CO2/N2 Separation Properties of Polyimide-Based Mixed-Matrix Membranes Comprising UiO-66 with Various Functionalities
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
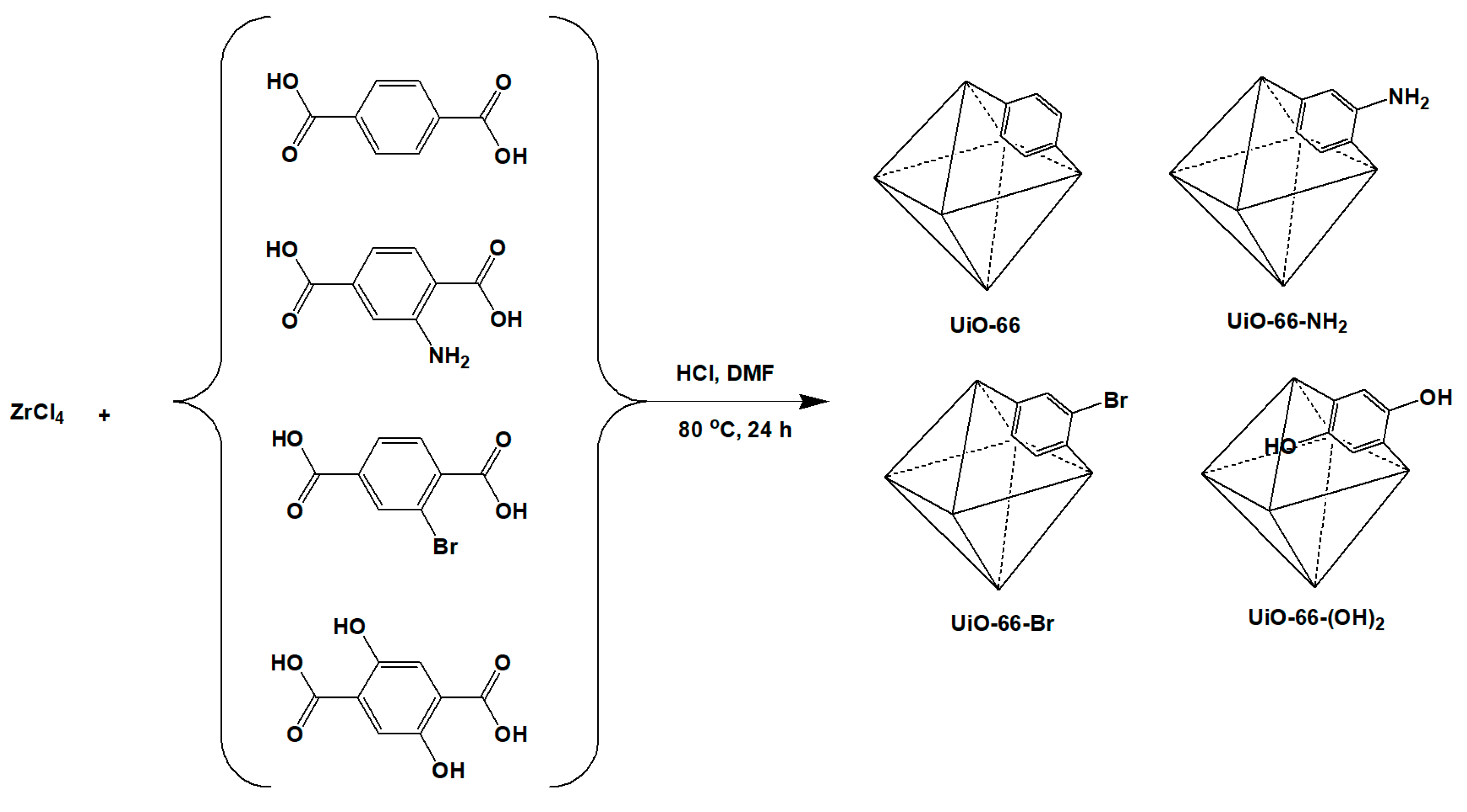
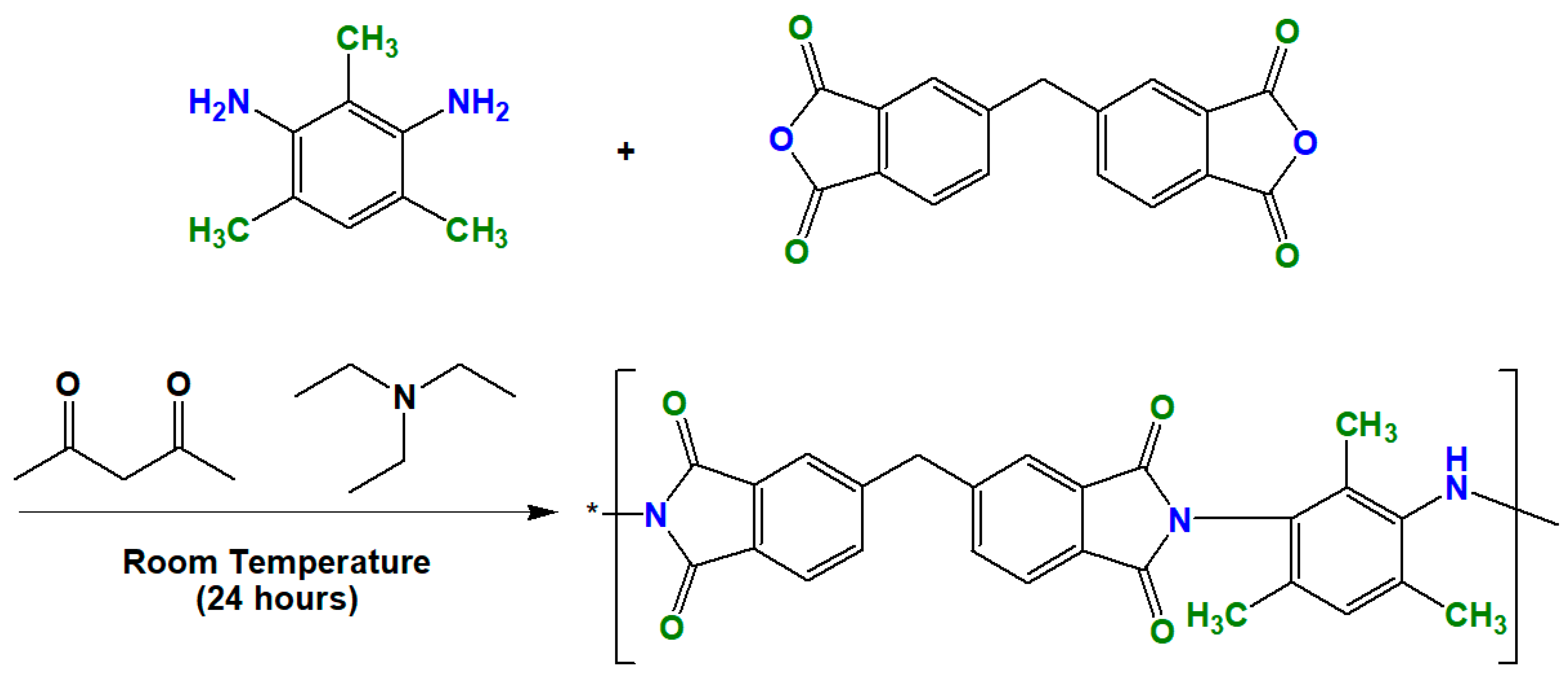
2.2. Synthesis of MOFs (UiO-66 and Its Derivatives) and Polymer (ODPA-TMPDA)
2.3. Membrane Fabrication
2.4. Characterization
2.4.1. Characterization of Nanocrystalline UiO-66 and Its Derivative
2.4.2. Characterization of Mixed-Matrix Membranes
2.4.3. Gas Permeation Test
2.4.4. Gas Adsorption Analysis
2.4.5. Filler Enhancement Index
3. Results and Discussion
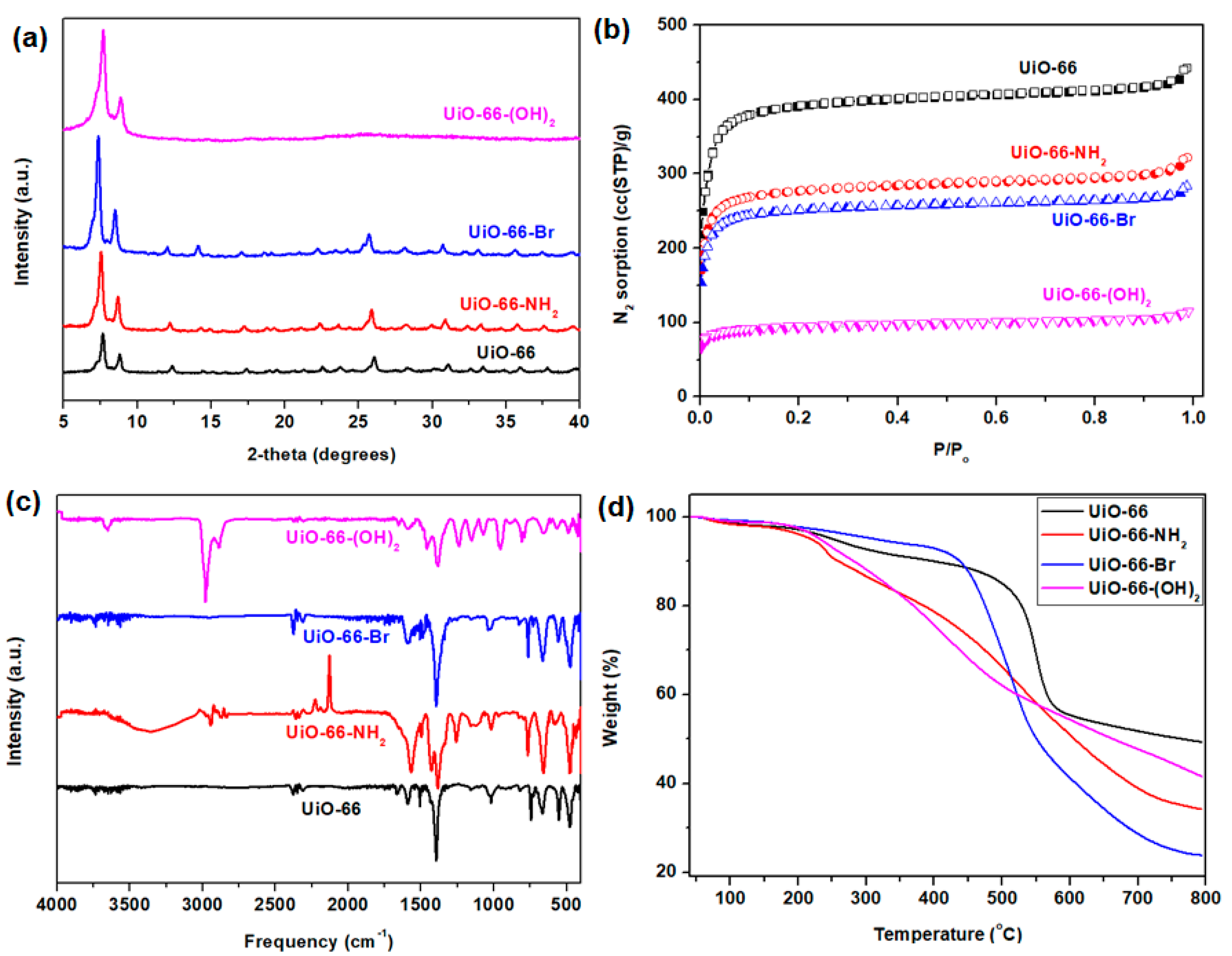
3.1. Synthesis of Nanocrystalline UiO-66 and Its Derivatives
3.2. CO2 and N2 Adsorption by UiO-66 and Its Derivatives
3.3. Fabrication of Mixed-matrix Membranes
3.4. Gas Permeation Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chuah, C.Y. Microporous Materials with Tailored Structural Properties for Enhanced Gas Separation; Nanyang Technological University: Singapore, 2019. [Google Scholar]
- Chuah, C.Y.; Kim, K.; Lee, J.; Koh, D.Y.; Bae, T.H. CO2 absorption using membrane contactors: Recent progress and future perspective. Ind. Eng. Chem. Res. 2020, 59, 6773–6794. [Google Scholar] [CrossRef]
- Aaron, D.; Tsouris, C. Separation of CO2 from flue gas: A review. Sep. Sci. Technol. 2005, 40, 321–348. [Google Scholar] [CrossRef]
- Chuah, C.Y.; Goh, K.; Yang, Y.; Gong, H.; Li, W.; Karahan, H.E.; Guiver, M.D.; Wang, R.; Bae, T.H. Harnessing filler materials for enhancing biogas separation membranes. Chem. Rev. 2018, 118, 8655–8769. [Google Scholar] [CrossRef] [PubMed]
- Mondal, M.K.; Balsora, H.K.; Varshney, P. Progress and trends in CO2 capture/separation technologies: A review. Energy 2012, 46, 431–441. [Google Scholar] [CrossRef]
- Zhang, Y.; Sunarso, J.; Liu, S.; Wang, R. Current status and development of membranes for CO2/CH4 separation: A review. Int. J. Greenh. Gas Con. 2013, 12, 84–107. [Google Scholar] [CrossRef]
- Yu, C.H.; Huang, C.H.; Tan, C.S. A review of CO2 capture by absorption and adsorption. Aerosol Air Qual. Res 2012, 12, 745–769. [Google Scholar] [CrossRef]
- Yang, H.; Xu, Z.; Fan, M.; Gupta, R.; Slimane, R.B.; Bland, A.E.; Wright, I. Progress in carbon dioxide separation and capture: A review. J. Environ. Sci. 2008, 20, 14–27. [Google Scholar] [CrossRef]
- Esposito, E.; Dellamuzia, L.; Moretti, U.; Fuoco, A.; Giorno, L.; Jansen, J.C. Simultaneous production of biomethane and food grade CO2 from biogas: An industrial case study. Energy Environ. Sci. 2019, 12, 281–289. [Google Scholar] [CrossRef]
- Sanders, D.F.; Smith, Z.P.; Guo, R.; Robeson, L.M.; McGrath, J.E.; Paul, D.R.; Freeman, B.D. Energy-efficient polymeric gas separation membranes for a sustainable future: A review. Polymer 2013, 54, 4729–4761. [Google Scholar] [CrossRef]
- Karahan, H.E.; Goh, K.; Zhang, C.; Yang, E.; Yıldırım, C.; Chuah, C.Y.; Ahunbay, M.G.; Lee, J.; Tantekin-Ersolmaz, Ş.B.; Chen, Y. MXene Materials for Designing Advanced Separation Membranes. Adv. Mater. 2020, 1906697. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Robeson, L.M. Correlation of separation factor versus permeability for polymeric membranes. J. Membr. Sci. 1991, 62, 165–185. [Google Scholar] [CrossRef]
- Li, W.; Goh, K.; Chuah, C.Y.; Bae, T.H. Mixed-matrix carbon molecular sieve membranes using hierarchical zeolite: A simple approach towards high CO2 permeability enhancements. J. Membr. Sci. 2019, 588, 117220. [Google Scholar] [CrossRef]
- Li, S.; Falconer, J.L.; Noble, R.D. SAPO-34 membranes for CO2/CH4 separation. J. Membr. Sci. 2004, 241, 121–135. [Google Scholar] [CrossRef]
- Pera-Titus, M. Porous inorganic membranes for CO2 capture: Present and prospects. Chem. Rev. 2013, 114, 1413–1492. [Google Scholar] [CrossRef]
- Zhang, X.; Chuah, C.Y.; Dong, P.; Cha, Y.H.; Bae, T.H.; Song, M.K. Hierarchically Porous Co-MOF-74 Hollow Nanorods for Enhanced Dynamic CO2 Separation. ACS Appl. Mater. Int. 2018, 10, 43316–43322. [Google Scholar] [CrossRef] [PubMed]
- Vinoba, M.; Bhagiyalakshmi, M.; Alqaheem, Y.; Alomair, A.A.; Pérez, A.; Rana, M.S. Recent progress of fillers in mixed matrix membranes for CO2 separation: A review. Sep. Purif. Technol. 2017, 188, 431–450. [Google Scholar] [CrossRef]
- Adatoz, E.; Avci, A.K.; Keskin, S. Opportunities and challenges of MOF-based membranes in gas separations. Sep. Purif. Technol. 2015, 152, 207–237. [Google Scholar] [CrossRef]
- Lin, Y.S. Metal organic framework membranes for separation applications. Curr. Opin. Chem. Eng. 2015, 8, 21–28. [Google Scholar] [CrossRef]
- Sumida, K.; Rogow, D.L.; Mason, J.A.; McDonald, T.M.; Bloch, E.D.; Herm, Z.R.; Bae, T.H.; Long, J.R. Carbon dioxide capture in metal–organic frameworks. Chem. Rev. 2012, 112, 724–781. [Google Scholar] [CrossRef]
- Chuah, C.Y.; Li, W.; Samarasinghe, S.; Sethunga, G.; Bae, T.H. Enhancing the CO2 separation performance of polymer membranes via the incorporation of amine-functionalized HKUST-1 nanocrystals. Micropor. Mesopor. Mater. 2019, 290, 109680. [Google Scholar] [CrossRef]
- McDonald, T.M.; Lee, W.R.; Mason, J.A.; Wiers, B.M.; Hong, C.S.; Long, J.R. Capture of carbon dioxide from air and flue gas in the alkylamine-appended metal–organic framework mmen-Mg2(dobpdc). J. Am. Chem. Soc. 2012, 134, 7056–7065. [Google Scholar] [CrossRef] [PubMed]
- Darunte, L.A.; Oetomo, A.D.; Walton, K.S.; Sholl, D.S.; Jones, C.W. Direct air capture of CO2 using amine functionalized MIL-101 (Cr). ACS Sust. Chem. Eng. 2016, 4, 5761–5768. [Google Scholar] [CrossRef]
- Gong, H.; Lee, S.S.; Bae, T.H. Mixed-matrix membranes containing inorganically surface-modified 5A zeolite for enhanced CO2/CH4 separation. Micropor. Mesopor. Mater. 2017, 237, 82–89. [Google Scholar] [CrossRef]
- Bae, T.H.; Liu, J.; Thompson, J.A.; Koros, W.J.; Jones, C.W.; Nair, S. Solvothermal deposition and characterization of magnesium hydroxide nanostructures on zeolite crystals. Micropor. Mesopor. Mater. 2011, 139, 120–129. [Google Scholar] [CrossRef]
- Li, Y.; Chung, T.S.; Cao, C.; Kulprathipanja, S. The effects of polymer chain rigidification, zeolite pore size and pore blockage on polyethersulfone (PES)-zeolite A mixed matrix membranes. J. Membr. Sci. 2005, 260, 45–55. [Google Scholar] [CrossRef]
- Li, Y.; Guan, H.M.; Chung, T.S.; Kulprathipanja, S. Effects of novel silane modification of zeolite surface on polymer chain rigidification and partial pore blockage in polyethersulfone (PES)–zeolite A mixed matrix membranes. J. Membr. Sci. 2006, 275, 17–28. [Google Scholar] [CrossRef]
- Bae, T.H.; Liu, J.; Lee, J.S.; Koros, W.J.; Jones, C.W.; Nair, S. Facile high-yield solvothermal deposition of inorganic nanostructures on zeolite crystals for mixed matrix membrane fabrication. J. Am. Chem. Soc. 2009, 131, 14662–14663. [Google Scholar] [CrossRef]
- Liu, J.; Benin, A.I.; Furtado, A.M.; Jakubczak, P.; Willis, R.R.; LeVan, M.D. Stability effects on CO2 adsorption for the DOBDC series of metal–organic frameworks. Langmuir 2011, 27, 11451–11456. [Google Scholar] [CrossRef]
- Li, W.; Chuah, C.Y.; Yang, Y.; Bae, T.H. Nanocomposites formed by in situ growth of NiDOBDC nanoparticles on graphene oxide sheets for enhanced CO2 and H2 storage. Micropor. Mesopor. Mater. 2018, 265, 35–42. [Google Scholar] [CrossRef]
- Garibay, S.J.; Cohen, S.M. Isoreticular synthesis and modification of frameworks with the UiO-66 topology. Chem. Commun. 2010, 46, 7700–7702. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.J.; Brown, Z.J.; Colón, Y.J.; Siu, P.W.; Scheidt, K.A.; Snurr, R.Q.; Hupp, J.T.; Farha, O.K. A facile synthesis of UiO-66, UiO-67 and their derivatives. Chem. Commun. 2013, 49, 9449–9451. [Google Scholar] [CrossRef] [PubMed]
- Samarasinghe, S.; Chuah, C.Y.; Karahan, H.E.; Sethunga, G.; Bae, T.H. Enhanced O2/N2 Separation of Mixed-Matrix Membrane Filled with Pluronic-Compatibilized Cobalt Phthalocyanine Particles. Membranes 2020, 10, 75. [Google Scholar] [CrossRef] [PubMed]
- Dorosti, F.; Omidkhah, M.; Abedini, R. Fabrication and characterization of Matrimid/MIL-53 mixed matrix membrane for CO2/CH4 separation. Chem. Eng. Res. Des. 2014, 92, 2439–2448. [Google Scholar] [CrossRef]
- Castro-Munoz, R.; Fila, V.; Martin-Gil, V.; Muller, C. Enhanced CO2 permeability in Matrimid® 5218 mixed matrix membranes for separating binary CO2/CH4 mixtures. Sep. Purif. Technol. 2019, 210, 553–562. [Google Scholar] [CrossRef]
- Yong, W.F.; Li, F.Y.; Xiao, Y.C.; Chung, T.S.; Tong, Y.W. High performance PIM-1/Matrimid hollow fiber membranes for CO2/CH4, O2/N2 and CO2/N2 separation. J. Membr. Sci. 2013, 443, 156–169. [Google Scholar] [CrossRef]
- Dai, Y.; Johnson, J.; Karvan, O.; Sholl, D.S.; Koros, W. Ultem®/ZIF-8 mixed matrix hollow fiber membranes for CO2/N2 separations. J. Membr. Sci. 2012, 401, 76–82. [Google Scholar] [CrossRef]
- Samarasinghe, S.; Chuah, C.Y.; Li, W.; Sethunga, G.; Wang, R.; Bae, T.H. Incorporation of CoIII acetylacetonate and SNW-1 nanoparticles to tailor O2/N2 separation performance of mixed-matrix membrane. Sep. Purif. Technol. 2019, 223, 133–141. [Google Scholar] [CrossRef]
- Samarasinghe, S.; Chuah, C.Y.; Yang, Y.; Bae, T.H. Tailoring CO2/CH4 separation properties of mixed-matrix membranes via combined use of two-and three-dimensional metal–organic frameworks. J. Membr. Sci. 2018, 557, 30–37. [Google Scholar] [CrossRef]
- Gong, H.; Chuah, C.Y.; Yang, Y.; Bae, T.H. High performance composite membranes comprising Zn(pyrz)2(SiF6) nanocrystals for CO2/CH4 separation. J. Ind. Eng. Chem. 2018, 60, 279–285. [Google Scholar] [CrossRef]
- Yang, Y.; Goh, K.; Weerachanchai, P.; Bae, T.H. 3D covalent organic framework for morphologically induced high-performance membranes with strong resistance toward physical aging. J. Membr. Sci. 2019, 574, 235–242. [Google Scholar] [CrossRef]
- Chuah, C.Y.; Samarasinghe, S.; Li, W.; Goh, K.; Bae, T.H. Leveraging Nanocrystal HKUST-1 in Mixed-Matrix Membranes for Ethylene/Ethane Separation. Membranes 2020, 10, 74. [Google Scholar] [CrossRef] [PubMed]
- Askari, M.; Chung, T.S. Natural gas purification and olefin/paraffin separation using thermal cross-linkable co-polyimide/ZIF-8 mixed matrix membranes. J. Membr. Sci. 2013, 444, 173–183. [Google Scholar] [CrossRef]
- Duan, C.; Jie, X.; Liu, D.; Cao, Y.; Yuan, Q. Post-treatment effect on gas separation property of mixed matrix membranes containing metal organic frameworks. J. Membr. Sci. 2014, 466, 92–102. [Google Scholar] [CrossRef]
- Chuah, C.Y.; Yang, Y.; Bae, T.H. Hierarchically porous polymers containing triphenylamine for enhanced SF6 separation. Micropor. Mesopor. Mater. 2018, 272, 232–240. [Google Scholar] [CrossRef]
- Chuah, C.Y.; Yu, S.; Na, K.; Bae, T.H. Enhanced SF6 recovery by hierarchically structured MFI zeolite. J. Ind. Eng. Chem. 2018, 62, 64–71. [Google Scholar] [CrossRef]
- Myers, A.; Prausnitz, J.M. Thermodynamics of mixed-gas adsorption. AIChE J. 1965, 11, 121–127. [Google Scholar] [CrossRef]
- Li, J.; Musho, T.; Bright, J.; Wu, N. Functionalization of a Metal–organic Framework Semiconductor for Tuned Band Structure and Catalytic Activity. J. Electrochem. Soc. 2019, 166, H3029–H3034. [Google Scholar] [CrossRef]
- Khdhayyer, M.R.; Esposito, E.; Fuoco, A.; Monteleone, M.; Giorno, L.; Jansen, J.C.; Attfield, M.P.; Budd, P.M. Mixed matrix membranes based on UiO-66 MOFs in the polymer of intrinsic microporosity PIM-1. Sep. Purif. Technol. 2017, 173, 304–313. [Google Scholar] [CrossRef]
- Shen, J.; Liu, G.; Huang, K.; Li, Q.; Guan, K.; Li, Y.; Jin, W. UiO-66-polyether block amide mixed matrix membranes for CO2 separation. J. Membr. Sci. 2016, 513, 155–165. [Google Scholar] [CrossRef]
- Rodrigues, M.A.; de Souza Ribeiro, J.; de Souza Costa, E.; de Miranda, J.L.; Ferraz, H.C. Nanostructured membranes containing UiO-66 (Zr) and MIL-101 (Cr) for O2/N2 and CO2/N2 separation. Sep. Purif. Technol. 2018, 192, 491–500. [Google Scholar] [CrossRef]
- Jadhav, N.; Kumar, S.N.; Tanvidkar, P.S.; Kuncharam, B.V.R. Synthesis and characterization of mixed-matrix material of Zirconium based metal organic framework (MOF: UiO-66-NH2) and poly (ether-urethane-urea). Mater. Today Proc. 2020, 28, 734–738. [Google Scholar] [CrossRef]
- Han, Y.; Liu, M.; Li, K.; Zuo, Y.; Wei, Y.; Xu, S.; Zhang, G.; Song, C.; Zhang, Z.; Guo, X. Facile synthesis of morphology and size-controlled zirconium metal–organic framework UiO-66: The role of hydrofluoric acid in crystallization. CrystEngComm 2015, 17, 6434–6440. [Google Scholar] [CrossRef]
- Bae, T.H.; Lee, J.S.; Qiu, W.; Koros, W.J.; Jones, C.W.; Nair, S. A high-performance gas-separation membrane containing submicrometer-sized metal–organic framework crystals. Angew. Chem. Int. Ed. 2010, 49, 9863–9866. [Google Scholar] [CrossRef]
- Li, J.R.; Kuppler, R.J.; Zhou, H.C. Selective gas adsorption and separation in metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.B.; Kim, K.M.; Kim, T.H.; Yoon, T.U.; Kim, E.J.; Kim, J.H.; Bae, Y.S. Highly selective adsorption of SF6 over N2 in a bromine-functionalized zirconium-based metal–organic framework. Chem. Eng. J. 2018, 339, 223–229. [Google Scholar] [CrossRef]
- Yang, Y.; Chuah, C.Y.; Bae, T.H. Polyamine-appended porous organic polymers for efficient post-combustion CO2 capture. Chem. Eng. J. 2019, 358, 1227–1234. [Google Scholar] [CrossRef]
- Li, W.; Samarasinghe, S.; Bae, T.H. Enhancing CO2/CH4 separation performance and mechanical strength of mixed-matrix membrane via combined use of graphene oxide and ZIF-8. J. Ind. Eng. Chem. 2018, 67, 156–163. [Google Scholar] [CrossRef]
- Goh, P.; Ismail, A.; Sanip, S.; Ng, B.; Aziz, M. Recent advances of inorganic fillers in mixed matrix membrane for gas separation. Sep. Purif. Technol. 2011, 81, 243–264. [Google Scholar] [CrossRef]
- Gong, H.; Nguyen, T.H.; Wang, R.; Bae, T.H. Separations of binary mixtures of CO2/CH4 and CO2/N2 with mixed-matrix membranes containing Zn(pyrz)2(SiF6) metal–organic framework. J. Membr. Sci. 2015, 495, 169–175. [Google Scholar] [CrossRef]
- Kim, K.; Ingole, P.G.; Kim, J.; Lee, H. Separation performance of PEBAX/PEI hollow fiber composite membrane for SO2/CO2/N2 mixed gas. Chem. Eng. J. 2013, 233, 242–250. [Google Scholar] [CrossRef]
- Esposito, E.; Clarizia, G.; Bernardo, P.; Jansen, J.C.; Sedláková, Z.; Izák, P.; Curcio, S.; de Cindio, B.; Tasselli, F. Pebax®/PAN hollow fiber membranes for CO2/CH4 separation. Chem. Eng. Process 2015, 94, 53–61. [Google Scholar] [CrossRef]
- Sutrisna, P.D.; Hou, J.; Zulkifli, M.Y.; Li, H.; Zhang, Y.; Liang, W.; D’Alessandro, D.M.; Chen, V. Surface functionalized UiO-66/Pebax-based ultrathin composite hollow fiber gas separation membranes. J. Mater. Chem. A 2018, 6, 918–931. [Google Scholar] [CrossRef]
- Prasetya, N.; Donose, B.C.; Ladewig, B.P. A new and highly robust light-responsive Azo-UiO-66 for highly selective and low energy post-combustion CO2 capture and its application in a mixed matrix membrane for CO2/N2 separation. J. Mater. Chem. A 2018, 6, 16390–16402. [Google Scholar] [CrossRef]
- Ghalei, B.; Sakurai, K.; Kinoshita, Y.; Wakimoto, K.; Isfahani, A.P.; Song, Q.; Doitomi, K.; Furukawa, S.; Hirao, H.; Kusuda, H. Enhanced selectivity in mixed matrix membranes for CO2 capture through efficient dispersion of amine-functionalized MOF nanoparticles. Nat. Energy 2017, 2, 17086. [Google Scholar] [CrossRef]
- Mason, J.A.; McDonald, T.M.; Bae, T.H.; Bachman, J.E.; Sumida, K.; Dutton, J.J.; Kaye, S.S.; Long, J.R. Application of a high-throughput analyzer in evaluating solid adsorbents for post-combustion carbon capture via multicomponent adsorption of CO2, N2, and H2O. J. Am. Chem. Soc. 2015, 137, 4787–4803. [Google Scholar] [CrossRef] [PubMed]
- Su, N.C.; Sun, D.T.; Beavers, C.M.; Britt, D.K.; Queen, W.L.; Urban, J.J. Enhanced permeation arising from dual transport pathways in hybrid polymer–MOF membranes. Energy Environ. Sci. 2016, 9, 922–931. [Google Scholar] [CrossRef]
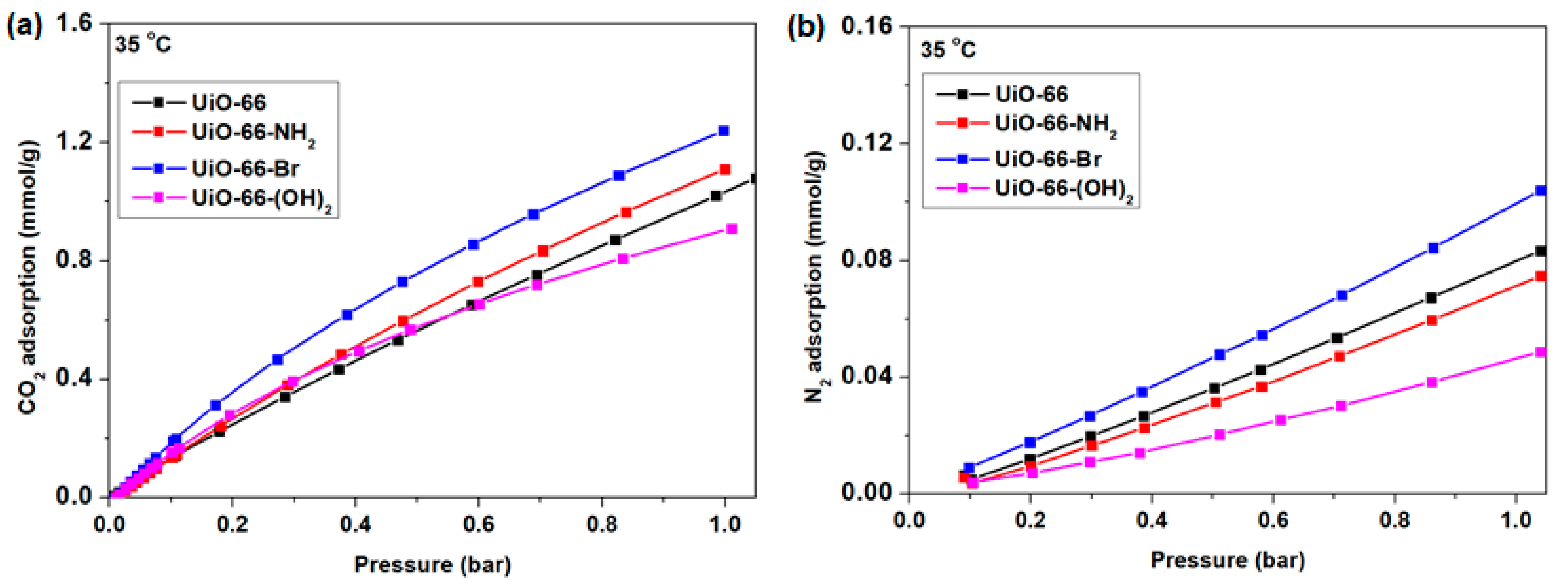


| Sample | SBET (a) (m2/g) | SLANG (a) (m2/g) | Smicro (b) (m2/g) | Vmicro (b) (cc/g) | Vtotal (c) (cc/g) |
|---|---|---|---|---|---|
| UiO-66 | 1733 | 2266 | 1662 | 0.809 | 0.938 |
| UiO-66-NH2 | 1218 | 1599 | 1160 | 0.569 | 0.682 |
| UiO-66-Br | 851 | 1117 | 818 | 0.380 | 0.440 |
| UiO-66-(OH)2 | 318 | 418 | 293 | 0.136 | 0.178 |
| Membrane | CO2 Permeability (Barrer) (b) | CO2/N2 Selectivity |
|---|---|---|
| ODPA-TMPDA | 88 ± 2 | 33.1 ± 1.2 |
| 10 wt% UiO-66 | 142 ± 5 | 29.0 ± 0.4 |
| 20 wt% UiO-66 | 169 ± 2 | 31.9 ± 0.2 |
| 10 wt% UiO-66-NH2 | 129 ± 3 | 36.1 ± 0.8 |
| 20 wt% UiO-66-NH2 | 142 ± 1 | 37.1 ± 2.3 |
| 10 wt% UiO-66-Br | 158 ± 2 | 33.7 ± 1.0 |
| 20 wt% UiO-66-Br | 200 ± 4 | 34.5 ± 1.9 |
| 10 wt% UiO-66-(OH)2 | 98 ± 2 | 35.2 ± 0.8 |
| 20 wt% UiO-66-(OH)2 | 125 ± 4 | 38.9 ± 0.9 |
| Membrane | Density (g/cm3) | CO2 Solubility (mol/m3.bar) | CO2 Diffusivity, × 10−12 (m2/s) | N2 Solubility (mol/m3.bar) | N2 Diffusivity, × 10−12 (m2/s) |
|---|---|---|---|---|---|
| ODPA-TMPDA | 1.286 | 1526 | 1.96 | 32.1 | 2.81 |
| 20 wt% UiO-66 | 1.210 | 1164 | 4.92 | 31.5 | 5.71 |
| 20 wt% UiO-66-NH2 | 1.330 | 1172 | 4.11 | 28.1 | 4.62 |
| 20 wt% UiO-66-Br | 1.354 | 1256 | 5.40 | 22.5 | 8.72 |
| 20 wt% UiO-66-(OH)2 | 1.380 | 1385 | 3.06 | 29.3 | 3.72 |
| Filler | Polymer | Filler Loading (wt%) (b) | Separation Performance | Findex | Yr (Ref.) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Testing Condition | P(CO2) Barrer | % En. | α (CO2/N2) | % En. | ||||||
| Pressure (bar) | Temp. (°C) | |||||||||
| UiO-66 | PEBA | 10 | - (c) | 25 | 96.3 | 87.0 | 56.6 | 34.4 | 1.48 | 16′ [51] |
| UiO-66 | PEBA | 10 | - (c, d) | 25 | 139.7 | 171 | 61.1 | 45.1 | 2.07 | 16′ [51] |
| UiO-66 | PSF | 20 | 3 | 35 | 16 | 186 | 26.2 | −11.0 | 0.71 | 16′ [68] |
| UiO-66-NH2 | PEBA | 20 | - (c) | 25 | 87.0 | 68.9 | 66.1 | 57.0 | 1.82 | 16′ [51] |
| UiO-66-NH2 | PEBA | 20 | - (c, d) | 25 | 130.2 | 153 | 72.2 | 71.5 | 2.48 | 16′ [51] |
| UiO-66-ref | PIM-1 | 20 | 4 | 25 | 6981 | 128.6 | 13.0 | −19.3 | 0.21 | 17′ [66] |
| UiO-66 | PIM-1 | 23.1 | 1 | 25 | 7610 | 59.5 | 20.7 | −5.1 | 0.31 | 17′ [50] |
| UiO-66 | PIM-1 (MeOH treated) | 23.1 | 1 | 25 | 9980 | 109.2 | 21.6 | −0.9 | 0.71 | 17′ [50] |
| UiO-66-COOH | PIM-1 | 23.1 | 1 | 25 | 5300 | 11.1 | 20 | −8.26 | −0.14 | 17′ [50] |
| UiO-66-H | PIM-1 | 20 | 4 | 25 | 2606 | −14.7 | 24.6 | 52.8 | 1.07 | 17′ [66] |
| UiO-66-NH2 | PIM-1 | 9.1 | 1 | 25 | 4810 | 0.83 | 22.3 | 2.29 | 0.07 | 17′ [50] |
| UiO-66-NH2 | PIM-1 | 10 | 4 | 25 | 2869 | −6.1 | 27.5 | 70.8 | 1.48 | 17′ [66] |
| UiO-66-NH2 | PIM-1 | 10 | 4 (c) | 25 | 1900 | −37.8 | 24 | 49.0 | 0.67 | 17′ [66] |
| UiO-66-NH2 | PIM-1 (3-month aging) | 9.1 | 1 (e) | 25 | 4835 | 1.36 | 28.2 | 29.4 | 0.75 | 17′ [50] |
| UiO-66 | PU | 24 | - | - | 75.2 | 95.8 | 34.2 | −12.8 | 0.27 | 18′ [52] |
| UiO-66 | Matrimid | 10 | 4 | 37 | 7.8 | 13.0 | 29.4 | −1.4 | 0.08 | 18′ [65] |
| Azo-UiO-66 | Matrimid | 10 | 4 | 37 | 10 | 44.9 | 37 | 24.0 | 0.99 | 18′ [65] |
| UiO-66 | ODPA-TMPDA | 20 | 1 (f) | 35 | 169 | 92.0 | 31.9 | −3.6 | 0.54 | This work |
| UiO-66-NH2 | ODPA-TMPDA | 20 | 1 (f) | 35 | 142 | 61.3 | 37.1 | 12.0 | 0.81 | This work |
| UiO-66-Br | ODPA-TMPDA | 20 | 1 (f) | 35 | 200 | 127 | 34.5 | 4.23 | 0.94 | This work |
| UiO-66-(OH)2 | ODPA-TMPDA | 20 | 1 (f) | 35 | 125 | 42.0 | 38.9 | 17.5 | 0.81 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuah, C.Y.; Lee, J.; Song, J.; Bae, T.-H. CO2/N2 Separation Properties of Polyimide-Based Mixed-Matrix Membranes Comprising UiO-66 with Various Functionalities. Membranes 2020, 10, 154. https://doi.org/10.3390/membranes10070154
Chuah CY, Lee J, Song J, Bae T-H. CO2/N2 Separation Properties of Polyimide-Based Mixed-Matrix Membranes Comprising UiO-66 with Various Functionalities. Membranes. 2020; 10(7):154. https://doi.org/10.3390/membranes10070154
Chicago/Turabian StyleChuah, Chong Yang, Junghyun Lee, Juha Song, and Tae-Hyun Bae. 2020. "CO2/N2 Separation Properties of Polyimide-Based Mixed-Matrix Membranes Comprising UiO-66 with Various Functionalities" Membranes 10, no. 7: 154. https://doi.org/10.3390/membranes10070154
APA StyleChuah, C. Y., Lee, J., Song, J., & Bae, T.-H. (2020). CO2/N2 Separation Properties of Polyimide-Based Mixed-Matrix Membranes Comprising UiO-66 with Various Functionalities. Membranes, 10(7), 154. https://doi.org/10.3390/membranes10070154








