature. J. Colloid Interface Sci. 2014, 424, 37–43. [Google Scholar] [CrossRef]
- Lu, G.; Li, S.; Guo, Z.; Farha, O.K.; Hauser, B.G.; Qi, X.; Wang, Y.; Wang, X.; Han, S.; Liu, X.; et al. Imparting functionality to a metal-organic framework material by controlled nanoparticle encapsulation. Nat. Chem. 2012, 4, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, X.; Yan, X.; Kong, L.; Zhang, G.; Liu, H.; Qiu, J.; Yeung, K.L. Synthesis of Fe3O4@ZIF−8 magnetic core-shell microspheres and their potential application in a capillary microreactor. Chem. Eng. J. 2013, 228, 398–404. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, H.Y.; Liu, L.L.; Qiu, L.G.; Jiang, X. Fe3O4 embedded ZIF−8 nanocrystals with ultra-high adsorption capacity towards hydroquinone. J. Alloy. Compd. 2015, 646, 1075–1082. [Google Scholar] [CrossRef]
- Venna, S.R.; Carreon, M.A. Metal organic framework membranes for carbon dioxide separation. Chem. Eng. Sci. 2015, 124, 3–19. [Google Scholar] [CrossRef]
- Guan, W.; Dai, Y.; Dong, C.; Yang, X.; Xi, Y. Zeolite imidazolate framework (ZIF)-based mixed matrix membranes for CO 2 separation: A review. J. Appl. Polym. Sci. 2020, 48968, 1–13. [Google Scholar] [CrossRef]
- Pimentel, B.R.; Parulkar, A.; Zhou, E.; Brunelli, N.A. Zeolitic Imidazolate Frameworks: Next-Generation Materials for Energy-Efficient Gas Separations. ChemSusChem 2014, 7, 3202–3240. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ozcan, A.; Yazaydin, A.O.; Ladewig, B.P. Gas permeation through single-crystal ZIF−8 membranes. J. Memb. Sci. 2019, 575, 209–216. [Google Scholar] [CrossRef] [Green Version]
- Shahid, S.; Nijmeijer, K.; Nehache, S.; Vankelecom, I.; Deratani, A.; Quemener, D. MOF-mixed matrix membranes: Precise dispersion of MOF particles with better compatibility via a particle fusion approach for enhanced gas separation properties. J. Memb. Sci. 2015, 492, 21–31. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, D.; Zhang, S.; Hu, L.; Jin, J. Interfacial Design of Mixed Matrix Membranes for Improved Gas Separation Performance. Adv. Mater. 2016, 28, 3399–3405. [Google Scholar] [CrossRef]
- Sánchez-Laínez, J.; Zornoza, B.; Friebe, S.; Caro, J.; Cao, S.; Sabetghadam, A.; Seoane, B.; Gascon, J.; Kapteijn, F.; Le Guillouzer, C.; et al. Influence of ZIF−8 particle size in the performance of polybenzimidazole mixed matrix membranes for pre-combustion CO2 capture and its validation through interlaboratory test. J. Memb. Sci. 2016, 515, 45–53. [Google Scholar] [CrossRef]
- Song, Q.; Nataraj, S.K.; Roussenova, M.V.; Tan, J.C.; Hughes, D.J.; Li, W.; Bourgoin, P.; Alam, M.A.; Cheetham, A.K.; Al-Muhtaseb, S.A.; et al. Zeolitic imidazolate framework (ZIF−8) based polymer nanocomposite membranes for gas separation. Energy Environ. Sci. 2012, 5, 8359–8369. [Google Scholar] [CrossRef]
- Mahdi, E.M.; Tan, J.C. Mixed-matrix membranes of zeolitic imidazolate framework (ZIF−8)/Matrimid nanocomposite: Thermo-mechanical stability and viscoelasticity underpinning membrane separation performance. J. Memb. Sci. 2016, 498, 276–290. [Google Scholar] [CrossRef]
- Cheng, F.; Marshall, E.S.; Young, A.J.; Robinson, P.J.; Bouillard, J.S.G.; Adawi, A.M.; Vermeulen, N.A.; Farha, O.K.; Reithofer, M.R.; Chin, J.M. Magnetic Control of MOF Crystal Orientation and Alignment. Chem. Eur. J. 2017, 23, 15578–15582. [Google Scholar] [CrossRef]
- Sharma, A.; Tripathi, B.; Vijay, Y.K. Dramatic Improvement in properties of magnetically aligned CNT/polymer nanocomposites. J. Memb. Sci. 2010, 361, 89–95. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, S.; Tripathi, B.; Singh, M.; Vijay, Y.K. Aligned CNT/Polymer nanocomposite membranes for hydrogen separation. Int. J. Hydrog. Energy 2009, 34, 3977–3982. [Google Scholar] [CrossRef]
- Qiao, Z.; Zhao, S.; Wang, J.; Wang, S.; Wang, Z.; Guiver, M.D. A Highly Permeable Aligned Montmorillonite Mixed-Matrix Membrane for CO2 Separation. Angew. Chem. 2016, 128, 9467–9471. [Google Scholar] [CrossRef]
- Jung, H.S.; Kwon, S.H.; Choi, H.J.; Jung, J.H.; Kim, Y.G. Magnetic carbonyl iron/natural rubber composite elastomer and its magnetorheology. Compos. Struct. 2016, 136, 106–112. [Google Scholar] [CrossRef]
- Yao, J.; Sun, Y.; Wang, Y.; Fu, Q.; Xiong, Z.; Liu, Y. Magnet-induced aligning magnetorheological elastomer based on ultra-soft matrix. Compos. Sci. Technol. 2018, 162, 170–179. [Google Scholar] [CrossRef]
- Weber, R.; Orsino, S.; Lallemant, N.; Verlaan, A. Combustion of natural gas with high-temperature air and large quantities of flue gas. Proc. Combust. Inst. 2000, 28, 1315–1321. [Google Scholar] [CrossRef]
- Ibbs, T.L.; Underwood, L. A comparison of the behaviour in thermal diffusion of nitrogen and carbon monoxide, and of nitrous oxide and carbon dioxide. Proc. Phys. Soc. 1926, 39, 227–237. [Google Scholar] [CrossRef]
- Bongers, W.; Bouwmeester, H.; Wolf, B.; Peeters, F.; Welzel, S.; van den Bekerom, D.; den Harder, N.; Goede, A.; Graswinckel, M.; Groen, P.W.; et al. Plasma-driven dissociation of CO2 for fuel synthesis. Plasma Process. Polym. 2017, 14, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Petcharoen, K.; Sirivat, A. Synthesis and characterization of magnetite nanoparticles via the chemical co-precipitation method. Mater. Sci. Eng. B 2012, 177, 421–427. [Google Scholar] [CrossRef]
- Nordin, N.A.H.M.; Ismail, A.F.; Mustafa, A.; Murali, R.S.; Matsuura, T. The impact of ZIF−8 particle size and heat treatment on CO2/CH4 separation using asymmetric mixed matrix membrane. RSC Adv. 2014, 4, 52530–52541. [Google Scholar] [CrossRef]
- Venkatasubramanian, A.; Navaei, M.; Bagnall, K.R.; McCarley, K.C.; Nair, S.; Hesketh, P.J. Gas Adsorption Characteristics of Metal-Organic Frameworks via Quartz Crystal Microbalance Techniques. J. Phys. Chem. C 2012, 116, 15313–15321. [Google Scholar] [CrossRef]
- Chokbunpiam, T.; Fritzsche, S.; Chmelik, C.; Caro, J.; Janke, W.; Hannongbua, S. Gate Opening, Diffusion, and Adsorption of CO2 and N2 Mixtures in ZIF−8. J. Phys. Chem. C 2016, 120, 23458–23468. [Google Scholar] [CrossRef]
- Ordoñez, M.J.C.; Balkus, K.J.; Ferraris, J.P.; Musselman, I.H. Molecular sieving realized with ZIF−8/Matrimid® mixed-matrix membranes. J. Memb. Sci. 2010, 361, 28–37. [Google Scholar] [CrossRef]
- Balçık, M.; Tantekin-Ersolmaz, S.B.; Ahunbay, M.G. Interfacial analysis of mixed-matrix membranes under exposure to high-pressure CO2. J. Memb. Sci. 2020, 607, 1–10. [Google Scholar] [CrossRef]
- Shahid, S.; Nijmeijer, K. Performance and plasticization behavior of polymer-MOF membranes for gas separation at elevated pressures. J. Memb. Sci. 2014, 470, 166–177. [Google Scholar] [CrossRef]
- Shahid, S.; Nijmeijer, K. High pressure gas separation performance of mixed-matrix polymer membranes containing mesoporous Fe(BTC). J. Memb. Sci. 2014, 459, 33–44. [Google Scholar] [CrossRef]

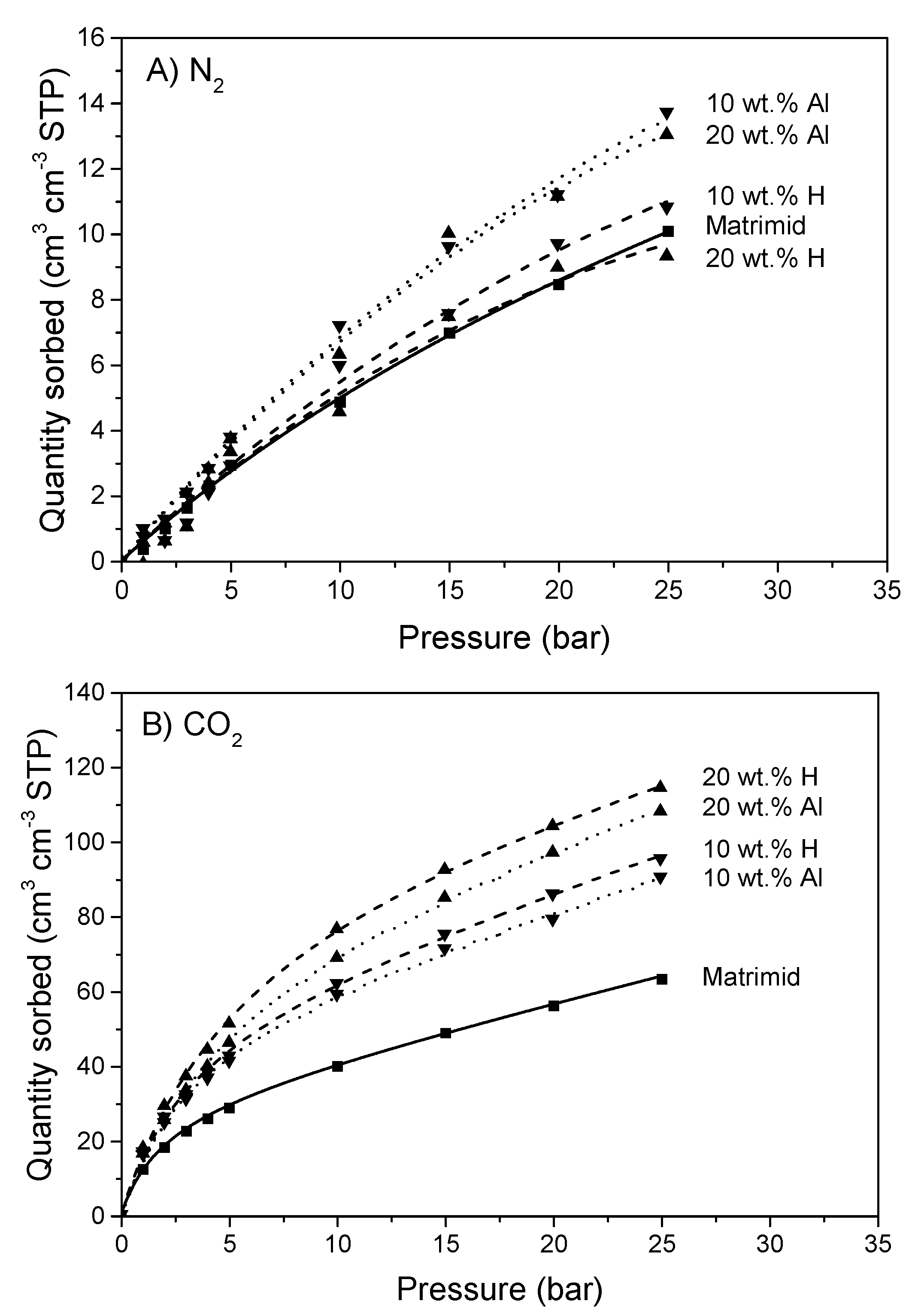
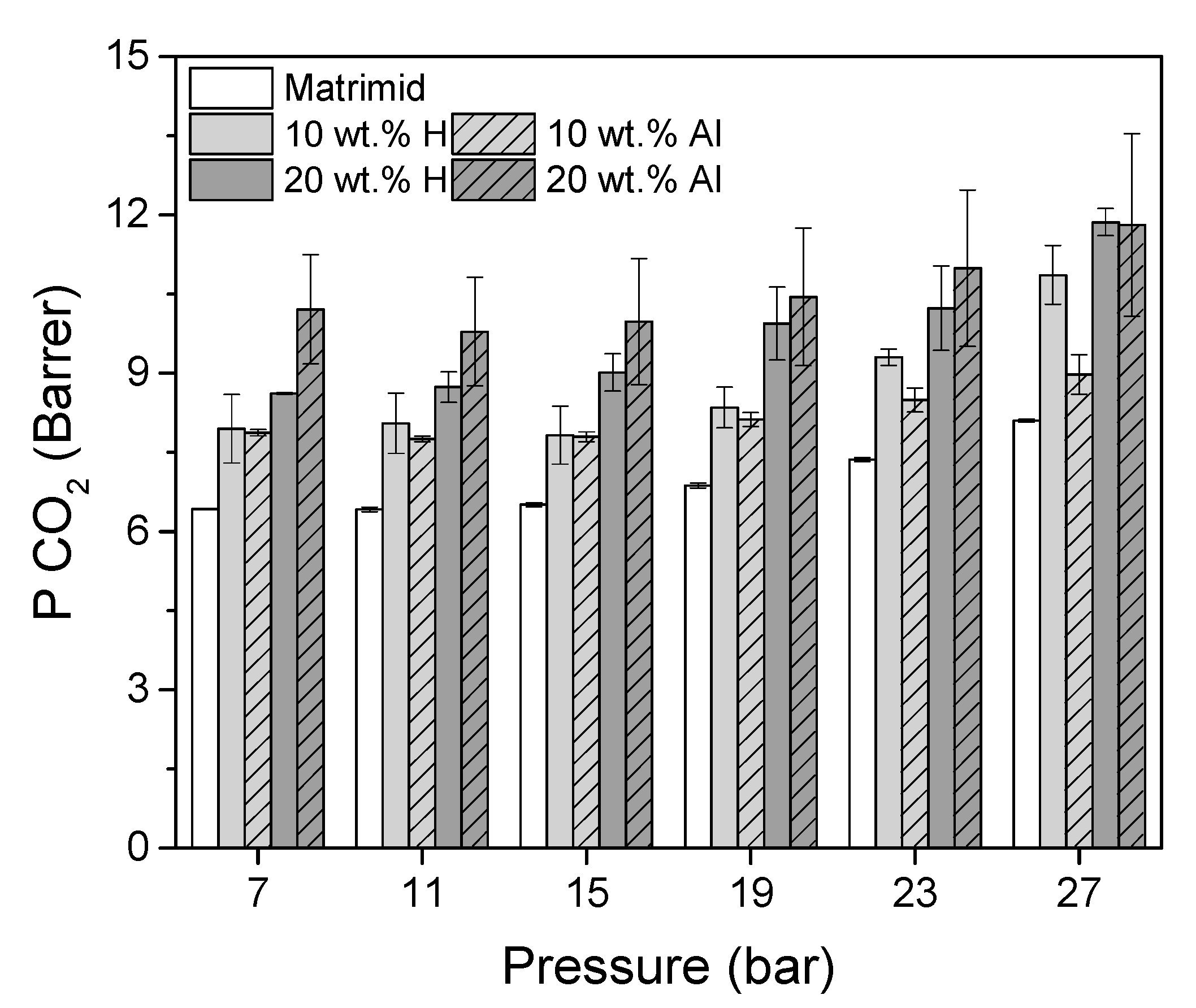
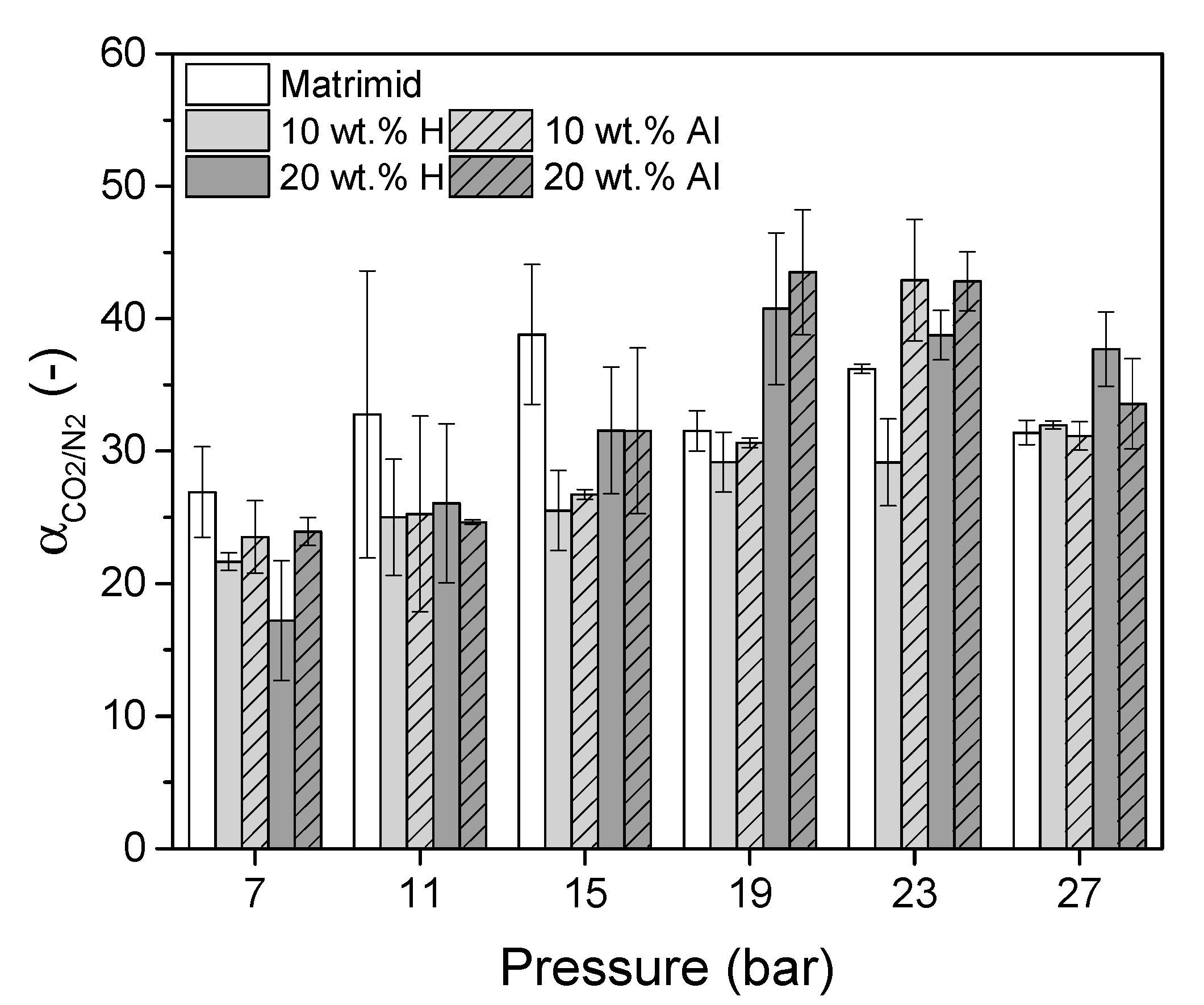
| Membrane | P [Barrer] | S [cm3 STP/(cm3 · cmHg)] | D [10−8 cm2/s] |
|---|---|---|---|
| Matrimid | 6.4 | 0.051 | 1.3 |
| 10 wt.% H | 8.1 | 0.078 | 1.0 |
| 10 wt.% Al | 7.8 | 0.074 | 1.0 |
| 20 wt.% H | 8.7 | 0.096 | 0.9 |
| 20 wt.% Al | 9.8 | 0.087 | 1.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Essen, M.; Montrée, E.; Houben, M.; Borneman, Z.; Nijmeijer, K. Magnetically Aligned and Enriched Pathways of Zeolitic Imidazolate Framework 8 in Matrimid Mixed Matrix Membranes for Enhanced CO2 Permeability. Membranes 2020, 10, 155. https://doi.org/10.3390/membranes10070155
van Essen M, Montrée E, Houben M, Borneman Z, Nijmeijer K. Magnetically Aligned and Enriched Pathways of Zeolitic Imidazolate Framework 8 in Matrimid Mixed Matrix Membranes for Enhanced CO2 Permeability. Membranes. 2020; 10(7):155. https://doi.org/10.3390/membranes10070155
Chicago/Turabian Stylevan Essen, Machiel, Esther Montrée, Menno Houben, Zandrie Borneman, and Kitty Nijmeijer. 2020. "Magnetically Aligned and Enriched Pathways of Zeolitic Imidazolate Framework 8 in Matrimid Mixed Matrix Membranes for Enhanced CO2 Permeability" Membranes 10, no. 7: 155. https://doi.org/10.3390/membranes10070155
APA Stylevan Essen, M., Montrée, E., Houben, M., Borneman, Z., & Nijmeijer, K. (2020). Magnetically Aligned and Enriched Pathways of Zeolitic Imidazolate Framework 8 in Matrimid Mixed Matrix Membranes for Enhanced CO2 Permeability. Membranes, 10(7), 155. https://doi.org/10.3390/membranes10070155







