Industrial Wastewater Treatment by Nanofiltration—A Case Study on the Anodizing Industry
Abstract
1. Introduction
2. Materials and Methods
2.1. Wastewater Analysis
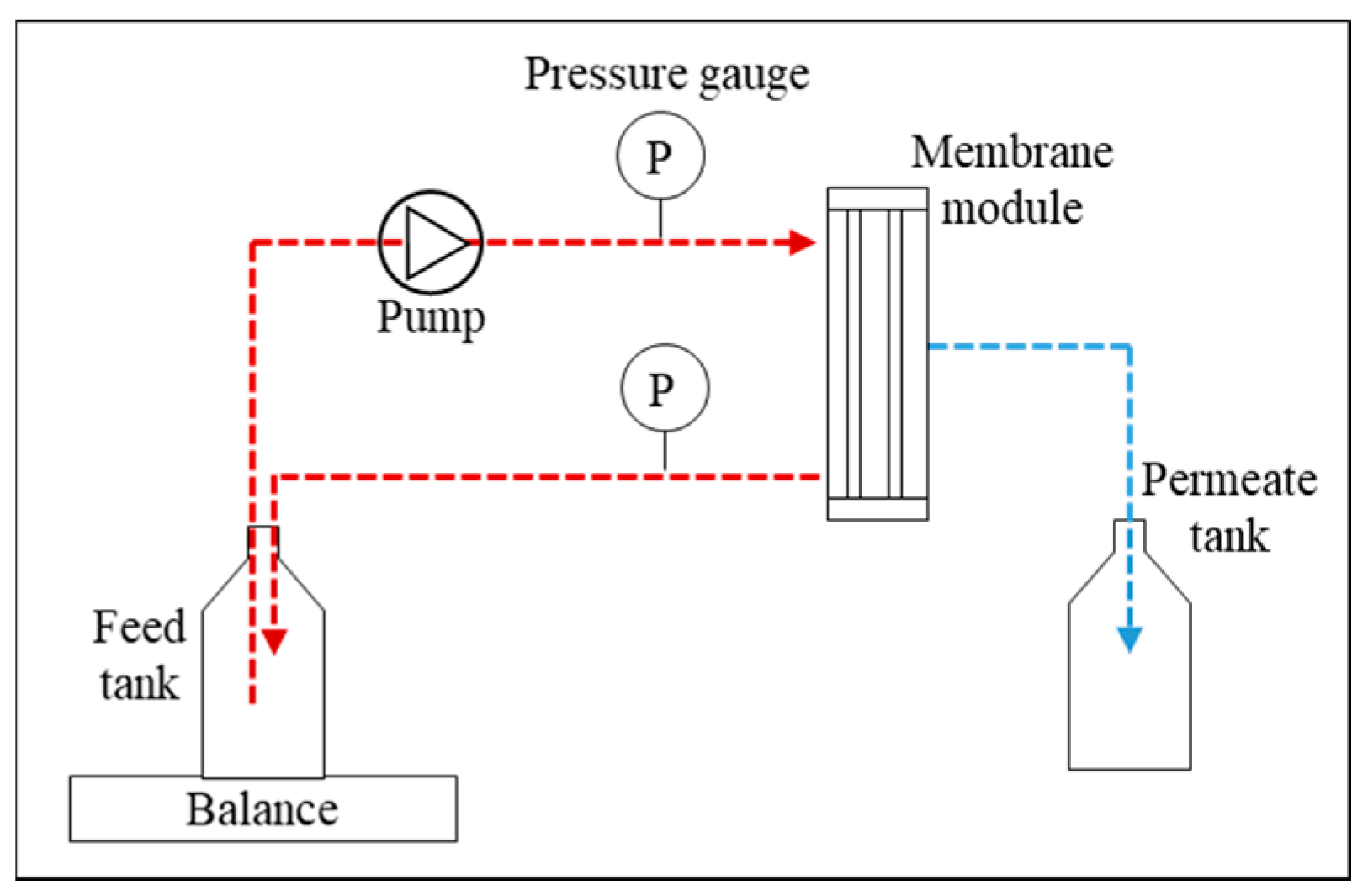
2.2. NF Tests
3. Results and Discussion
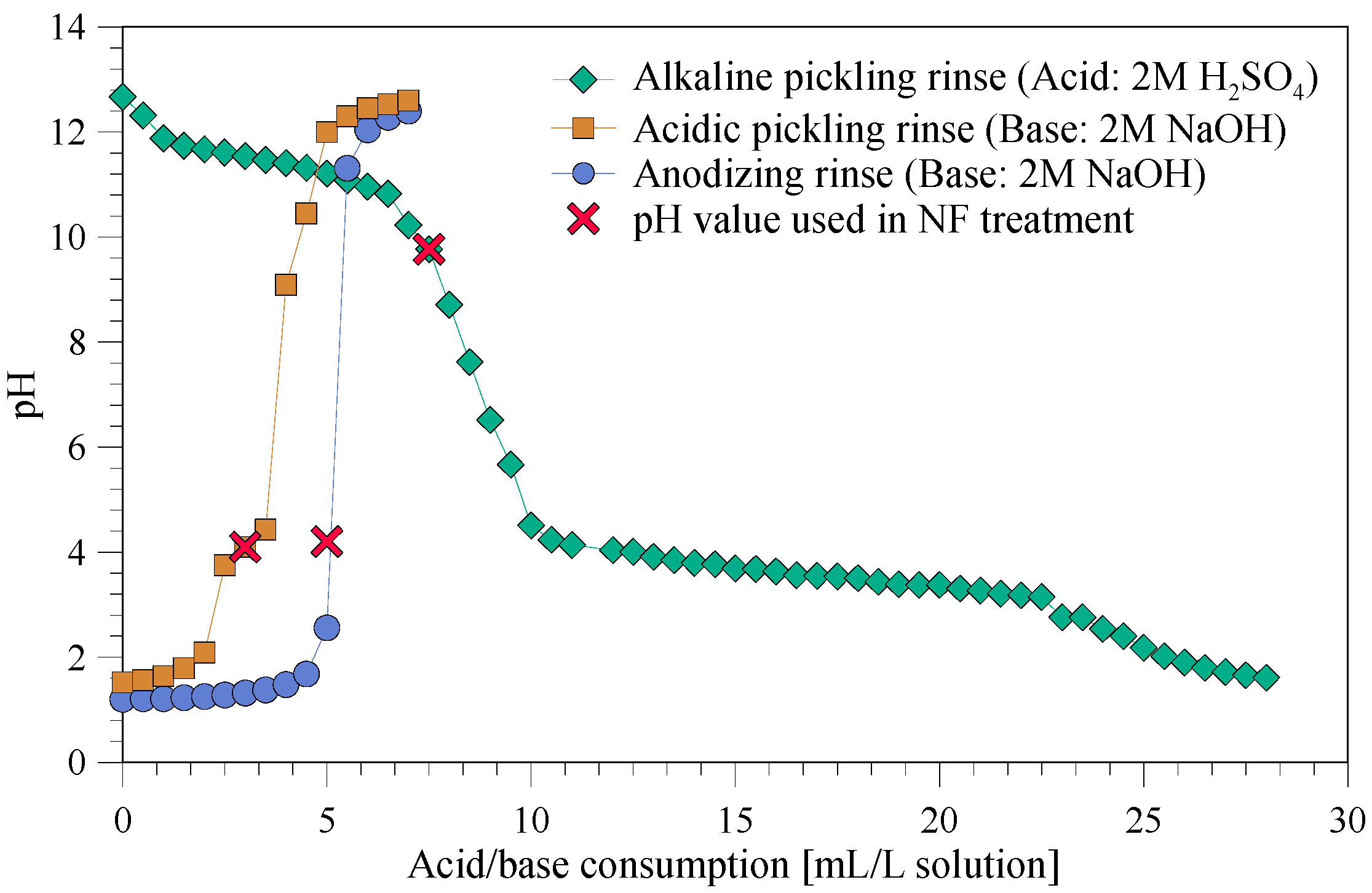
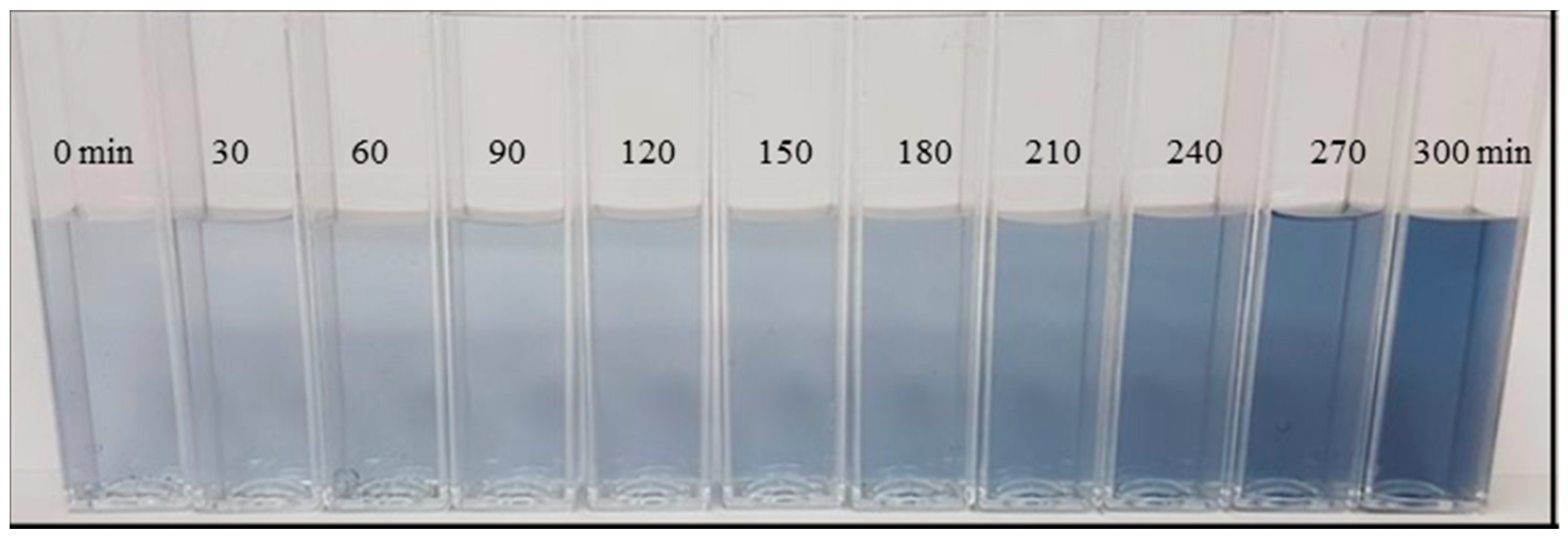
3.1. Effect of pH Adjustment
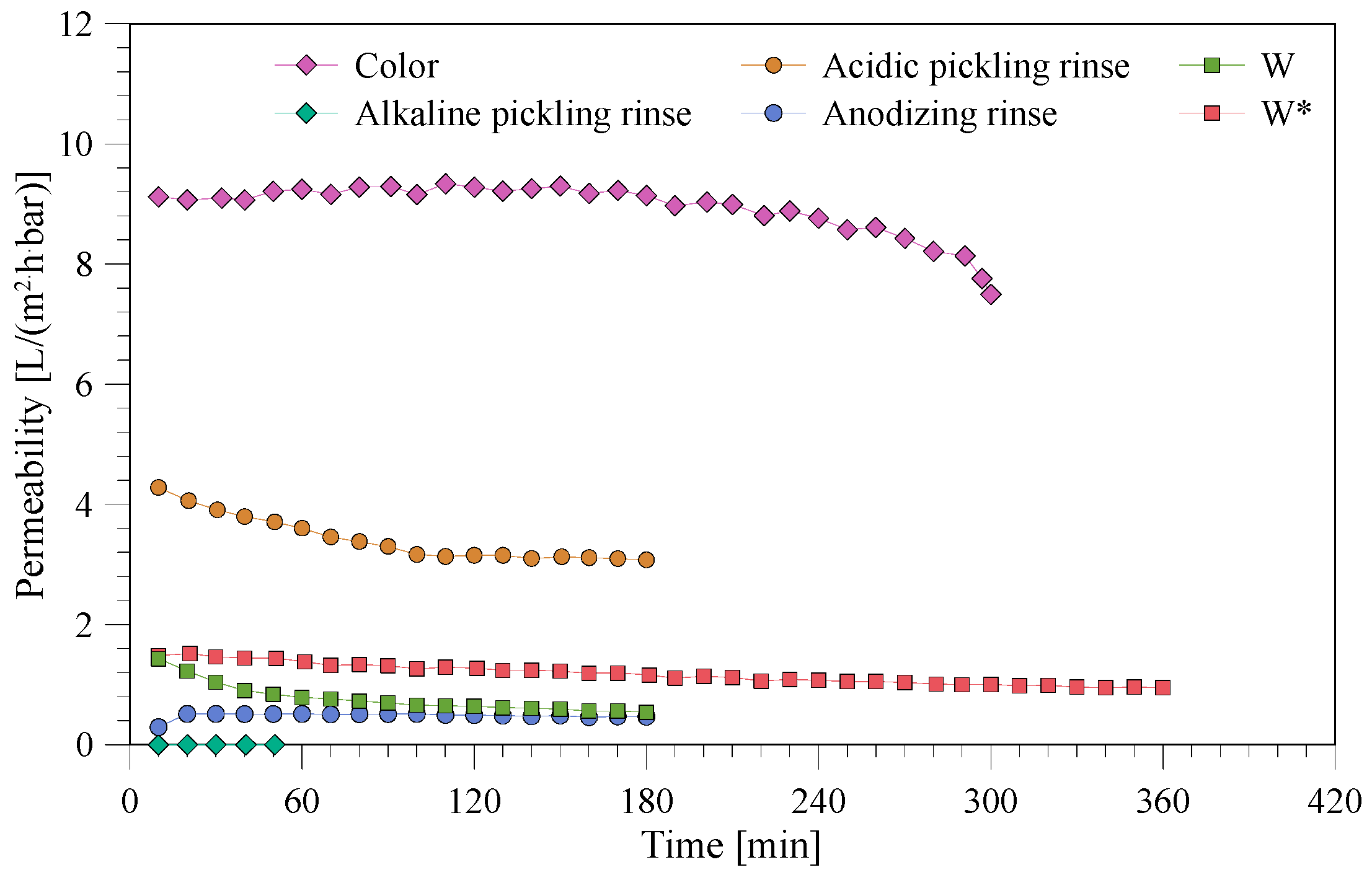
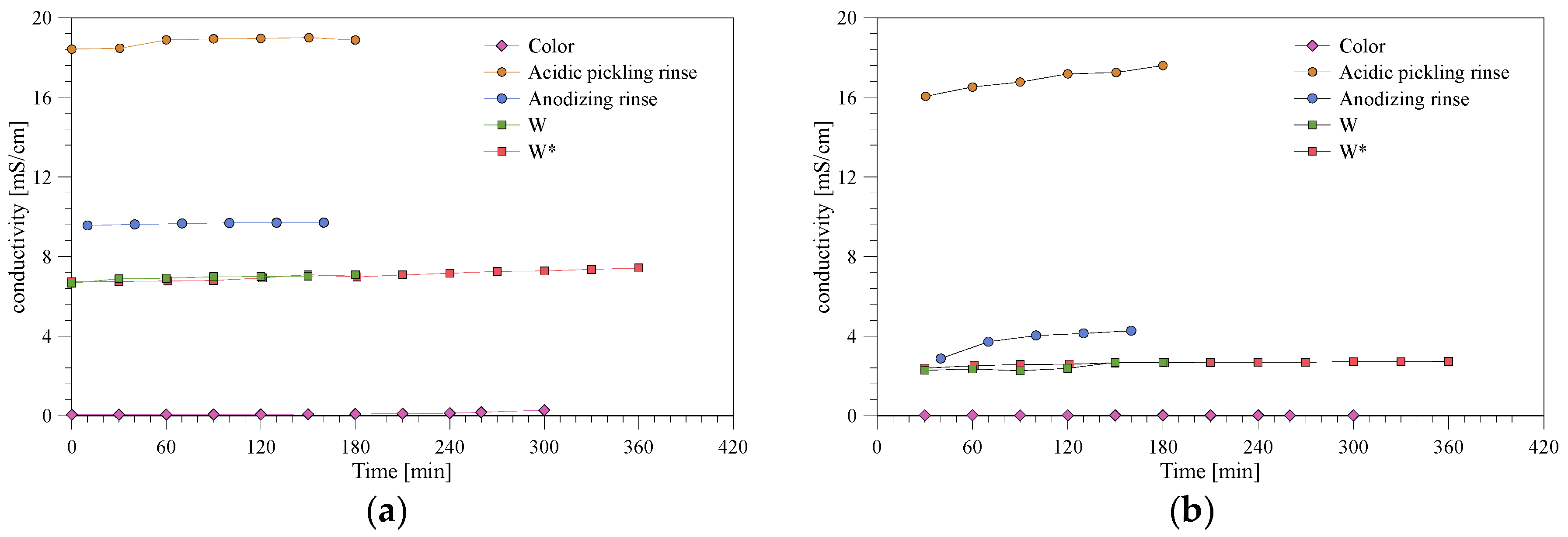
3.2. Nanofiltration of Various Wastewaters
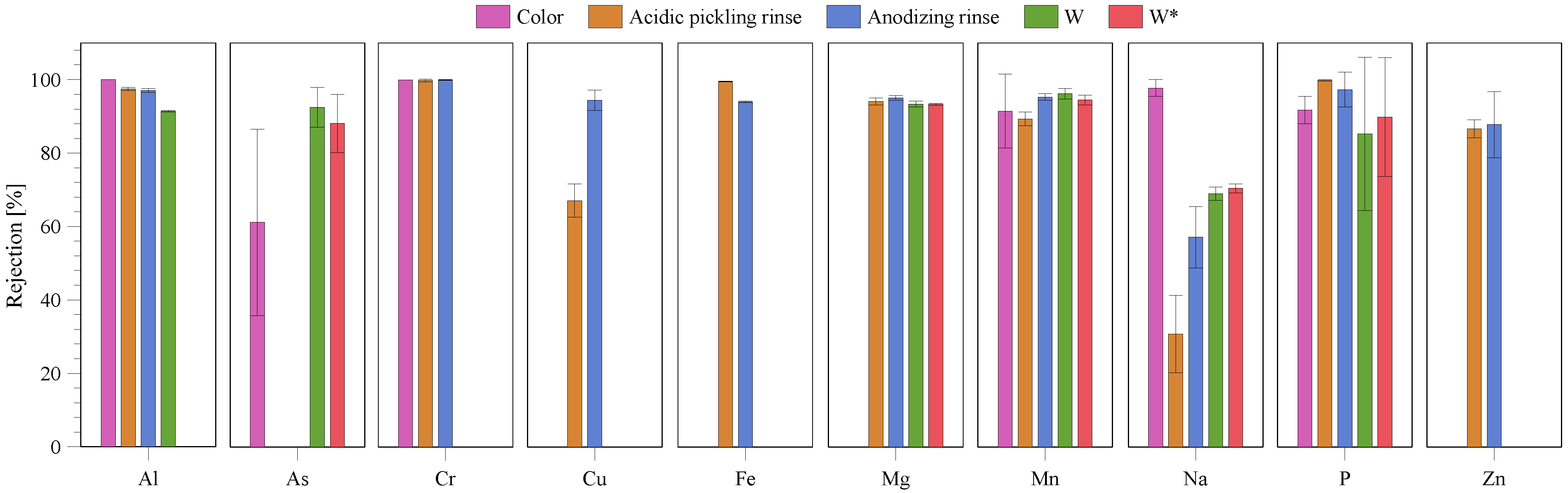
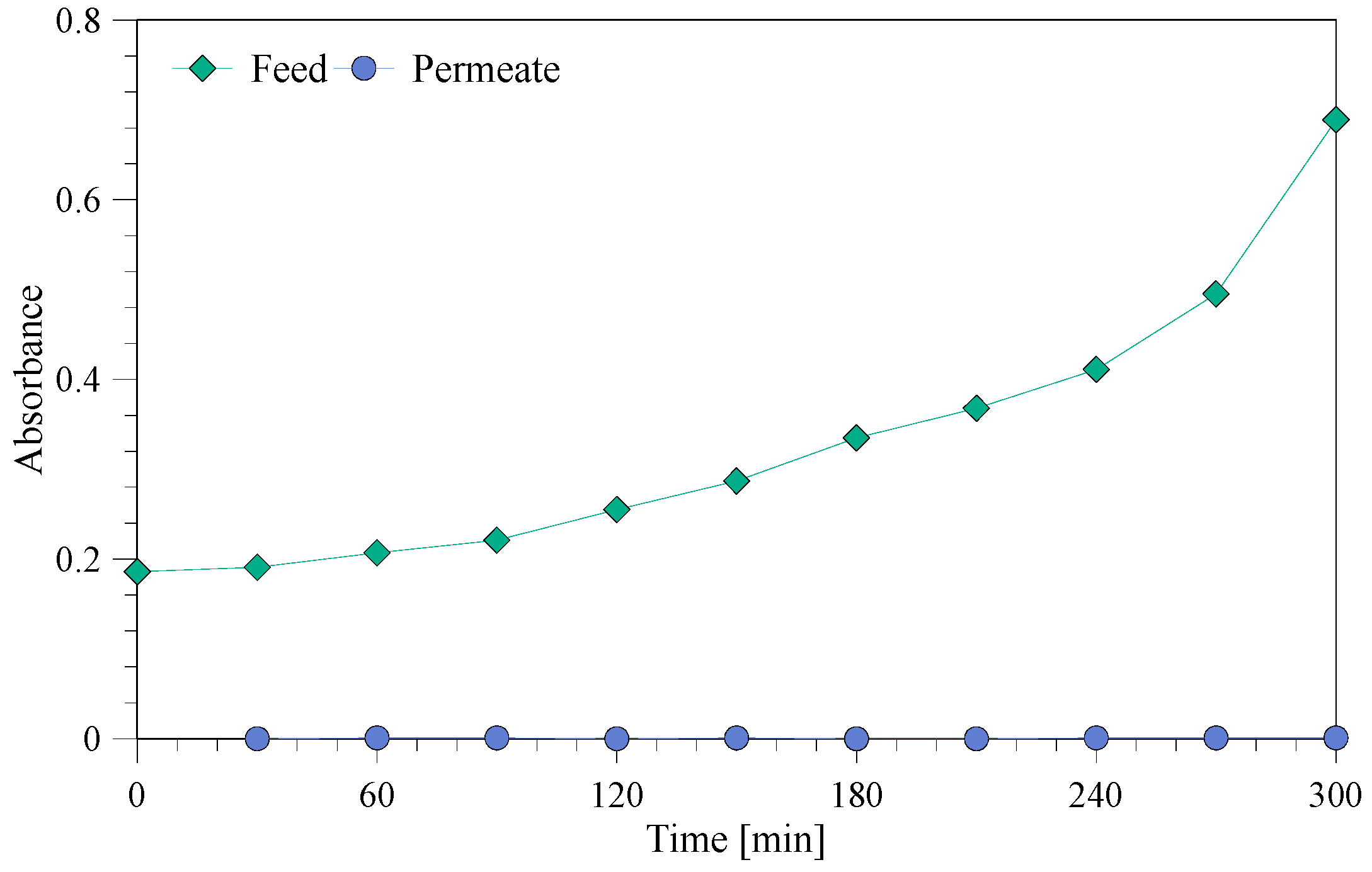
3.3. Permeate Quality
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stevenson, M.F. Anodizing. In ASM Handbook; ASM International: Materials Park, OH, USA, 1994; Volume 5, pp. 482–493. [Google Scholar]
- Grubbs, C.A. Anodizing of aluminum. Met. Finish. 1999, 97, 476–493. [Google Scholar] [CrossRef]
- Barakat, M. New trends in removing heavy metals from wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Lin, X.; Burns, R.C.; Lawrance, G.A. Heavy Metals in Wastewater: The effect of electrolyte composition on the precipitation of cadmium (II) using lime and magnesia. Water. Air. Soil Pollut. 2005, 165, 131–152. [Google Scholar] [CrossRef]
- Update, T.I. The Environmental Impact of Anodizing. Available online: https://www.thomasnet.com/articles/custom-manufacturing-fabricating/anodizing-environmental (accessed on 20 September 2019).
- Correia, A.; Chambino, T.; Gonc, L.; Franco, A.; Gonc, R.; Limpo, V.; Delmas, F.; Nogueira, C.; Bartolomeu, F. Municipal wastewater treatment with anodizing solid waste. Desalination 2005, 185, 341–350. [Google Scholar] [CrossRef]
- Chambino, T.; Correia, A.; Barany, S. Aluminium salts hydrolysis products from industrial anodising sludges in wastewater treatment. Prog. Colloid Polym. Sci. 2008, 5, 65–69. [Google Scholar]
- Magalhães, J.M.; Silva, J.E.; Castro, F.P.; Labrincha, J.A. Physical and chemical characterisation of metal finishing industrial wastes. J. Environ. Manag. 2005, 75, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Matlock, M.M.; Howerton, B.S.; Atwood, D.A. Chemical precipitation of heavy metals from acid mine drainage. Water Res. 2002, 36, 4757–4764. [Google Scholar] [CrossRef]
- Amer, S. Treating Metal Finishing Wastewater; AQUACHEM INC.: Denver, CO, USA, 1998; pp. 1–7. [Google Scholar]
- Da̧browski, A.; Robens, E. Selective removal of the heavy metal ions from waters and industrial wastewaters by ion-exchange method. Chemosphere 2004, 56, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Drioli, E.; Ali, A.; Macedonio, F. Membrane Operations for Process Intensification in Desalination. Appl. Sci. 2017, 7, 100. [Google Scholar] [CrossRef]
- Fane, A.G.; Awang, A.R.; Bolko, M.; Macoun, R.; Schofield, R.; Shen, Y.R.; Zha, F. Metal recovery from wastewater using membranes. Water Sci. Technol. 1992, 25, 5–18. [Google Scholar] [CrossRef]
- Andrade, L.H.; Aguiar, A.O.; Pires, W.L.; Miranda, G.A.; Teixeira, L.P.T.; Almeida, G.C.C.; Amaral, M.C.S. Nanofiltration and reverse osmosis applied to gold mining effluent treatment and reuse. Braz. J. Chem. Eng. 2017, 34, 93–107. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, G.; Meng, Q.; Zhang, H. Performance of Nanofiltration and Reverse Osmosis Membranes in Metal Effluent Treatment. Chin. J. Chem. Eng. 2008, 16, 441–445. [Google Scholar] [CrossRef]
- Muthukrishnan, M.; Guha, B.K. Effect of pH on rejection of hexavalent chromium by nanofiltration. Desalination 2008, 219, 171–178. [Google Scholar] [CrossRef]
- Mnif, A.; Bejaoui, I.; Mouelhi, M.; Hamrouni, B. Hexavalent Chromium Removal from Model Water and Car Shock Absorber Factory Effluent by Nanofiltration and Reverse Osmosis Membrane. Int. J. Anal. Chem. 2017, 2017, 7415708. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, A.W.; Othaman, R.; Hilal, N. Potential use of nanofiltration membranes in treatment of industrial wastewater from Ni-P electroless plating. Desalination 2004, 168, 241–252. [Google Scholar] [CrossRef]
- Lin, S.; Wang, T.; Juang, R. Metal Rejection by Nanofiltration from Diluted Solutions in the Presence of Complexing Agents. Sep. Sci. Technol. 2005, 39, 363–376. [Google Scholar] [CrossRef]
- Ates, N.; Uzal, N. Removal of heavy metals from aluminum anodic oxidation wastewaters by membrane filtration. Environ. Sci. Pollut. Res. 2018, 25, 22259–22272. [Google Scholar] [CrossRef] [PubMed]
- Al-zoubi, H.; Rieger, A.; Steinberger, P.; Pelzc, W.; Haseneder, R.; Hartel, G. Nanofiltration of Acid Mine Drainage Nanofiltration of Acid Mine Drainage. Desalin. Water Treatmen 2010, 21, 1–14. [Google Scholar] [CrossRef]
- Oatley, D.L.; Llenas, L.; Pérez, R.; Williams, P.M.; Martínez-Lladó, X.; Rovira, M. Review of the dielectric properties of nanofiltration membranes and verification of the single oriented layer approximation. Adv. Colloid Interface Sci. 2012, 173, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Alfa, L. Alfa Laval NF and RO Flat Sheet Membranes. Available online: https://www.alfalaval.com/globalassets/documents/products/separation/membranes/flat-sheet-membranes/nf-and-ro-flat-sheet-membranes_200000076-1-en-gb.pdf (accessed on 12 February 2020).
- Meschke, K.; Hansen, N.; Hofmann, R.; Haseneder, R.; Repke, J.U. Characterization and performance evaluation of polymeric nanofiltration membranes for the separation of strategic elements from aqueous solutions. J. Memb. Sci. 2018, 546, 246–257. [Google Scholar] [CrossRef]
- The Danish Environmental Protection Agency. Tilslutning af Industrispildevand til Offentlige Spildevandsanlæg; The Danish Environmental Protection Agency: København, Denmark, 2006. [Google Scholar]
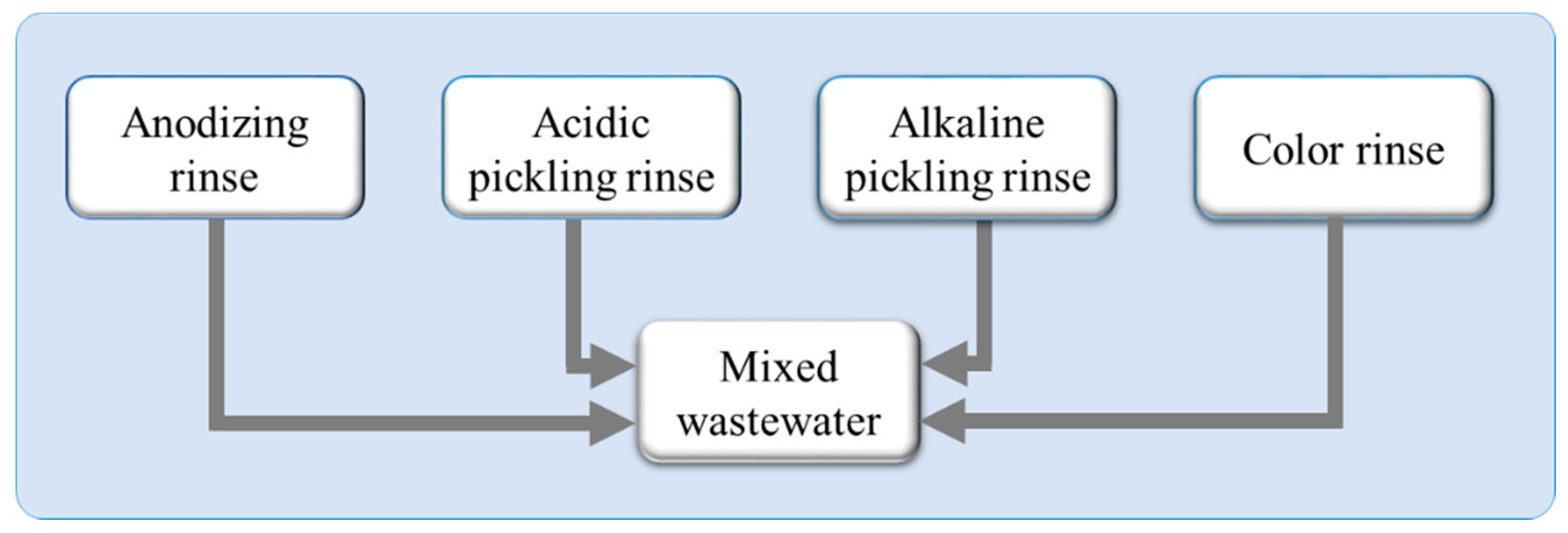
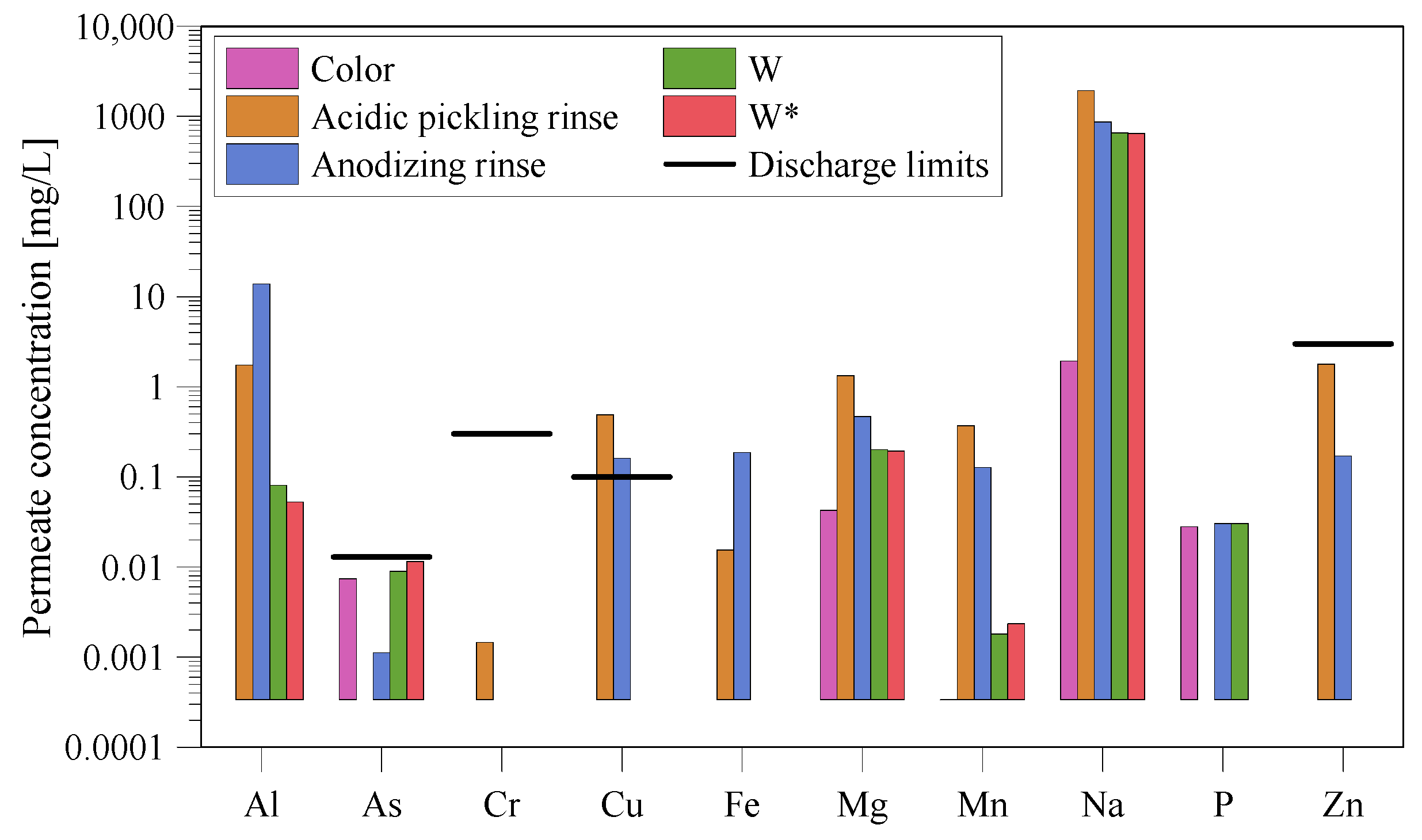
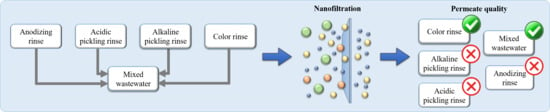
| Color Rinse | Alkaline Pickling Rinse | Acidic Pickling Rinse | Anodizing Rinse | Mixed Wastewater (W) | |
|---|---|---|---|---|---|
| pH | 7.4 | 12.7 | 1.2 | 1.52 | 7.5 |
| Conductivity [mS/cm] | 0.06 | 35.9 | 76.1 | 24.1 | 6.7 |
| Dry matter [%] | 0 | 6.0 | 0 | 0.7 | 0.7 |
| Water activity | N.A. | 0.9837 | 0.9964 | 0.9992 | 0.9977 |
| Al [ppm] | 0.0838 | 9040.6 | 56.78 | 510.84 | 0.678 |
| As [ppm] | 0.149 | N.D | N.D | N.D | 0.093 |
| Cr [ppm] | 0.962 | 2.36 | 0.56 | 0.38 | N.D |
| Cu [ppm] | N.D | 0.36 | 1.33 | 2.05 | N.D |
| Fe [ppm] | N.D | 3.35 | 5.06 | 2.95 | N.D |
| Mg [ppm] | N.D | 1.31 | 18.43 | 8.62 | 2.74 |
| Mn [ppm] | 0.0108 | 2.23 | 2.82 | 2.39 | 0.092 |
| Na [ppm] | 11.19 | 4275.7 | 43.07 | 20.65 | N.A |
| Ni [ppm] | N.D | N.D | 4.69 | N.D | N.D |
| P [ppm] | 0.183 | 46.30 | 0.48 | 0.63 | N.D |
| Pb [ppm] | N.D | N.D | N.D | N.D | N.D |
| Zn [ppm] | N.D | 0.24 | 10.51 | 1.34 | N.D. |
| Cl [ppm] | 8 | 623.6 | 33.78 | 5.68 | N.A |
| NO [ppm] | N.D | N.D | 9.66 | N.D | N.A |
| NO3 [ppm] | N.D | 150.8 | 3858 | 36.7 | N.A |
| SO4 [ppm] | 37.64 | 64.2 | 16.1 | 3570 | N.A |
| Alkaline Pickling Rinse a | Acidic Pickling Rinse a | Anodizing Rinse a | Mixed Wastewater (W*) b | |
|---|---|---|---|---|
| pH | 9.5 | 4.2 | 4.0 | 7.5 |
| Conductivity [mS/cm] | 36 | 18 | 10 | 7 |
| Dry matter [%] | 3.6 | 1.6 | 1.1 | 0.5 |
| Water activity | 0.9924 | 0.9959 | 0.9991 | 0.9993 |
| Al [ppm] | 37.11 | 50.02 | 488.34 | N.D |
| As [ppm] | N.D. | N.D. | N.D. | 0.022 |
| Cr [ppm] | N.D. | 0.48 | 0.37 | N.D |
| Cu [ppm] | N.D. | 1.22 | 1.99 | N.D |
| Fe [ppm] | 0.021 | 4.02 | 2.93 | N.D |
| Mg [ppm] | N.D. | 16.97 | 8.60 | 2.68 |
| Mn [ppm] | N.D. | 2.61 | 2.40 | 0.035 |
| Na [ppm] | 4205.6 | 3138.6 | 1882.1 | 2016.0 |
| Ni [ppm] | N.D. | N.D. | N.D. | N.D |
| P [ppm] | 0.16 | 3.7 | 0.65 | 0.068 |
| Pb [ppm] | N.D. | N.D. | N.D. | N.D |
| Zn [ppm] | N.D. | 10.06 | 1.31 | N.D |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, A.; Nymann, M.C.; Christensen, M.L.; Quist-Jensen, C.A. Industrial Wastewater Treatment by Nanofiltration—A Case Study on the Anodizing Industry. Membranes 2020, 10, 85. https://doi.org/10.3390/membranes10050085
Ali A, Nymann MC, Christensen ML, Quist-Jensen CA. Industrial Wastewater Treatment by Nanofiltration—A Case Study on the Anodizing Industry. Membranes. 2020; 10(5):85. https://doi.org/10.3390/membranes10050085
Chicago/Turabian StyleAli, Aamer, Maria C. Nymann, Morten L. Christensen, and Cejna A. Quist-Jensen. 2020. "Industrial Wastewater Treatment by Nanofiltration—A Case Study on the Anodizing Industry" Membranes 10, no. 5: 85. https://doi.org/10.3390/membranes10050085
APA StyleAli, A., Nymann, M. C., Christensen, M. L., & Quist-Jensen, C. A. (2020). Industrial Wastewater Treatment by Nanofiltration—A Case Study on the Anodizing Industry. Membranes, 10(5), 85. https://doi.org/10.3390/membranes10050085