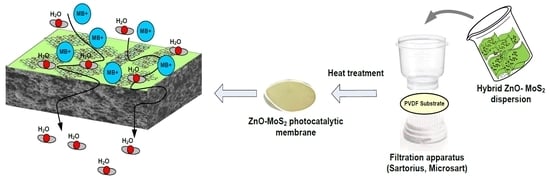
Improved Surface Functional and Photocatalytic Properties of Hybrid ZnO-MoS2-Deposited Membrane for Photocatalysis-Assisted Dye Filtration
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
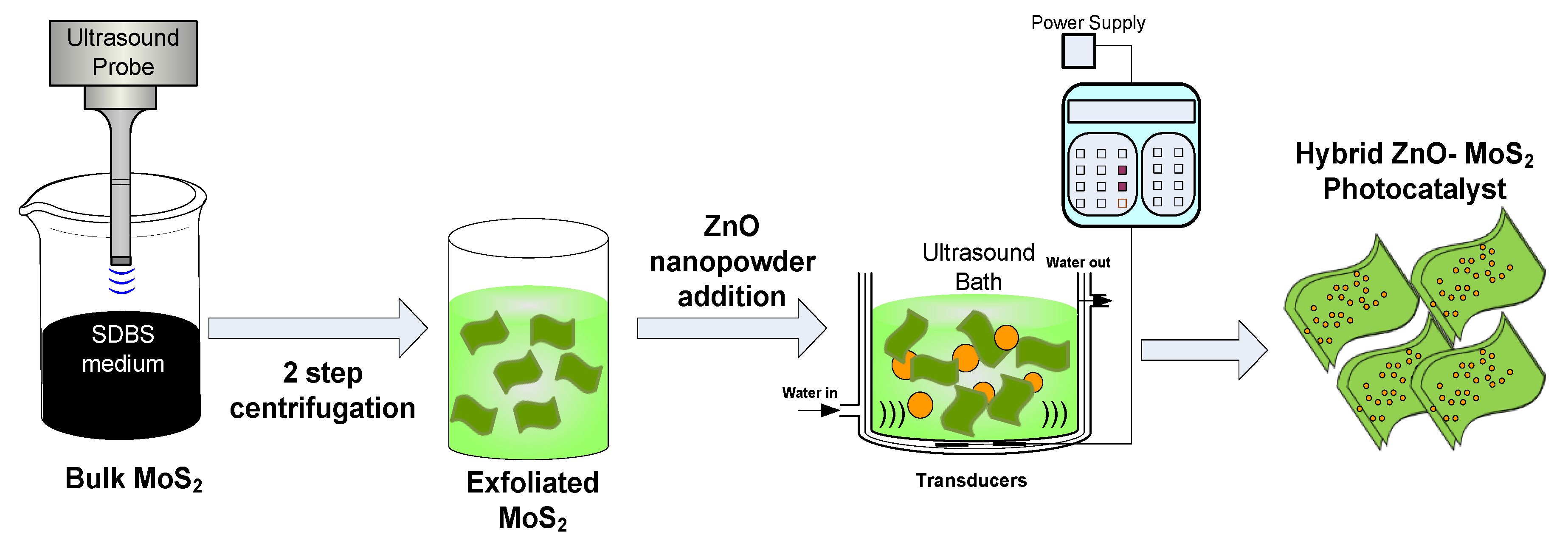
2.2. Surfactant Assisted Exfoliation of Bulk MoS2
2.3. Preparation of Hybrid ZnO-MoS2 Nanocomposite
2.4. Characterisation of E-MoS2 Dispersion and MoS2 Doped ZnO
2.5. Modification of PVDF Membrane with Hybrid ZnO-MoS2 Photocatalysts
2.6. Characterisation of ZnO-MoS2 PCM
2.6.1. Hydrophilicity Characteristics
2.6.2. Morphological Characteristics
2.6.3. Structural Characteristics
2.7. Studies on Photocatalytic Degradation of MB dye
2.7.1. Hybrid ZnO Doped MoS2 Photocatalysts for MB Dye Treatment
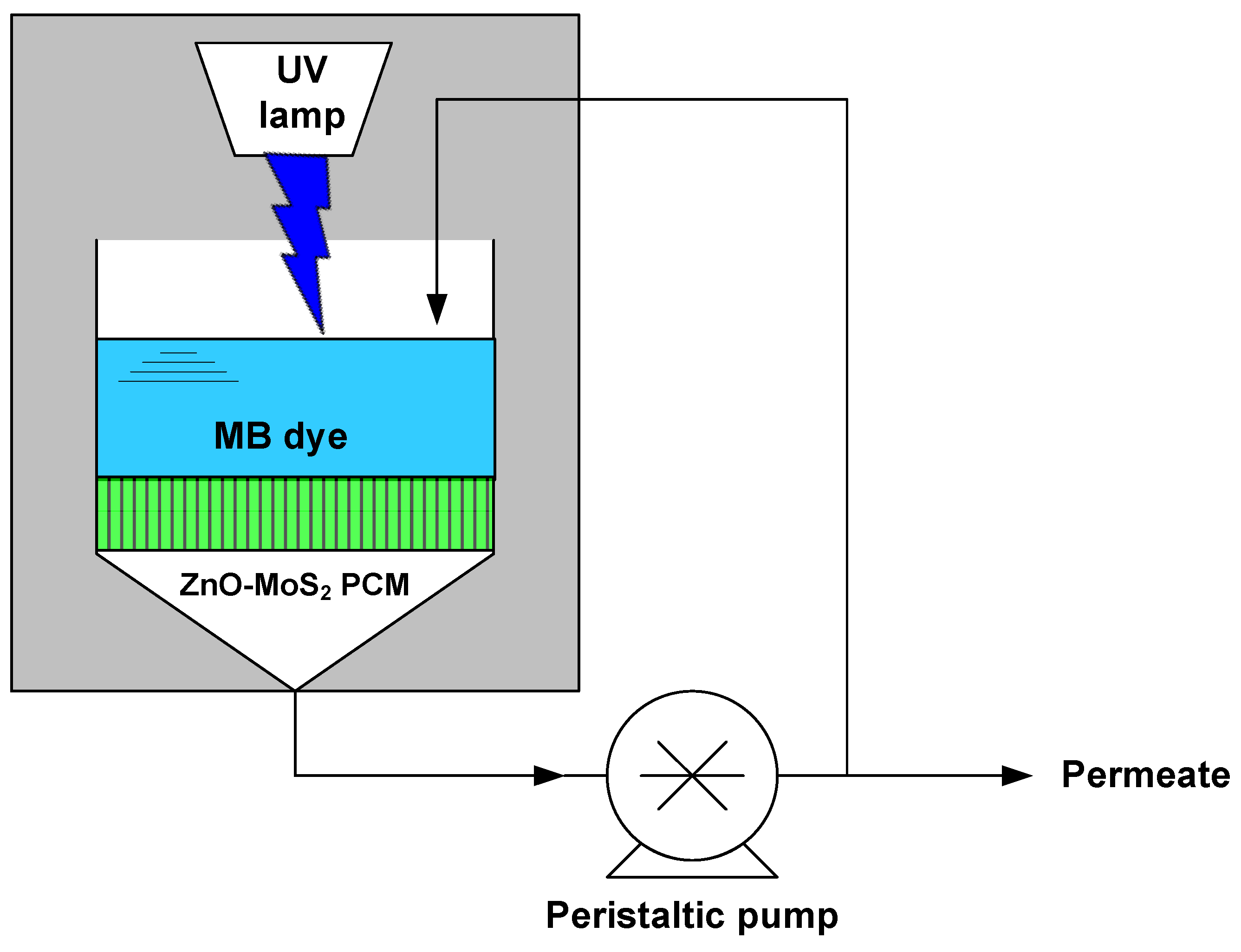
2.7.2. Photocatalysis Assisted Membrane Filtration Studies for MB Dye Treatment
3. Results and Discussion
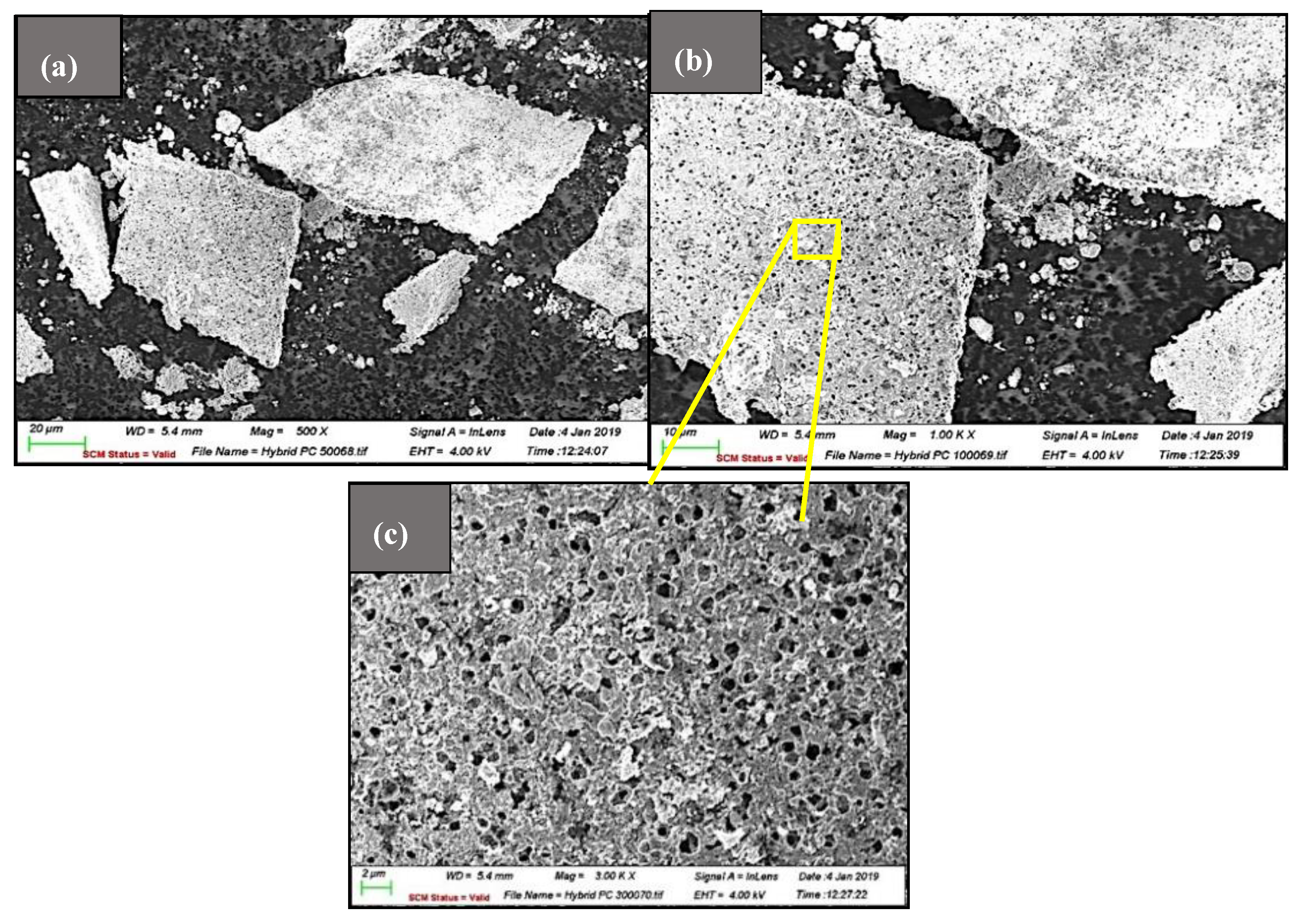
3.1. Morphology of ZnO-MoS2 Photocatalyst
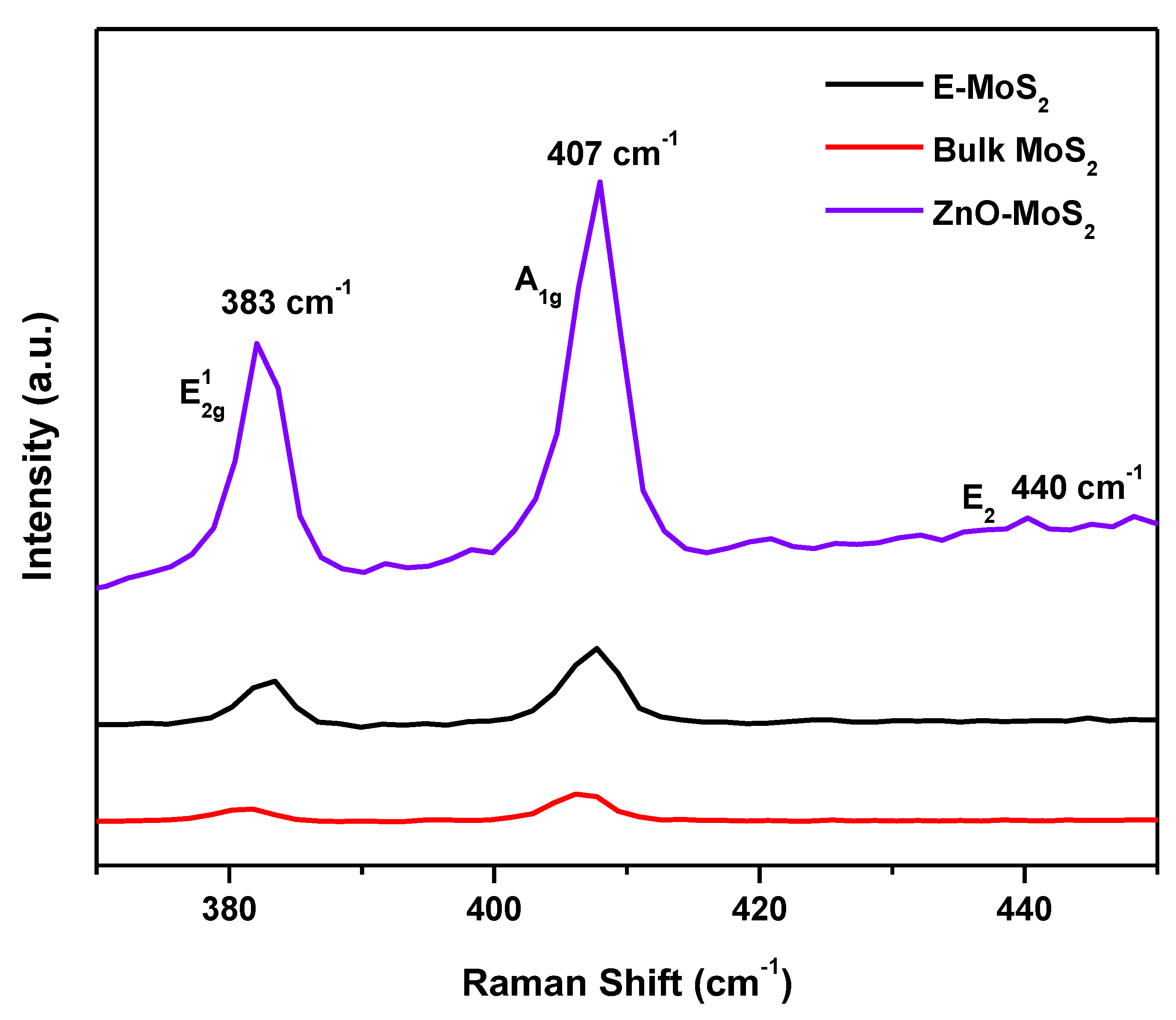
3.2. Raman Spectral Analysis of MoS2 and ZnO-MoS2 Photocatalyst
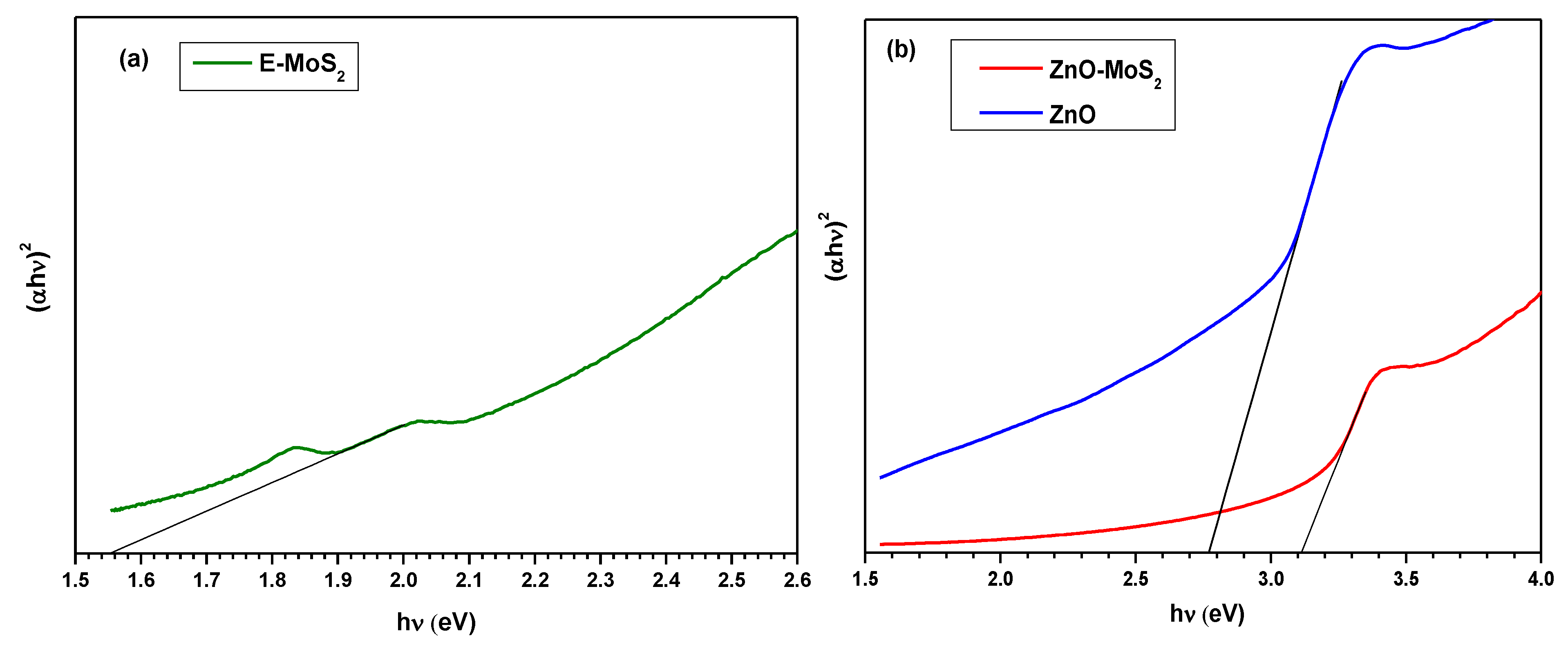
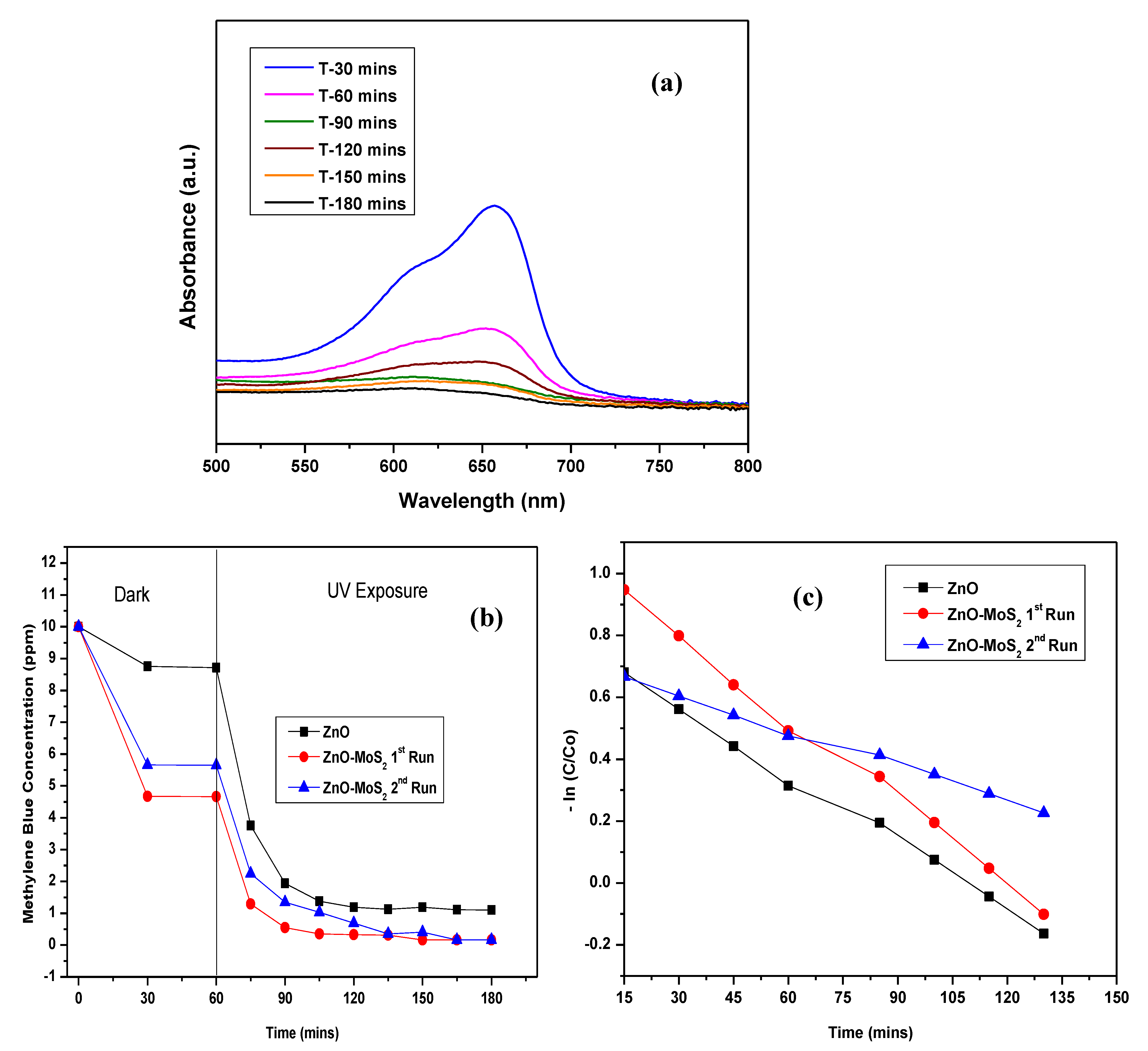
3.3. Band Gap Energy and Stability Analysis of ZnO-MoS2 Photocatalyst
3.4. Effect of ZnO-MoS2 Photocatalyst Deposition on PVDF Membrane Properties
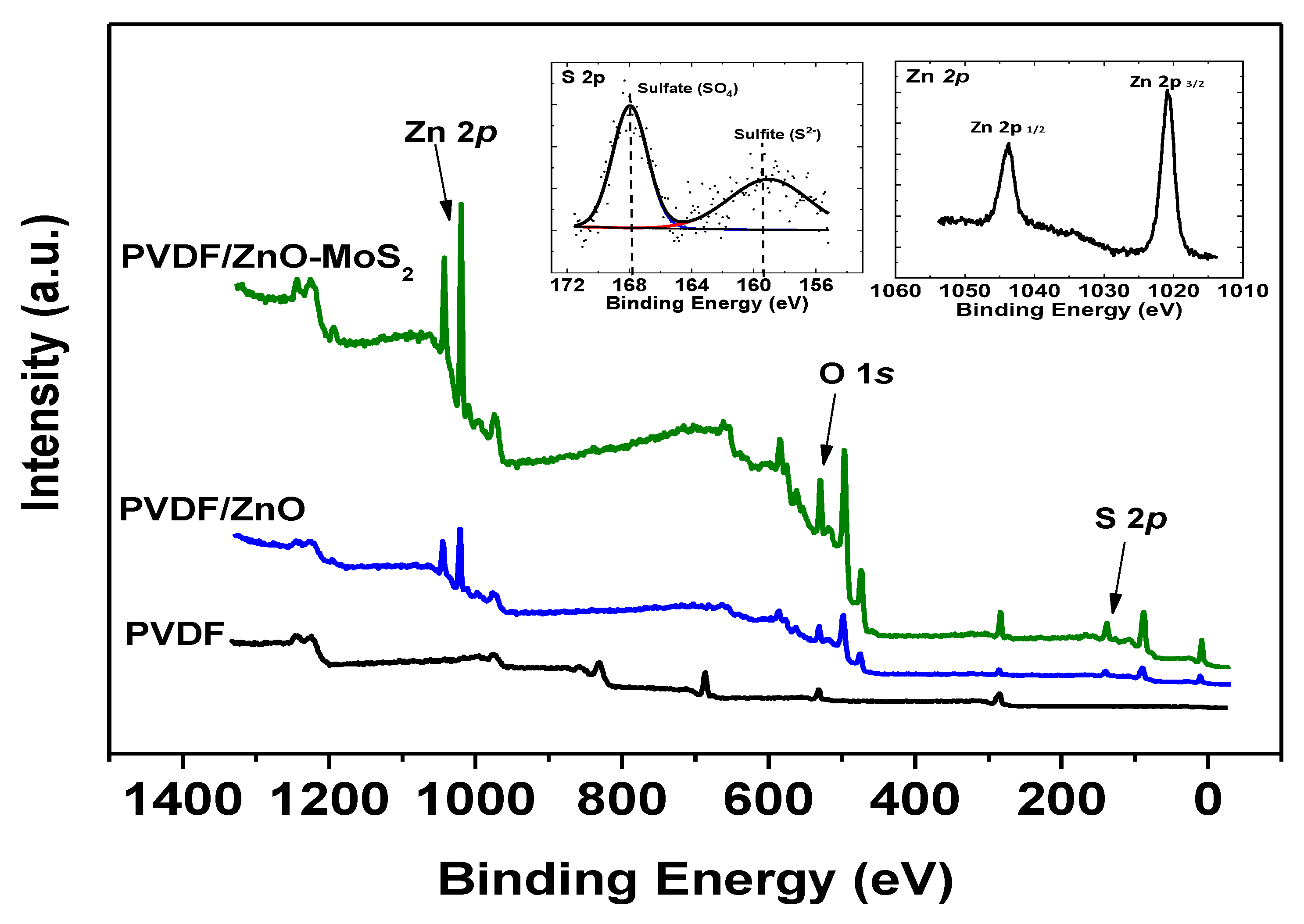
3.4.1. XPS Structural Analysis
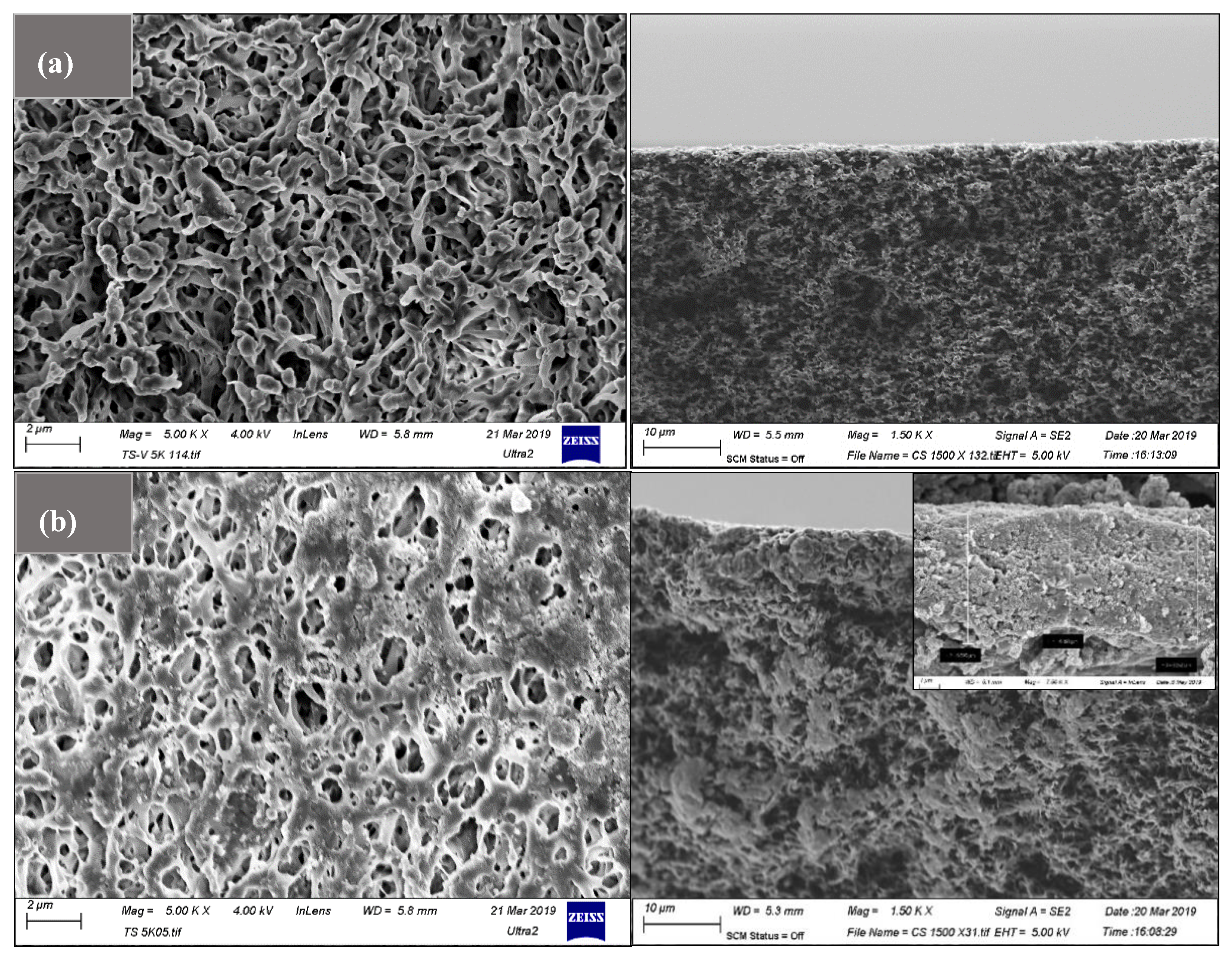
3.4.2. Influence of ZnO-MoS2 on Morphological Characteristics
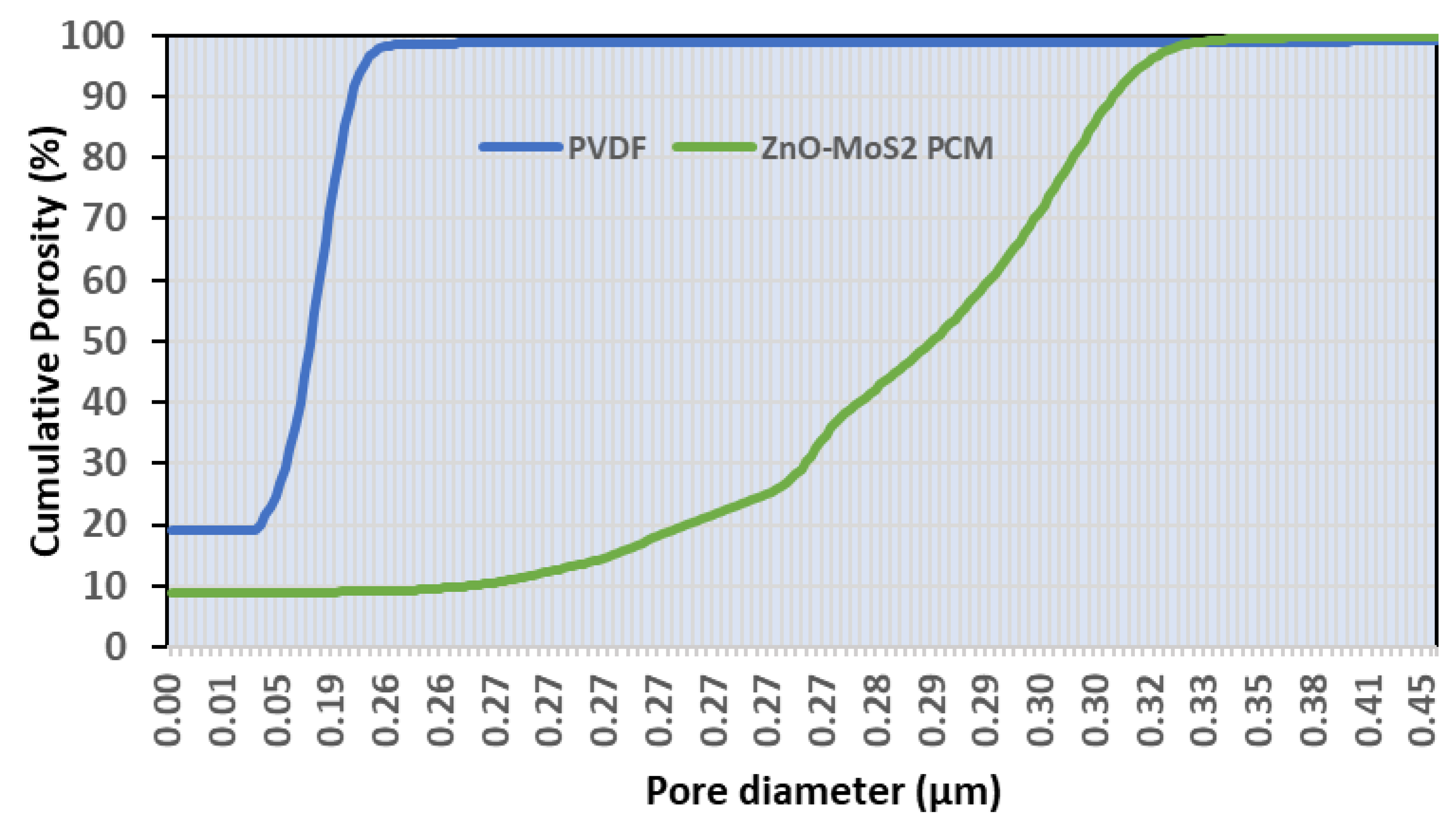
3.4.3. Influence of ZnO-MoS2 on Hydrophilicity and Porosity Characteristics
3.5. Performance Evaluation of ZnO-MoS2 on MB Dye Degradation
3.6. Performance Evaluation of ZnO-MoS2 Deposited Photocatalytic Membrane
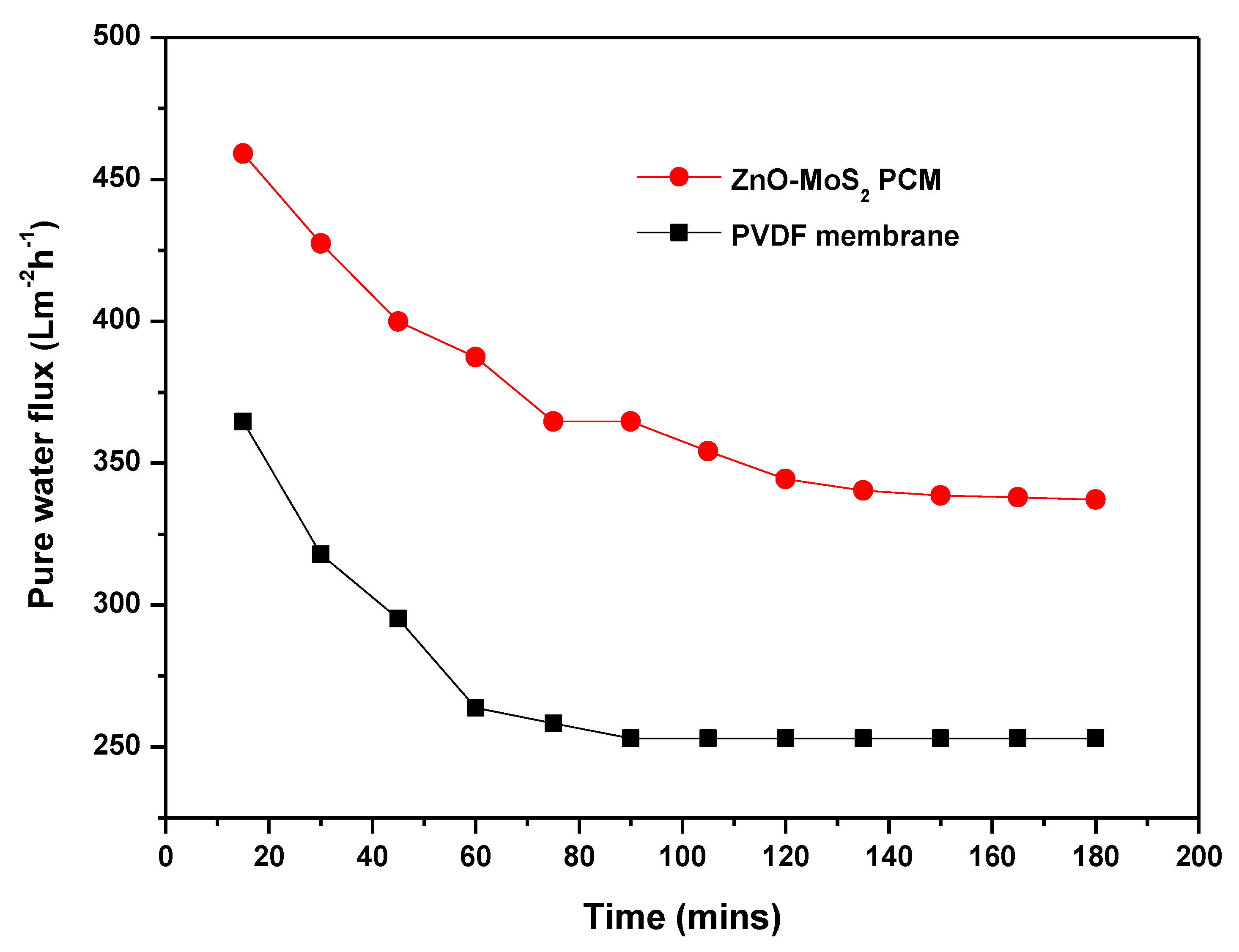
3.6.1. Flux Performance
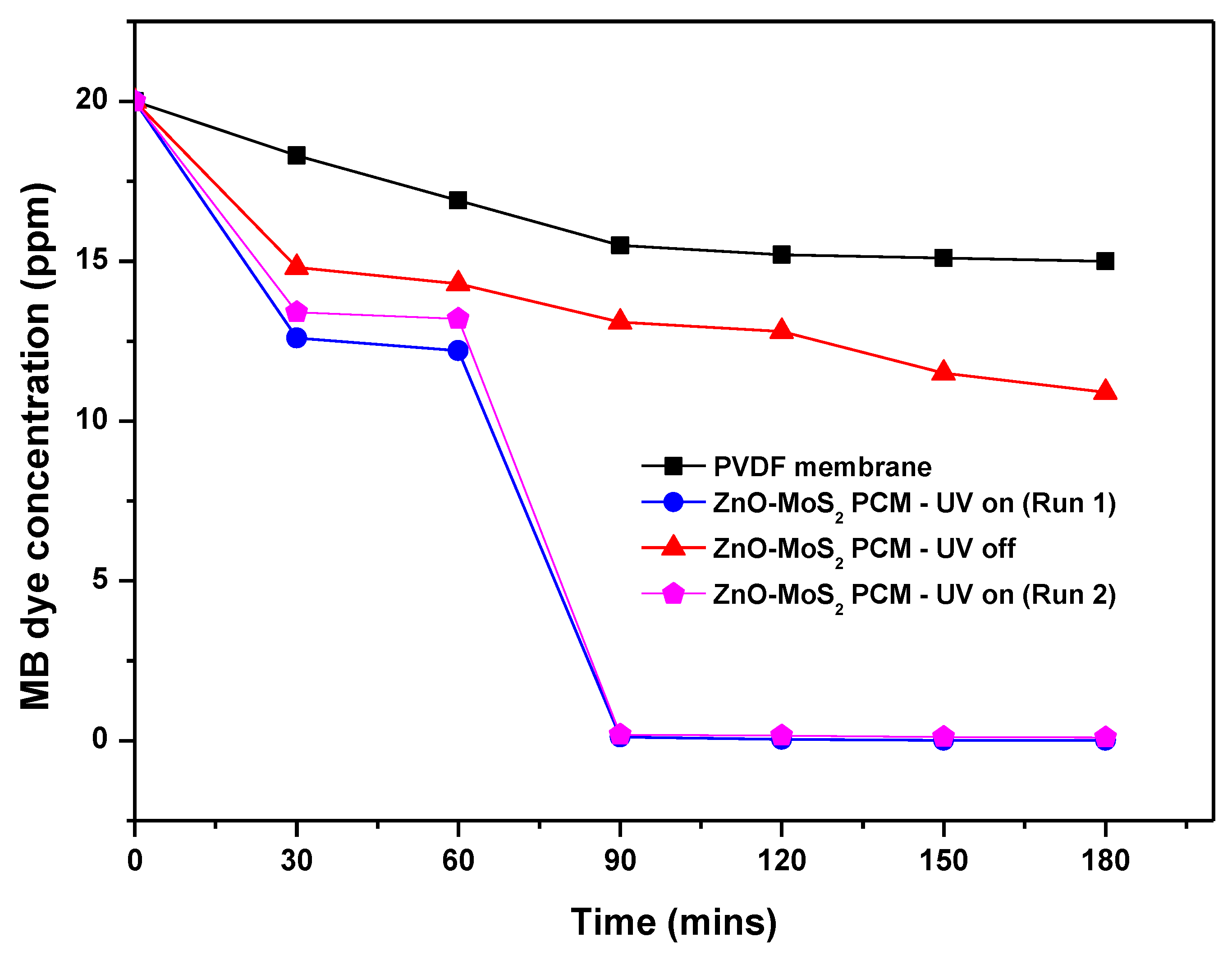
3.6.2. Synergistic Effect of ZnO-MoS2 PCM for Improved MB Dye Treatment
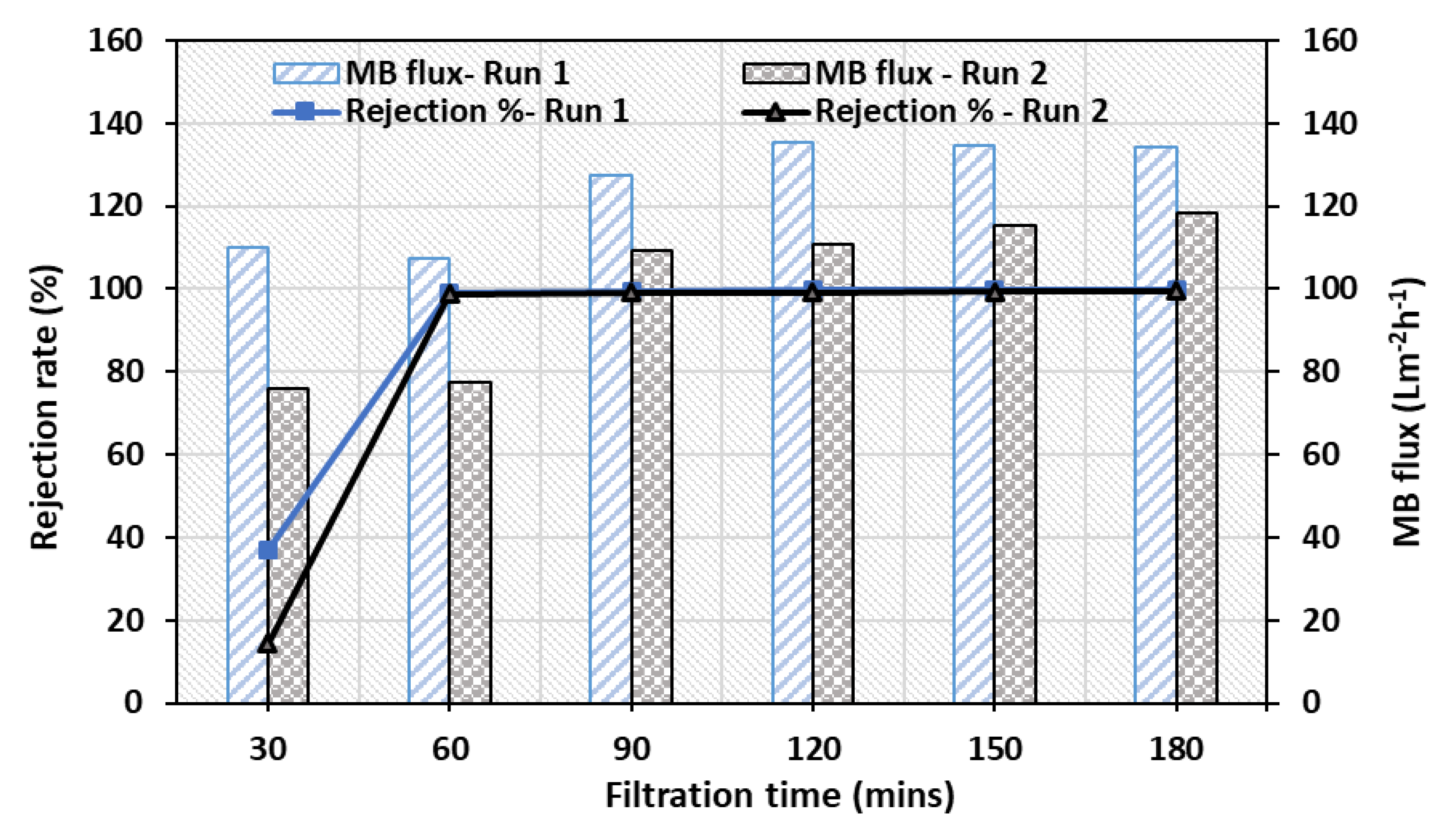
3.6.3. Reusability of ZnO-MoS2 PCM
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gao, Y.; Hu, M.; Mi, B. Membrane surface modification with TiO2–graphene oxide for enhanced photocatalytic performance. J. Membr. Sci. 2014, 455, 349–356. [Google Scholar] [CrossRef]
- Ang, W.L.; Mohammad, A.W.; Hilal, N.; Leo, C.P. A review on the applicability of integrated/hybrid membrane processes in water treatment and desalination plants. Desalination 2015, 363, 2–18. [Google Scholar] [CrossRef]
- Yang, E.; Chae, K.; Choi, M.; He, Z.; Kim, I.S. Critical review of bioelectrochemical systems integrated with membrane-based technologies for desalination, energy self-sufficiency, and high-efficiency water and wastewater treatment. Desalination 2019, 452, 40–67. [Google Scholar] [CrossRef]
- Plakas, K.V.; Sarasidis, V.C.; Patsios, S.I.; Lambropoulou, D.A.; Karabelas, A.J. Novel pilot scale continuous photocatalytic membrane reactor for removal of organic micropollutants from water. Chem. Eng. J. 2016, 304, 335–343. [Google Scholar] [CrossRef]
- Ramesh Babu, P.; Gaikar, V.G. Preparation, structure, and transport properties of ultrafiltration membranes of poly (vinyl chloride) (PVC), carboxylated poly (vinyl chloride) (CPVC), and PVC/CPVC blends. J. Appl. Poly. Sci. 1999, 73, 1117–1130. [Google Scholar] [CrossRef]
- Iglesias, O.; Rivero, M.J.; Urtiaga, A.M.; Ortiz, I. Membrane-based photocatalytic systems for process intensification. Chem. Eng. J. 2016, 305, 136–148. [Google Scholar] [CrossRef]
- Zheng, X.; Shen, Z.; Shi, L.; Cheng, R.; Yuan, D. Photocatalytic Membrane Reactors (PMRs) in Water Treatment: Configurations and Influencing Factors. Catalysts 2017, 7, 224. [Google Scholar] [CrossRef]
- Hassanpour, A.; Nahar, S.; Tong, X.; Zhang, G.; Gauthier, M.A.; Sun, S. Photocatalytic interlayer spacing adjustment of a graphene oxide/zinc oxide hybrid membrane for efficient water filtration. Desalination 2020, 475, 114174. [Google Scholar] [CrossRef]
- Luo, B.; Liu, G.; Wang, L. Recent advances in 2D materials for photocatalysis. Nanoscale 2016, 8, 6904–6920. [Google Scholar] [CrossRef]
- Saha, S.; Chaudhary, N.; Mittal, H.; Gupta, G.; Khanuja, M. Inorganic–organic nanohybrid of MoS2-PANI for advanced photocatalytic application. Int. Nano Lett. 2019, 9, 127–139. [Google Scholar] [CrossRef]
- Dervin, S.; Dionysiou, D.D.; Pillai, S.C. 2D nanostructures for water purification: Graphene and beyond. Nanoscale 2016, 8, 15115–15131. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Zhang, M.; Yin, H.; Liu, X. Transition Metal Dichalcogenides in Photocatalysts. In Two Dimensional Transition Metal Dichalcogenides; Arul, N., Nithya, V., Eds.; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Vattikuti, S.V.P.; Byon, C.; Reddy, C.V.; Ravikumar, R.V.S.S.N. Improved photocatalytic activity of MoS2 nanosheets decorated with SnO2 nanoparticles. RSC Adv. 2015, 5, 86675–86684. [Google Scholar] [CrossRef]
- Song, F.; Hu, X. Exfoliation of layered double hydroxides for enhanced oxygen evolution catalysis. Nat. Commun. 2014, 5, 4477. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Li, H.; Xiao, J.; Lu, Y.; Zhang, M.; Hu, K.; Yan, K. Integration of Theory and Experiment on Mesoporous Nickel Sulfide Microsphere for Hydrogen Evolution Reaction. ACS Sustain. Chem. Eng. 2018, 6, 15995–16000. [Google Scholar] [CrossRef]
- Zhang, M.; He, Y.; Yan, D.; Xu, H.; Wang, A.; Chen, Z.; Wang, S.; Luo, H.; Yan, K. Multifunctional 2H-TaS2 nanoflakes for efficient supercapacitors and electrocatalytic evolution of hydrogen and oxygen. Nanoscale 2019, 11, 22255–22260. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.; Wang, C.; Li, W.; Luo, S.; Yao, Y.; Yu, S.; Sun, R.; Wong, C.-P. A facile and clean process for exfoliating MoS2 nanosheets assisted by a surface active agent in aqueous solution. Nanotechnol 2018, 29, 425702. [Google Scholar] [CrossRef]
- Hirunpinyopas, W.; Prestat, E.; Worrall, S.D.; Haigh, S.J.; Dryfe, R.A.W.; Bissett, M.A. Desalination and Nanofiltration through Functionalized Laminar MoS2 Membranes. ACS Nano 2017, 11, 11082–11090. [Google Scholar] [CrossRef]
- Wang, Z.; Tu, Q.; Zheng, S.; Urban, J.J.; Li, S.; Mi, B. Understanding the Aqueous Stability and Filtration Capability of MoS2 Membranes. Nano Lett. 2017, 17, 7289–7298. [Google Scholar] [CrossRef]
- Mishra, A.K.; Lakshmi, K.V.; Huang, L. Eco-friendly synthesis of metal dichalcogenides nanosheets and their environmental remediation potential driven by visible light. Sci. Rep. 2015, 5, 15718. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, M.; Wang, J.; Liu, G.; Liu, H.; Jiang, Y. Bioinspired Modification of Layer-Stacked Molybdenum Disulfide (MoS2) Membranes for Enhanced Nanofiltration Performance. ACS Omega 2019, 4, 4012–4022. [Google Scholar] [CrossRef]
- Benavente, E.; Durán, F.; Sotomayor-Torres, C.; González, G. Heterostructured layered hybrid ZnO/MoS2 nanosheets with enhanced visible light photocatalytic activity. J. Phys. Chem. Solids 2018, 113, 119–124. [Google Scholar] [CrossRef]
- Tan, Y.; Yu, K.; Li, J.; Fu, H.; Zhu, Z. MoS2@ZnO nano-heterojunctions with enhanced photocatalysis and field emission properties. J. Appl. Phys. 2014, 116, 064305. [Google Scholar] [CrossRef]
- Tian, N.; Li, Z.; Xu, D.; Li, Y.; Peng, W.; Zhang, G.; Zhang, F.; Fan, X. Utilization of MoS2 Nanosheets To Enhance the Photocatalytic Activity of ZnO for the Aerobic Oxidation of Benzyl Halides under Visible Light. Ind. Eng. Chem. Res. 2016, 55, 8726–8732. [Google Scholar] [CrossRef]
- Guan, W.; Zhang, Z.; Tian, S.; Du, J. Ti4O7/g-C3N4 for Visible Light Photocatalytic Oxidation of Hypophosphite: Effect of Mass Ratio of Ti4O7/g-C3N4. Front. Chem. 2018, 6, 313. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, K.; Moradi, M.; Dehghan, R.; Yazdani, A. Enhancement of sunlight-induced photocatalytic activity of ZnO nanorods by few-layer MoS2 nanosheets. Mater. Lett. 2019, 234, 134–137. [Google Scholar] [CrossRef]
- Dong, H.; Li, J.; Chen, M.; Wang, H.; Jiang, X.; Xiao, Y.G.; Tian, B.; Zhang, X. High-throughput Production of ZnO-MoS2-Graphene Heterostructures for Highly Efficient Photocatalytic Hydrogen Evolution. Materials 2019, 12, 2233. [Google Scholar] [CrossRef]
- Faglia, G.; Ferroni, M.; Dang, T.T.T.; Donarelli, M.; Rigoni, F.; Baratto, C. Vertically Coupling ZnO Nanorods onto MoS2 Flakes for Optical Gas Sensing. Chemosensors 2020, 8, 19. [Google Scholar] [CrossRef]
- Hinnemann, B.; Moses, P.G.; Bonde, J.; Jørgensen, K.P.; Nielsen, J.H.; Horch, S.; Chorkendorff, I.; Nørskov, J.K. Biomimetic Hydrogen Evolution: MoS2 Nanoparticles as Catalyst for Hydrogen Evolution. J. Am. Chem. Soc. 2005, 127, 5308–5309. [Google Scholar] [CrossRef]
- Gupta, A.; Arunachalam, V.; Vasudevan, S. Water Dispersible, Positively and Negatively Charged MoS2 Nanosheets: Surface Chemistry and the Role of Surfactant Binding. J. Phys. Chem. Lett. 2015, 6, 739–744. [Google Scholar] [CrossRef]
- Zhao, Y.; Xie, Y.; Liu, Z.; Wang, X.; Chai, Y.; Yan, F. Two-Dimensional Material Membranes: An Emerging Platform for Controllable Mass Transport Applications. Small 2014, 10, 4521–4542. [Google Scholar] [CrossRef]
- Xu, G.; Xu, J.; Su, H.; Liu, X.; Li, L.; Zhao, H.L.; Feng, H.; Das, R. Two-dimensional (2D) nanoporous membranes with sub-nanopores in reverse osmosis desalination: Latest developments and future directions. Desalination 2019, 451, 18–34. [Google Scholar] [CrossRef]
- Yan, K.; Lu, Y. Direct Growth of MoS2 Microspheres on Ni Foam as a Hybrid Nanocomposite Efficient for Oxygen Evolution Reaction. Small 2016, 12, 2975–2981. [Google Scholar] [CrossRef] [PubMed]
- Berean, K.J.; Ou, J.Z.; Daeneke, T.; Carey, B.J.; Nguyen, E.P.; Wang, Y.; Russo, S.P.; Kaner, R.B.; Kalantar-Zadeh, K. 2D MoS2 PDMS Nanocomposites for NO2 Separation. Small 2015, 11, 5035–5040. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xie, Y.; Ling, Y.; Jiao, J.; Li, X.; Zhao, J. Facile construction of a molybdenum disulphide/zinc oxide nanosheet hybrid for an advanced photocatalyst. J. Alloys Compd. 2019, 778, 761–767. [Google Scholar] [CrossRef]
- Ye, M.; Winslow, D.; Zhang, D.; Pandey, R.; Yoke, K.Y. Recent Advancement on the Optical Properties of Two-Dimensional Molybdenum Disulfide (MoS2) Thin Films. Photonics 2015, 2, 288–307. [Google Scholar] [CrossRef]
- Sheng, Y.; Jiang, S.; Yang, C.; Liu, M.; Liu, A.; Zhang, C.; Li, Z.; Huo, Y.; Wang, M.; Man, B. Three-dimensional nanoporous MoS2 framework decorated with Au nanoparticles for surface-enhanced Raman scattering. Chem. Phys. Lett. 2017, 682, 64–70. [Google Scholar] [CrossRef]
- De-Mello, G.B.; Smith, L.; Rowley-Neale, S.J.; Gruber, J.; Hutton, S.J.; Banks, C.E. Surfactant-exfoliated 2D molybdenum disulphide (2D-MoS2): The role of surfactant upon the hydrogen evolution reaction. RSC Adv. 2017, 7, 36208. [Google Scholar] [CrossRef]
- Lee, C.; Yan, H.; Brus, L.E.; Heinz, T.F.; Hone, J.; Ryu, S. Anomalous Lattice Vibrations of Single- and Few-Layer MoS2. ACS Nano 2010, 4, 2695–2700. [Google Scholar] [CrossRef]
- Dinesh, G.K.; Saranya, R. Facile approach for synthesis of stable, efficient, and recyclable ZnO through pulsed sonication and its application for degradation of recalcitrant azo dyes in wastewater. Can. J. Chem. 2018, 96, 897–905. [Google Scholar] [CrossRef]
- Mauro, A.D.; Cantarella, M.; Nicotra, G.; Pellegrino, G.; Gulino, A.; Brundo, M.V.; Privitera, V.; Impellizzeri, G. Novel synthesis of ZnO/PMMA nanocomposites for photocatalytic applications. Sci. Rep. 2017, 7, 40895. [Google Scholar] [CrossRef]
- Viezbicke, B.D.; Patel, S.; Davis, B.E.; Birnie, D.P. Evaluation of the Tauc method for optical absorption edge determination: ZnO thin films as a model system. Phys. Status Solidi 2015, 252, 1700–1710. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, A.; Kumar, A.; Krishnan, V. Nanoscale zinc oxide based heterojunctions as visible light active photocatalysts for hydrogen energy and environmental remediation. Catal. Rev. 2019, 3, 1–60. [Google Scholar]
- Sabarinathan, M.; Harish, S.; Archana, J.; Navaneethan, M.; Ikeda, H.; Hayakawa, Y. Highly efficient visible-light photocatalytic activity of MoS2–TiO2 mixtures hybrid photocatalyst and functional properties. RSC Adv. 2017, 7, 24754. [Google Scholar] [CrossRef]
- Samadi, M.; Asghari, H.; Shivaee, M.; Zanetti, M.; Pourjavadi, A.; Moshfegh, A. Visible light photocatalytic activity of novel MWCNT-doped ZnO electrospun nanofibers. J. Mol. Catal. A. 2012, 359, 42–48. [Google Scholar] [CrossRef]
- Xu, Z.; Wu, T.; Shi, J.; Teng, K.; Wang, W.; Ma, M.; Li, J.; Qian, X.; Li, C.; Fan, J. Photocatalytic anti-fouling PVDF ultrafiltration membranes based on synergy of graphene oxide and TiO2 for water treatment. J. Membr. Sci. 2016, 20, 281–293. [Google Scholar] [CrossRef]
- Chen, W.; Ting, Y.; Xu, H.; Chen, T.; Geng, N.; Gao, X. An ultrafiltration membrane with enhanced photocatalytic performance from grafted N–TiO2/graphene oxide. RSC Adv. 2017, 7, 9880–9887. [Google Scholar] [CrossRef]
- Saranya, R.; Arthanareeswaran, G.; Dionysiou, D.D. Treatment of paper mill effluent using Polyethersulfone/functionalised multiwalled carbon nanotubes based nanocomposite membranes. Chem. Eng. J. 2014, 236, 369–377. [Google Scholar] [CrossRef]
- Filpo, G.D.; Pantuso, E.; Armentano, K.; Formoso, P.; Profio, G.D.; Poerio, T.; Fontananova, E.; Meringolo, C.; Mashin, A.I.; Nicoletta, F.P. Chemical vapor deposition of photocatalyst nanoparticles on PVDF membranes for advanced oxidation processes. Membranes 2018, 8, 35. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rameshkumar, S.; Henderson, R.; Padamati, R.B. Improved Surface Functional and Photocatalytic Properties of Hybrid ZnO-MoS2-Deposited Membrane for Photocatalysis-Assisted Dye Filtration. Membranes 2020, 10, 106. https://doi.org/10.3390/membranes10050106
Rameshkumar S, Henderson R, Padamati RB. Improved Surface Functional and Photocatalytic Properties of Hybrid ZnO-MoS2-Deposited Membrane for Photocatalysis-Assisted Dye Filtration. Membranes. 2020; 10(5):106. https://doi.org/10.3390/membranes10050106
Chicago/Turabian StyleRameshkumar, Saranya, Rory Henderson, and Ramesh Babu Padamati. 2020. "Improved Surface Functional and Photocatalytic Properties of Hybrid ZnO-MoS2-Deposited Membrane for Photocatalysis-Assisted Dye Filtration" Membranes 10, no. 5: 106. https://doi.org/10.3390/membranes10050106
APA StyleRameshkumar, S., Henderson, R., & Padamati, R. B. (2020). Improved Surface Functional and Photocatalytic Properties of Hybrid ZnO-MoS2-Deposited Membrane for Photocatalysis-Assisted Dye Filtration. Membranes, 10(5), 106. https://doi.org/10.3390/membranes10050106