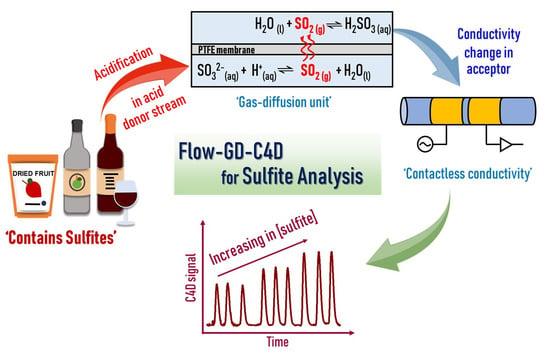
Simple Flow-Based System with an In-Line Membrane Gas–Liquid Separation Unit and a Contactless Conductivity Detector for the Direct Determination of Sulfite in Clear and Turbid Food Samples
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Preparation of Sulfite Standard Solutions
2.2. Preparation of Samples
2.3. Flow Systems and Operation
3. Results and Discussion
3.1. Flow System Design
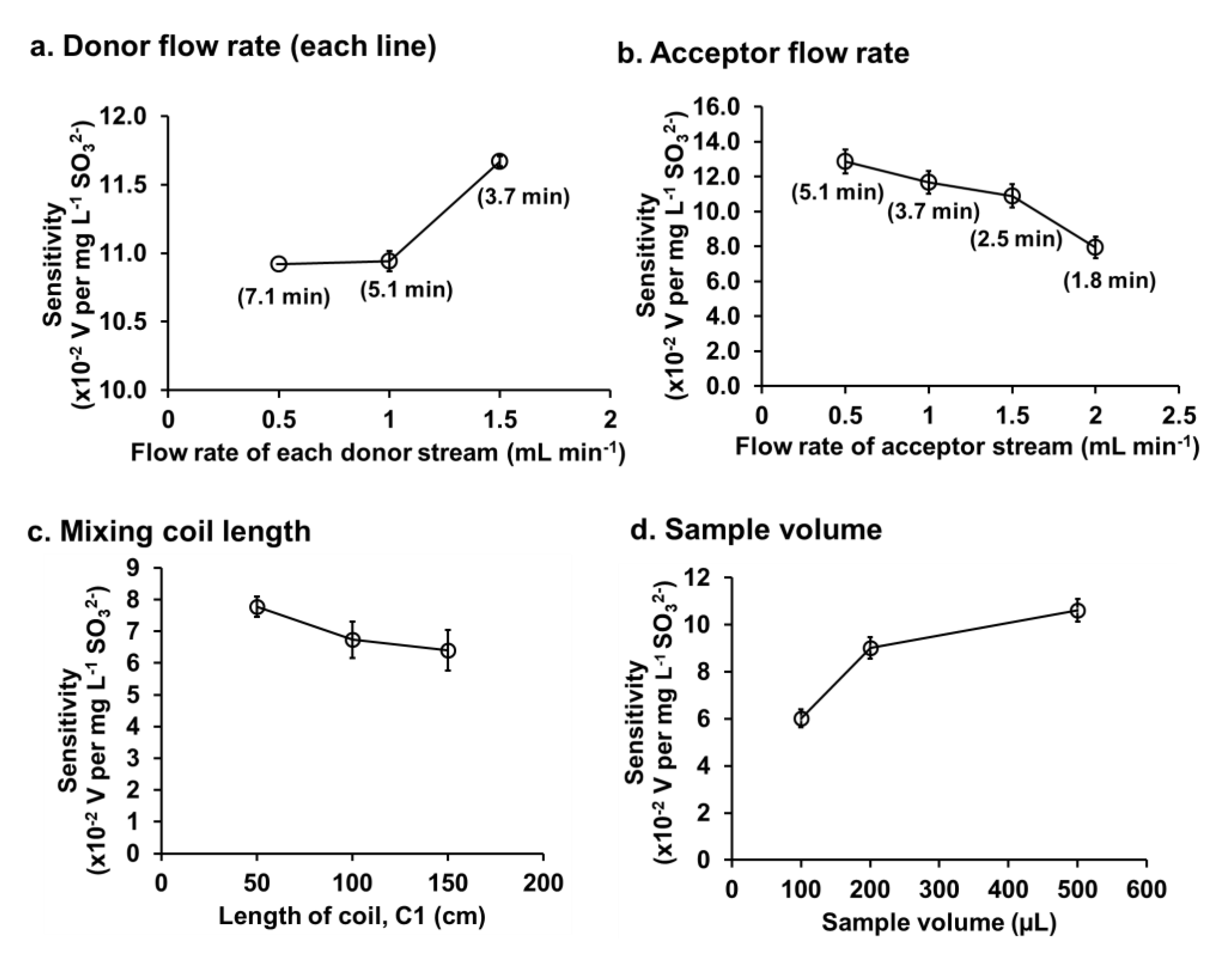
3.2. Optimization
3.2.1. Concentration of the Sulfuric Acid
3.2.2. Selection of Type of Acceptor Liquid
3.2.3. Flow Rate of Donor and Acceptor Streams
3.2.4. Length of Mixing Coil C1
3.2.5. Sample Volume
3.3. Interference Study
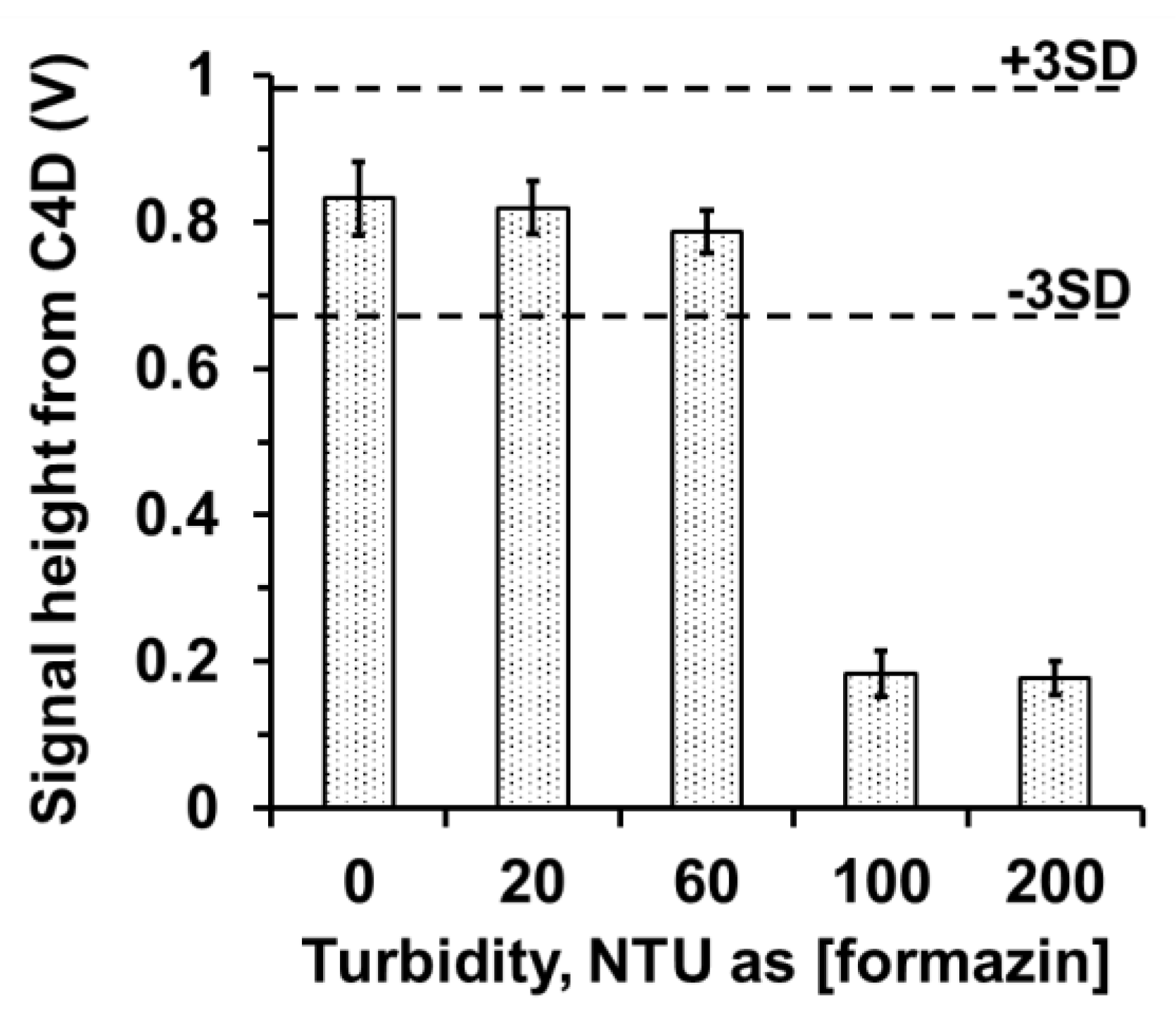
3.4. Capability of the Proposed System for Turbid Samples
3.5. Analytical Performances
3.6. Application to Wine, Dried Fruit and Fruit Juice
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
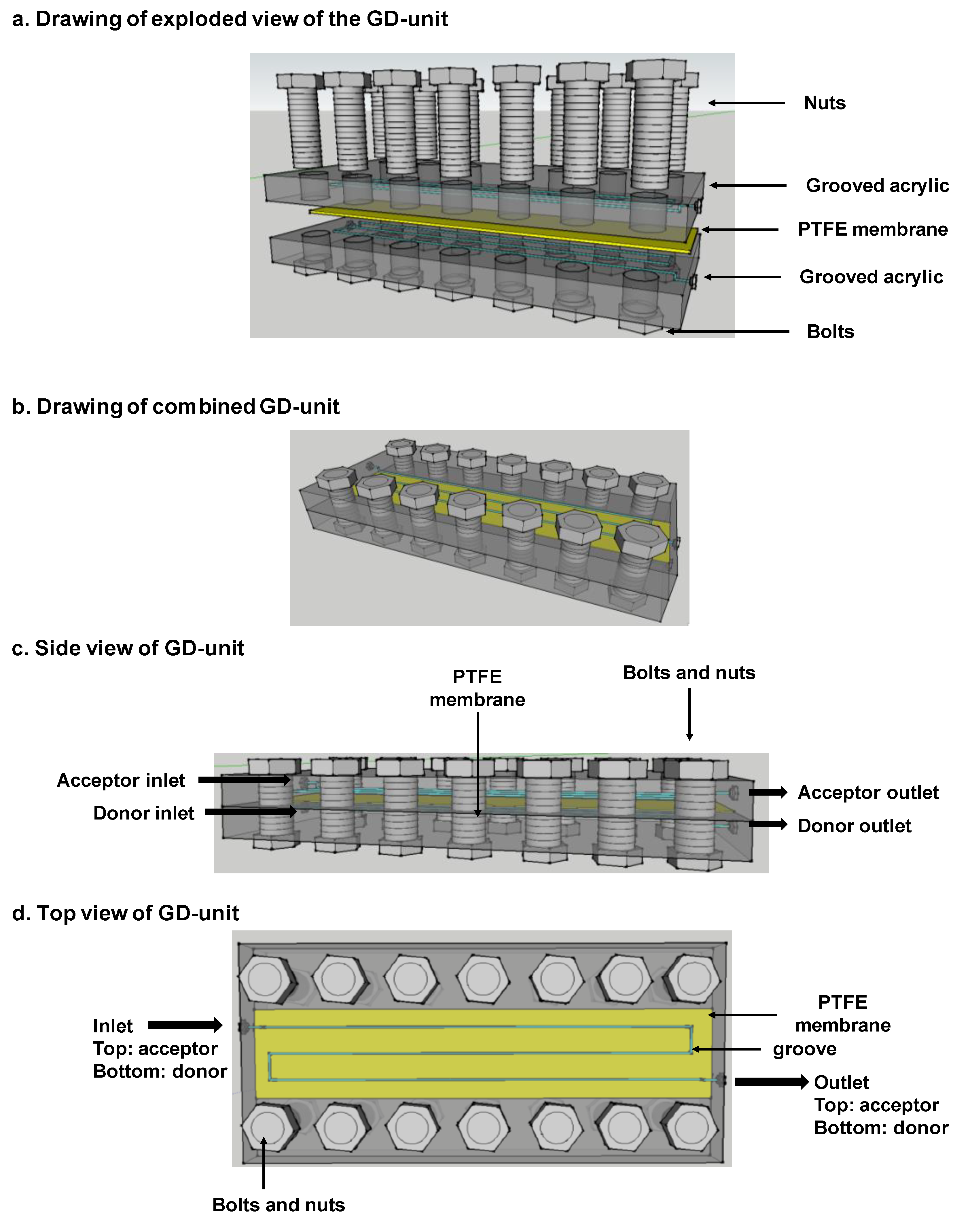
Appendix A. Gas-Diffusion Unit
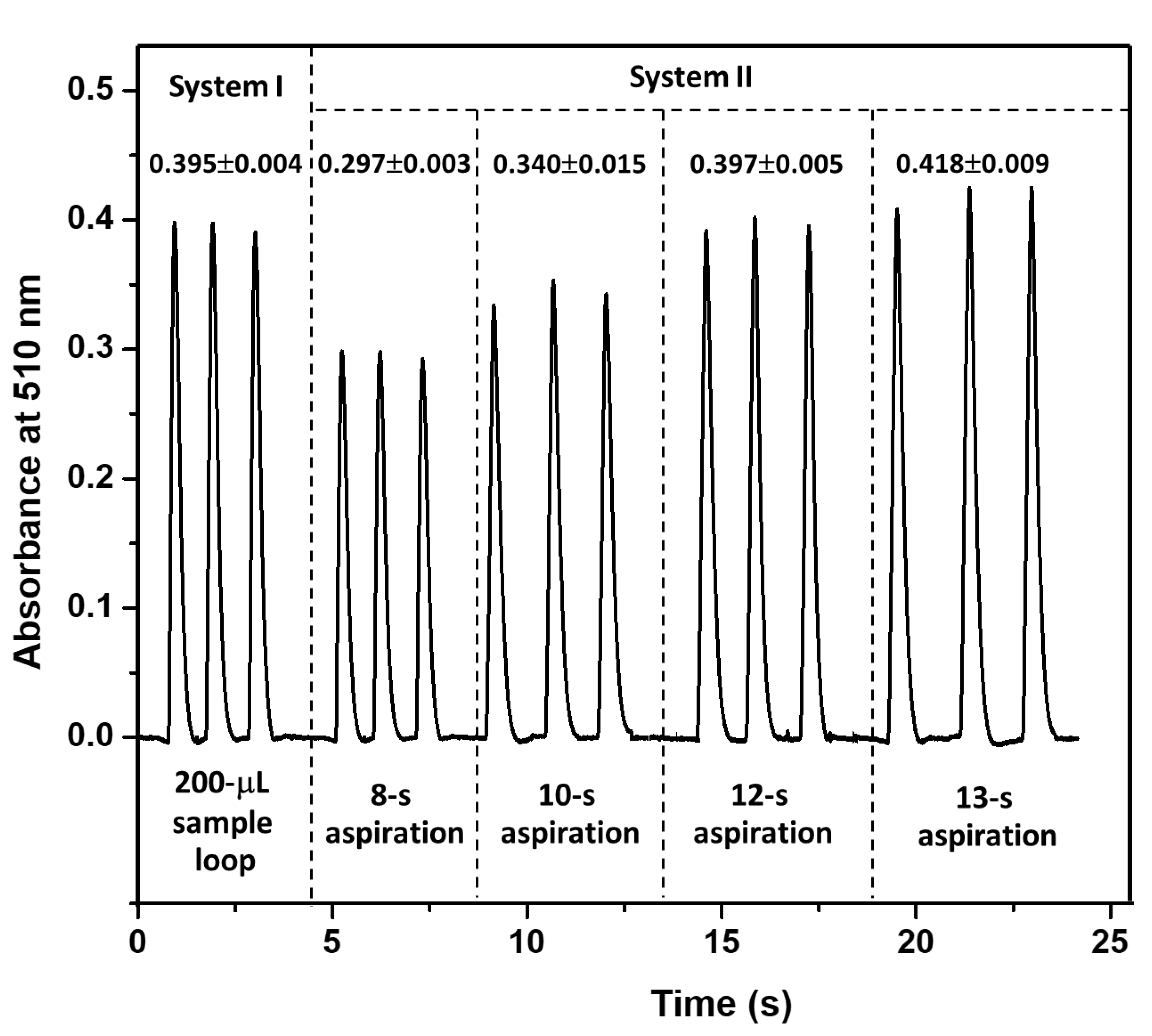
Appendix B. Study of Aspiration Time for Sample Introduction in System II
Appendix C. Back-Calculated Concentrations from Calibration Graphs Obtained from the Systems I and II
| Nominal Concentration | Back-Calculated Concentration (mg L−1 SO32−) | |
|---|---|---|
| System I | System II | |
| 5.0 | 5.16 ± 0.19 | 4.97 ± 0.28 |
| 10.0 | 9.96 ± 0.09 | 10.71 ± 0.51 |
| 15.0 | 13.53 ± 0.32 | 15.10 ± 0.63 |
| 20.0 | 20.86 ± 0.21 | 19.51 ± 0.80 |
| 25.0 | 24.40 ± 0.20 | 25.11 ± 0.24 |
Appendix D. Investigation of Tolerance Limit for Turbidity Suitable for Diluted Cloudy Juice Samples
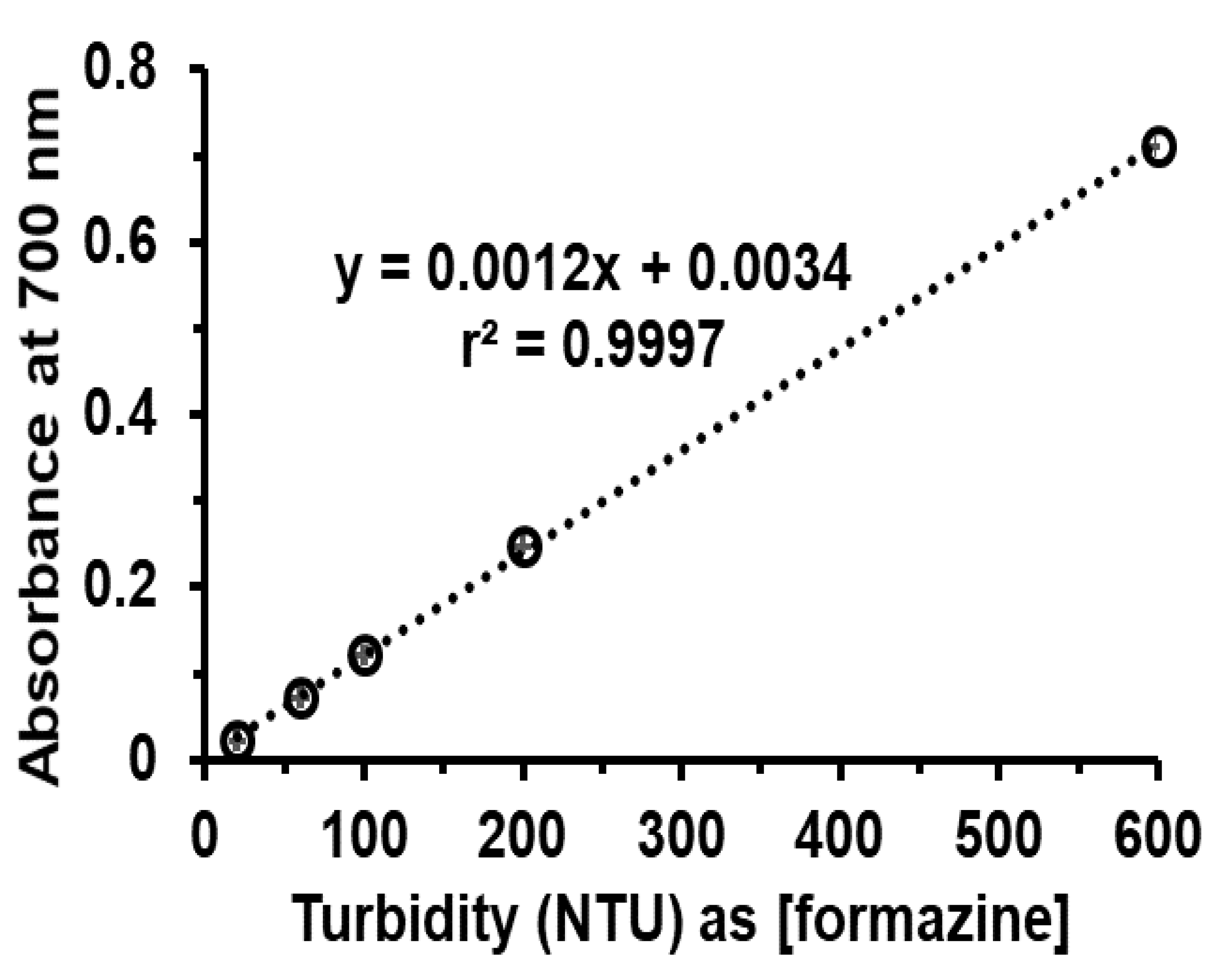
| Sample | Turbidity, NTU (n = 3) | |
|---|---|---|
| Tenfold Diluted Sample | Original Sample | |
| Apple juice #1 | 19.5 ± 0.4 | 194.7 ± 4.2 |
| Apple juice #2 | 19.3 ± 0.2 | 193.1 ± 1.7 |
| Lime juice | 50.1 ± 0.5 | 501.4 ± 5.4 |
Appendix E. Results of the Determination of Free and Total Sulfite
| Sample | Free sulfite (mg L−1 SO32−) | Total sulfite (mg L−1 SO32−) |
|---|---|---|
| White wine #1 | 67.4 ± 2.2 | 183.5 ± 1.1 |
| White wine #2 | 78.9 ± 5.4 | 211.6 ± 1.6 |
| Red wine #1 | 88.6 ± 1.2 | 113.0 ± 2.1 |
| Red wine #2 | 102.6 ± 1.6 | 137.7 ± 6.4 |
| Dried fruit | n.d. | 341.7 ± 6.5 |
| Juice | 107.8 ± 3.5 | 244.8 ± 3.2 |
References
- Iammarino, M.; Ientile, A.R.; Di Taranto, A. Sulphur dioxide in meat products: 3-year control results of an accredited Italian laboratory. Food Addit. Contam. Part B 2017, 10, 99–104. [Google Scholar] [CrossRef]
- Taylor, S.L.; Higley, N.A.; Bush, R.K. Sulfites in Foods: Uses, Analytical Methods, Residues, Fate, Exposure Assessment, Metabolism, Toxicity, and Hypersensitivity. In Advances in Food Research; Chichester, C.O., Mrak, E.M., Schweigert, B.S., Eds.; Academic Press: Cambridge, MA, USA, 1986; Volume 30, pp. 1–76. [Google Scholar]
- Brody, T.O.M. 10—Inorganic nutrients. In Nutritional Biochemistry, 2nd ed.; Brody, T.O.M., Ed.; Academic Press: San Diego, CA, USA, 1999; pp. 693–878. [Google Scholar]
- Vally, H.; Misso, N.L.A.; Madan, V. Clinical effects of sulphite additives. Clin. Exp. Allergy 2009, 39, 1643–1651. [Google Scholar] [CrossRef]
- Gunnison, A.F.; Jacobsen, D.W.; Schwartz, H.J. Sulfite Hypersensitivity. A Critical Review. Crc Crit. Rev. Toxicol. 1987, 17, 185–214. [Google Scholar] [CrossRef] [PubMed]
- WHO. Safety Evaluation of Certain Food Additives and Contaminants/Prepared by the Fifty-Seventh Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA); World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- Timbo, B.; Koehler, K.M.; Wolyniak, C.; Klontz, K.C. Sulfites—A Food and Drug Administration Review of Recalls and Reported Adverse Events. J. Food Prot. 2004, 67, 1806–1811. [Google Scholar] [CrossRef]
- Vahl, J.M.; Converse, J.E. Ripper procedure for determining sulfur dioxide in wine: Collaborative study. J. Assoc. Off. Anal. Chem. 1980, 63, 194–199. [Google Scholar] [CrossRef]
- De Paula, N.T.G.; Barbosa, E.M.O.; da Silva, P.A.B.; de Souza, G.C.S.; Nascimento, V.B.; Lavorante, A.F. In-line electrochemical reagent generation coupled to a flow injection biamperometric system for the determination of sulfite in beverage samples. Food Chem. 2016, 203, 183–189. [Google Scholar] [CrossRef]
- Total Sulfite Analyzer | Labcompare.com. Available online: https://www.labcompare.com/6184-Total-Sulfite-Analyzer/56827-Total-Sulfite-and-SO2-H2S-Analyzer/?pda=6184|56827_2_0|||#productdetails (accessed on 8 May 2020).
- Danchana, K.; Clavijo, S.; Cerda, V. Conductometric Determination of Sulfur Dioxide in Wine Using a Multipumping System Coupled to a Gas-Diffusion cell. Anal. Lett. 2019, 52, 1363–1378. [Google Scholar] [CrossRef]
- Henriquez, C.; Horstkotte, B.; Cerda, V. A highly reproducible solenoid micropump system for the analysis of total inorganic carbon and ammonium using gas-diffusion with conductimetric detection. Talanta 2014, 118, 186–194. [Google Scholar] [CrossRef]
- Gimenez-Gomez, P.; Gutierrez-Capitan, M.; Puig-Pujol, A.; Capdevila, F.; Munoz, S.; Tobena, A.; Miro, A.; Jimenez-Jorquera, C. Analysis of free and total sulfur dioxide in wine by using a gas-diffusion analytical system with pH detection. Food Chem. 2017, 228, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Bartroli, J.; Escalada, M.; Jorquera, C.J.; Alonso, J. Determination of Total and Free Sulfur Dioxide in Wine by Flow Injection Analysis and Gas-diffusion using p-aminoazobenzene as the Colorimetric Reagent. Anal. Chem. 1991, 63, 2532–2535. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.C.C.; Korn, M. Exploiting Sulphide Generation and Gas Diffusion Separation in a Flow System for Indirect Sulphite Determination in Wines and Fruit Juices. Microchim. Acta 2006, 153, 87–94. [Google Scholar] [CrossRef]
- Silva, C.R.; Gomes, T.F.; Barros, V.A.; Zagatto, E.A. A multi-purpose flow manifold for the spectrophotometric determination of sulphide, sulphite and ethanol involving gas diffusion: Application to wine and molasses analysis. Talanta 2013, 113, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Hobo, T. Flow-injection analysis with chemiluminescent detection of sulphite using Na2CO3-NaHCO3-Cu2+ system. Anal. Chim. Acta 1996, 323, 69–74. [Google Scholar] [CrossRef]
- Araujo, A.N.; Couto, C.M.C.M.; Lima, J.F.C.L.; Montenegro, M.C.B.S.M. Determination of SO2 in Wines Using a Flow Injection Analysis System with Potentiometric Detection. J. Agric. Food Chem. 1998, 46, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, L.M.; Grosso Pacheco, J.; Jorge Magalhães, P.; António Rodrigues, J.; Araújo Barros, A. Determination of free and total sulfites in wine using an automatic flow injection analysis system with voltammetric detection. Food Addit. Contam. Part A 2010, 27, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Chinvongamorn, C.; Pinwattana, K.; Praphairaksit, N.; Imato, T.; Chailapakul, O. Amperometric Determination of Sulfite by Gas Diffusion-Sequential Injection with Boron-Doped Diamond Electrode. Sensors 2008, 8, 1846–1857. [Google Scholar] [CrossRef] [PubMed]
- Martins, P.R.; Popolim, W.D.; Nagato, L.A.F.; Takemoto, E.; Araki, K.; Toma, H.E.; Angnes, L.; Penteado, M.D.V.C. Fast and reliable analyses of sulphite in fruit juices using a supramolecular amperometric detector encompassing in flow gas diffusion unit. Food Chem. 2011, 127, 249–255. [Google Scholar] [CrossRef]
- Ratanawimarnwong, N.; Pluangklang, T.; Chysiri, T.; Nacapricha, D. New membraneless vaporization unit coupled with flow systems for analysis of ethanol. Anal. Chim. Acta 2013, 796, 61–67. [Google Scholar] [CrossRef]
- Alahmad, W.; Pluangklang, T.; Mantim, T.; Cerda, V.; Wilairat, P.; Ratanawimarnwong, N.; Nacapricha, D. Development of flow systems incorporating membraneless vaporization units and flow-through contactless conductivity detector for determination of dissolved ammonium and sulfide in canal water. Talanta 2018, 177, 34–40. [Google Scholar] [CrossRef]
- Chantipmanee, N.; Alahmad, W.; Sonsa-ard, T.; Uraisin, K.; Ratanawimarnwong, N.; Mantim, T.; Nacapricha, D. Green analytical flow method for the determination of total sulfite in wine using membraneless gas–liquid separation with contactless conductivity detection. Anal. Methods 2017, 9, 6107–6116. [Google Scholar] [CrossRef]
- Lin, J.; Zhu, Y.; Cheng, W.; Wang, J.; Wu, B.; Wang, J. Determination of Free and Total Sulfite in Red Globe Grape by Ion Chromatography. Food Sci. Technol. Res. 2014, 20, 1079–1085. [Google Scholar] [CrossRef]
- Kraikaew, P.; Pluangklang, T.; Ratanawimarnwong, N.; Uraisin, K.; Wilairat, P.; Mantim, T.; Nacapricha, D. Simultaneous determination of ethanol and total sulfite in white wine using on-line cone reservoirs membraneless gas-liquid separation flow system. Microchem. J. 2019, 149, 1–10. [Google Scholar] [CrossRef]
- Varela, C.; Dry, P.R.; Kutyna, D.R.; Francis, I.L.; Henschke, P.A.; Curtin, C.D.; Chambers, P.J. Strategies for reducing alcohol concentration in wine. Aust. J. Grape Wine Res. 2015, 21, 670–679. [Google Scholar] [CrossRef]
- Araujo, C.; Decarvalho, J.; Mota, D.; Dearaujo, C.; Coelho, N. Determination of sulphite and acetic acid in foods by gas permeation flow injection analysis. Food Chem. 2005, 92, 765–770. [Google Scholar] [CrossRef]



| Interfering Species (Unit) | Reported Level | Tolerance Limit for FIA-GD-C4D |
|---|---|---|
| Ethanol (%v/v) | 16 a | 30 |
| Sucrose (%w/w) | 0.05–0.5 b | 0.05 |
| Fructose (%w/w) | 0.05–0.5 b | 0.05 |
| Glucose (%w/w) | 0.05–0.5 b | 0.01 |
| Ascorbic acid (mg L−1) | 1100–1200 b | 500 |
| Tartaric acid (mg L−1) | 1000–6,000 b | 1000 |
| Citric acid (mg L−1) | Less than 1,000 b | 500 |
| Glycerol (%w/v) | 0.8–1 b | 0.5 |
| CO2 (from HCO3−) (mg L−1) | Less than 2,000 b | 100 |
| Analytical parameter | System I: FIA-GD-C4D | System II: Flow-C4D-G4D |
|---|---|---|
| 1. Working range | 5–25 mg L−1 SO32− | 5–25 mg L−1 SO32− |
| 2. Example of linear calibration, r2 | y = [(8.73 ± 0.26) × 10−2]x – (0.20 ± 0.04), r2 = 0.997 | y = [(8.48 ± 0.36) × 10-2]x – (0.12 ± 0.06), r2 = 0.995 |
| 3. Precision a (as RSD) | 1.4 % | 1.6 % |
| 4. Limit of detection b | 2.4 mg L−1 SO32− | 3.3 mg L−1 SO32− |
| 5. Injection throughput | 24 injections h-1 | 24 injections h-1 |
| 6. Type of samples | Wine | Wine, extracts of dried fruit, fruit juices |
| 7. Sample pretreatment (see Section 2.2) | Addition of EDTA and NaOH Dilution with deionized water | Extraction with NaOH Dilution with deionized water |
| Method | Gas–Liquid Separation Unit/Type of Membrane | Acidification Solution | Acceptor Liquid | Sample(s) | Throughput (h−1) | Linear Range and Limit of Quantitation/Detection | Ref. |
|---|---|---|---|---|---|---|---|
| MPFS Conductometry | GD/Tubular PTFE | 2 M H3PO4 | 1 mM H2O2 | Wines (white, red and rose) | 12 | 12.75-95.25 mg L−1 SO32− LOQ: 12.75 mg L−1 SO32− | [11] |
| FIA Conductometry | GD/PTFE membrane | 2 M HCl | Water | Wines (white, red and rose) and juices | 120 | 1-50 mg L−1 SO32− LOQ: 0.10 mg L−1 SO32− | [28] |
| FIA-SIA Contactless conductivity (C4D) | MBL-VP/- | 1.5 M H2SO4 | Water | Wines (white, and red) | 26 | 10-200 mg L−1 SO32−L LOQ: 0.30 mg L−1 SO32− | [24] |
| FIA-SIA Contactless conductivity (C4D) | MBL-VP/- | 1.5 M H2SO4 | Water | White wines | 24 | 10-200 mg L−1 SO32−L LOQ: 7.68 mg L−1 SO32− | [26] |
| FIA Contactless conductivity (C4D) | GD/PTFE membrane | 1 M H2SO4 | Water | Wines (white, and red) | 24 | 5-25 mg L−1 SO32− LOD: 2.4 mg L−1 SO32− | This work |
| Dried fruits and juices | 24 | 5-25 mg L−1 SO32− LOD: 3.3 mg L−1 SO32− |
| Sample | Total Sulfite | |
|---|---|---|
| This Work | Iodimetric Titration [8] | |
| White wine #1 * | 91.8 ± 1.2 a | 108.7 ± 8.4 a |
| White wine #2 * | 105.6 ± 0.4 a | 92.6 ± 8.8 a |
| White wine #3 * | 105.8 ± 1.6 a | 110.3 ± 2.3 a |
| White wine #4 * | 83.3 ± 0.8 a | 93.9 ± 0.9 a |
| Red wine #1 * | 101.2 ± 3.7 a | 112.8 ± 0.5 a |
| Red wine #2 * | 56.5 ± 2.1 a | 80.1 ± 1.7 a |
| Red wine #3 * | 78.8 ± 0.1 a | 79.7 ± 6.0 a |
| Red wine #4 * | 80.0 ± 0.1 a | 86.4 ± 8.8 a |
| Dried guava | 170.8 ± 0.7 b | 159.6 ± 4.0 b |
| Dried mango | 96.9 ± 0.8 b | 206.2 ± 2.3 b |
| Apple juice #1 | 81.4 ± 0.3 a | 205.4 ± 2.4 a |
| Apple juice #2 | 122.4 ± 1.6 a | 163.1 ± 0.5 a |
| Lime juice | 137.2 ± 1.8 a | 184.1 ± 8.8 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayuning Tyas, A.; Sonsa-ard, T.; Uraisin, K.; Nacapricha, D.; Saetear, P. Simple Flow-Based System with an In-Line Membrane Gas–Liquid Separation Unit and a Contactless Conductivity Detector for the Direct Determination of Sulfite in Clear and Turbid Food Samples. Membranes 2020, 10, 104. https://doi.org/10.3390/membranes10050104
Ayuning Tyas A, Sonsa-ard T, Uraisin K, Nacapricha D, Saetear P. Simple Flow-Based System with an In-Line Membrane Gas–Liquid Separation Unit and a Contactless Conductivity Detector for the Direct Determination of Sulfite in Clear and Turbid Food Samples. Membranes. 2020; 10(5):104. https://doi.org/10.3390/membranes10050104
Chicago/Turabian StyleAyuning Tyas, Aulia, Thitaporn Sonsa-ard, Kanchana Uraisin, Duangjai Nacapricha, and Phoonthawee Saetear. 2020. "Simple Flow-Based System with an In-Line Membrane Gas–Liquid Separation Unit and a Contactless Conductivity Detector for the Direct Determination of Sulfite in Clear and Turbid Food Samples" Membranes 10, no. 5: 104. https://doi.org/10.3390/membranes10050104
APA StyleAyuning Tyas, A., Sonsa-ard, T., Uraisin, K., Nacapricha, D., & Saetear, P. (2020). Simple Flow-Based System with an In-Line Membrane Gas–Liquid Separation Unit and a Contactless Conductivity Detector for the Direct Determination of Sulfite in Clear and Turbid Food Samples. Membranes, 10(5), 104. https://doi.org/10.3390/membranes10050104