The Effect of Emulsifiers on the Emulsion Stability and Extraction Efficiency of Cr(VI) Using Emulsion Liquid Membranes (ELMs) Formulated with a Green Solvent
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. ELM Preparation
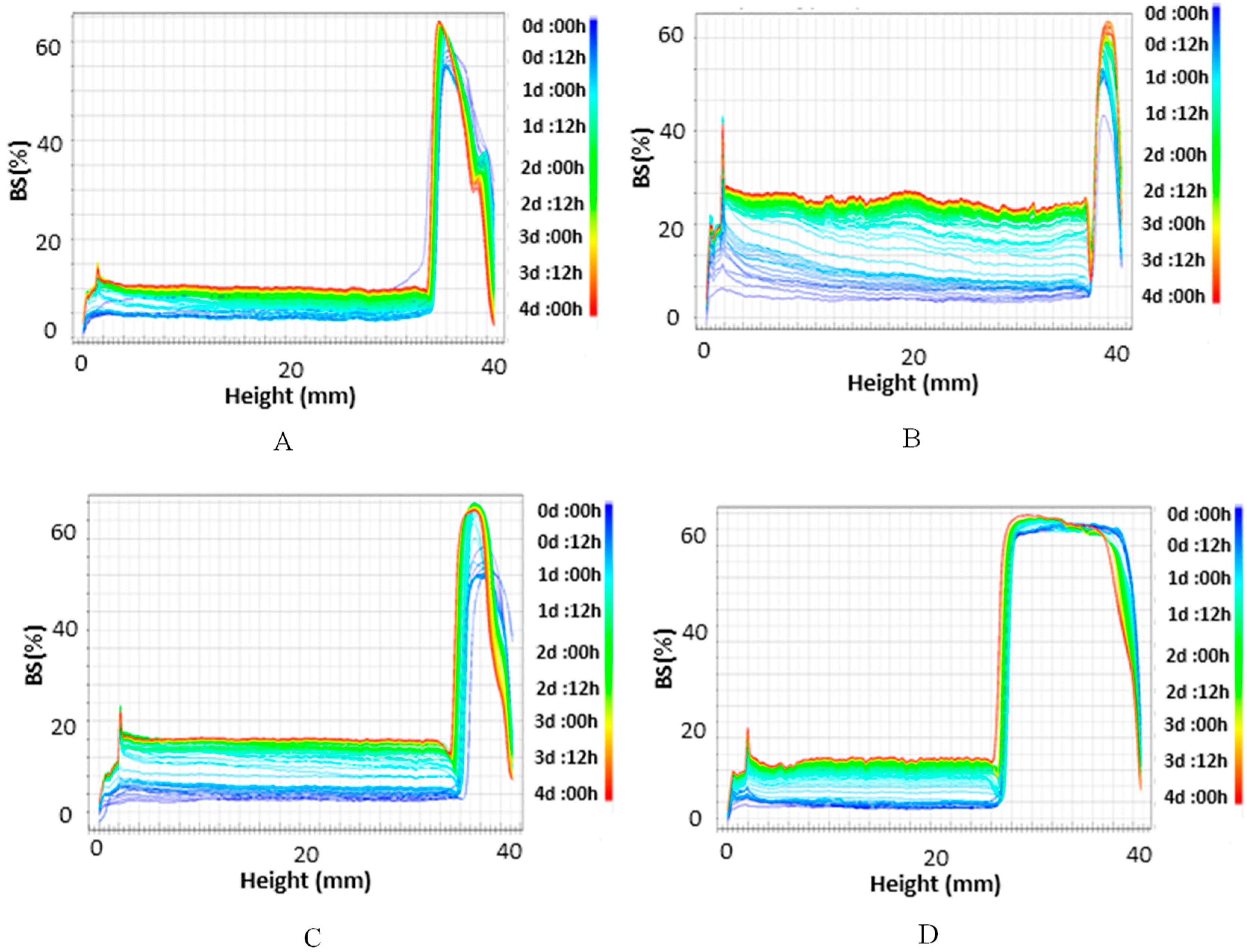
2.2.2. Double Emulsion Characterization
2.2.3. Extraction Efficiency
3. Results and Discussion
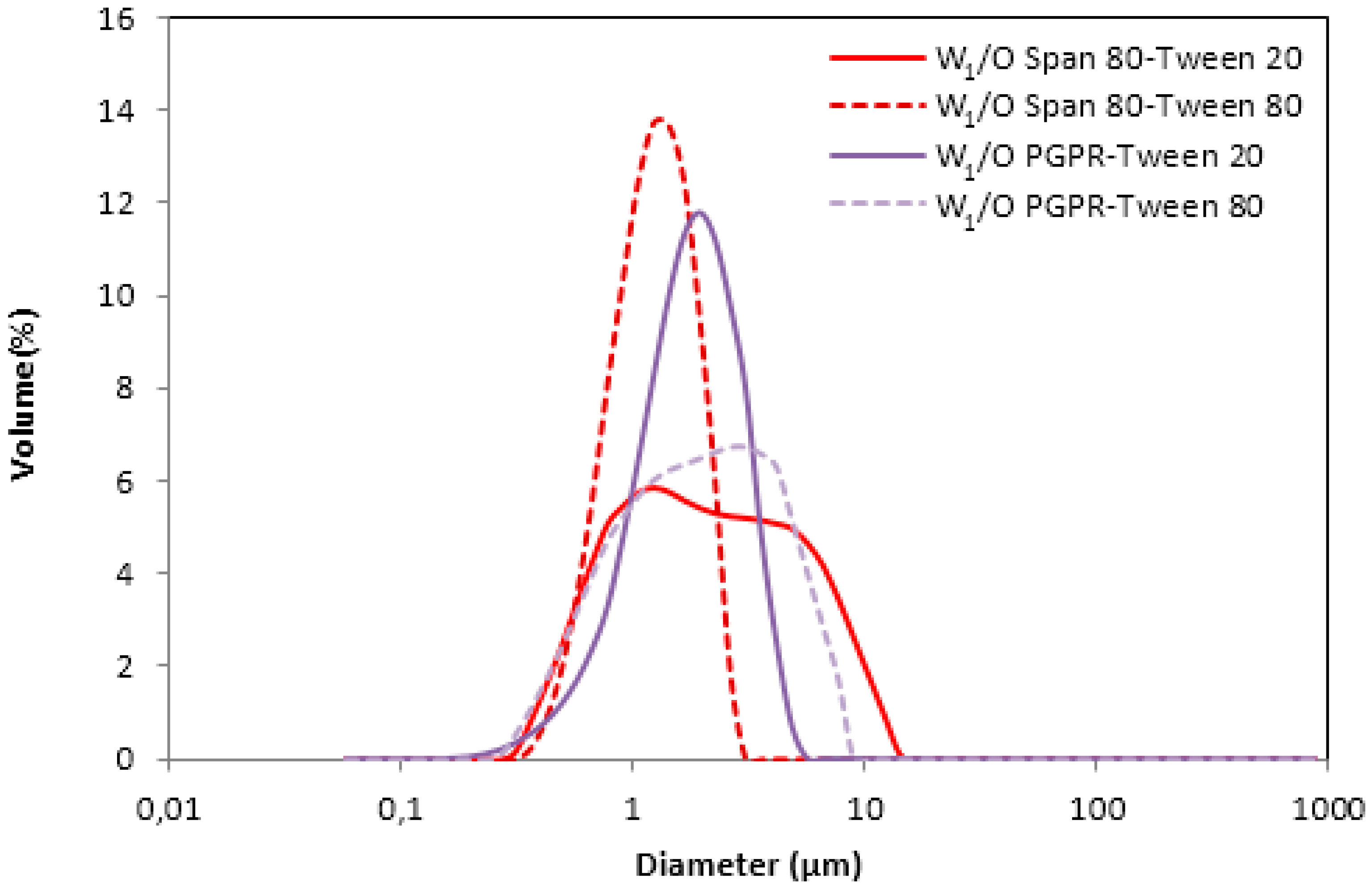
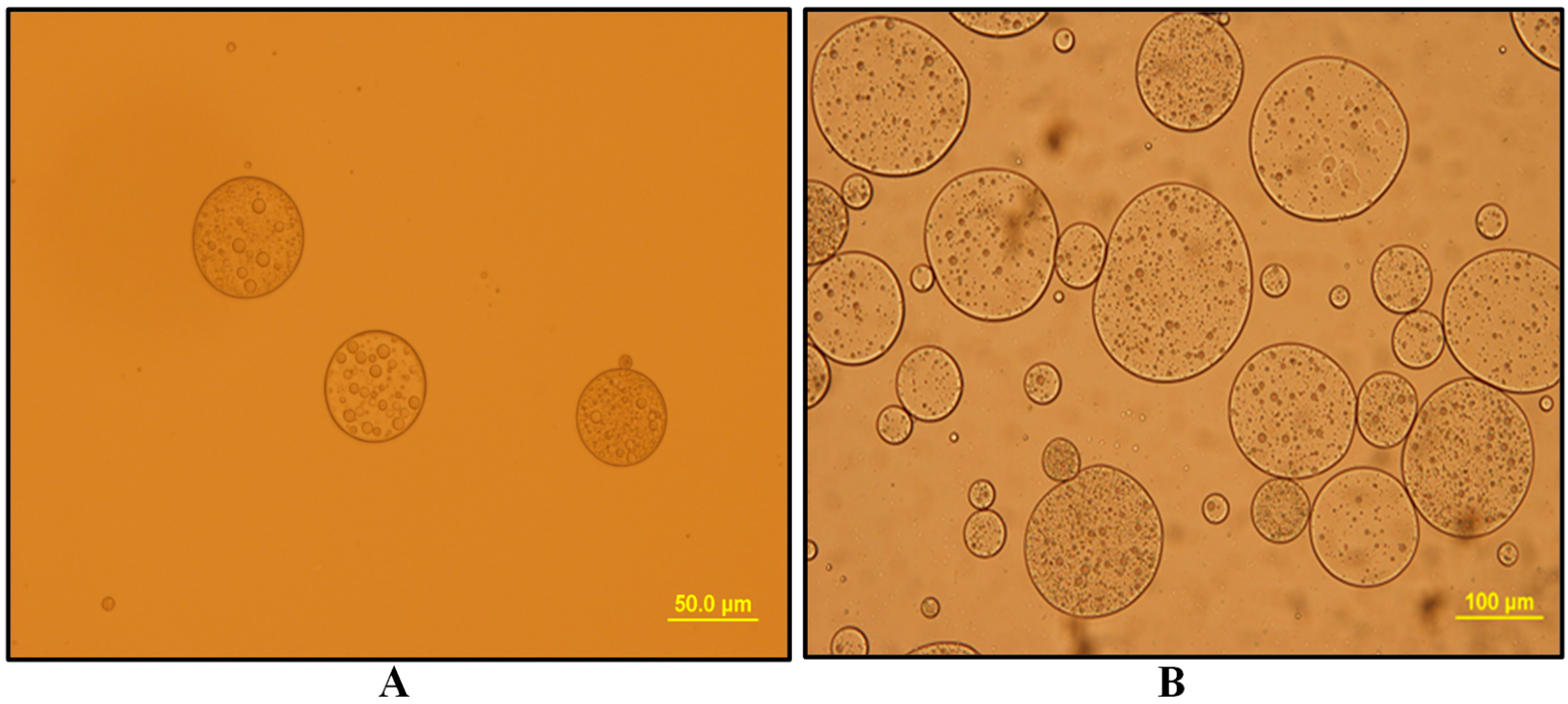
3.1. Water-in-Oil (W1/O) Emulsions
3.1.1. W1/O Emulsions Stabilized with Span 80
3.1.2. W1/O Emulsions Stabilized with PGPR
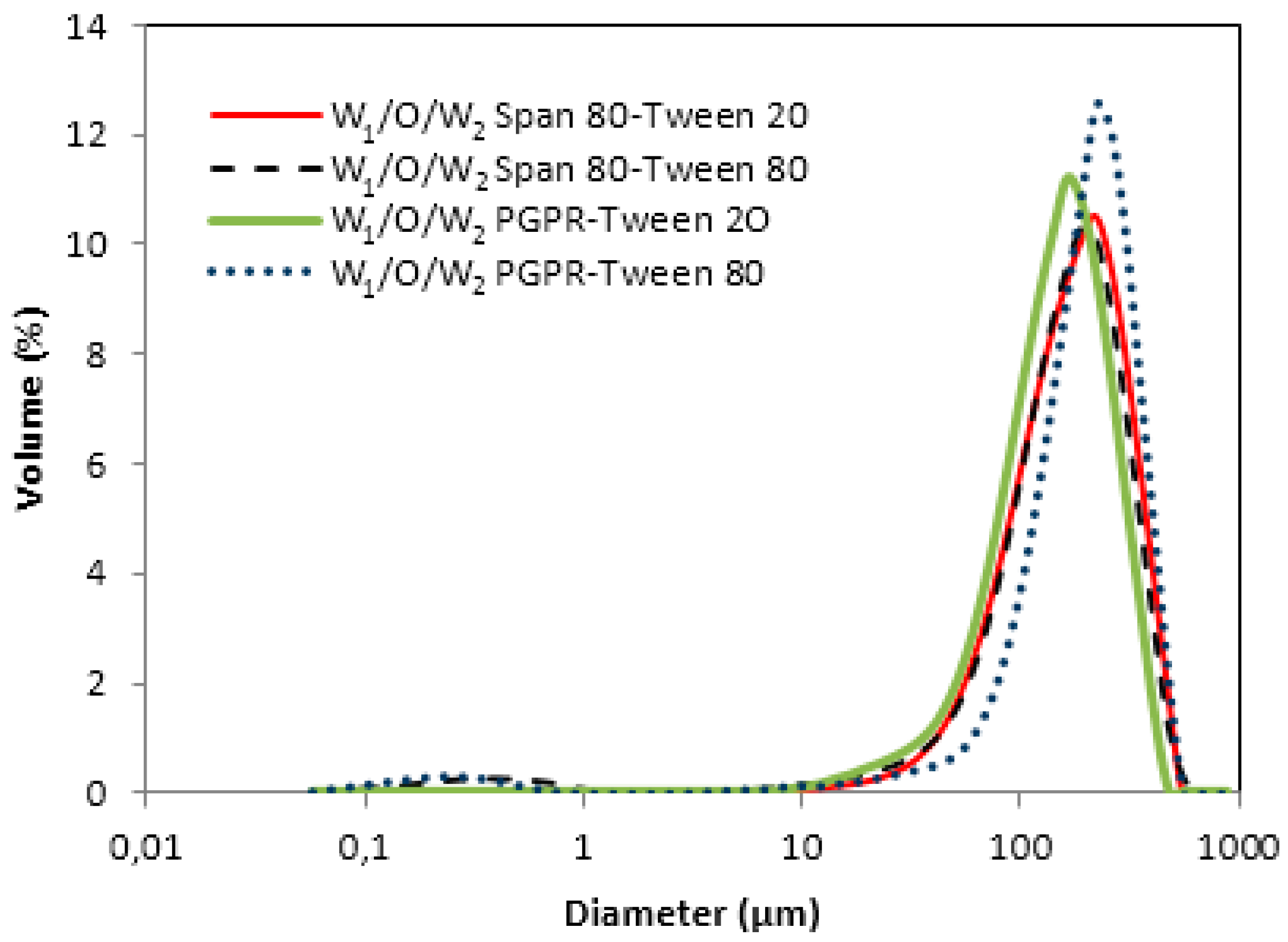
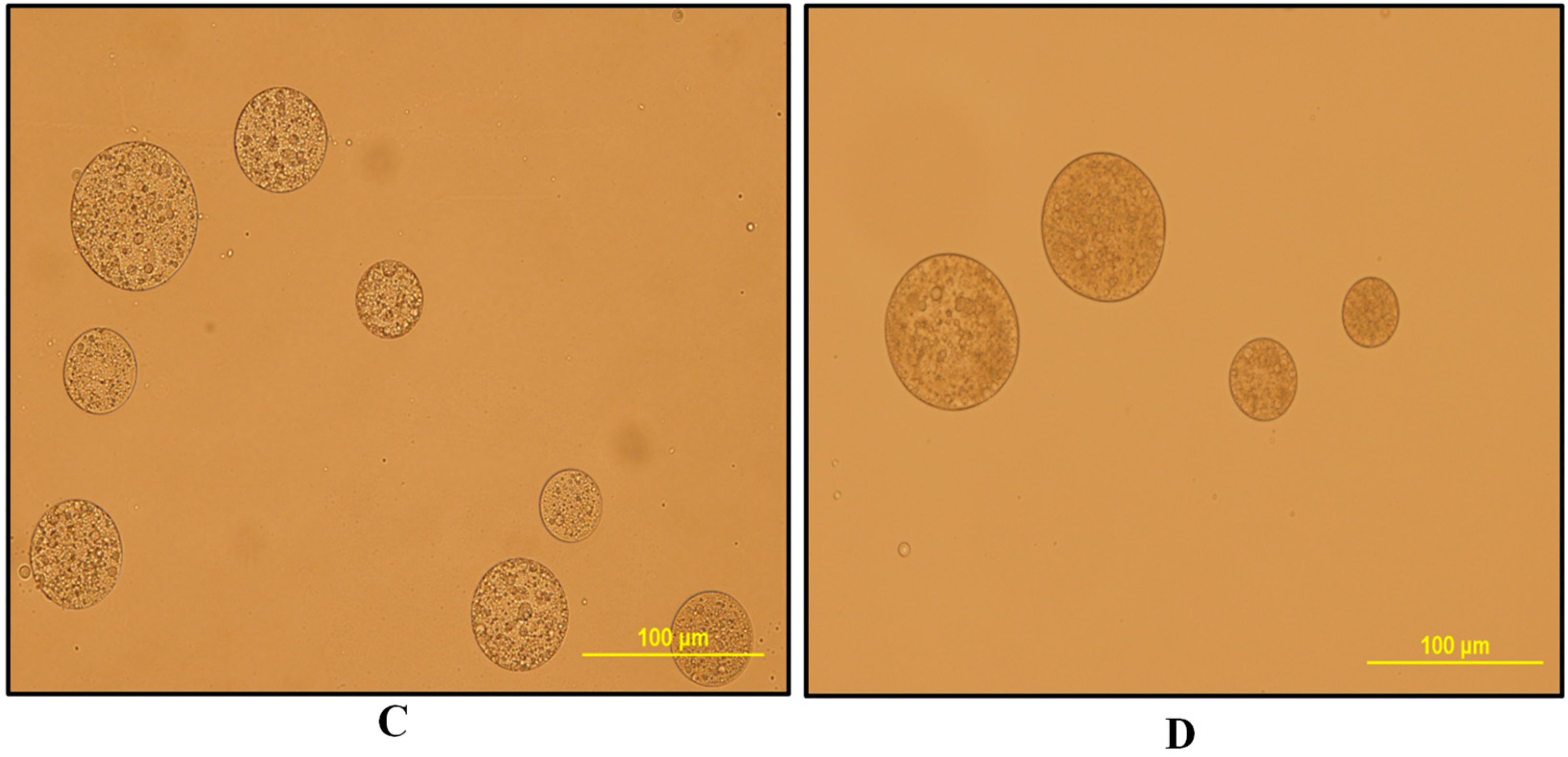
3.2. Water-in-Oil-in-Water Emulsions (W1/O/W2)
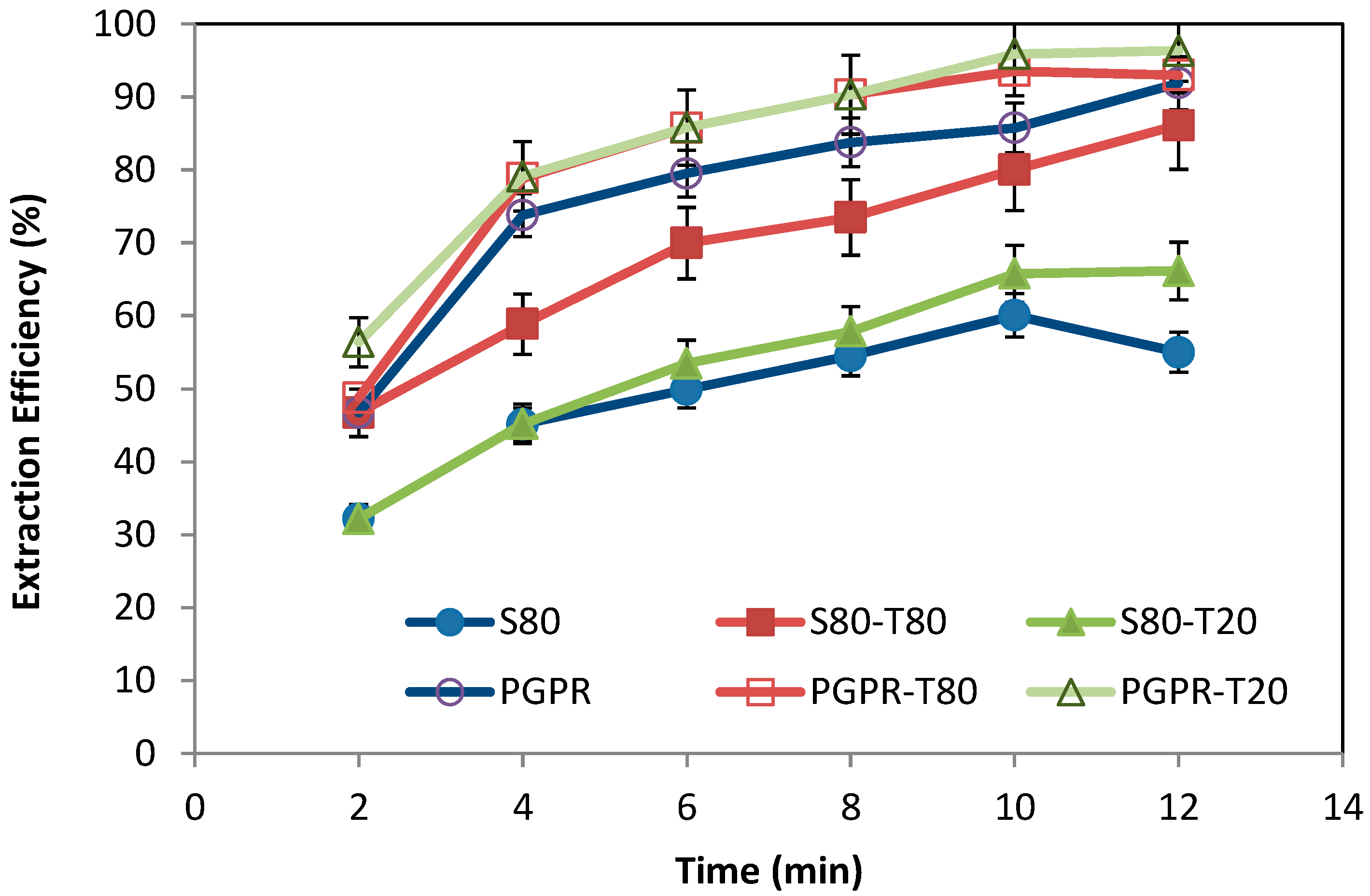
3.3. Effect of the Surfactants on the Extraction Efficiency of Cr(VI)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aserin, A. Multiple Emulsions: Technology and Applications; Wiley-Interscience: Hoboken, NJ, USA, 2008. [Google Scholar]
- Dickinson, E. Double emulsions stabilized by food biopolymers. Food Biophys. 2011, 6, 1–11. [Google Scholar] [CrossRef]
- Mine, Y.; Shimizu, M.; Nakashima, T. Preparation and stabilization of simple and multiple emulsions using a microporous glass membrane. Colloids Surf. B Biointerfaces 1996, 6, 261–268. [Google Scholar] [CrossRef]
- Frasch-Melnik, S.; Spyropoulos, F.; Norton, I.T. W1/O/W2 double emulsions stabilised by fat crystals-formulation. stability and salt release. J. Colloid Interface Sci. 2010, 350, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Matos, M.; Timgren, A.; Sjöö, M.; Dejmek, P.; Rayner, M. Preparation and encapsulation properties of double Pickering emulsions stabilized by quinoa starch granules. Colloids Surf. A Physicochem. Eng. Asp. 2013, 423, 147–153. [Google Scholar] [CrossRef]
- Li, N.N.; Chan, R.P.; Naden, D.; Lai, R.W.M. Liquid membrane processes for copper extraction. Hydrometallurgy 1983, 9, 277–305. [Google Scholar] [CrossRef]
- Jiao, J.; Burgess, D.J. Rheology and stability of water-in-oil-in-water multiple emulsions containing Span 83 and Tween 80. Aaps Pharmsci. 2003, 5, 62–73. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Kusumastuti, A.; Derek, C.J.C.; Ooi, B.S. Emulsion liquid membrane for cadmium removal: Studies on emulsion diameter and stability. Desalination 2012, 287, 30–34. [Google Scholar] [CrossRef]
- Djenouhat, M.; Hamdaoui, O.; Chiha, M.; Samar, M.H. Ultrasonication-assisted preparation of water-in-oil emulsions and application to the removal of cationic dyes from water by emulsion liquid membrane Part 1: Membrane stability. Seperation Purif. Technol. 2008, 62, 636–641. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Kusumastuti, A.; Derek, C.J.C.; Ooi, B.S. Emulsion liquid membrane for heavy metal removal: An overview on emulsion stabilization and destabilization. Chem. Eng. J. 2011, 171, 870–882. [Google Scholar] [CrossRef]
- Berrama, T.; Pareau, D.; Durand, G. Batch and dynamic study of lactic acid extraction using emulsion liquid membrane. Membr. Water Treat. 2015, 6, 277–292. [Google Scholar] [CrossRef]
- Ankit, S.; Ankush, B.; Utkarsh, S.; Rajeev, K.D.; Kailash, S.; Sushant, U. Comparative study of arsenic (V) removal from aqueous solution using Aliquat-336 and 2-ethyl hexanol through emulsion liquid membrane. J. Water Process Eng. 2017, 16, 64–68. [Google Scholar]
- Othman, N.; Zailani, S.N.; Mili, N. Recovery of synthetic dye from simulated wastewater using emulsion liquid membrane process containing tri-dodecyl amine as a mobile carrier. J. Hazard. Mater. 2011, 198, 103–112. [Google Scholar] [CrossRef]
- Othman, N.; Noah, N.F.M.; Shu, L.Y.; Ooi, Z.Y.; Jusoh, N.; Idroas, M.; Goto, M. Easy removing of phenol from wastewater using vegetable oil-based organic solvent in emulsion liquid membrane process. Chin. J. Chem. Eng. 2017, 25, 45–52. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Huang, J.; Liu, T.; Shi, Q. Optimization of vanadium (IV) extraction from stone coal leaching solution by emulsion liquid membrane using response surface methodology. Chem. Eng. Res. Des. 2017, 123, 111–119. [Google Scholar] [CrossRef]
- Vafaei, F.; Torkaman, R.; Moosavian, M.A.; Zaheri, P. Optimization of extraction conditions using central composite design for the removal of Co(II) from chloride solution by supported liquid membrane. Chem. Eng. Res. Des. 2018, 133, 126–136. [Google Scholar] [CrossRef]
- Goyal, R.K.; Jayakumar, N.S.; Hashim, M.A. Chromium removal by emulsion liquid membrane using [BMIM]+[NTf2]− as stabilizer and TOMAC as extractant. Desalination 2011, 278, 50–56. [Google Scholar] [CrossRef]
- Kumbasar, R.A. Selective separation of chromium (VI) from acidic solutions containing various metal ions through emulsion liquid membrane using trioctylamine as extractant. Seperation Purif. Technol. 2008, 64, 56–62. [Google Scholar] [CrossRef]
- Nosrati, S.; Jayakumar, N.S.; Hashim, M.A. Extraction performance of chromium (VI) with emulsion liquid membrane by Cyanex 923 as carrier using response surface methodology. Desalination 2011, 266, 286–290. [Google Scholar] [CrossRef]
- Hasan, M.A.; Selim, Y.T.; Mohamed, K.M. Removal of chromium from aqueous waste solution using liquid emulsion membrane. J. Hazard. Mater. 2009, 168, 1537–1541. [Google Scholar] [CrossRef]
- Kumbasar, R.A. Extraction of chromium (VI) from multicomponent acidic solutions by emulsion liquid membranes using TOPO as extractant. J. Hazard. Mater. 2009, 167, 1141–1147. [Google Scholar] [CrossRef]
- Pfeiffer, R.M.; Bunge, A.L. Calculating leakage in emulsion liquid membrane systems from pH measurement. Sep. Purif. Technol. 2020, 235, 116162. [Google Scholar] [CrossRef]
- Dang, N.T.T.; Wang, D.-M.; Huang, S.-Y.; Tran, K.T. Indium recovery from aqueous solution containing oxalic acid–Enhancement by using hydrophobic membranes. Sep. Purif. Technol. 2020, 235, 116300. [Google Scholar] [CrossRef]
- Kumar, A.; Thakur, A.; Panesar, P.S. Recent developments on sustainable solvents for emulsion liquid membrane processes. J. Clean. Prod. 2019, 240, 118250. [Google Scholar] [CrossRef]
- Jager-Lezer, N.; Terrisse, I.; Bruneau, F.; Tokgoz, S.; Ferreira, L.; Clausse, D.; Seiler, M.; Grossiord, J.L. Influence of lipophilic surfactant on the release kinetics of water-soluble molecules entrapped in a W/O/W multiple emulsion. J. Control. Release 1997, 45, 1–13. [Google Scholar] [CrossRef]
- Jiao, H.; Peng, W.; Zhao, J.; Xu, C. Extraction performance of bisphenol A from aqueous solutions by emulsion liquid membrane using response surface methodology. Desalination 2013, 313, 36–43. [Google Scholar] [CrossRef]
- Patnaik, P.R. Liquid emulsion membranes: Principles. problems and applications in fermentation processes. Biotechnol. Adv. 1995, 13, 175–208. [Google Scholar] [CrossRef]
- Chakraborty, M.; Bhattacharya, C.; Datta, S. Emulsion liquid membranes: Definitions and classification. theories, module design, applications new directions and perspectives. In Liquid Membranes: Principles and Applications in Chemical Separations and Wastewater Treatment; Kislik, V.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Ahmad, A.L.; Kusumastuti, A.; Derek, C.J.C.; Ooi, B.S. Emulsion liquid membranes for cadmium removal: Studies of extraction efficiency. Membr. Water Treat. 2013, 4, 11–24. [Google Scholar] [CrossRef]
- Bart, H.J.; Stevens, G.W. Reactive solvent extraction. In Ion Exchange and Solvent Extraction; Marcus, Y., Sengupta, A.K., Eds.; Marcel Dekker: New York, NY, USA, 2004; Volume 17, pp. 37–82. [Google Scholar]
- Zhao, L.; Fei, D.; Dang, Y.; Zhou, X.; Xiao, J. Studies on the extraction of chromium(III) by emulsion liquid membrane. J. Hazard. Mater. 2010, 178, 130–135. [Google Scholar] [CrossRef]
- Kumbasar, R.A. Selective extraction of chromium (VI) from multicomponent acidic solutions by emulsion liquid membranes using tributhylphosphate as carrier. J. Hazard. Mater. 2010, 178, 875–882. [Google Scholar] [CrossRef]
- Venkateswaran, P.; Palanivelu, K. Recovery of phenol from aqueous solution by supported liquid membrane using vegetable oils as liquid membrane. J. Hazard. Mater. 2006, 131, 146–152. [Google Scholar] [CrossRef]
- Ehtash, M.; Fournier-Salaün, M.C.; Dimitrov, K.; Salaün, P.; Saboni, A. Phenol removal from aqueous media by pertraction using vegetable oil as a liquid membrane. Chem. Eng. J. 2014, 250, 42–47. [Google Scholar] [CrossRef]
- Chang, S.H.; Teng, T.T.; Ismail, N. Extraction of Cu(II) from aqueous solutions by vegetable oil-based organic solvents. J. Hazard. Mater. 2010, 181, 868–872. [Google Scholar] [CrossRef]
- Hossain, M.M. Treatment of ground waters in a hollow-fibre liquid membrane contactor for removal of ions. Membr. Water Treat. 2013, 4, 95–108. [Google Scholar] [CrossRef]
- Muthuraman, G.; Teng, T.T. Use of vegetable oil in supported liquid membrane for the transport of Rhodamine B. Desalination 2009, 249, 1062–1066. [Google Scholar] [CrossRef]
- Muthuraman, G.; Palanivelu, K. Transport of textile dye in vegetable oils based supported liquid membrane. Dye. Pigment. 2006, 70, 99–104. [Google Scholar] [CrossRef]
- Matos, M.; Gutiérrez, G.; Coca, J.; Pazos, C. Preparation of water-in-oil-in-water (W1/O/W2) double emulsions containing trans-resveratrol. Colloids Surf. A Physicochem. Eng. Asp. 2014, 442, 111–122. [Google Scholar] [CrossRef]
- Márquez, A.L.; Medrano, A.; Panizzolo, L.A.; Wagner, J.R. Effect of calcium salts and surfactant concentration on the stability of water-in-oil (w/o) emulsions prepared with polyglycerol polyricinoleate. J. Colloid Interface Sci. 2010, 341, 101–108. [Google Scholar] [CrossRef]
- Basualto, C.; Poblete, M.; Marchese, J.; Ochoa, A.; Acosta, A.; Sapag, J.; Valenzuela, F. Extraction of cadmium from aqueous solutions by emulsion liquid membranes using a stirred transfer cell contactor. J. Braz. Chem. Soc. 2006, 17, 1347–1354. [Google Scholar] [CrossRef]
- Gutiérrez, G.; Benito, J.M.; Coca, J.; Pazos, C. Evaporation of aqueous dispersed systems and concentrated emulsions formulated with non-ionic surfactants. Int. J. Heat Mass Transf. 2014, 69, 117–128. [Google Scholar] [CrossRef]
- Wan, Y.; Zhang, X. Swelling determination of W/O/W emulsion liquid membranes. J. Membr. Sci. 2002, 196, 185–201. [Google Scholar] [CrossRef]
- Björkegren, S.; Karimi, R.F.; Martinelli, A.; Jayakumar, N.S.; Hashim, M.A. A New Emulsion Liquid Membrane Based on a Palm Oil for the Extraction of Heavy Metals. Membranes 2015, 5, 168–179. [Google Scholar] [CrossRef] [PubMed]


| Lipophilic | Hydrophilic | D[3,2] (µm) | D[4,3] (µm) |
|---|---|---|---|
| Span 80 | Tween 80 | 1.0 | 1.2 |
| Tween 20 | 1.4 | 2.8 | |
| PGPR | Tween 80 | 1.3 | 2.3 |
| Tween 20 | 1.3 | 1.8 |
| Different Combinations of Surfactants | |
|---|---|
| 1 | 4% (v/v) Span 80 and 1% (v/v) Tween 80 |
| 2 | 4% (v/v) Span 80 and 1% (v/v) Tween 20 |
| 3 | 4% (v/v) PGPR and 1% (v/v) Tween 80 |
| 4 | 4% (v/v) PGPR and 1% (v/v) Tween 20 |
| HLBmixture | |||
|---|---|---|---|
| Span 80–Tween 80 | Span 80–Tween 20 | PGPR–Tween 80 | PGPR–Tween 20 |
| 6.4 | 6.8 | 5.4 | 5.7 |
| Lipophilic | Hydrophilic | D[3,2] (µm) | D[4,3] (µm) | Zeta Potential (mV) |
|---|---|---|---|---|
| Span 80 | Tween 80 | 6.76 | 167 | 5.1 ± 2.3 |
| Tween 20 | 117 | 179 | 1.3 ± 2.1 | |
| PGPR | Tween 80 | 4.92 | 193 | −0.2 ± 3.5 |
| Tween 20 | 100 | 150 | 4.6 ± 2.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anarakdim, K.; Gutiérrez, G.; Cambiella, Á.; Senhadji-Kebiche, O.; Matos, M. The Effect of Emulsifiers on the Emulsion Stability and Extraction Efficiency of Cr(VI) Using Emulsion Liquid Membranes (ELMs) Formulated with a Green Solvent. Membranes 2020, 10, 76. https://doi.org/10.3390/membranes10040076
Anarakdim K, Gutiérrez G, Cambiella Á, Senhadji-Kebiche O, Matos M. The Effect of Emulsifiers on the Emulsion Stability and Extraction Efficiency of Cr(VI) Using Emulsion Liquid Membranes (ELMs) Formulated with a Green Solvent. Membranes. 2020; 10(4):76. https://doi.org/10.3390/membranes10040076
Chicago/Turabian StyleAnarakdim, Katia, Gemma Gutiérrez, Ángel Cambiella, Ounissa Senhadji-Kebiche, and María Matos. 2020. "The Effect of Emulsifiers on the Emulsion Stability and Extraction Efficiency of Cr(VI) Using Emulsion Liquid Membranes (ELMs) Formulated with a Green Solvent" Membranes 10, no. 4: 76. https://doi.org/10.3390/membranes10040076
APA StyleAnarakdim, K., Gutiérrez, G., Cambiella, Á., Senhadji-Kebiche, O., & Matos, M. (2020). The Effect of Emulsifiers on the Emulsion Stability and Extraction Efficiency of Cr(VI) Using Emulsion Liquid Membranes (ELMs) Formulated with a Green Solvent. Membranes, 10(4), 76. https://doi.org/10.3390/membranes10040076