Oily Wastewater Treatment Using Polyamide Thin Film Composite Membrane Technology
Abstract
1. Introduction
2. Materials and Methods
2.1. Measuring and Analysis
2.1.1. Chemical Oxygen Demand (COD)
2.1.2. Turbidity
2.1.3. Thermogravimetric Analysis (TGA)
2.1.4. Fourier-Transform Infrared Spectroscopy (FTIR)
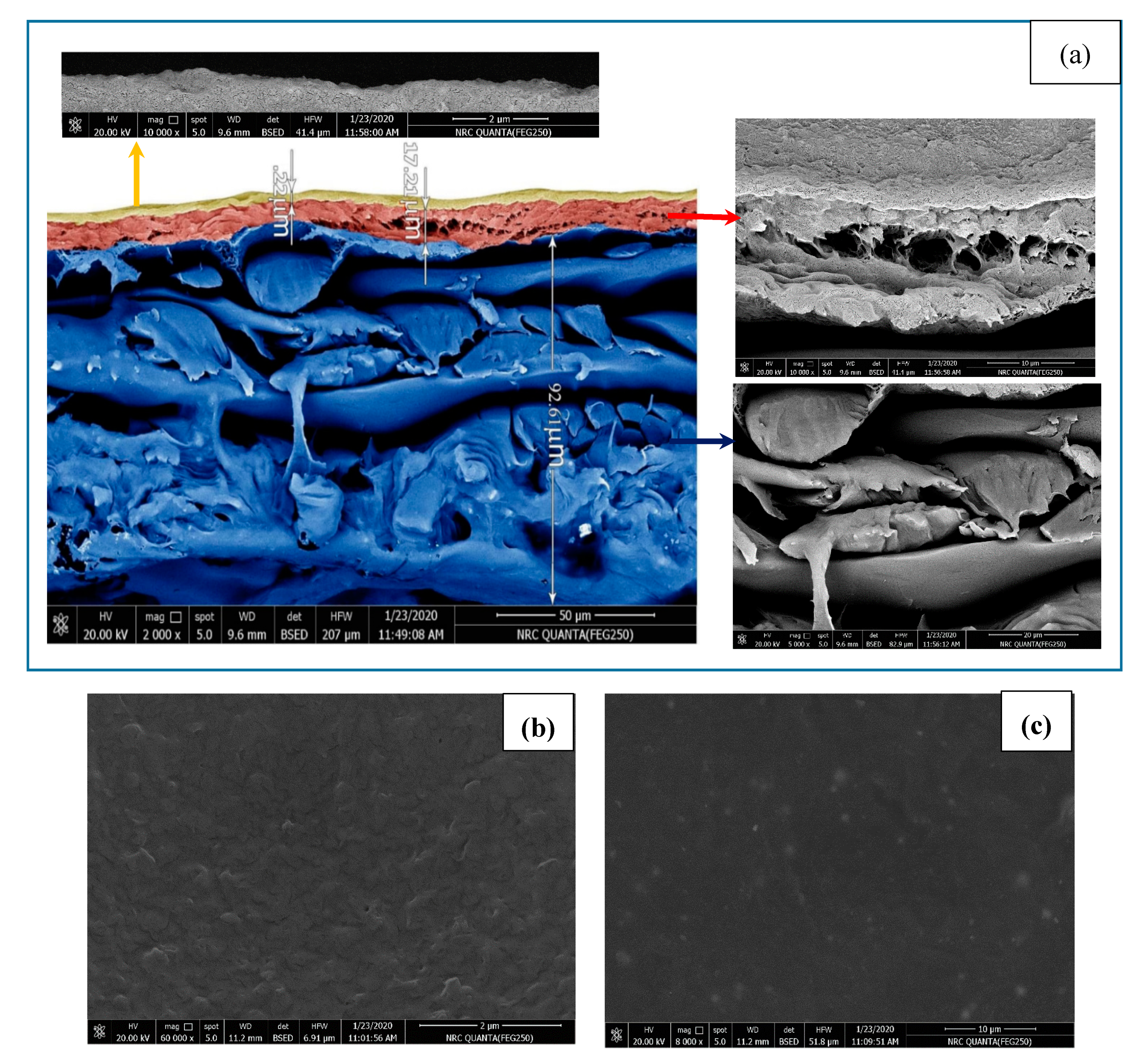
2.1.5. Scanning Electron Microscopy (SEM)
2.1.6. Mechanical Testing
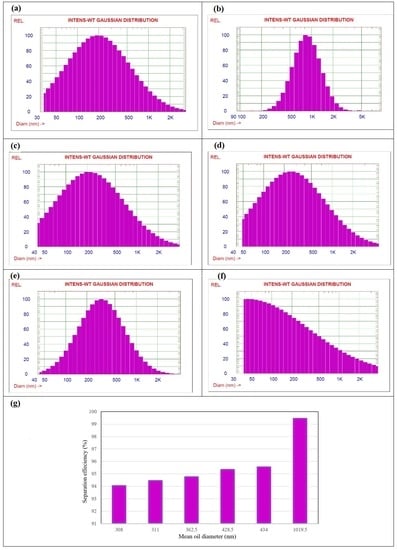
2.1.7. Average oil Particle Size
2.1.8. Dynamic Wetting
2.1.9. Contact Angle
2.1.10. Oil and Grease
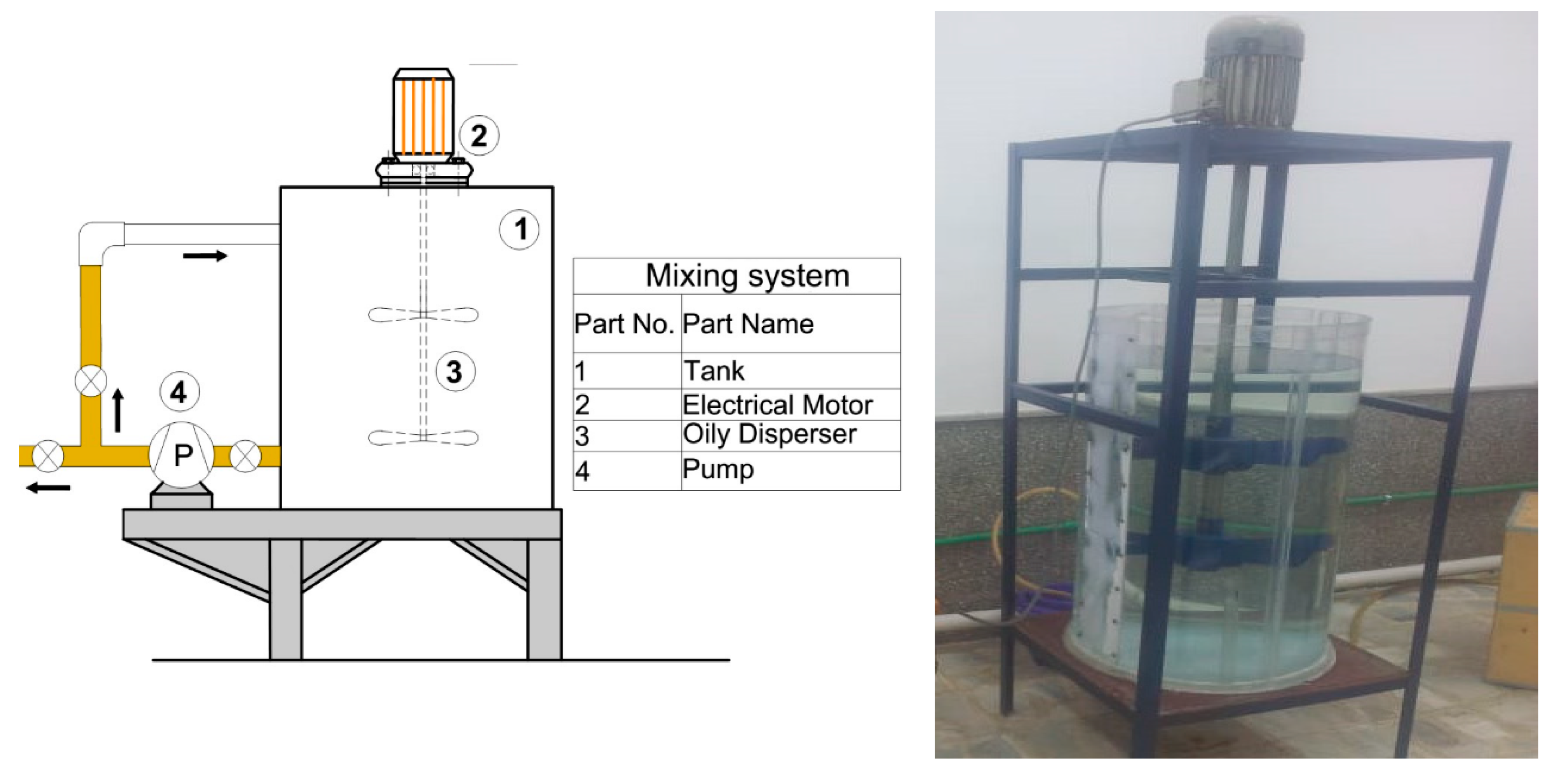
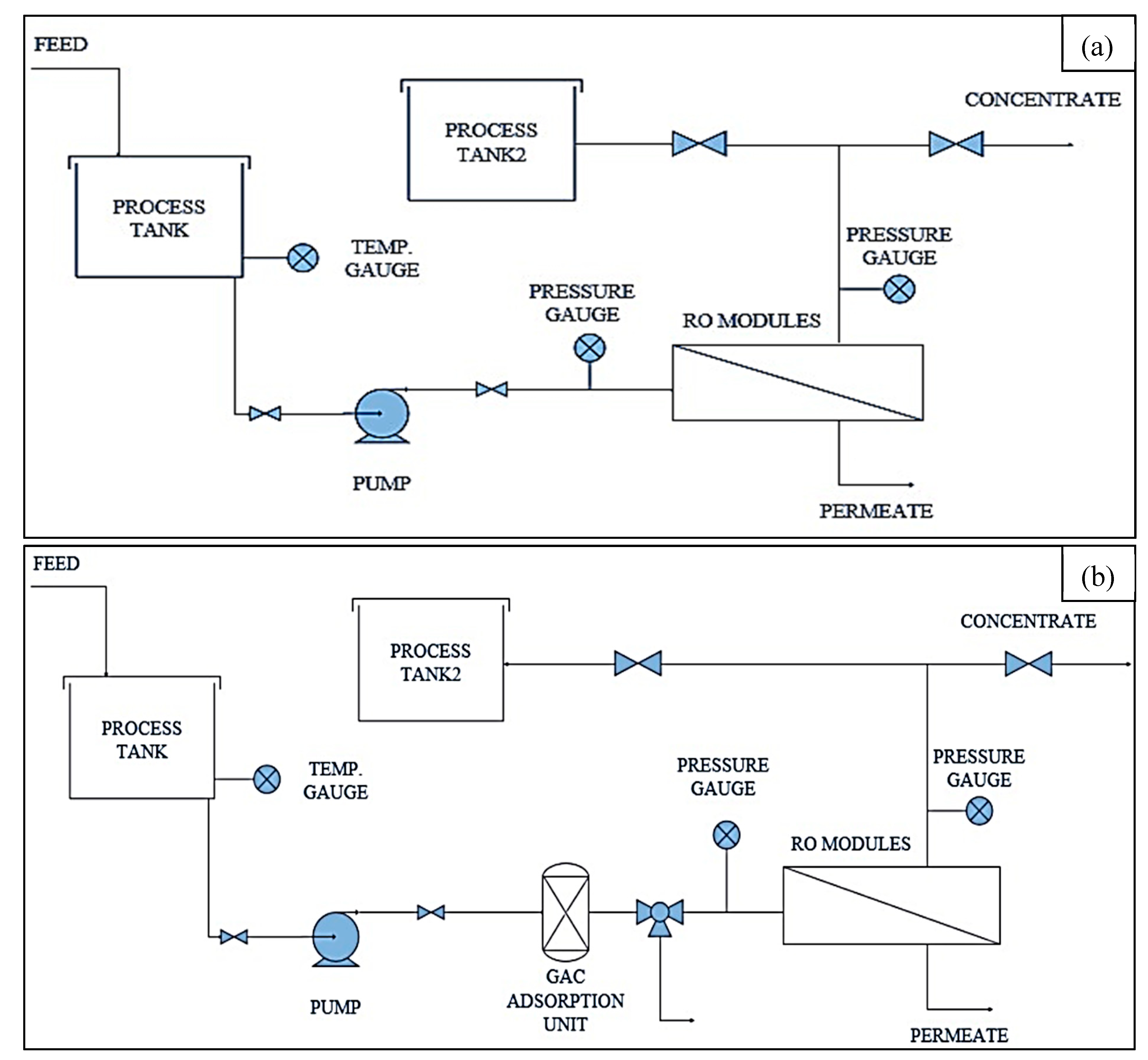
2.2. Experimental Setup and Operation
Percentage Oil Rejection and Flux
3. Results and Discussion
3.1. Membrane Characterization
3.1.1. Surface Morphology
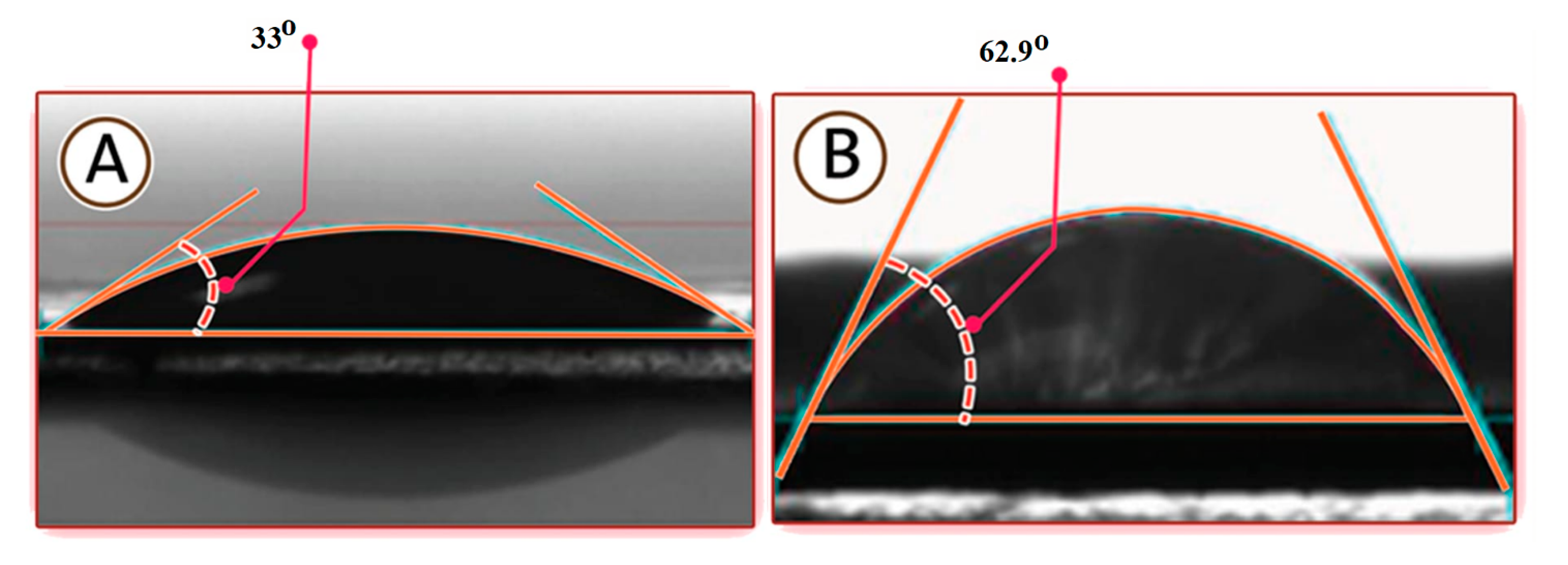
3.1.2. Contact Angle
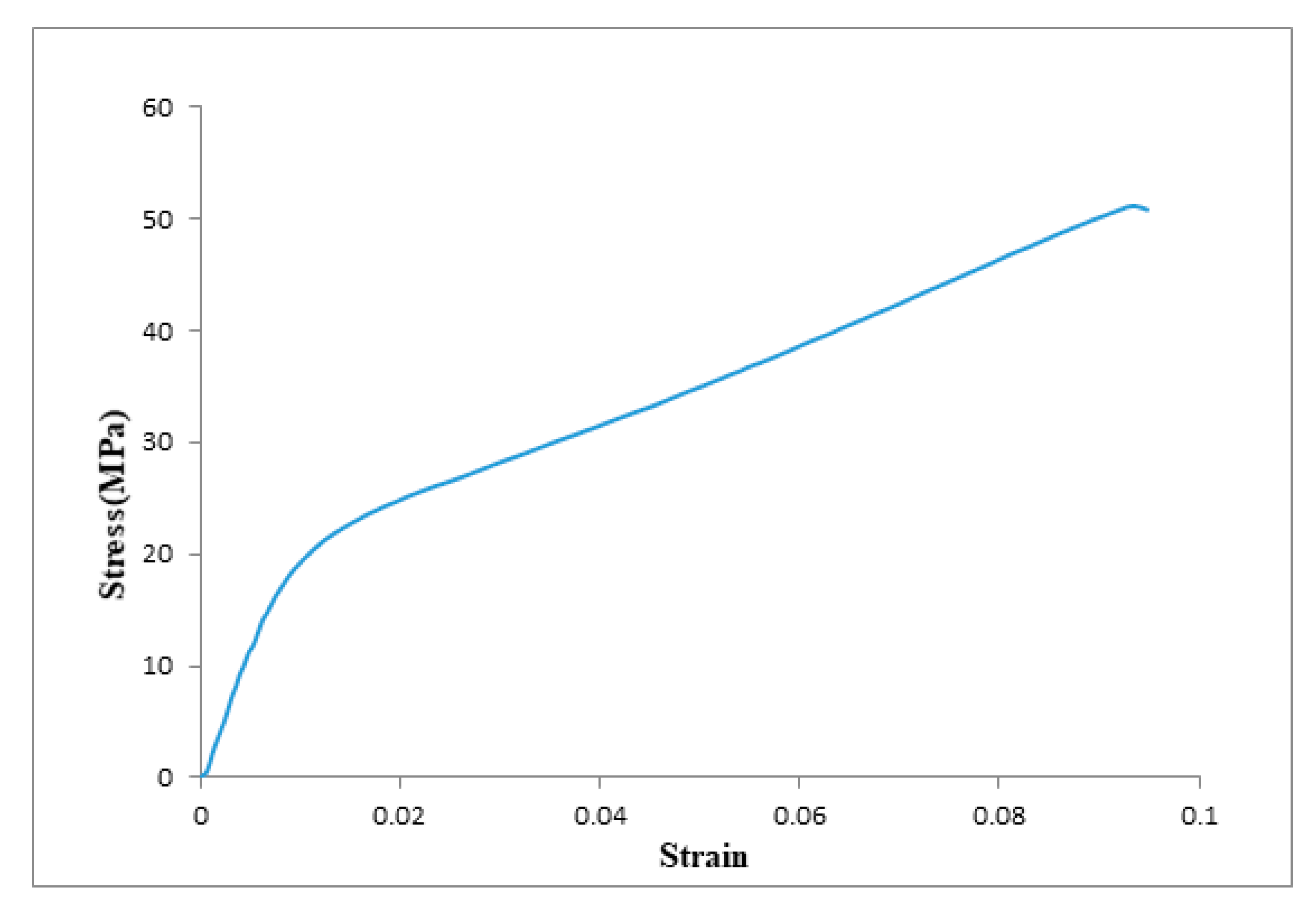
3.1.3. Mechanical Test
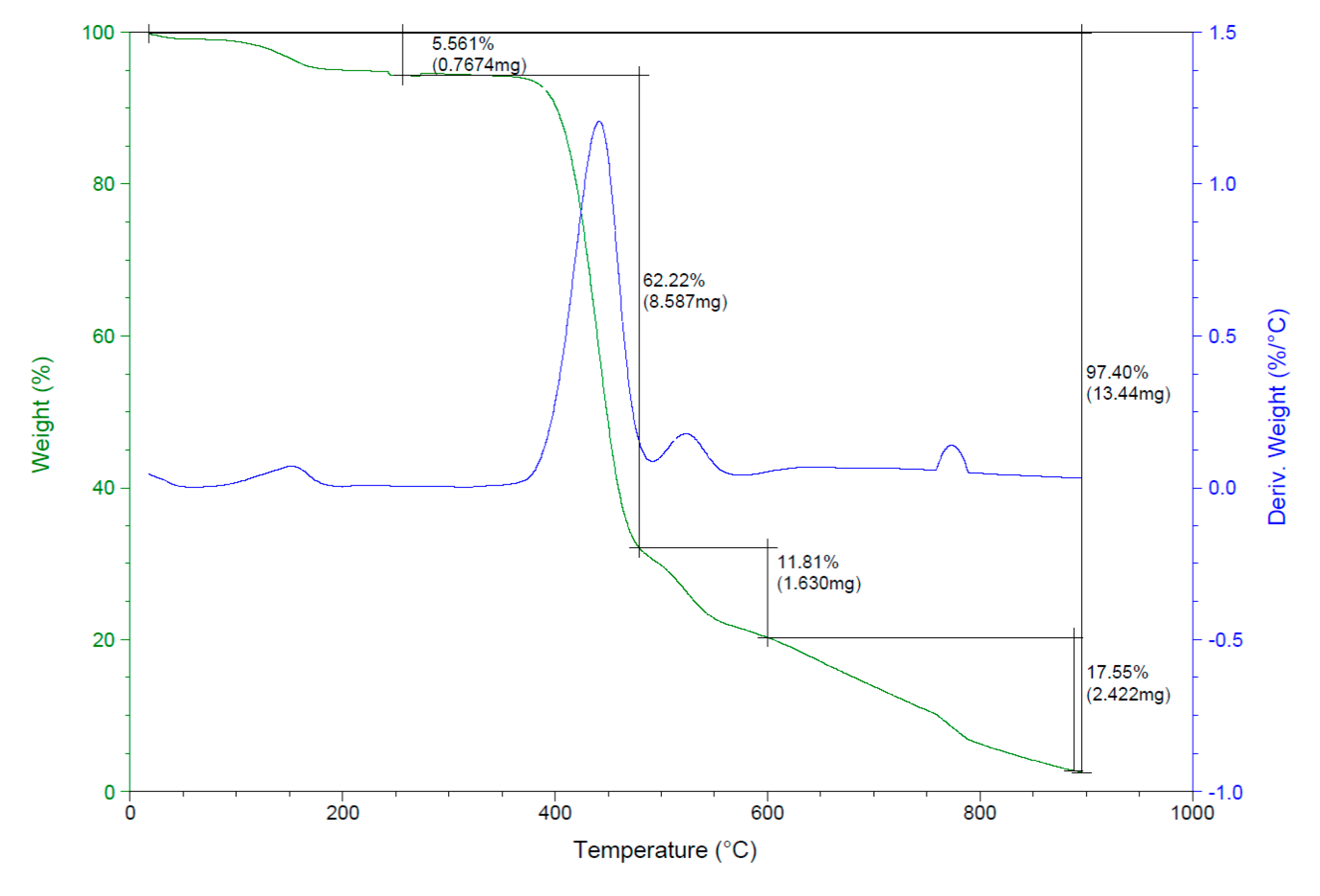
3.1.4. Thermogravimetric Analysis (TGA)
3.1.5. Fourier Transform-Infrared Spectroscopy FTIR
3.2. Effect of Oil Particle Size on Separation Efficiency
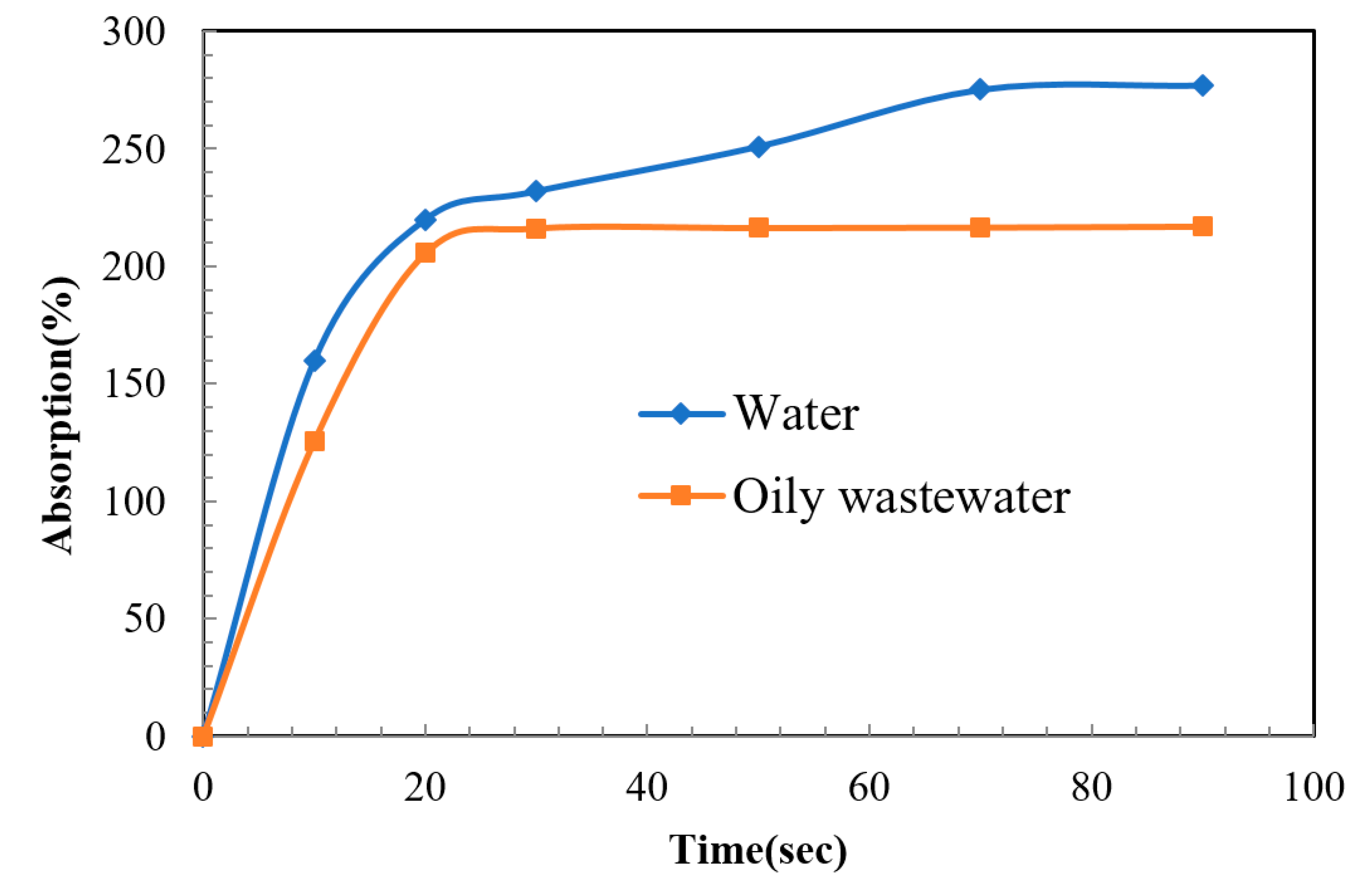
3.3. Dynamic Wetting
3.4. Effect of Time on Permeate Flux
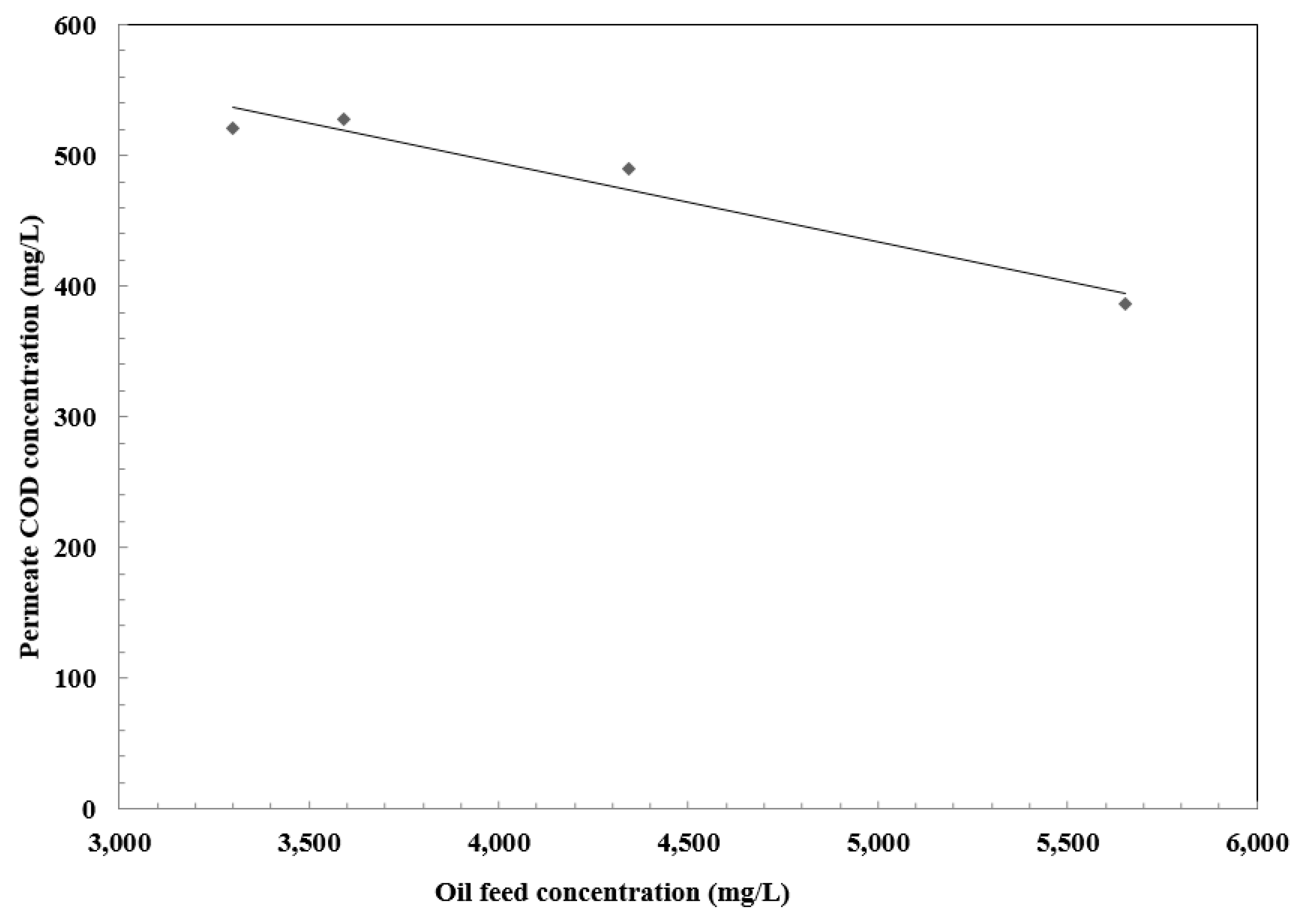
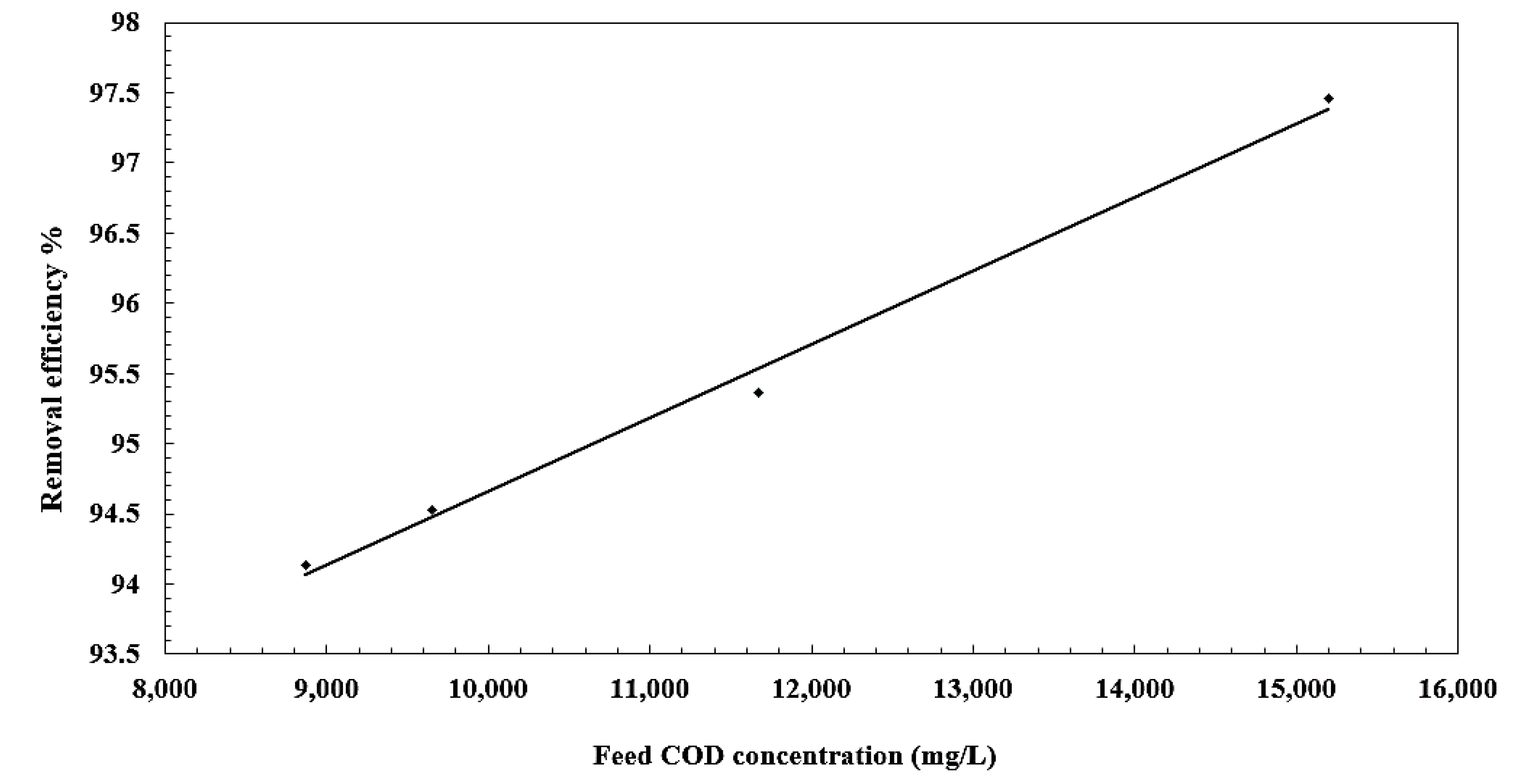
3.5. Effect of Oil Concentration on Separation Efficiency
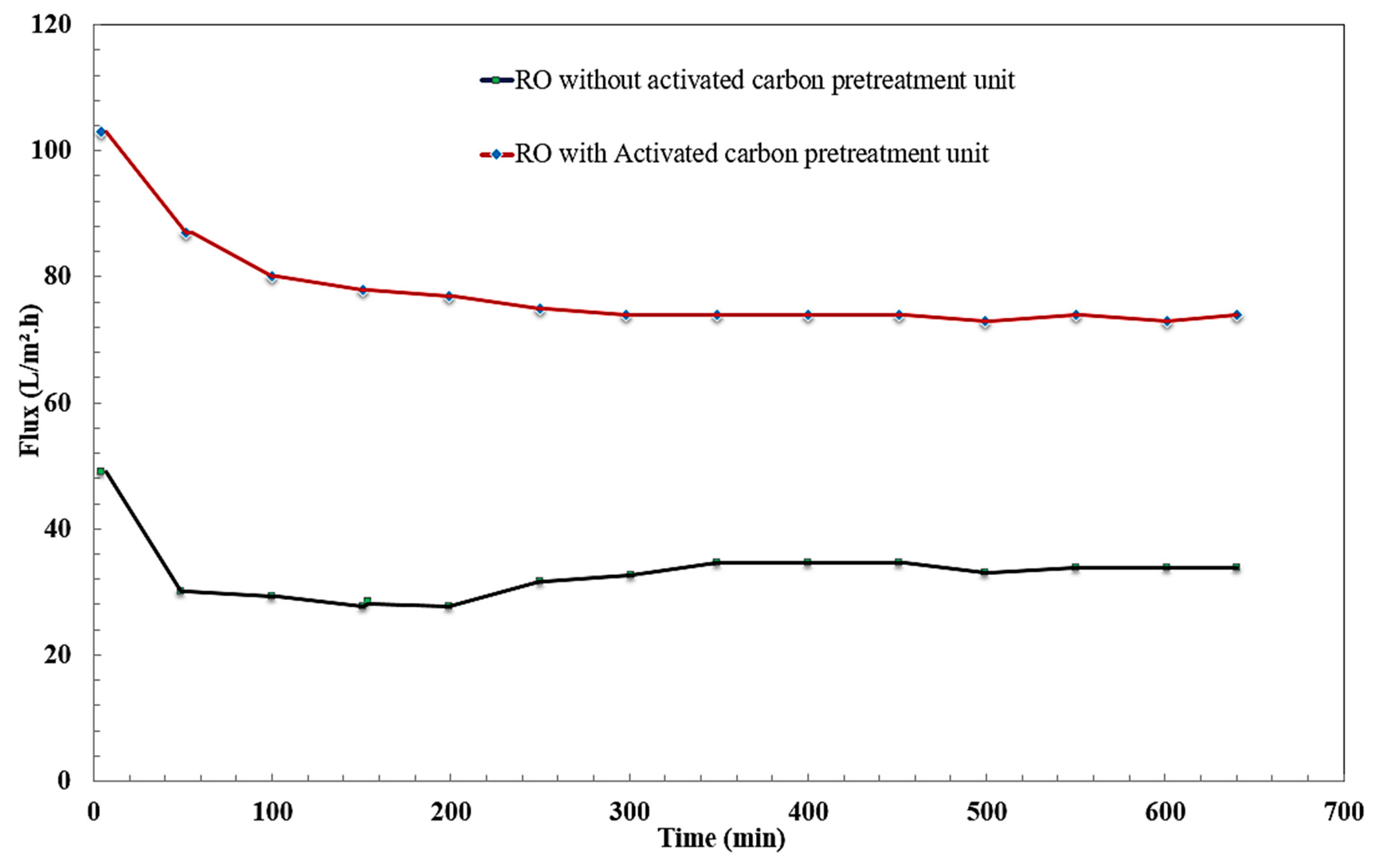
3.6. Effect of Adsorption Pretreatment unit on Membrane Filtration Performance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lee, Z.S.; Chin, S.Y.; Lim, J.W.; Witoon, T.; Cheng, C.K. Treatment technologies of palm oil mill effluent (POME) and olive mill wastewater (OMW): A brief review. Environ. Technol. Innov. 2019, 15, 100377. [Google Scholar] [CrossRef]
- Talha, A.; Belwal, T.; Li, L.; Ramola, S.; Aadil, R.M.; Xu, Y.; Luo, Z. Utilization of wastewater from edible oil industry, turning waste into valuable products: A review. Trends Food Sci. Technol. 2020, 99, 21–33. [Google Scholar]
- Ahmad, N.A.; Goh, P.S.; Abdul Karim, Z.; Ismail, A.F. Thin film composite membrane for oily waste water treatment: Recent advances and challenges. Membranes 2018, 8, 86. [Google Scholar] [CrossRef]
- Daramola, M.O.; Hlanyane, P.; Sadare, O.O.; Oluwasina, O.O.; Iyuke, S.E. Performance of carbon nanotube/polysulfone (CNT/Psf) composite membranes during Oil–water mixture separation: Effect of CNT dispersion method. Membranes 2017, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Kale, V.; Katikaneni, S.S.P.; Cheryan, M. Deacidification of ricebran oil by solvent extraction and membrane technology. JAOCS 1999, 76, 723. [Google Scholar] [CrossRef]
- Han, M.; Zhang, J.; Chu, W.; Chen, J.; Zhou, G. Research Progress and Prospects of Marine Oily Wastewater Treatment: A Review. Water 2019, 11, 2517. [Google Scholar] [CrossRef]
- Shao, S.; Li, Y.; Lü, T.; Qi, D.; Zhang, D.; Zhao, H. Removal of Emulsified Oil from Aqueous Environment by Using Polyvinylpyrrolidone-Coated Magnetic Nanoparticles. Water 2019, 11, 1993. [Google Scholar] [CrossRef]
- Capodici, M.; Cosenza, A.; Di Trapani, D.; Mannina, G.; Torregrossa, M.; Viviani, G. Treatment of Oily Wastewater with Membrane Bioreactor Systems. Water 2017, 9, 412. [Google Scholar] [CrossRef]
- Song, Y.C.; Kim, I.S.; Koh, S.C. Demulsification of oily wastewater througha synergistic effect of ozone and salt. Water Sci. Technol. 1998, 38, 247–253. [Google Scholar] [CrossRef]
- Liu, Z.; Qin, D.; Zhao, J.; Feng, Q.; Li, Z.; Bai, H.; Sun, D.D. Efficient Oil/Water Separation Membrane Derived from Super-Flexible and Superhydrophilic Core–Shell Organic/Inorganic Nanofibrous Architectures. Polymers 2019, 11, 974. [Google Scholar] [CrossRef]
- Deng, S.; Yu, G.; Jiang, Z.; Zhang, R.; Ting, Y.P. Destabilization of oil droplets in produced water from ASP flooding. Colloid Surf. A Physicochem. Eng. Aspects 2005, 252, 113–119. [Google Scholar] [CrossRef]
- Zouboulis, A.I.; Avranas, A. Treatment of oil-in-water emulsions by coag-ulation and dissolved-air flotation. Colloid Surf. A Physicochem. Eng. Asp. 2000, 172, 153–161. [Google Scholar] [CrossRef]
- Chen, G. Electrochemical technologies in wastewater treatment. Sep. Purif. Technol. 2004, 38, 11–41. [Google Scholar] [CrossRef]
- Iskandar, M.J.; Baharum, A.; Anuar, F.H.; Othaman, R. Palm oil industry in South East Asia and the effluent treatment technology—A review. Environ. Technol. Innov. 2018, 9, 169–185. [Google Scholar] [CrossRef]
- Elhenawy, Y.; Nabil, A.S.; Elminshawy, M.; Bassyouni, A.A.A.; Drioli, E. Experimental and theoretical investigation of a new air gap membrane distillation module with a corrugated feed channel. J. Membr. Sci. 2020, 594, 117461. [Google Scholar] [CrossRef]
- Bassyouni, M.; Abdel-Aziz, M.H.; Zoromba, M.S.; Abdel-Hamid, S.M.S.; Drioli, E. A review of polymeric nanocomposite membranes for water purification. J. Ind. Eng. Chem. 2019, 73, 19–46. [Google Scholar] [CrossRef]
- Asmaa, E.; Nady, N.; Bassyouni, M.; El-Shazly, A. Metal organic framework based polymer mixed matrix membranes: Review on applications in water purification. Membranes 2019, 9, 88. [Google Scholar]
- Ali, I.; Bamaga, O.A.; Gzara, L.; Bassyouni, M.; Abdel-Aziz, M.H.; Soliman, M.F.; Drioli, E.; Albeirutty, M. Assessment of blend PVDF membranes, and the effect of polymer concentration and blend composition. Membranes 2018, 8, 13. [Google Scholar] [CrossRef]
- Soliman, M.F.; Abdel-Aziz, M.H.; Bamaga, O.A.; Gzara, L.; Sharaf, F.; Al-Sharif, M.; Bassyouni, Z.; Ahmad, R. Performance evaluation of blended PVDF membranes for desalination of seawater RO brine using direct contact membrane distillation. Desalin. Water Treat. 2017, 63, 6. [Google Scholar] [CrossRef]
- Maddah, H.A.; Alzhrani, A.S.; Almalki, A.M.; Bassyouni, M.; Abdel-Aziz, M.H.; Zoromba, M.; Shihon, M.A. Determination of the treatment efficiency of different commercial membrane modules for the treatment of groundwater. J. Mater. Environ. Sci. 2017, 8, 2006–2012. [Google Scholar]
- Hemmati, M.; Rekabdar, F.; Gheshlaghi, A.; Salahi, A.; Mohammadi, T. Effects of air sparging, cross flow velocity and pressure on permeation flux enhancement in industrial oily wastewater treatment using microfi ltration. Desalin. Water Treat. 2012, 39, 33–40. [Google Scholar] [CrossRef]
- Wu, C.; Li, A.; Li, L.; Zhang, L.; Wang, H.; Qi, X.; Zhang, Q. Treatment of oily water by a poly (vinyl alcohol) ultrafiltration membrane. Desalination 2008, 225, 312–321. [Google Scholar] [CrossRef]
- Masoudnia, K.; Raisi, A.; Aroujalian, A.; Fathizadeh, M. A hybrid microfiltra-tion/ultrafiltration membrane process for treatment of oily wastewater. Desalin. Water Treat. 2005, 55, 901–912. [Google Scholar] [CrossRef]
- Pulido, J.M.O. A review on the use of membrane technology and fouling control for olive mill wastewater treatment. Sci. Total Environ. 2016, 563, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Gryta, M.; Karakulski, K.; Morawski, A.W. Purification of oily wastewater by hybrid UF/MD. Water Res. 2001, 35, 3665–3669. [Google Scholar] [CrossRef]
- Yan, L.; Hong, S.; Li, M.L.; Li, Y.S. Application of the Al2O3–PVDF nanocompositetubular ultrafiltration (UF) membrane for oily wastewater treatment and its antifouling research. Sep. Purif. Technol. 2009, 66, 347–352. [Google Scholar] [CrossRef]
- Kaur, H.; Bulasara, V.K.; Gupta, R.K. Influence of pH and temperature ofdip-coating solution on the properties of cellulose acetate-ceramic compositemembrane for ultrafiltration. Carbohydr. Polym. 2018, 195, 613–621. [Google Scholar] [CrossRef]
- Schwarze, M. Micellar-enhanced ultrafiltration (MEUF)—State of the art. Environ. Sci. Water Res. Technol. 2017, 3, 598–624. [Google Scholar] [CrossRef]
- Gholamzadeh, N.; Peyravi, M.; Jahanshahi, M. Study on olive oil wastewater treatment: Nanotechnology impact. J. Water Environ. Nanotechnol. 2016, 1, 145–161. [Google Scholar]
- Wu, P.; Jiang, L.Y.; He, Z.; Song, Y. Treatment of metallurgical industrywastewater for organic contaminant removal in China: Status, challenges, andperspectives. Environ. Sci. Water Res. Technol. 2017, 3, 1015–1031. [Google Scholar] [CrossRef]
- Bassyouni, M.; Mansi, A.E.; Elgabry, A.; Ibrahim, B.A.; Kassem, O.A.; Alhebeshy, R. Utilization of carbon nanotubes in removal of heavy metals from wastewater: A review of the CNTs’ potential and current challenges. Appl. Phys. A 2020, 126, 38. [Google Scholar] [CrossRef]
- Gao, W.J.; Leung, K.T.; Qin, W.S.; Liao, B.Q. Effects of temperature andtemperature shock on the performance and microbial community structureof a submerged anaerobic membrane bioreactor. Bioresour. Technol. 2011, 102, 8733–8740. [Google Scholar] [CrossRef] [PubMed]
- Xiangli, Q.; Zhenjia, Z.; Nongcun, W.; Wee, V.; Low, M.; Loh, C.S.; Hing, N.T. Coagulation pretreatment for a large-scale ultrafiltration process treating water from the Taihu River. Desalination 2008, 230, 305–313. [Google Scholar] [CrossRef]
- Ahmed, Y.; Yaakob, Z.; Akhtar, P.; Sopian, K. Production of biogas and performance evaluation of existing treatment processes in palm oil mill effluent (POME). Renew. Sustain. Energy Rev. 2015, 42, 1260–1278. [Google Scholar] [CrossRef]
- Louhıchı, G.; Bousselmı, L.; Ghrabı, A.; Khounı, I. Process optimization via response surface methodology in the physico-chemical treatment of vegetable oil refinery wastewater. Environ. Sci. Pollut. Res. 2018, 26, 18993–19011. [Google Scholar]
- Šereš, Z.; Simović, D.Š.; Dokić, L.; Giorno, L.; Pajin, B.; Hodur, C.; Maravić, N. Edible oilindustry wastewater treatment by microfiltration with ceramic membrane. Int. J. Civ. Mech. Eng. 2016, 10, 410–413. [Google Scholar]
- Stoller, M.; Vilardia, G.; Di, L.; Palmaa, A.C. Treatment of olive oil processing wastewater by ultrafiltration, nanofiltration, reverse osmosis and biofiltration. Chem. Eng. 2016, 47, 409–414. [Google Scholar]
- Azmi, N.S.; Yunos KF, M.; Baharuddin, A.S.; Dom, Z.M. The effect of operating parameters on ultrafiltration and reverse osmosis of palm oil mill effluent for reclamation and reuse of water. BioResources 2012, 8, 76–87. [Google Scholar] [CrossRef][Green Version]
- Azmi, N.A.; Yunos, K.M.; Zakaria, R. Application of sandwich membrane for the treatment of palm oil mill effluent (POME) for water reuse. Procedia Eng. 2012, 44, 1980–1981. [Google Scholar] [CrossRef]
- Košutić, K.; Dolar, D.; Kunst, B. On experimental parameters characterizing the reverse osmosis and nanofiltration membranes’ active layer. J. Membr. Sci. 2006, 282, 109–114. [Google Scholar]
- Khouni, I.; Louhichi, G.; Ghrabi, A.; Moulin, P. Efficiency of a coagulation/flocculation–membrane filtration hybrid process for the treatment of vegetable oil refinery wastewater for safe reuse and recovery. Process Saf. Environ. Prot. 2020, 135, 323–341. [Google Scholar] [CrossRef]
- Zhao, F.-B.; Yu, Z.-J.; Park, H.-D.; Liu, X.-Y.; Song, X.-R.; Li, Z.-S. Polyvinylchloride ultrafiltration membranes modified with different SiO2 particles and their antifouling mechanism for oil extraction wastewater. J. Environ. Eng. 2015, 141, 04015009. [Google Scholar] [CrossRef]
- Chakrabarty, B.; Ghoshal, A.K.; Purkait, M.K. Ultrafiltration of stable oil-in-wateremulsion by polysulfone membrane. J. Membr. Sci. 2008, 325, 427. [Google Scholar] [CrossRef]
- Mittal, P.; Jana, S.; Mohanty, K. Synthesis of low-cost hydrophilic ceramic–polymeric composite membrane for treatment of oily wastewater. Desalination 2011, 282, 54–62. [Google Scholar] [CrossRef]
- He, Z.; Miller, D.J.; Kasemset, S.; Paul, D.R.; Freeman, B.D. The effect of permeate flux on membrane fouling during microfiltration of oily water. J. Membr. Sci. 2017, 525, 25–34. [Google Scholar] [CrossRef]
- Iranizadeh, S.T.; Chenar, M.P.; Mahboub, M.N.; Namaghi, H.A. Preparation and characterization of thin-film composite reverse osmosis membrane on a novel aminosilane-modified polyvinyl chloride support. Braz. J. Chem. Eng. 2019, 36, 251–264. [Google Scholar] [CrossRef]
- Yee, T.L.; Rathnayake, T.; Visvanathan, C. Performance Evaluation of a Thermophilic Anaerobic Membrane Bioreactor for Palm Oil Wastewater Treatment. Membranes 2019, 9, 55. [Google Scholar] [CrossRef]
- Al-Ani, F.H.; Alsalhy, Q.F.; Raheem, R.S.; Rashid, K.T.; Figoli, A. Experimental Investigation of the Effect of Implanting TiO2-NPs on PVC for Long-Term UF Membrane Performance to Treat Refinery Wastewater. Membranes 2020, 10, 77. [Google Scholar] [CrossRef]
- Vu, A.; Mark, N.S.; Ramon, G.Z.; Qian, X.; Sengupta, A.; Wickramasinghe, S.R. Oil Deposition on Polymer Brush-Coated NF Membranes. Membranes 2019, 9, 168. [Google Scholar] [CrossRef]


| Property | Polyamide Thin Film Composite |
|---|---|
| Tensile strength, MPa | 51.15 |
| Young’s modulus, MPa | 1000 |
| PA | PSF | ||
|---|---|---|---|
| Range (cm−1) | Assignment | Range (cm−1) | Assignment |
| 840.39 | aromatic hydrogen, isolated | 727.89 | Aromatic hydrogen |
| 1105.05 | - | 870.12 | Hydrogen deformation of para-substituted phenyl groups. |
| 1155.67 | C–N bending | 1248.53 | C–O–C asymmetric stretching vibration of the aryl–O–aryl group |
| 1242.98 | C–N bending | 1581.03 | Aromatic in-plane ring bend stretching vibration |
| 1493.59 | Aromatic ring breathing | 3064.54 | O–H aromatic stretching |
| 1590.13 | C=O band of an amide group, C–N stretching, and C–N–C deformation vibration in a secondary amide group | - | - |
| 3420.93 | N–H (and O–H) | - | - |
| 3647.56 | O–H aromatic stretching bands | - | - |
| Feed COD Concentration (mg/L) | Permeate COD Concentration (mg/L) | Separation Efficiency % | Feed Turbidity (NTUs) | Permeate Turbidity (NTUs) |
|---|---|---|---|---|
| 5653 | 386 | 97.4 | 990 | 1.28 |
| 4343 | 490 | 95.35 | 980 | 1.22 |
| 3591 | 528 | 94.53 | 870 | 1.84 |
| 3299 | 521 | 94.12 | 850 | 1.6 |
| Oil Concentration of Feed (mg/L) | Oil Concentration of Permeate before Pretreatment (mg/L) | Oil Concentration of Permeate after Pretreatment and RO (mg/L) | Rejection % |
|---|---|---|---|
| 5653 | 105 | 4.8 | 99.91 |
| 4343 | 219 | 5.3 | 99.87 |
| 3591 | 186 | 4.3 | 99.88 |
| 3299 | 235 | 3.2 | 99.90 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elhady, S.; Bassyouni, M.; Mansour, R.A.; Elzahar, M.H.; Abdel-Hamid, S.; Elhenawy, Y.; Saleh, M.Y. Oily Wastewater Treatment Using Polyamide Thin Film Composite Membrane Technology. Membranes 2020, 10, 84. https://doi.org/10.3390/membranes10050084
Elhady S, Bassyouni M, Mansour RA, Elzahar MH, Abdel-Hamid S, Elhenawy Y, Saleh MY. Oily Wastewater Treatment Using Polyamide Thin Film Composite Membrane Technology. Membranes. 2020; 10(5):84. https://doi.org/10.3390/membranes10050084
Chicago/Turabian StyleElhady, Sarah, Mohamed Bassyouni, Ramadan A. Mansour, Medhat H. Elzahar, Shereen Abdel-Hamid, Yasser Elhenawy, and Mamdou Y. Saleh. 2020. "Oily Wastewater Treatment Using Polyamide Thin Film Composite Membrane Technology" Membranes 10, no. 5: 84. https://doi.org/10.3390/membranes10050084
APA StyleElhady, S., Bassyouni, M., Mansour, R. A., Elzahar, M. H., Abdel-Hamid, S., Elhenawy, Y., & Saleh, M. Y. (2020). Oily Wastewater Treatment Using Polyamide Thin Film Composite Membrane Technology. Membranes, 10(5), 84. https://doi.org/10.3390/membranes10050084