An Experimental Study of Membrane Contactor Modules for Recovering Cyanide through a Gas Membrane Process
Abstract
1. Introduction
2. Materials and Methods
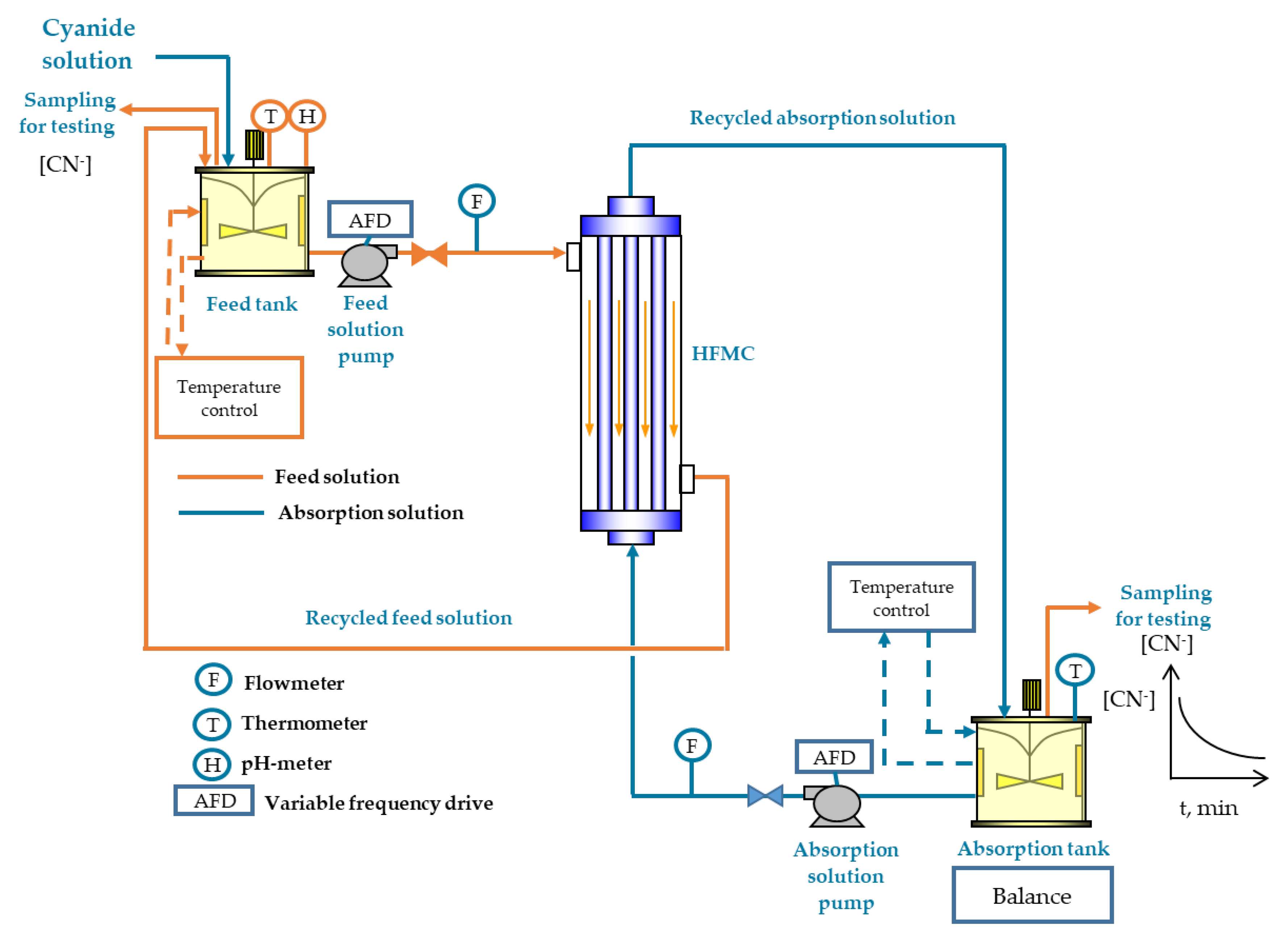
2.1. Experimental Set-Up
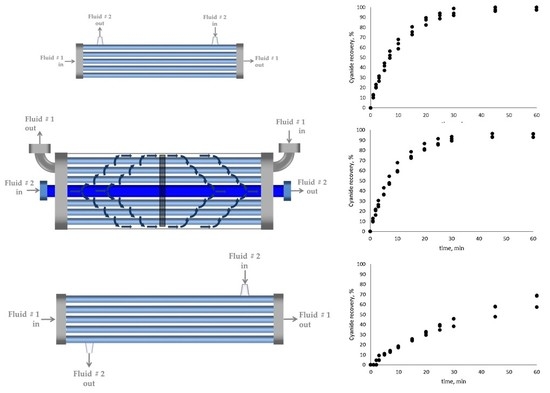
2.2. Hollow-Fiber Membrane Contactor (HFMC) Modules
2.3. Determination of Mass-Transfer Correlations
3. Results and Discussion
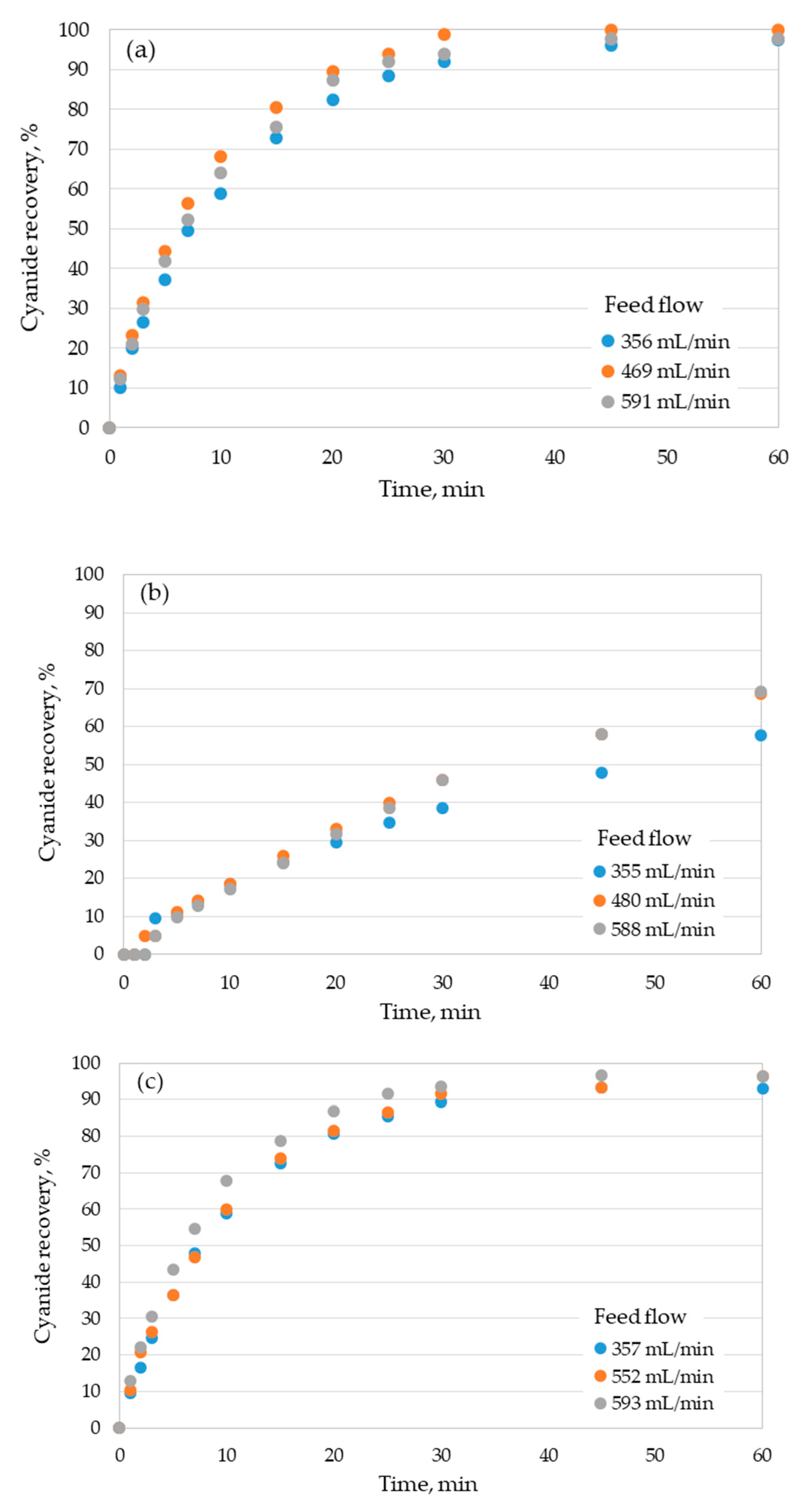
3.1. Cyanide Recovery
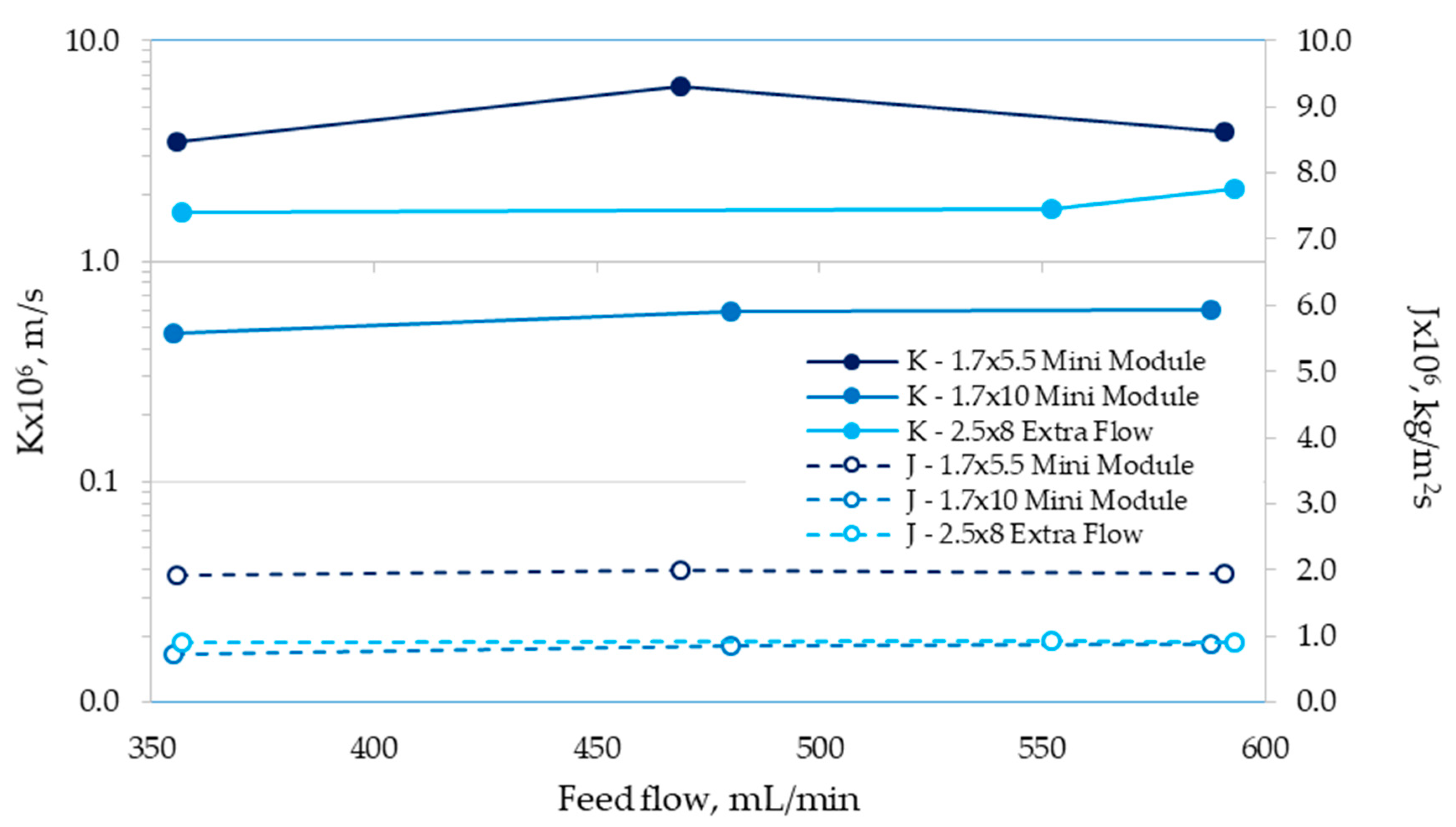
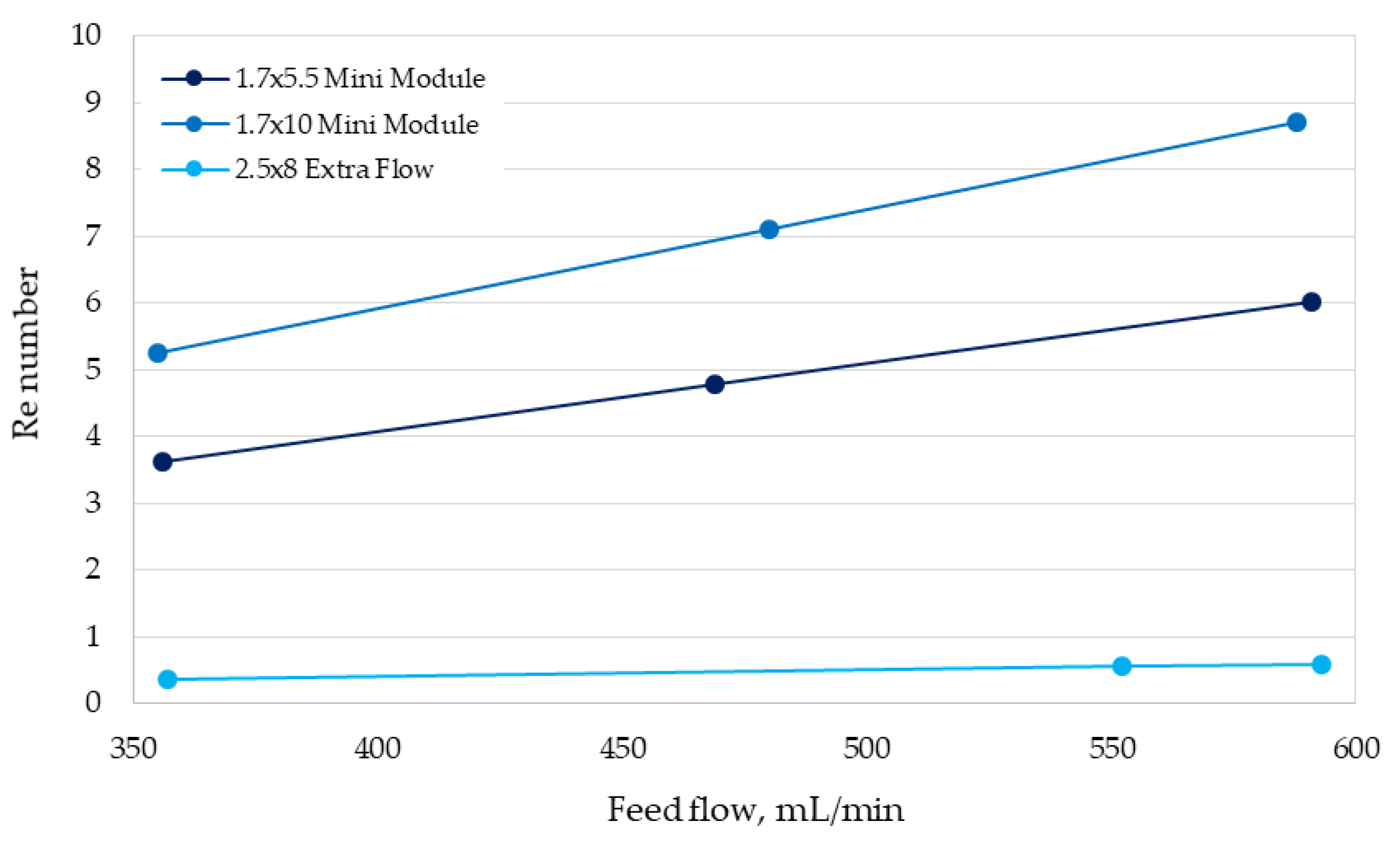
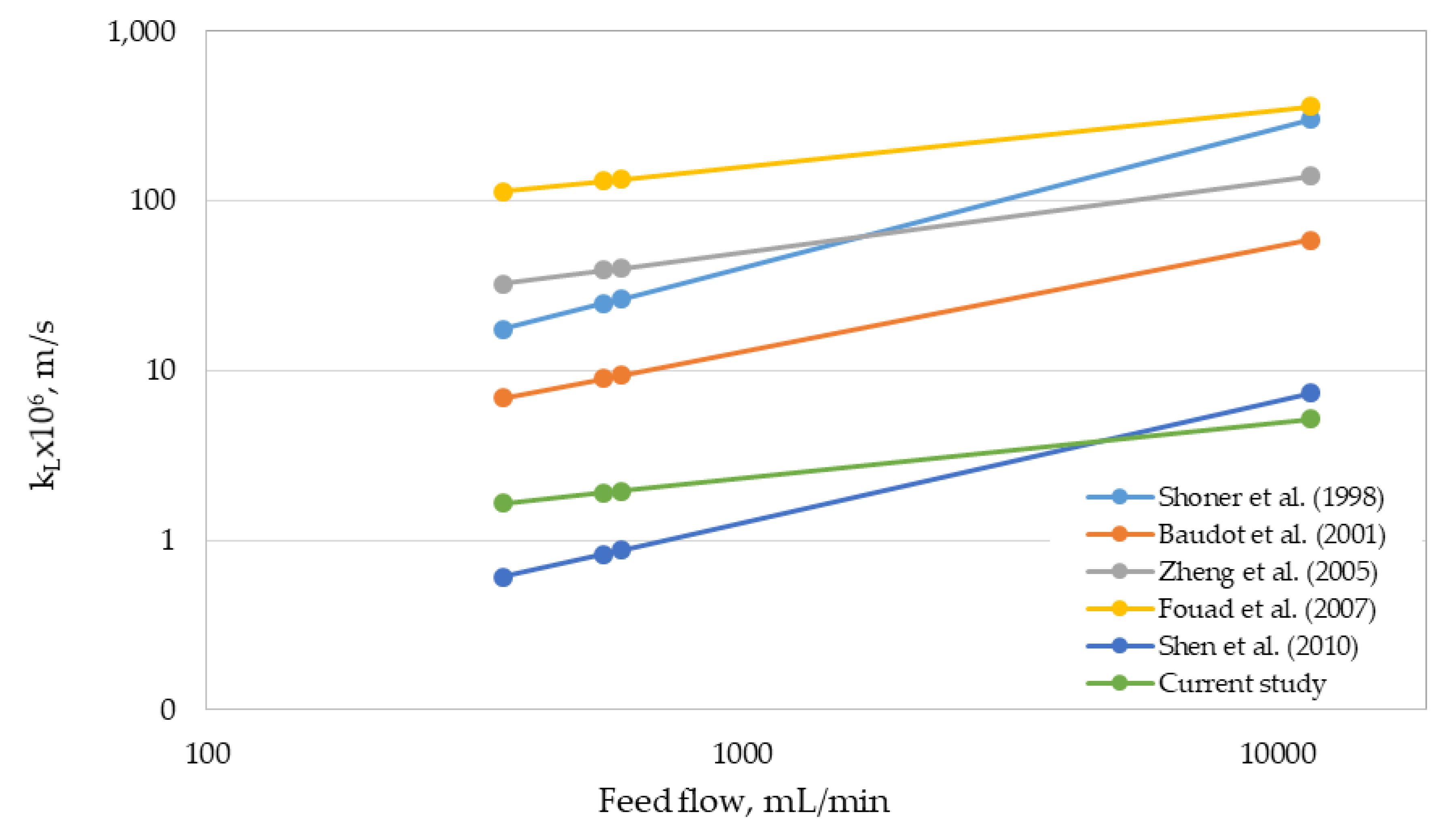
3.2. Determination of Mass-Transfer Coefficients
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Aligwe, P.A.; Sirkar, K.K.; Canlas, C.J. Hollow fiber gas membrane-based removal and recovery of ammonia from water in three different scales and types of modules. Sep. Pur. Tech. 2019, 224, 580–590. [Google Scholar] [CrossRef]
- Chuah, C.Y.; Kim, K.; Lee, J.; Koh, D.Y.; Bae, T.H. CO2 absorption using membrane contactors: Recent progress and future perspective. Ind. Eng. Chem. Res. 2020, in press. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, M.K.; Park, J.H.; Magnone, E. Temperature and pressure dependence of the CO2 absorption through a ceramic fiber membrane contactor module. Chem. Eng. Process. 2020, 150, 107871. [Google Scholar] [CrossRef]
- Dardor, D.; Minier-Matar, J.; Janson, A.; AlShamari, E.; Adham, S. The effect of Hydrogen sulfide oxidation with ultraviolet light and aeration on sour water treatment via membrane contactors. Sep. Pur. Tech. 2020, 236, 116262. [Google Scholar] [CrossRef]
- Scholes, C.A. Hydrogen cyanide recovery by membrane gas separation. Chem. Eng. J. 2020, 386, 124049. [Google Scholar] [CrossRef]
- Hidalgo, D.; Sanz-Bedate, S.; Martín-Marroquín, J.M.; Castro, J.; Antolín, G. Selective separation of CH4 and CO2 using membrane contactors. Renew. Energ. 2020, 150, 935–942. [Google Scholar] [CrossRef]
- Gabelman, A.; Hwang, S. Hollow fiber membrane contactors. J. Memb. Sci. 1999, 159, 61–106. [Google Scholar] [CrossRef]
- Estay, H.; Ortiz, M.; Romero, J. A novel process based on gas filled membrane absorption to recover cyanide in gold mining. Hydrometallurgy 2013, 134–135, 166–176. [Google Scholar] [CrossRef]
- Dai, X.; Breuer, P. Comparison of AVR and gas membrane technology in cyanide recovery. In Proceedings of the ALTA 2013 Gold Conference, Perth, Australia, 25 May–1 June 2013. [Google Scholar]
- Estay, H.; Troncoso, E.; Romero, J. Design and cost estimation of a gas-filled membrane absorption (GFMA) process as alternative for cyanide recovery in gold mining. J. Memb. Sci. 2014, 466, 253–264. [Google Scholar] [CrossRef]
- Estay, H.; Troncoso, E.; Ruby-Figueroa, R.; Romero, J. Performance evaluation of mass transfer correlations in the GFMA process: A review with perspectives to the design. J. Memb. Sci. 2018, 554, 140–155. [Google Scholar] [CrossRef]
- Estay, H.; Troncoso, E.; Ruby-Figueroa, R.; Romero, J. Assessment of Industrial Modules to Design a GFMA Process for Cyanide Recovery Based on a Phenomenological Model. Processes 2018, 6, 34. [Google Scholar] [CrossRef]
- Gonen, N.; Kabasakal, O.S.; Ozdil, G. Recovery of cyanide in gold leaching waste solution by volatilization and absorption. J. Hazard. Mater. 2004, B113, 231–236. [Google Scholar] [CrossRef] [PubMed]
- LiquiCel, LiquiCel Catalogue. Available online: https://www.3m.com/3M/en_US/company-us/all-3m-products/~/All-3M-Products/Filtration/Gas-Transfer-Membrane-Contactors/?N=5002385+8710655+8711017+8758377+3294857497&rt=r3 (accessed on 1 April 2020).
- Kenfield, C.F.; Qin, R.; Semmens, M.J.; Cussler, E.L. Cyanide recovery across hollow fiber gas membranes. Environ. Sci. Technol. 1988, 22, 1151–1155. [Google Scholar] [CrossRef] [PubMed]
- Pozzobon, V.; Perré, P. Mass transfer in hollow fiber membrane contactor: Computational fluid dynamics determination of the shell side resistance. Sep. Pur. Tech. 2020, 241, 116674. [Google Scholar] [CrossRef]
- Basu, R.; Prasad, R.; Sirkar, K.K. Nondispersive membrane solvent back extraction of phenol. AICHE J. 1990, 36, 450–460. [Google Scholar] [CrossRef]
- Yun, C.H.; Prasad, R.; Guha, A.K.; Sirkar, K.K. Hollow fiber solvent extraction removal of toxic heavy metals from aqueous waste streams. Ind. Eng. Chem. Res. 1993, 32, 1186–1195. [Google Scholar] [CrossRef]
- Schoner, P.; Plucinski, P.; Nitsch, W.; Daiminger, U. Mass transfer in the shell side of cross flow hollow fiber modules. Chem. Eng. Sci. 1998, 53, 2319–2326. [Google Scholar] [CrossRef]
- Baudot, A.; Floury, J.; Smorenburg, H.E. Liquid-liquid extraction of aroma compounds with hollow fiber contactor. AIChE J. 2001, 47, 1780–1793. [Google Scholar] [CrossRef]
- Zheng, J.M.; Dai, Z.W.; Wong, F.S.; Xu, Z.K. Shell side mass transfer in a transverse flow hollow fiber membrane contactor. J. Memb. Sci. 2005, 261, 114–120. [Google Scholar] [CrossRef]
- Fouad, E.A.; Bart, H.J. Separation of zinc by a non-dispersion solvent extraction process in a hollow fiber contactor. Solvent Extr. Ion. Exch. 2007, 25, 857–877. [Google Scholar] [CrossRef]
- Shen, S.; Kentish, S.E.; Stevens, G.W. Solvent extraction and ion exchange shell-side mass-transfer performance in hollow-fiber membrane contactors. Solvent Extr. Ion. Exch. 2010, 28, 817–844. [Google Scholar] [CrossRef]
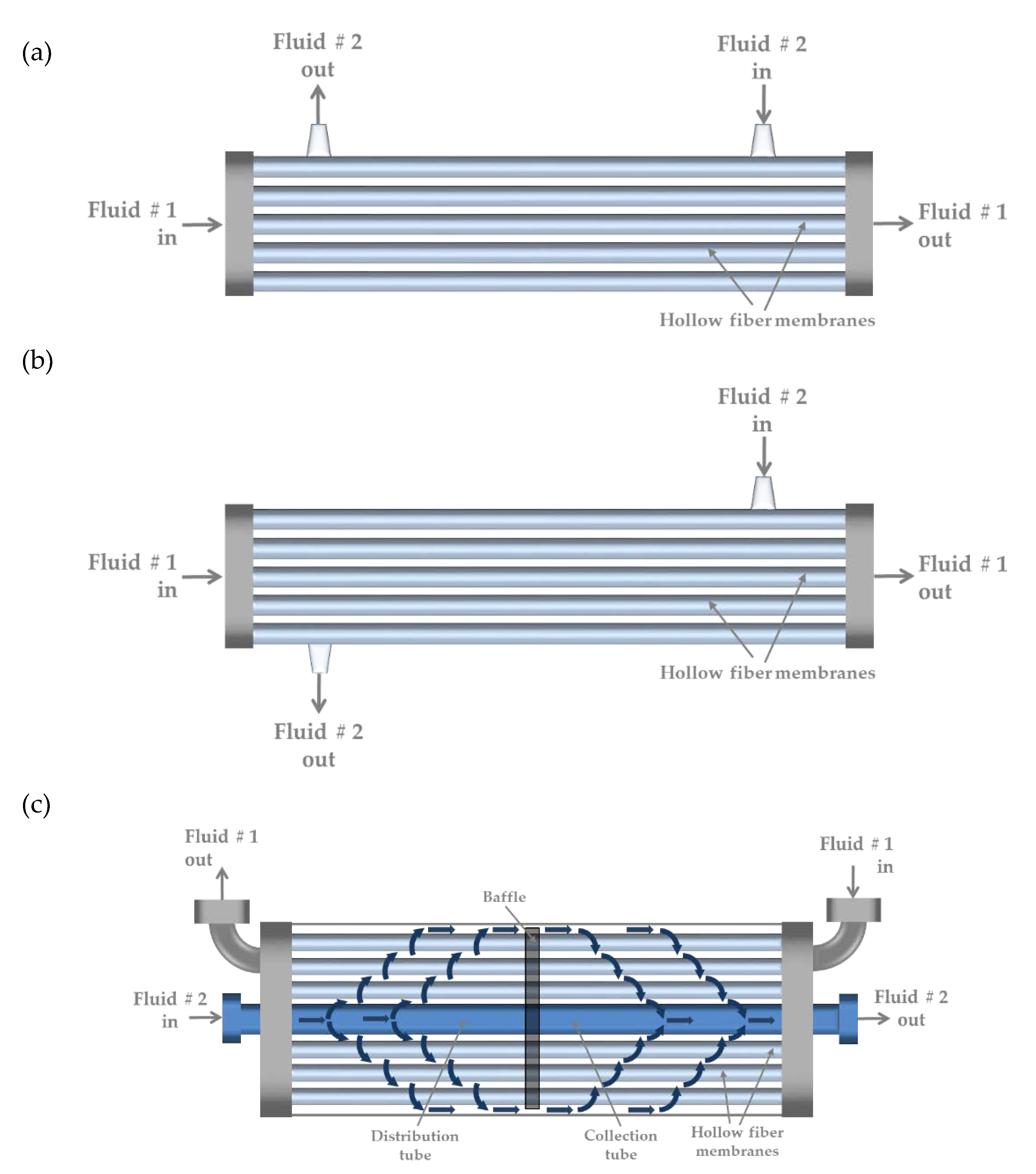
| Description | Unit | 1.7 × 5.5 Mini Module | 1.7 × 10 Mini Module | 2.5 × 8 Extra Flow |
|---|---|---|---|---|
| Feed flow capacity | m³/h | <0.15 | <0.12 | 0.1–0.7 |
| Membrane material | - | Polypropylene | Polypropylene | Polypropylene |
| Fiber outer diameter | µm | 300 | 300 | 300 |
| Fiber inner diameter | µm | 220 | 220 | 220 |
| Fiber length | m | 0.12 | 0.25 | 0.15 |
| Shell inner diameter | m | 0.043 | 0.045 | 0.056 |
| Membrane porosity | - | 0.5 | 0.4 | 0.5 |
| Mass-transfer area | m² | 0.51 | 0.81 | 1.42 |
| Centre tube diameter | m | - | - | 0.0222 |
| Module | Equivalent Diameter | Velocity | |
|---|---|---|---|
| 1.7 × 5.5 Mini Module | |||
| 1.7 × 10 Mini Module | |||
| 2.5 × 8 Extra Flow | |||
| Nomenclature: | |||
| ds | : inner diameter of the shell (m) | ||
| dout | : outer diameter of the fibers (m) | ||
| dct | : outer diameter of the center tube in the Extra-Flow module (m) | ||
| n | : number of fibers contained in the HFMC module | ||
| Qs | : cyanide solution flow fed into the shell side (m3/s) | ||
| As | : cross-sectional area of the flow in the shell side (m2) | ||
| L | : length of the fibers (m) | ||
| Module | Mass-Transfer Correlation | Validity Range Based on the Re Number |
|---|---|---|
| 1.7 × 5.5 Mini Module | 3.5–6.0 | |
| 1.7 × 10 Mini Module | 5.0–9.0 | |
| 2.5 × 8 Extra Flow | 0.3–0.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quilaqueo, M.; Seriche, G.; Valetto, S.; Barros, L.; Díaz-Quezada, S.; Ruby-Figueroa, R.; Troncoso, E.; Estay, H. An Experimental Study of Membrane Contactor Modules for Recovering Cyanide through a Gas Membrane Process. Membranes 2020, 10, 105. https://doi.org/10.3390/membranes10050105
Quilaqueo M, Seriche G, Valetto S, Barros L, Díaz-Quezada S, Ruby-Figueroa R, Troncoso E, Estay H. An Experimental Study of Membrane Contactor Modules for Recovering Cyanide through a Gas Membrane Process. Membranes. 2020; 10(5):105. https://doi.org/10.3390/membranes10050105
Chicago/Turabian StyleQuilaqueo, Michelle, Gabriel Seriche, Sicely Valetto, Lorena Barros, Simón Díaz-Quezada, René Ruby-Figueroa, Elizabeth Troncoso, and Humberto Estay. 2020. "An Experimental Study of Membrane Contactor Modules for Recovering Cyanide through a Gas Membrane Process" Membranes 10, no. 5: 105. https://doi.org/10.3390/membranes10050105
APA StyleQuilaqueo, M., Seriche, G., Valetto, S., Barros, L., Díaz-Quezada, S., Ruby-Figueroa, R., Troncoso, E., & Estay, H. (2020). An Experimental Study of Membrane Contactor Modules for Recovering Cyanide through a Gas Membrane Process. Membranes, 10(5), 105. https://doi.org/10.3390/membranes10050105