Metal Organic Framework Based Polymer Mixed Matrix Membranes: Review on Applications in Water Purification
Abstract
1. Introduction
2. Timeline and Synthesis Routes
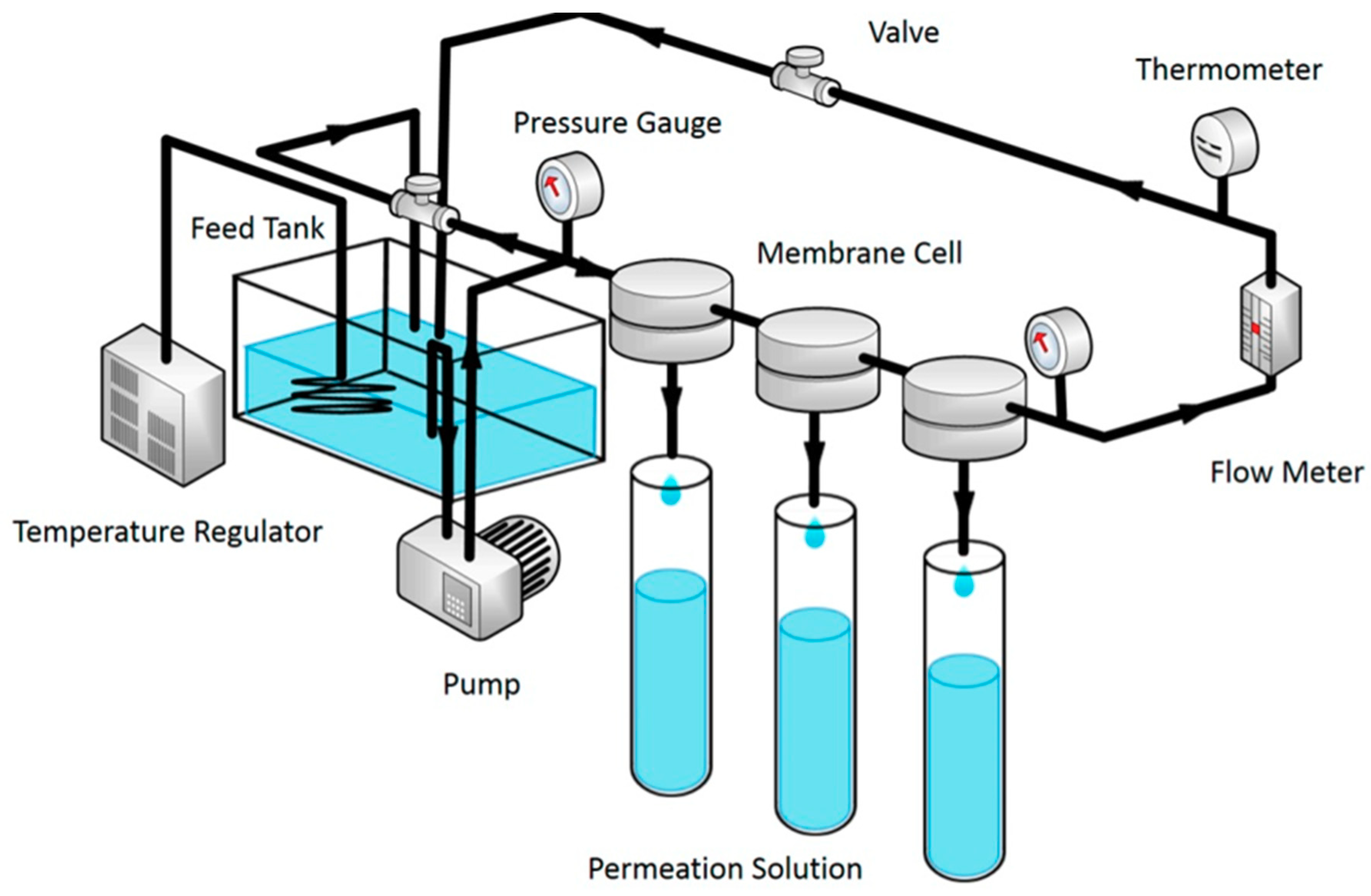
3. MOFs Applications in Water Purification Membranes
3.1. Zeolite Imidazolate Frameworks (ZIFs)
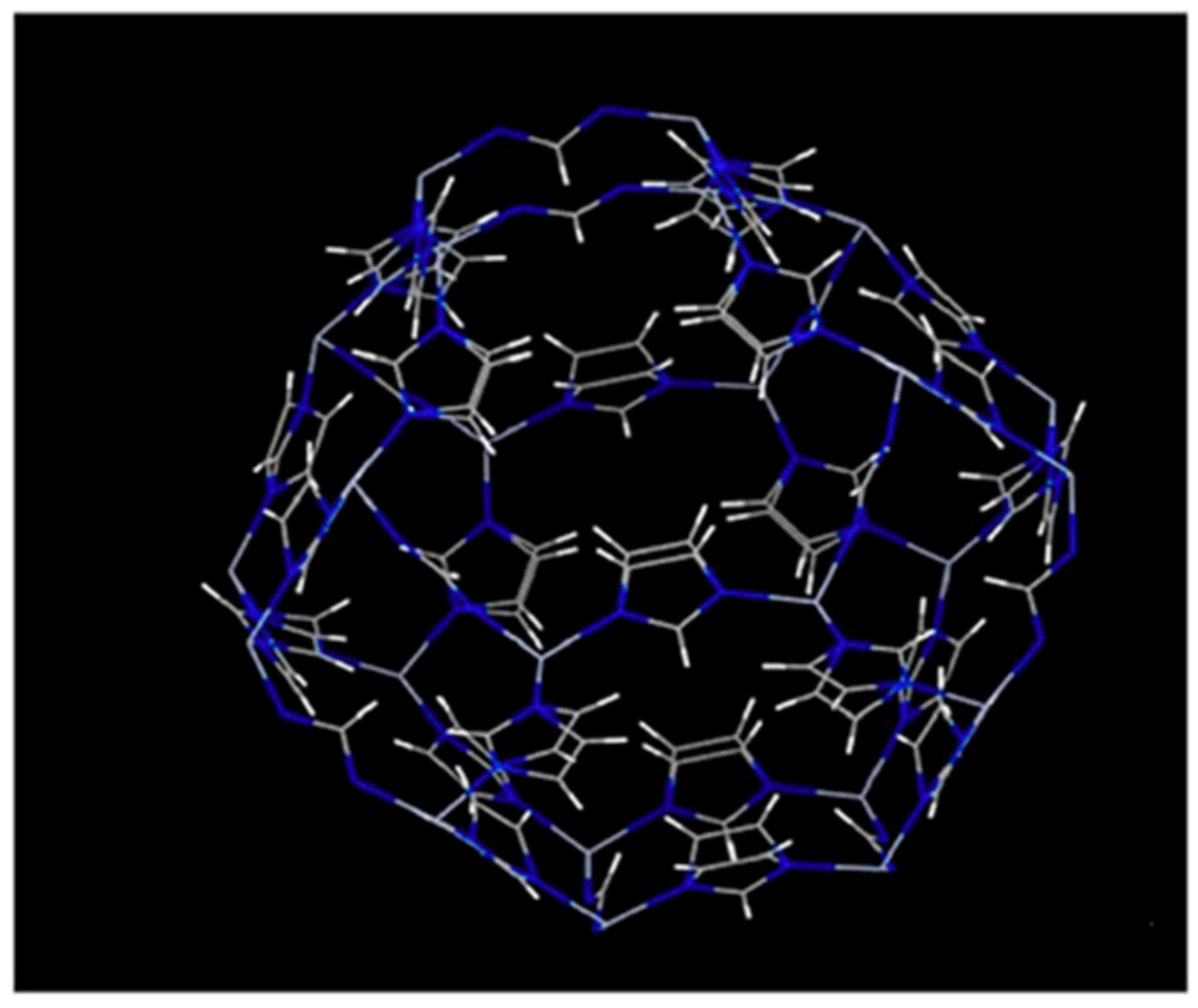
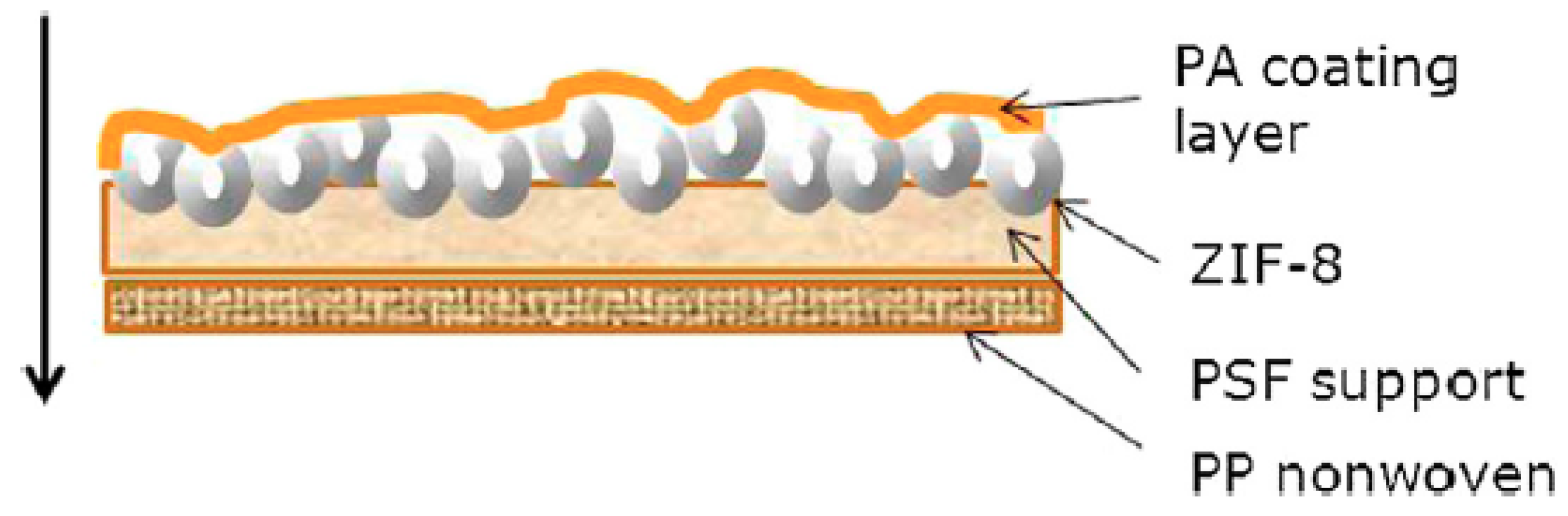
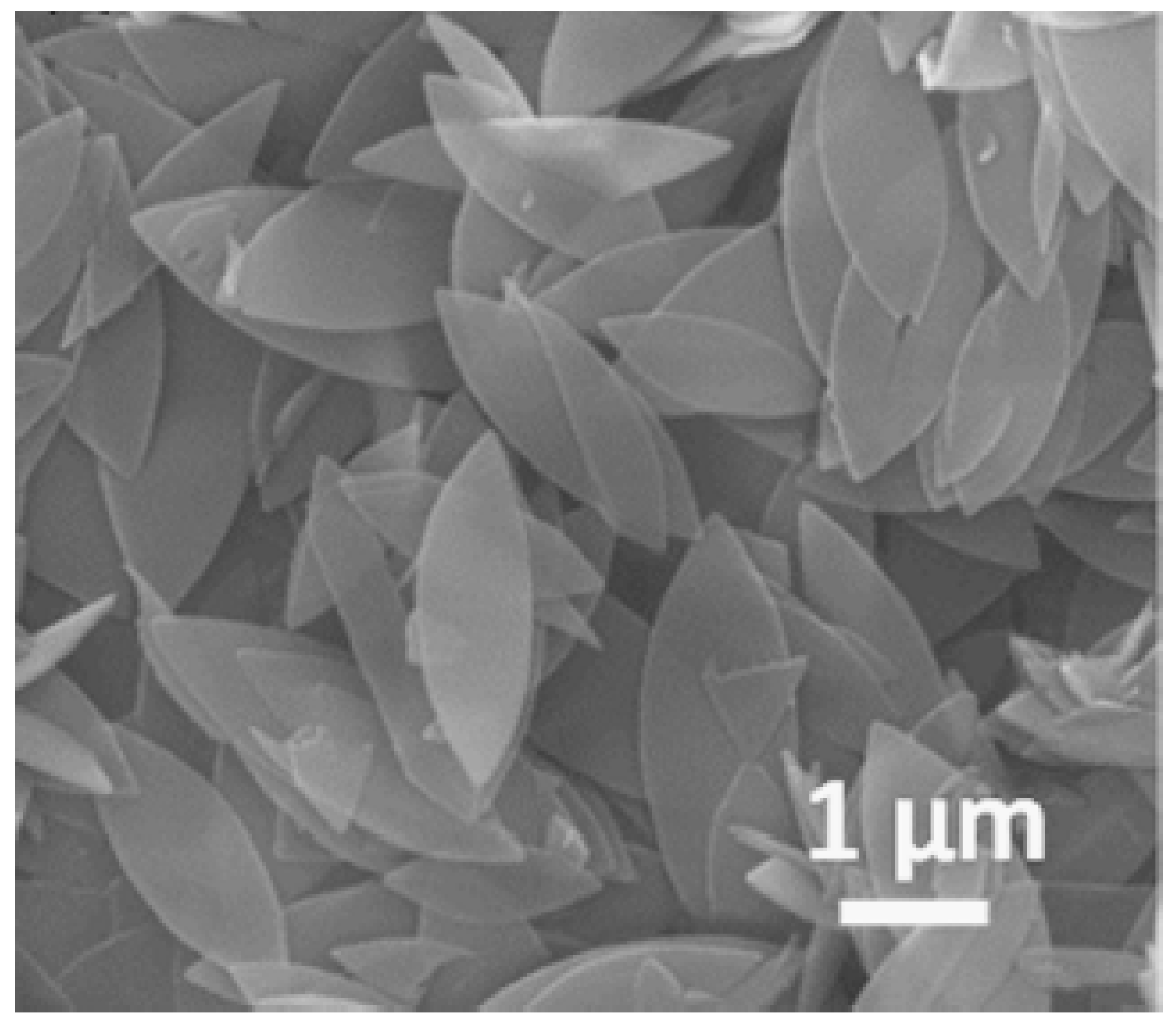
ZIF-8
3.2. Materials of Institute Lavoisier (MIL)
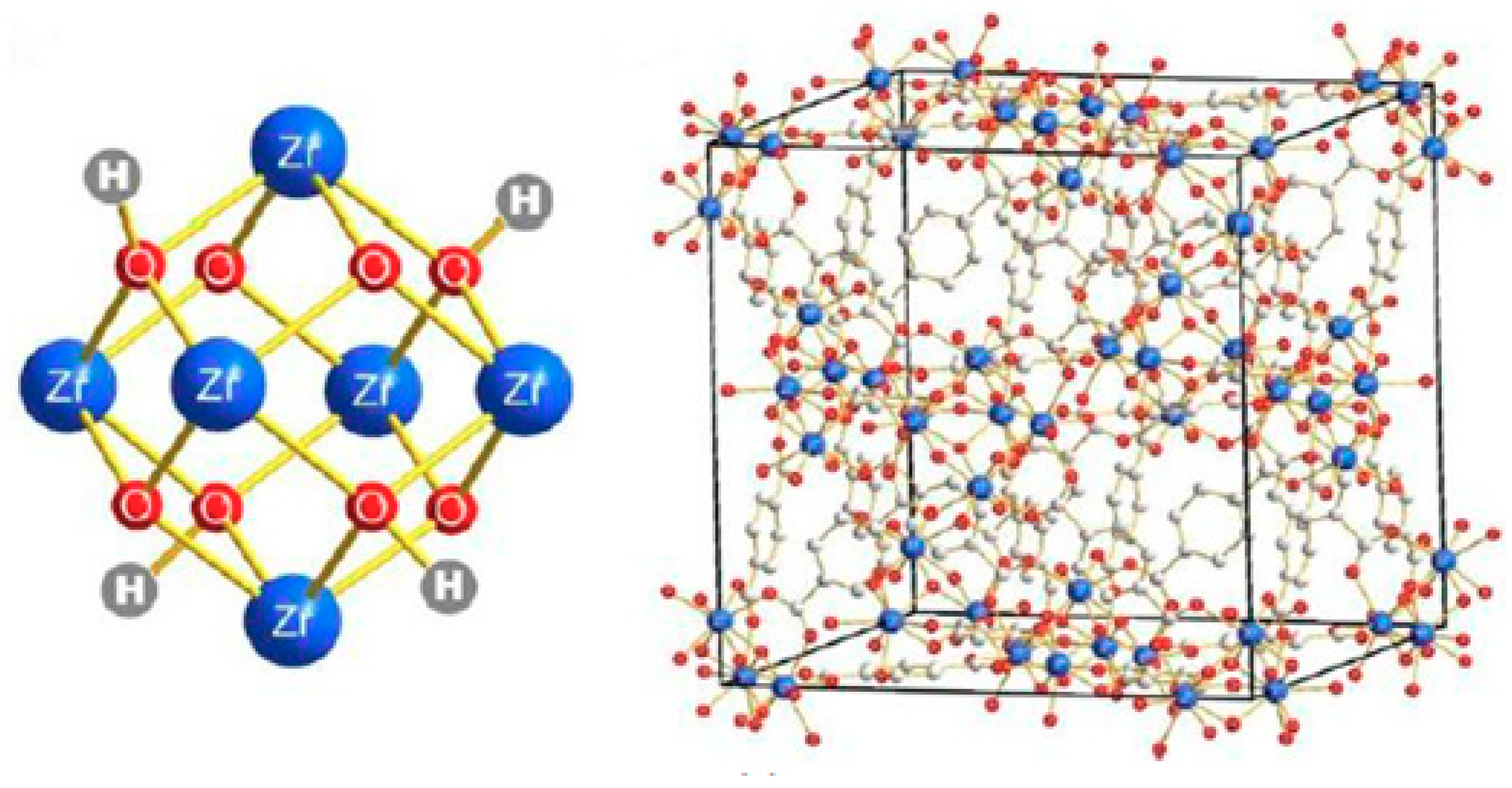
3.3. University of Oslo (UiO-66)
3.4. Pore Forming MOFs
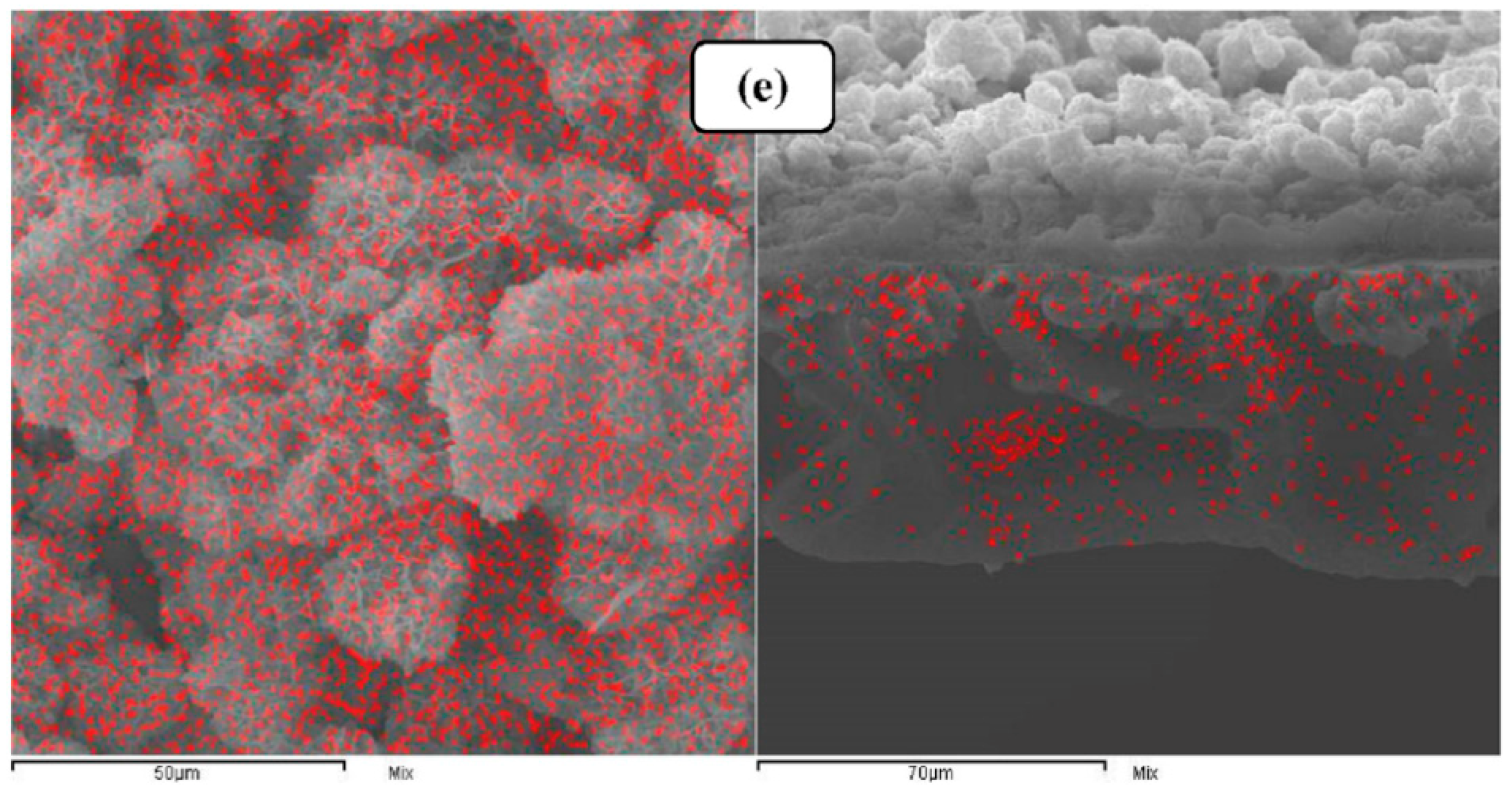
4. Effect of MOFs Incorporation Technique on the Membrane Performance
5. MOFs-MMMs Challenges, Solutions and Future Prospective
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zoromba, M.S.; Mohamed, I.M.; Bassyouni, M.; Abdel-Aziz, M.H.; Salah, N.; Alshahrie, A.; Memic, A. Fabrication and characterization of poly (aniline-co-o-anthranilic acid)/magnetite nanocomposites and their application in wastewater treatment. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 121–130. [Google Scholar] [CrossRef]
- Soliman, M.F.; Abdel-Aziz, M.H.; Bamaga, O.A.; Gzara, L.; Sharaf, F.; Al-Sharif, M.; Bassyouni, M.; Ahmad, R. Performance evaluation of blended PVDF membranes for desalination of seawater RO brine using direct contact membrane distillation. Desalin. Water Treat. 2017, 63, 6–14. [Google Scholar] [CrossRef]
- Ali, I.; Bamaga, O.; Gzara, L.; Bassyouni, M.; Abdel-Aziz, M.; Soliman, M.; Drioli, E.; Albeirutty, M. Assessment of blend PVDF membranes, and the effect of polymer concentration and blend composition. Membranes 2018, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Bassyouni, M.; Abdel-Aziz, M.H.; Zoromba, M.S.; Abdel-Hamid, S.M.S.; Drioli, E. A review of polymeric nanocomposite membranes for water purification. J. Ind. Eng. Chem. 2019, 73, 19–46. [Google Scholar] [CrossRef]
- Gutub, S.A.; Bassyouni, M.; Abdel-Hamid, S.M.S. Dissolved solids adsorption of freshwater using synthesized bio-foam composite. Life Sci. J. 2013, 2, 464–471. [Google Scholar]
- Loeb, S. Reverse Osmosis: Introduction. In Desalination and Water Resources: Membrane Processes; UNESCO: London, UK, 2010; Volume 1, pp. 269–282. [Google Scholar]
- Nady, N.; Franssen, M.; Zuilhof, H.; Boom, R.; Schroën, K. Enzymatic modification of polyethersulfone membranes. Water 2012, 4, 932–943. [Google Scholar] [CrossRef]
- Nady, N. PES surface modification using green chemistry: New generation of antifouling membranes. Membranes 2016, 6, 23. [Google Scholar] [CrossRef]
- Goh, P.S.; Ismail, A.F. A review on inorganic membranes for desalination and wastewater treatment. Desalination 2018, 434, 60–80. [Google Scholar] [CrossRef]
- Kadhom, M.; Deng, B. Metal-organic frameworks (MOFs) in water filtration membranes for desalination and other applications. Appl. Mater. Today 2018, 11, 219–230. [Google Scholar] [CrossRef]
- Beisl, S.; Monteiro, S.; Santos, R.; Figueiredo, A.S.; Sánchez-Loredoe, M.G.; Lemos, M.A.; Lemos, F.; Minhalma, M.; Norberta de Pinhoc, M. Synthesis and bactericide activity of nanofiltration composite membranes—Cellulose acetate/silver nanoparticles and cellulose acetate/silver ion exchanged zeolites. Water Res. 2019, 149, 225–231. [Google Scholar] [CrossRef]
- Dong, L.X.; Yang, H.W.; Liu, S.T.; Wang, X.M.; Xie, Y.F. Fabrication and anti-biofouling properties of alumina and zeolite nanoparticle embedded ultrafiltration membranes. Desalination 2015, 365, 70–78. [Google Scholar] [CrossRef]
- Peng, L.; Xu, X.; Yao, X.; Liu, H.; Gu, X. Fabrication of novel hierarchical ZSM-5 zeolite membranes with tunable mesopores for ultrafiltration. J. Membr. Sci. 2018, 549, 446–455. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Rafiei, S.; Hamidi, A.R.; Moghadassi, A.R.; Madaeni, S.S. Preparation and electrochemical characterization of mixed matrix heterogeneous cation exchange membranes filled with zeolite nanoparticles: Ionic transport property in desalination. Desalination 2014, 351, 138–144. [Google Scholar] [CrossRef]
- Dong, H.; Zhao, L.; Zhang, L.; Chen, H.; Gao, C.; Ho, W.W. High-flux reverse osmosis membranes incorporated with NaY zeolite nanoparticles for brackish water desalination. J. Membr. Sci. 2015, 476, 373–383. [Google Scholar] [CrossRef]
- Liu, R.; Raman, A.K.Y.; Shaik, I.; Aichele, C.; Kim, S.J. Inorganic microfiltration membranes incorporated with hydrophilic silica nanoparticles for oil-in-water emulsion separation. J. Water Process Eng. 2018, 26, 124–130. [Google Scholar] [CrossRef]
- Jalali, A.M.; Shariatinia, Z.; Taromi, F.A. Desulfurization efficiency of polydimethylsiloxane/silica nanoparticle nanocomposite membranes: MD simulations. Comput. Mater. Sci. 2017, 139, 115–124. [Google Scholar] [CrossRef]
- Albertini, F.; Ribeiro, T.; Alves, S.; Baleizão, C.; Farinha, J.P.S. Boron-chelating membranes based in hybrid mesoporous silica nanoparticles for water purification. Mater. Des. 2018, 141, 407–413. [Google Scholar] [CrossRef]
- Abadikhah, H.; Kalali, E.N.; Behzadi, S.; Khan, S.A.; Xu, X.; Agathopoulos, S. Amino functionalized silica nanoparticles incorporated thin film nanocomposite membrane with suppressed aggregation and high desalination performance. Polymer 2018, 154, 200–209. [Google Scholar] [CrossRef]
- Abdelsamad, A.M.; Khalil, A.S.; Ulbricht, M. Influence of controlled functionalization of mesoporous silica nanoparticles as tailored fillers for thin-film nanocomposite membranes on desalination performance. J. Membr. Sci. 2018, 563, 149–161. [Google Scholar] [CrossRef]
- Behboudi, A.; Jafarzadeh, Y.; Yegani, R. Incorporation of silica grafted silver nanoparticles into polyvinyl chloride/polycarbonate hollow fiber membranes for pharmaceutical wastewater treatment. Chem. Eng. Res. Des. 2018, 135, 153–165. [Google Scholar] [CrossRef]
- Wanda, E.M.; Nyoni, H.; Mamba, B.B.; Msagati, T.A. Application of silica and germanium dioxide nanoparticles/polyethersulfone blend membranes for removal of emerging micropollutants from water. Phys. Chem. Earth Parts A/B/C 2018, 108, 28–47. [Google Scholar] [CrossRef]
- Nie, G.; Wu, L.; Du, Y.; Wang, H.; Xu, Y.; Ding, Z.; Liu, Z. Efficient removal of phosphate by a millimeter-sized nanocomposite of titanium oxides encapsulated in positively charged polymer. Chem. Eng. J. 2019, 360, 1128–1136. [Google Scholar] [CrossRef]
- Dasgupta, J.; Chakraborty, S.; Sikder, J.; Kumar, R.; Pal, D.; Curcio, S.; Drioli, E. The effects of thermally stable titanium silicon oxide nanoparticles on structure and performance of cellulose acetate ultrafiltration membranes. Sep. Purif. Technol. 2014, 133, 55–68. [Google Scholar] [CrossRef]
- Kutuzau, M.; Shumskaya, A.; Kaniukov, E.; Alisienok, O.; Shidlouskaya, V.; Melnikova, G.; shemukhin, A.; Nazarov, A.; Kozlovsky, A.; Zdorovets, M. Photocatalytically active filtration systems based on modified with titanium dioxide PET-membranes. Nucl. Instrum. Methods Phys. Res. 2019, in press. [Google Scholar] [CrossRef]
- Casado, C.; Mesones, S.; Adán, C.; Marugán, J. Comparing Potentiostatic and Galvanostatic Anodization of Titanium Membranes for Hybrid Photocatalytic/Microfiltration Processes. Appl. Catal. A 2019, 578, 40–52. [Google Scholar] [CrossRef]
- Betard, A.; Fischer, R.A. Metal–organic framework thin films: from fundamentals to applications. Chem. Rev. 2012, 112, 1055–1083. [Google Scholar] [CrossRef]
- Shekhah, O.; Liu, J.; Fischer, R.A.; Wöll, C. MOF thin films: existing and future applications. Chem. Soc. Rev. 2011, 40, 1081–1106. [Google Scholar] [CrossRef]
- Basu, S.; Balakrishnan, M. Polyamide thin film composite membranes containing ZIF-8 for the separation of pharmaceutical compounds from aqueous streams. Sep. Purif. Technol. 2017, 179, 118–125. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, X.; Hu, P.; Peng, X. ZIF-8 coated polyvinylidenefluoride (PVDF) hollow fiber for highly efficientseparation of small dye molecules. Appl. Mater. Today 2016, 5, 103–110. [Google Scholar] [CrossRef]
- Liu, X.; Demir, N.K.; Wu, Z.; Li, K. Highly water-stable zirconium metal–organic framework UiO-66 membranes supported on alumina hollow fibers for desalination. J. Am. Chem. Soc. 2015, 137, 6999–7002. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, D.; Kong, C.; Zhou, F.; Jiang, T.; Chen, L. Design of thin and tubular MOFs-polymer mixed matrix membranes for highly selective separation of H2 and CO2. Sep. Purif. Technol. 2019, 220, 197–205. [Google Scholar] [CrossRef]
- Kadioglu, O.; Keskin, S. Efficient separation of helium from methane using MOF membranes. Sep. Purif. Technol. 2018, 191, 192–199. [Google Scholar] [CrossRef]
- Mohideen, M.I.H.; Pillai, R.S.; Adil, K.; Bhatt, P.M.; Belmabkhout, Y.; Shkurenko, A.; Maurin, G.; Eddaoudi, M. A fine-tuned MOF for gas and vapor separation: A multipurpose adsorbent for acid gas removal, dehydration, and BTX sieving. Chem 2017, 3, 822–833. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Navarro, M.; Lhotka, M.; Zornoza, B.; Téllez, C.; de Vos, W.M.; Benes, N.E.; Konnertz, N.M.; Visser, T.; Semino, R.; et al. Enhanced gas separation performance of 6FDA-DAM based mixed matrix membranes by incorporating MOF UiO-66 and its derivatives. J. Membr. Sci. 2018, 558, 64–77. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, Y.; Jiang, J. Water desalination through zeolite imidazolate framework-8 as a reverse osmosis membrane for water desalination: Insight from molecular simulation. J. Chem. Phys. 2011, 134, 134705. [Google Scholar] [CrossRef]
- Gupta, K.M.; Zhang, K.; Jiang, J. Water desalination through zeolite imidazole framework membranes: Significant role of functional groups. Langmuir 2015, 31, 13230–13237. [Google Scholar] [CrossRef]
- Kebria, M.R.S.; Rahimour, A.; Bakeri, G.; Abedini, R. Experimental and theoretical investigation of thin ZIF-8/chitosan coated layer on air gap membrane distillation performance of PVDF membrane. Desalination 2019, 450, 21–32. [Google Scholar] [CrossRef]
- Duan, J.; Pan, Y.; Pacheco, F.; Litwiller, E.; Lai, Z.; Pinnau, I. High-performance polyamide thin-film nanocomposite reverse osmosis membranes containing Hydrophobic zeolitic imidazolate framework-8. J. Membr. Sci. 2015, 476, 303–310. [Google Scholar] [CrossRef]
- Xu, Y.; Gao, X.; Wang, X.; Wang, Q.; Ji, Z.; Wang, X.; Wu, T.; Gao, C. Highly and stably water permeable thin film nanocomposite membranes doped withMIL-101 (Cr) nanoparticles for reverse osmosis application. Materials 2016, 9, 870. [Google Scholar] [CrossRef]
- Yang, F.; Efome, J.E.; Rana, D.; Matsuura, T.; Lan, C. Metal–organic frameworks supported on nanofiber for desalination by direct contact membrane distillation. ACS Appl. Mater. Interfaces 2018, 10, 11251–11260. [Google Scholar] [CrossRef]
- Kadhom, M.; Hu, W.; Deng, B. Thin Film Nanocomposite Membrane Filled with Metal-Organic Frameworks UiO-66 and MIL-125 Nanoparticles for Water Desalination. Membranes 2017, 7, 31. [Google Scholar] [CrossRef]
- Zhou, H.C.; Long, J.R.; Yaghi, O.M. Introduction to metal–organic frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef]
- Li, J.R.; Sculley, J.; Zhou, H.C. Metal–organic frameworks for separations. Chem. Rev. 2012, 112, 869–932. [Google Scholar] [CrossRef]
- Hu, J.; Liu, Y.; Liu, J.; Gu, C.; Wu, D. High CO2 adsorption capacities in UiO type MOFs comprising heterocyclic ligand. Microporous Mesoporous Mater. 2018, 256, 25–31. [Google Scholar] [CrossRef]
- Rostami, S.; Pour, A.N.; Salimi, A.; Abolghasempour, A. Hydrogen adsorption in metal-organic frameworks (MOFs): Effects of adsorbent architecture. Int. J. Hydrog. Energy 2018, 43, 7072–7080. [Google Scholar] [CrossRef]
- Abazari, R.; Mahjoub, A.R.; Shariati, J. Synthesis of a nanostructured pillar MOF with high adsorption capacity towards antibiotics pollutants from aqueous solution. J. Hazard. Mater. 2019, 366, 439–451. [Google Scholar] [CrossRef]
- Sumer, Z.; Keskin, S. Adsorption-and membrane-based CH4/N2 separation performances of MOFs. Ind. Eng. Chem. Res. 2017, 56, 8713–8722. [Google Scholar] [CrossRef]
- Hossain, M.I.; Udoh, A.; Grabicka, B.E.; Walton, K.S.; Ritchie, S.M.; Glover, T.G. Membrane-Coated UiO-66 MOF Adsorbents. Ind. Eng. Chem. Res. 2018, 58, 1352–1362. [Google Scholar] [CrossRef]
- Szczęśniak, B.; Choma, J.; Jaroniec, M. Ultrahigh benzene adsorption capacity of graphene-MOF composite fabricated via MOF crystallization in 3D mesoporous graphene. Microporous Mesoporous Mater. 2019, 279, 387–394. [Google Scholar] [CrossRef]
- Ahsan, A.; Jabbari, V.; Islam, T.; Turley, R.S.; Dominguez, N.; Kim, H.; Castro, E.; Hernandez-Viezcas, J.A.; Curry, M.L.; Lopez, J.; et al. Sustainable synthesis and remarkable adsorption capacity of MOF/graphene oxide and MOF/CNT based hybrid nanocomposites for the removal of bisphenol a from water. Sci. Total Environ. 2019, 673, 306–317. [Google Scholar] [CrossRef]
- Dhaka, S.; Kumar, R.; Deep, A.; Kurade, M.B.; Ji, S.W.; Jeon, B.H. Metal–organic frameworks (MOFs) for the removal of emerging contaminants from aquatic environments. Coord. Chem. Rev. 2019, 380, 330–352. [Google Scholar] [CrossRef]
- Gao, G.; Nie, L.; Yang, S.; Jin, P.; Chen, R.; Ding, D.; Wang, X.C.; Wang, W.; Wu, K.; Zhang, Q. Well-defined strategy for development of adsorbent using metal organic frameworks (MOF) template for high performance removal of hexavalent chromium. Appl. Surf. Sci. 2018, 457, 1208–1217. [Google Scholar] [CrossRef]
- Sun, D.T.; Peng, L.; Reeder, W.S.; Moosavi, S.M.; Tiana, D.; Britt, D.K.; Oveisi, E.; Queen, W.L. Rapid, selective heavy metal removal from water by a metal–organic framework/polydopamine composite. ACS Cent. Sci. 2018, 4, 349–356. [Google Scholar] [CrossRef]
- Abu Tarboush, B.J.; Chouman, A.; Jonderian, A.; Ahmad, M.; Hmadeh, M.; Al-Ghoul, M. Metal–Organic Framework-74 for Ultratrace Arsenic Removal from Water: Experimental and Density Functional Theory Studies. ACS Appl. Nano Mater. 2018, 1, 3283–3292. [Google Scholar] [CrossRef]
- Liu, J.; Zou, R.; Zhao, Y. Recent developments in porous materials for H2 and CH4 storage. Tetrahedron Lett. 2016, 57, 4873–4881. [Google Scholar] [CrossRef]
- Ubaid, S.; Zacharia, R.; Xiao, J.; Chahine, R.; Bénard, P.; Tessier, P. Charge–discharge cycling, flowthrough cooling and para-ortho conversion for cooling bulk hydrogen storage tank filled with MOF-5. Int. J. Hydrogen Energy 2016, 41, 1044–1052. [Google Scholar] [CrossRef]
- Bambalaza, S.E.; Langmi, H.W.; Musyoka, N.M.; Ren, J.; Khotseng, L.E. Incorporation of UiO-66 into graphene foam for hydrogen storage applications. Mater. Today 2018, 5, 10431–10439. [Google Scholar] [CrossRef]
- Yang, J.; Grzech, A.; Mulder, F.M.; Dingemans, T.J. The hydrogen storage capacity of mono-substituted MOF-5 derivatives: An experimental and computational approach. Microporous Mesoporous Mater. 2013, 171, 65–71. [Google Scholar] [CrossRef]
- Gangu, K.K.; Maddila, S.; Mukkamala, S.B.; Jonnalagadda, S.B. Characteristics of MOF, MWCNT and graphene containing materials for hydrogen storage: A review. J. Energy Chem. 2018, 30, 132–144. [Google Scholar] [CrossRef]
- Avci, G.; Velioglu, S.; Keskin, S. High-throughput screening of MOF adsorbents and membranes for H2 purification and CO2 capture. ACS Appl. Mater. Interfaces 2018, 10, 33693–33706. [Google Scholar] [CrossRef]
- Zhu, J.; Wu, L.; Bu, Z.; Jie, S.; Li, B.G. Polyethyleneimine-Grafted HKUST-type MOF/polyHIPE Porous Composites (PEI@ PGD-H) as Highly Efficient CO2 Adsorbents. Ind. Eng. Chem. Res. 2019, 58, 4257–4266. [Google Scholar] [CrossRef]
- Saha, D.; Bao, Z.; Jia, F.; Deng, S. Adsorption of CO2, CH4, N2O, and N2 on MOF-5, MOF-177, and zeolite 5A. Environ. Sci. Technol. 2010, 44, 1820–1826. [Google Scholar] [CrossRef]
- Sun, J.; Li, Q.; Chen, G.; Duan, J.; Liu, G.; Jin, W. MOF-801 incorporated PEBA mixed-matrix composite membranes for CO2 capture. Sep. Purif. Technol. 2019, 217, 229–239. [Google Scholar] [CrossRef]
- Bahamon, D.; Díaz-Márquez, A.; Gamallo, P.; Vega, L.F. Energetic evaluation of swing adsorption processes for CO2 capture in selected MOFs and zeolites: Effect of impurities. Chem. Eng. J. 2018, 342, 458–473. [Google Scholar] [CrossRef]
- Lestari, W.W.; Wibowo, A.H.; Astuti, S.; Pamungkas, A.Z.; Krisnandi, Y.K. Fabrication of hybrid coating material of polypropylene itaconate containing MOF-5 for CO2 capture. Prog. Org. Coat. 2018, 115, 49–55. [Google Scholar] [CrossRef]
- Ma, X.; Li, L.; Chen, R.; Wang, C.; Li, H.; Wang, S. Heteroatom-doped nanoporous carbon derived from MOF-5 for CO2 capture. Appl. Surf. Sci. 2018, 435, 494–502. [Google Scholar] [CrossRef]
- Kazemi, S.; Safarifard, V. Carbon dioxide capture in MOFs: The effect of ligand functionalization. Polyhedron 2018, 154, 236–251. [Google Scholar] [CrossRef]
- Elsabawy, K.M.; Fallatah, A.M. Synthesis of newly wings like structure non-crystalline Ni++-1, 3, 5-tribenzyl-1, 3, 5-triazine-2, 4, 6-(1H, 3H, 5H)-trione coordinated MOFs for CO2-Capture. J. Mol. Struct. 2019, 1177, 255–259. [Google Scholar] [CrossRef]
- Gaikwad, S.; Kim, S.J.; Han, S. CO2 capture using amine-functionalized bimetallic MIL-101 MOFs and their stability on exposure to humid air and acid gases. Microporous Mesoporous Mater. 2019, 277, 253–260. [Google Scholar] [CrossRef]
- Lázaro, I.A.; Forgan, R.S. Application of zirconium MOFs in drug delivery and biomedicine. Coord. Chem. Rev. 2019, 380, 230–259. [Google Scholar] [CrossRef]
- Pander, M.; Żelichowska, A.; Bury, W. Probing mesoporous Zr-MOF as drug delivery system for carboxylate functionalized molecules. Polyhedron 2018, 156, 131–137. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Y.; Shi, R.; Kang, T.; Pang, G.; Wang, B.; Zhao, Y.; Zeng, X.; Zou, C.; Wu, P.; et al. Synthesis of hollow nanocages MOF-5 as drug delivery vehicle to solve the load-bearing problem of insoluble antitumor drug oleanolic acid (OA). Inorg. Chem. Commun. 2018, 96, 20–23. [Google Scholar] [CrossRef]
- Javanbakht, S.; Pooresmaeil, M.; Namazi, H. Green one-pot synthesis of carboxymethylcellulose/Zn-based metal-organic framework/graphene oxide bio-nanocomposite as a nanocarrier for drug delivery system. Carbohydr. Polym. 2019, 208, 294–301. [Google Scholar] [CrossRef]
- Nadizadeh, Z.; Naimi-Jamal, M.R.; Panahi, L. Mechanochemical solvent-free in situ synthesis of drug-loaded {Cu2 (1, 4-bdc)2(dabco)} n MOFs for controlled drug delivery. J. Solid State Chem. 2018, 259, 35–42. [Google Scholar] [CrossRef]
- Singh, R. Metal organic frameworks for drug delivery. In Applications of Nanocomposite Materials in Drug Delivery; Woodhead Publishing: Cambridge, UK, 2018; pp. 605–617. [Google Scholar]
- Liu, X.L.; Yin, Q.; Huang, G.; Liu, T.F. Stable pyrazolate-based metal-organic frameworks for drug delivery. Inorg. Chem. Commun. 2018, 94, 21–26. [Google Scholar] [CrossRef]
- Su, F.; Jia, Q.; Li, Z.; Wang, M.; He, L.; Peng, D.; Song, Y.; Zhang, Z.; Fang, S. Aptamer-templated silver nanoclusters embedded in zirconium metal–organic framework for targeted antitumor drug delivery. Microporous Mesoporous Mater. 2019, 275, 152–162. [Google Scholar] [CrossRef]
- Liu, D.; Xiang, L.; Chang, H.; Chen, K.; Wang, C.; Pan, Y.; Li, Y.; Jiang, Z. Rational matching between MOFs and polymers in mixed matrix membranes for propylene/propane separation. Chem. Eng. Sci. 2019, 204, 151–160. [Google Scholar] [CrossRef]
- Du, J.; Zhang, C.; Pu, H.; Li, Y.; Jin, S.; Tan, L.; Zhou, C.; Dong, L. HKUST-1 MOFs decorated 3D copper foam with superhydrophobicity/superoleophilicity for durable oil/water separation. Colloids Surf. A 2019, 573, 222–229. [Google Scholar] [CrossRef]
- Liang, W.; Bhatt, P.M.; Shkurenko, A.; Adil, K.; Mouchaham, G.; Aggarwal, H.; Mallick, A.; Jamal, A.; Belmabkhout, Y.; Eddaoudi, M. A Tailor-Made Interpenetrated MOF with Exceptional Carbon-Capture Performance from Flue Gas. Chem 2019, 5, 950–963. [Google Scholar] [CrossRef]
- Liu, G.; Labreche, Y.; Chernikova, V.; Shekhah, O.; Zhang, C.; Belmabkhout, Y.; Eddaoudi, M.; Koros, W.J. Zeolite-like MOF nanocrystals incorporated 6FDA-polyimide mixed-matrix membranes for CO2/CH4 separation. J. Membr. Sci. 2018, 565, 186–193. [Google Scholar] [CrossRef]
- Bunzen, H.; Javed, A.; Klawinski, D.; Lamp, A.; Grzywa, M.; Kalytta-Mewes, A.; Tiemann, M.; Krug von Nodda, H.; Wagner, T.; Volkmer, D. Anisotropic Water-Mediated Proton Conductivity in Large Iron (II) Metal–Organic Framework Single Crystals for Proton-Exchange Membrane Fuel Cells. ACS Appl. Nano Mater. 2018, 2, 291–298. [Google Scholar] [CrossRef]
- Campbell, J.; Davies, R.P.; Braddock, D.C.; Livingston, A.G. Improving the permeance of hybrid polymer/metal–organic framework (MOF) membranes for organic solvent nanofiltration (OSN)–Development of MOF thin films via interfacial synthesis. J. Mater. Chem. A 2015, 3, 9668–9674. [Google Scholar] [CrossRef]
- Campbell, J.; Székely, G.; Davies, R.P.; Braddock, D.C.; Livingston, A.G. Fabrication of hybrid polymer/metal organic framework membranes: Mixed matrix membranes versus in situ growth. J. Mater. Chem. A 2014, 2, 9260–9271. [Google Scholar] [CrossRef]
- Zhu, L.; Yu, H.; Zhang, H.; Shen, J.; Xue, L.; Gao, C.; van der Bruggen, B. Mixed matrix membranes containing MIL-53 (Al) for potential application in organic solvent nanofiltration. RSC Adv. 2015, 5, 73068–73076. [Google Scholar] [CrossRef]
- Campbell, J.; Burgal, J.D.S.; Szekely, G.; Davies, R.P.; Braddock, D.C.; Livingston, A. Hybrid polymer/MOF membranes for Organic Solvent Nanofiltration (OSN): Chemical modification and the quest for perfection. J. Membr. Sci. 2016, 503, 166–176. [Google Scholar] [CrossRef]
- Ren, J.; Musyoka, N.M.; Annamalai, P.; Langmi, H.W.; North, B.C.; Mathe, M. Electrospun MOF nanofibers as hydrogen storage media. Int. J. Hydrogen Energy 2015, 40, 9382–9387. [Google Scholar] [CrossRef]
- Qasem, N.A.; Ben-Mansour, R.; Habib, M.A. An efficient CO2 adsorptive storage using MOF-5 and MOF-177. Appl. Energy 2018, 210, 317–326. [Google Scholar] [CrossRef]
- Wang, Z.; Ge, L.; Li, M.; Lin, R.; Wang, H.; Zhu, Z. Orientated growth of copper-based MOF for acetylene storage. Chem. Eng. J. 2019, 357, 320–327. [Google Scholar] [CrossRef]
- Hou, X.X.; Sulic, M.; Ortmann, J.P.; Cai, M.; Chakraborty, A. Experimental and numerical investigation of the cryogenic hydrogen storage processes over MOF-5. Int. J. Hydrogen Energy 2016, 41, 4026–4038. [Google Scholar] [CrossRef]
- Sorribas, S.; Gorgio, P.; Tellez, C.; Coronas, J.; Livingston, A.G. High flux thin film nanocomposite membranes based on metal-organic frameworks for organic solvent nanofiltration. J. Am. Chem. Soc. 2013, 135, 15201–15208. [Google Scholar] [CrossRef]
- Cook, T.R.; Zheng, Y.R.; Stang, P.J. Metal–organic frameworks and self-assembled supramolecular coordination complexes: Comparing and contrasting the design, synthesis, and functionality of metal–organic materials. Chem. Rev. 2012, 113, 734–777. [Google Scholar] [CrossRef]
- Stock, N.; Biswas, S. Synthesis of metal-organic frameworks (MOFs): Routes to various MOF topologies, morphologies and composites. Chem. Rev. 2012, 112, 933–969. [Google Scholar] [CrossRef]
- Reinsch, H. “Green” Synthesis of Metal-Organic Frameworks. Eur. J. Inorg. Chem. 2016, 2016, 4290–4299. [Google Scholar] [CrossRef]
- Lee, J.Y.; Tang, C.Y.; Huo, F. Fabrication of porous matrix membrane (PMM) using metal-organic framework as green template for water treatment. Sci. Rep. 2014, 4, 3740. [Google Scholar] [CrossRef]
- Lee, J.Y.; She, Q.; Huo, F.; Tang, C.Y. Metal–organic framework-based porous matrix membranes for improving mass transfer in forward osmosis membranes. J. Membr. Sci. 2015, 492, 392–399. [Google Scholar] [CrossRef]
- Mao, H.; Zhen, H.G.; Ahmad, A.; Zhang, A.S.; Zhao, Z.P. In situ fabrication of MOF nanoparticles in PDMS membrane via interfacial synthesis for enhanced ethanol permselective pervaporation. J. Membr. Sci. 2019, 573, 344–358. [Google Scholar] [CrossRef]
- Lee, T.H.; Oh, J.Y.; Hong, S.P.; Lee, J.M.; Roh, S.M.; Kim, S.H.; Park, H.B. ZIF-8 particle size effects on reverse osmosis performance of polyamide thin-film nanocomposite membranes: Importance of particle deposition. J. Membr. Sci. 2019, 570, 23–33. [Google Scholar] [CrossRef]
- Karimi, A.; Vatanpour, V.; Khataee, A.; Safarpour, M. Contra-diffusion synthesis of ZIF-8 layer on polyvinylidene fluoride ultrafiltration membranes for improved water purification. J. Ind. Eng. Chem. 2019, 73, 95–105. [Google Scholar] [CrossRef]
- Zhang, R.; Ji, S.; Wang, N.; Wang, L.; Zhang, G.; Li, J.-R. Coordination-driven in situ self-assembly strategy for the preparation of metal-organic framework hybrid membranes. Angew. Chem. Int. Ed. 2014, 53, 9775–9779. [Google Scholar] [CrossRef]
- Maroofi, S.M.; Mahmoodi, N.M. Zeolitic imidazolate framework-polyvinylpyrrolidone-polyethersulfone composites membranes: From synthesis to the detailed pollutant removal from wastewater using cross flow system. Colloids Surf. A Physicochem. Eng. Asp. 2019, 572, 211–220. [Google Scholar] [CrossRef]
- Ragab, D.; Gomaa, H.G.; Sabouni, R.; Salem, M.; Ren, M.; Zhu, J. Micropollutants removal from water using microfiltration membrane modified with ZIF-8 metal organic frameworks (MOFs). Chem. Eng. J. 2016, 300, 273–279. [Google Scholar] [CrossRef]
- Zhu, J.; Qin, L.; Uliana, A.; Hou, J.; Wang, J.; Zhang, Y.; Li, X.; Yuan, S.; Li, J.; Tian, M.; et al. Elevated Performance of Thin Film Nanocomposite Membranes Enabled by Modified Hydrophilic MOFs for Nanofiltration. ACS Appl. Mater. Interfaces 2017, 9, 1975–1986. [Google Scholar] [CrossRef]
- Aljundi, I.H. Desalination characteristics of TFN-RO membrane incorporated with ZIF-8 nanoparticles. Desalination 2017, 420, 12–20. [Google Scholar] [CrossRef]
- Low, Z.X.; Razmjou, A.; Wang, K.; Gray, S.; Duke, M.; Wang, H. Effect of addition of two-dimensional ZIF-L nanoflakes on the properties of polyethersulfone ultrafiltration membrane. J. Membr. Sci. 2014, 460, 9–17. [Google Scholar] [CrossRef]
- Devic, T.; Serre, C. High valence 3p and transition metal based MOFs. Chem. Soc. Rev. 2014, 43, 6097–6115. [Google Scholar] [CrossRef]
- Moghaddam, Z.S.; Kaykhaii, M.; Khajeh, M.; Oveisi, A.R. Synthesis of UiO-66-OH zirconium metal-organic framework and its application for selective extraction and trace determination of thorium in water samples by spectrophotometry. Spectrochim. Acta Part A 2018, 194, 76–82. [Google Scholar] [CrossRef]
- Yang, F.; Li, W.; Tang, B. Facile synthesis of amorphous UiO-66 (Zr-MOF) for supercapacitor application. J. Alloys Compd. 2018, 733, 8–14. [Google Scholar] [CrossRef]
- Albuquerque, G.H.; Herman, G.S. Chemically modulated microwave-assisted synthesis of MOF-74 (Ni) and preparation of metal–organic framework-matrix based membranes for removal of metal ions from aqueous media. Crystal Growth Design 2016, 17, 156–162. [Google Scholar] [CrossRef]
- Wen, Y.; Chen, Y.; Wu, Z.; Liu, M.; Wang, Z. Thin-film nanocomposite membranes incorporated with water stable metal-organic framework CuBTTri for mitigating biofouling. J. Membr. Sci. 2019, 582, 289–297. [Google Scholar] [CrossRef]
- Venna, S.R.; Carreon, M.A. Highly Permeable Zeolite Imidazolate Framework-8 Membranes for CO2/CH4 Separation. J. Am. Chem. Soc. 2010, 132, 76–78. [Google Scholar] [CrossRef]
- Xu, Q. Nanoporous Metal-Organic Frameworks, Nanoporous Materials: Synthesis and Applications; CRC Press: Boca Raton, FL, USA, 2013; p. 75. [Google Scholar]
- Janiak, C.; Vieth, J.K. MOFs, MILs and more: Concepts, properties and applications for porous coordination networks (PCNs). N. J. Chem. 2010, 34, 2366–2388. [Google Scholar] [CrossRef]
- Dan-Hardi, M.; Serre, C.; Frot, T.; Rozes, L.; Maurin, G.; Sanchez, C.; Ferey, G. A New Photoactive Crystalline Highly Porous Titanium(IV) Dicarboxylate. J. Am. Chem. Soc. 2009, 131, 10857–10859. [Google Scholar] [CrossRef]
- Ma, X.-H.; Yang, Z.; Yao, Z.-L.; Xua, Z.-L.; Tang, C.Y. A facile preparation of novel positively charged MOF/chitosan nanofiltration membranes. J. Membr. Sci. 2017, 525, 269–276. [Google Scholar] [CrossRef]
- Ma, D.; Peh, S.B.; Han, G.; Chen, S.B. Thin-film nanocomposite (TFN) membranesincorporated with super-hydrophilic metal-organic framework (MOF) UiO-66: Toward enhancement of water flux and salt rejection. ACS Appl. Mater. Interfaces 2017, 9, 7523–7534. [Google Scholar] [CrossRef]
- Wang, C.; Liu, X.; Chen, J.P.; Li, K. Superior removal of arsenic from water with zirconium metal-organic framework UiO-66. Sci. Rep. 2015, 5, 16613. [Google Scholar] [CrossRef]
- Liu, L.; Xie, X.; Qi, S.; Li, R.; Zhang, X.; Song, X.; Gao, C. Thin film nanocomposite reverse osmosis membrane incorporated with UiO-66 nanoparticles for enhanced boron removal. J. Membr. Sci. 2019, 580, 101–109. [Google Scholar] [CrossRef]
- Gonzalez-Salamoa, J.; Gonzalez-Curbelob, J.A.; Hernandez-Borgesa, J.; Rodriguez-Delgadoa, M.A. Use of Basolite® F300 metal-organic framework for the dispersive solid phase extraction of phthalic acid esters from water samples prior to LC-MS determination. Talanta 2019, 195, 236–244. [Google Scholar] [CrossRef]
- Du, M.; Li, L.; Li, M.; Si, R. Adsorption mechanism on metal organic frameworks of Cu-BTC, Fe-BTC and ZIF-8 for CO2 capture investigated by X-ray absorption fine structure. RCS Adv. 2016, 6, 62705–62716. [Google Scholar] [CrossRef]
- Chopra, S.; Dhumal, S.; Abeli, P.; Beaudry, R.; Almenar, E. Metal-organic frameworks have utility in adsorption and release of ethylene and 1-methylcyclopropene in fresh produce packaging. Postharvest Biol. Technol. 2017, 130, 48–55. [Google Scholar] [CrossRef]
- Chui, S.S.Y.; Lo, S.M.F.; Charmant, J.P.H.; Orpen, A.G.; Williams, I.D. A chemically functionalizable nanoporous material [Cu3(TMA)2(H2O)3]n. Science 1999, 283, 1148–1150. [Google Scholar] [CrossRef]
- Arjmandi, M.; Peyravi, M.; Chenar, M.P.; Jahanshahi, M. A new concept of MOF-based PMM by modification of conventional dense film casting method: Significant impact on the performance of FO process. J. Membr. Sci. 2019, 579, 253–265. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Y.; Wang, X.-M.; Huang, X.; Xie, Y.F. Impacts of Metal-organic Frameworks on Structure and Performance of Polyamide Thin-film Nanocomposite Membranes. ACS Appl. Mater. Interfaces 2019, 11, 13724–13734. [Google Scholar] [CrossRef]
- Lin, R.; Hernandez, B.V.; Ge, L.; Zhu, Z. Metal organic framework based mixed matrix membranes: An overview on filler/polymer interfaces. J. Mater. Chem. A 2018, 6, 293–312. [Google Scholar] [CrossRef]
- Keskin, S.; Sholl, D.S. Selecting metal organic frameworks as enabling materials in mixed matrix membranes for high efficiency natural gas purification. Energy Environ. Sci. 2010, 3, 343–351. [Google Scholar] [CrossRef]
- Erucar, I.; Yilmaz, G.; Keskin, S. Recent advances in metal–organic framework-based mixed matrix membranes. Chem. Asian J. 2013, 8, 1692–1704. [Google Scholar] [CrossRef]
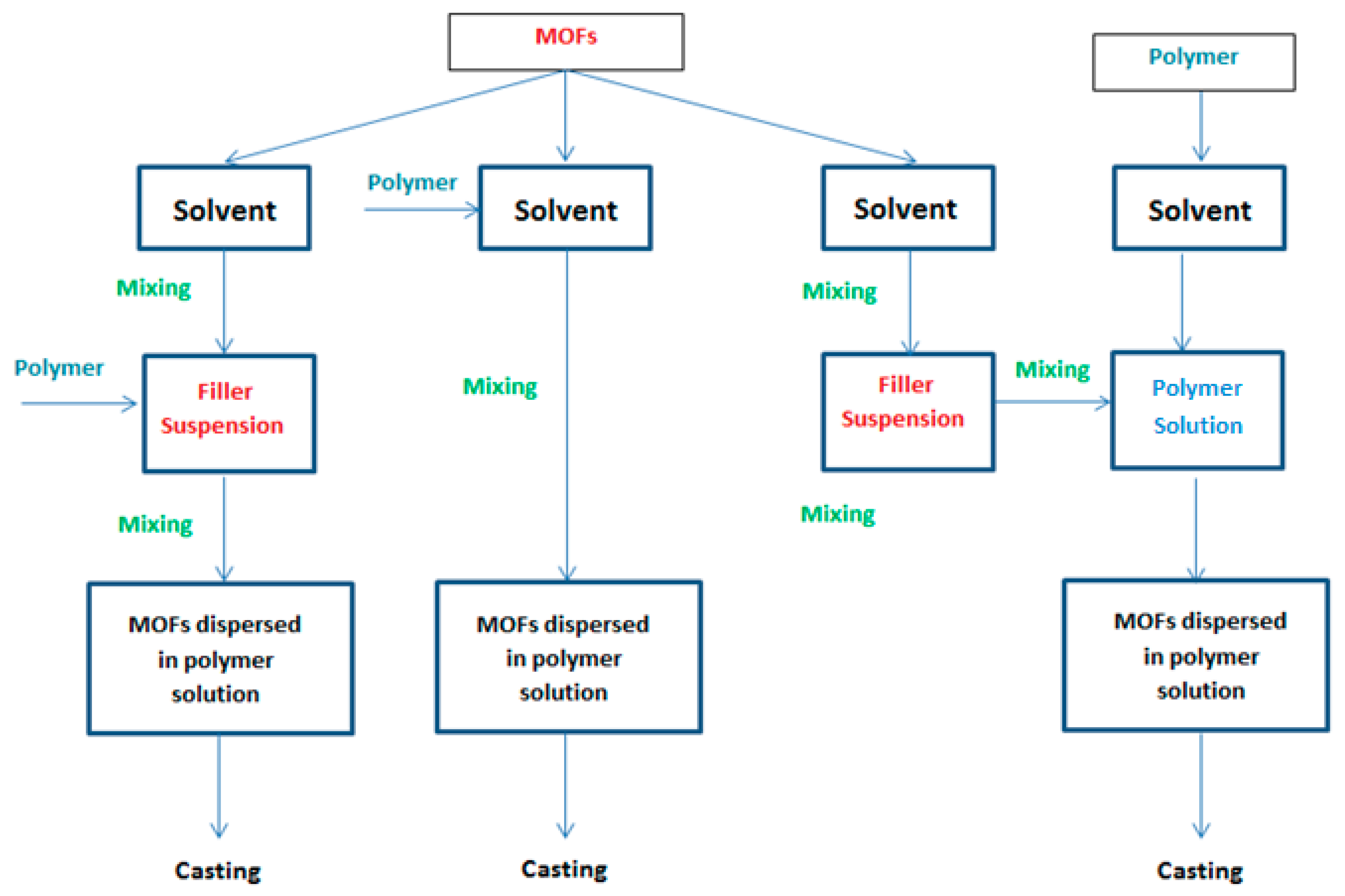



| Blending | In Situ Growth | Layer-By-Layer | Gelatin-Assisted Seed Growth |
|---|---|---|---|
The blending technique is divided into three methods:
| In this process, MOFs are produced by covalent coordination between the metal clusters and the organic ligand together with the membrane formation or within the pores of an already prepared membrane structure, which result in better dispersion and compatibility of the produced MOFs in the polymer matrix. | The LBL method involves the successive immersion of the substrate in solutions containing the metal salt and solutions of the organic ligands. After each cycle of deposition, the substrate is washed by an adequate solvent to remove any traces of unreacted compounds or any physico-sorbed components. Hence a layer of well-intergrown continuous dense film of the targeted MOFs is created on the substrate surface. | The substrate is immersed in a gelatin solution containing the MOFs seeds. This method was developed to overcome the limitations of the organic solvents synthesis that hindered growth of MOFs at elevated temperature thus enabling the growth of a uniform crack free MOFs thin layer at room temperature. |
| Type of Filler | Polymer Matrix | Support | Composite Membrane Fabrication Technique | Optimum Conditions | Permeation Flux | Separation/Rejection Factor | Filler Loading/Particle Size | Selection Criteria | Application Process | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
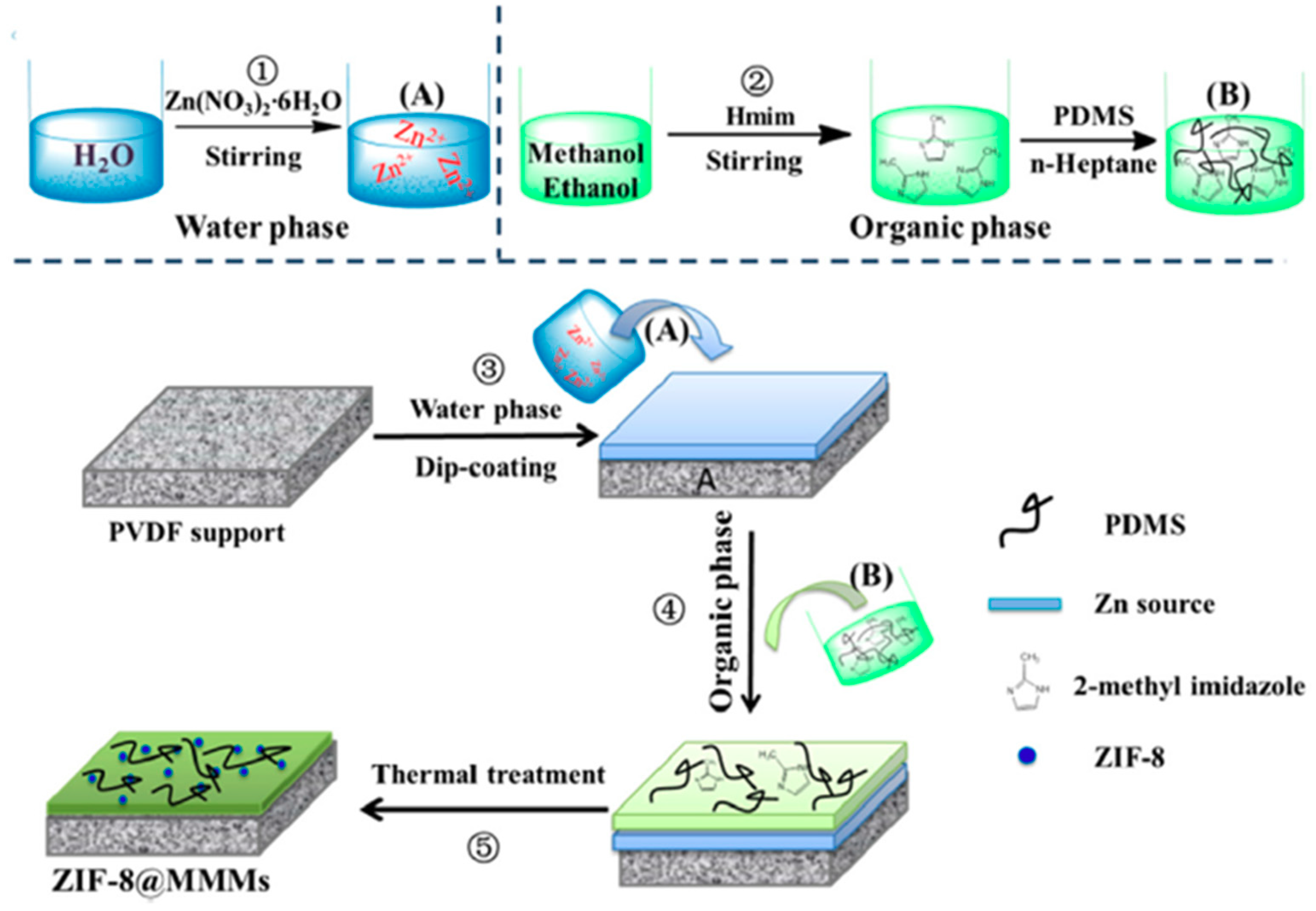
| ZIF-8 | PDMS | Polyvinylidene fluoride (PVDF) | In situ fabrication (growth) of MOFs within the polymer matrix. | Time: 10 min Conc.: 0.05 M of Zn(NO3)2 | 1868 g/m2·h | Ethanol separation factor 12.1 | 12.2–20.4 wt% based on starting Zn(NO3)2 concentration of 0.01–0.09 M |
| Pervaporation | [98] |
| ZIF-8 | PA | PSF | Deposition of ZIF-8 particles dispersed in m-Phenylene diamine (MPD) solution on the microporous support prior to interfacial polymerization of the PA layer. | Mean particle size ZIF-8 (150 nm) at filler loading of 0.2 wt%/vol% | 3.95 L/m2·h·bar | NaCl rejection 99.2% | 0.2 wt% and 0.4 vol% 60, 150 and 250 nm |
| RO | [99] |
| ZIF-8/chitosan | PVDF | PVDF membrane was immersed in a coating solution of ZIF-8 particles, chitosan, PEG and DI water. | 137 L/m2·h | rejection up to 97.5% | The gelatin-assisted technique was chosen to overcome the limitations of the organic solvent synthesis of the hindered growth of MOFs at elevated temperature that enabled the growth of a uniform crack-free ZIF-8 thin layer at room temperature. | Removal of Rhodamine B dye | [38] | |||
| ZIF-8 | PA | PSF | ZIF-8 particles were dispersed in TMC/hexane solution used in the IP. | 0.05% w/v to 0.40% w/v |
| Desalination by RO | [39] | |||
| ZIF-8 | Porous (PVDF) | Contra-diffusion synthesis method was used to create a uniform layer of zeolitic imidazolate framework- 8 (ZIF-8) on the porous polyvinylidene fluoride. | 5 h contra-diffusion synthesis time | 134 L/m2·h | 98.32% for reactive blue 21 dye and 82.25% for direct yellow 19 dye | Continuous layer |
| Dye removal | [100] | |
| ZIF-8 | PAN | PSS | Coordination-driven in situ self-assembly for the synthesis of hybrid ZIF-8/PSS membrane on the surface of a polyacrylonitrile (PAN) support. | Starting solution of 0.05 M concentration of Zn(NO3)2 | 265 L/m2·h·MPa | 98.6% of MB dye | Uniformly dispersed layer on the membrane— 150 nm at 0.05 M Zn(NO3)2 |
| Nanfiltration of MB dye from water | [101] |
| ZIF-8 | PVP/PES | Blending of previously prepared ZIF-8 particles with the polymer matrix. | 99.6% dye removal at 3% filler loading | 1–3% ˂100 nm | High separation ability of ZIF-8 particles due to its zeolite like structure | Malachite green dye removal in a cross-flow system | [102] | |||
| ZIF-8 | PA | PSF | Two different membrane structures were obtained by in situ growth of ZIF-8 particles in the PSF support then followed by deposition of a PA separation layer on top of the modified membrane. LBL assembly of ZIF-8/PA on top of PSF support. | 4 L/m2·h·bar | 0.02, 0.04, 0.06, 0.08, 0.1 g/100 mL | ZIF-8 significant separation ability | Separation of pharmaceuticals from aqueous streams | [29] | ||
| ZIF-8/Gelatin | PVDF hollow fiber | Gelatin-assisted growth technique. | 30 min reaction time to produce well inter-grown, uniform, continuous and dense ZIF-8/gelatin layer | 137 L/m2·h·bar | 97.5% dye rejection | Uniform, continuous and dense ZIF-8/gelatin layer on the inner and outer surface of the PVDF hollow fiber |
| Rhodamine B dye removal from waste water and AGMD | [30] | |
| ZIF-8 | PTFE | The modified membrane was prepared by solvent evaporation technique. The PTFE membrane was immersed in solutions of ZIF-8 of different concentrations to synthesize PTFE membranes with different ZIF-8 loading up to 20 wt%. | 10 wt% ZIF-8 filler | 5.48 × 104 L/m2·h·bar | The capacity of adsorption was increased by about 40% | Different ZIF-8 loading up to 20 wt% |
| Micropollutants removal (progesterone (PGS)) | [103] | |
| mZIF | PA | Hydrolyzed PAN | Modified ZIF particles were dispersed in the polypiperazine (PIP) phase used for the IP process. | Filler loading of 0.1% w/v | 14.9 L/m2·h·bar | Rejection values were over 99% | 0.05%, 0.10% and 0.20% w/v | Hydrothermal, thermal and chemical stability of ZIF-8 particles. | Reactive black 5 and reactive blue 2 dyes nanofiltration | [104] |
| ZIF-8 | PA | PSF | ZIF-8 nanoparticles were dispersed in the TMC hexane solution used for the IP process. | Filler loading of 0.4% (w/v) | 34.5 L/m2·h | 99.4 | 0.1 wt%, 0.2 wt%, 0.4 wt%, 0.6 wt% and 0.8 wt% |
| Desalination by RO | [105] |
| ZIF_L nanoflakes | PES | Non-solvent induced phase separation. | 0.5% filler loading | 378 ± 10 L/m2·h | 0.25%, 0.5%, and 1% PES/ZIF-L |
| UF | [106] | ||
| MIL-101(Cr) | PA | PSF | MIL-101 (Cr) nanoparticles was added into a 0.1% w/v TMC hexane solution used in the IP process. | Filler loading of 0.05% w/v | 2.25 L/m2·h·bar | ˃99% | 0.025% to 0.1% w/v |
| RO desalination | [40] |
| chitosan | PSF | Solvent casting of solution containing MOF particles were dispersed in chitosan on top of the PSF. | 15 wt% filler loading | NH2-MIL-101(Al) possessed a higher flux than the grainy NH2-MIL-101(Cr) by 200% with the same salt rejection. | 93% MgCl2 rejection | 0%, 5%, 10%, 15% and 20% |
| NF | [107] |
| UiO-66 | PA | PSU | UiO-66 particles were dispersed in TMC/n-hexane phase of the IP process constituents. | 0.1 wt% filler loading | 3.33 L/m2·h·bar | 95.3% salt rejection | 0.05 wt%, 0.1 wt%, 0.15 wt% and 0.2 wt%; around 512 nm |
| FO | [108] |
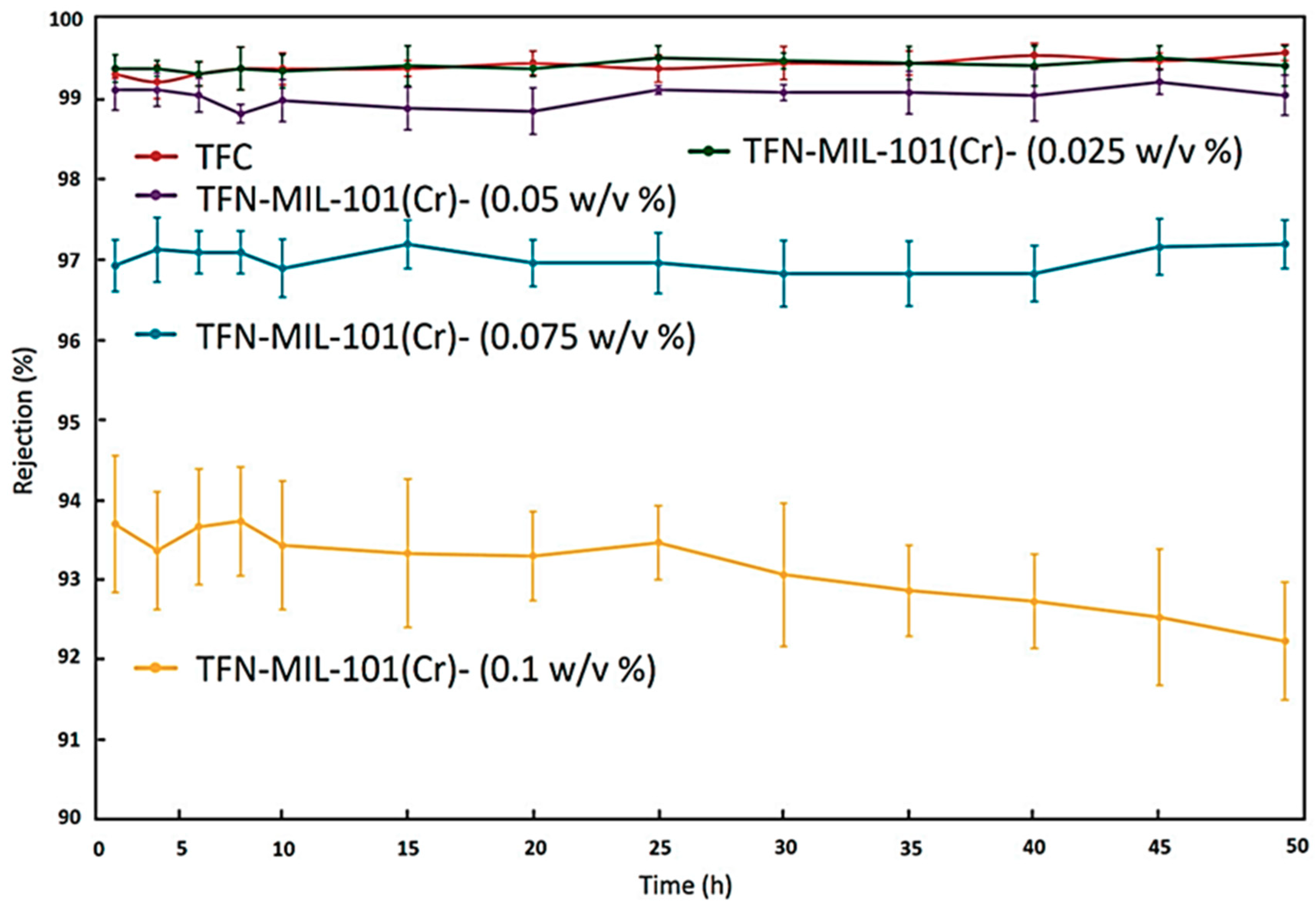
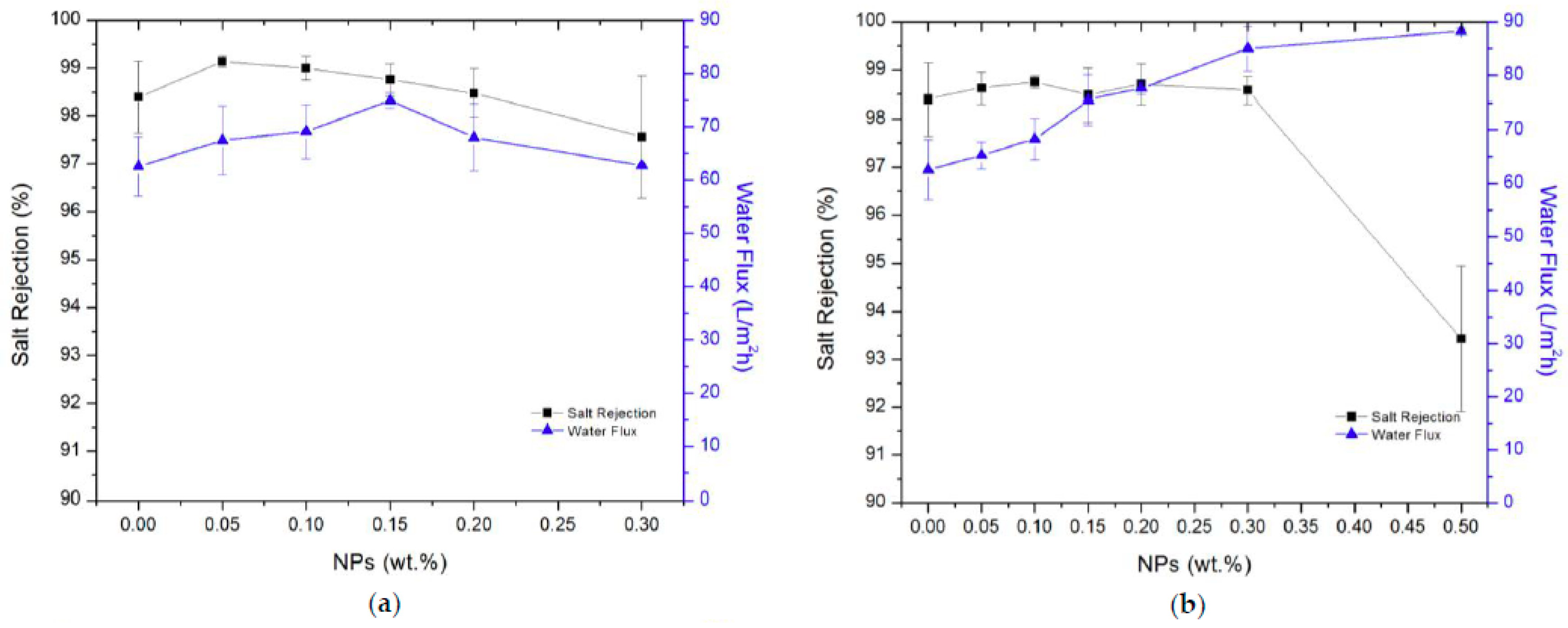
| UiO-66 | PA | PSF | UiO-66 particles were dispersed in TMC/n-hexane phase of the IP process constituents. | 0.05% w/v | 56.83 L/m2·h for BW desalination tests and 61.32 L/m2·h for the SW desalination tests | 99.35% salt rejection for BW desalination tests and remained unchanged for the SW desalination tests 91.2% boron rejection | 0.025%, 0.05%, 0.075% and 0.1% (w/v) UiO-66 Loading—50 nm |
| SW and BW desalination Boron removal | [109] |
| F300, A100 and C300 | PAN | Casting of well dispersed MOFs in PAN phase. | 0.1 wt% | Membrane doped with C300 scored the highest membrane permeability of 260.5 L/m2·h | Stable MOFs in polar organic phase but have very low water stability so easily dissolves in aqueous phase | PMM manufacture | [96] | |||
| F300, A100 and C300 | PAN | Alternative immersion of the MOF based PMM in PSS and PAH solution for the target of fabricating rejection layer via LBL method. | Membrane doped with C300 scored the optimum membrane permeability of 132 L/m2·h | MOF particles incorporated as removable fillers to synthesize FO membranes with high porosity | PMM manufacture to be utilized in FO | [97] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elrasheedy, A.; Nady, N.; Bassyouni, M.; El-Shazly, A. Metal Organic Framework Based Polymer Mixed Matrix Membranes: Review on Applications in Water Purification. Membranes 2019, 9, 88. https://doi.org/10.3390/membranes9070088
Elrasheedy A, Nady N, Bassyouni M, El-Shazly A. Metal Organic Framework Based Polymer Mixed Matrix Membranes: Review on Applications in Water Purification. Membranes. 2019; 9(7):88. https://doi.org/10.3390/membranes9070088
Chicago/Turabian StyleElrasheedy, Asmaa, Norhan Nady, Mohamed Bassyouni, and Ahmed El-Shazly. 2019. "Metal Organic Framework Based Polymer Mixed Matrix Membranes: Review on Applications in Water Purification" Membranes 9, no. 7: 88. https://doi.org/10.3390/membranes9070088
APA StyleElrasheedy, A., Nady, N., Bassyouni, M., & El-Shazly, A. (2019). Metal Organic Framework Based Polymer Mixed Matrix Membranes: Review on Applications in Water Purification. Membranes, 9(7), 88. https://doi.org/10.3390/membranes9070088






