Scale-Up of Membrane-Based Zinc Recovery from Spent Pickling Acids of Hot-Dip Galvanizing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Recovery of Zinc by Membrane-Based Solvent Extraction at Pilot Plant Scale
3. Results and Discussion
3.1. Mechanism of Zinc Extraction
3.2. Selective Zinc Extraction at Pilot Plant Scale
3.2.1. Selective Zinc Separation in Pilot Plant Experiments
3.2.2. Analysis of Zinc Separation Mechanism
3.2.3. Scale-Up from Laboratory to Pilot Scale
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abad, J.C.; García-Gabaldón, M.; Pérez-Herranz, V. pH effect on zinc recovery from the spent pickling baths of hot dip galvanizing industries. Sep. Purif. Technol. 2017, 177, 21–28. [Google Scholar] [CrossRef]
- Ortiz, I.; Bringas, E.; Román, M.F.S.; Urtiaga, A. Selective separation of zinc and iron from spent pickling solutions by membrane-based solvent extraction: Process viability. Sep. Sci. Technol. 2005, 39, 2441–2455. [Google Scholar] [CrossRef]
- Pietrelli, L.; Ferro, S.; Vocciante, M. Raw materials recovery from spent hydrochloric acid-based galvanizing wastewater. Chem. Eng. J. 2018, 341, 539–546. [Google Scholar] [CrossRef]
- Yang, F.; Wu, Y.; Fang, X.; Ma, L. Experimental and theoretical study on the behaviour of a pickling solution: The role of ferrous ions. J. Clean. Prod. 2020, 243, 118631. [Google Scholar] [CrossRef]
- Gueccia, R.; Aguirre, A.R.; Randazzo, S.; Cipollina, A.; Micale, G. Diffusion dialysis for separation of hydrochloric acid, iron and zinc ions from highly concentrated pickling solutions. Membranes 2020, 10, 129. [Google Scholar] [CrossRef] [PubMed]
- Regel-Rosocka, M. A review on methods of regeneration of spent pickling solutions from steel processing. J. Hazard. Mater. 2010, 177, 57–69. [Google Scholar] [CrossRef]
- Devi, A.; Singhal, A.; Gupta, R.; Panzade, P. A study on treatment methods of spent pickling liquor generated by pickling process of steel. Clean Technol. Environ. Policy 2014, 16, 1515–1527. [Google Scholar] [CrossRef]
- European Comission-Integrated Pollution Prevention and Control. Reference Document on Best Available Techniques in the Ferrous Metals Processing Industry; European Comission: Brussels, Belgium, 2001. [Google Scholar]
- Abad, J.C.; Gabaldón, M.G.; Ortega, E.M.; Pérez-Herranz, V. Electrochemical recovery of zinc from the spent pickling baths coming from the hot dip galvanizing industry. Potentiostatic operation. Sep. Purif. Technol. 2011, 81, 200–207. [Google Scholar] [CrossRef]
- Carrera, J.; Bringas, E.; Román, M.S.; Ortiz, I. Selective membrane alternative to the recovery of zinc from hot-dip galvanizing effluents. J. Membr. Sci. 2009, 326, 672–680. [Google Scholar] [CrossRef]
- Csicsovszki, G.; Kékesi, T.; Török, T.I. Selective recovery of Zn and Fe from spent pickling solutions by the combination of anion exchange and membrane electrowinning techniques. Hydrometallurgy 2005, 77, 19–28. [Google Scholar] [CrossRef]
- Ortiz, I.; Bringas, E.; Samaniego, H.; Roman, M.F.S.; Urtiaga, A. Membrane processes for the efficient recovery of anionic pollutants. Desalination 2006, 193, 375–380. [Google Scholar] [CrossRef]
- Samaniego, H.; Román, M.F.S.; Ortiz, I. Kinetics of zinc recovery from spent pickling effluents. Ind. Eng. Chem. Res. 2007, 46, 907–912. [Google Scholar] [CrossRef]
- Carrera, J.; Munoz, E.; Bringas, E.; Román, M.S.; Ortiz, I. Influence of operation variables on the recovery of zinc from spent pickling effluents using the emulsion pertraction technology. Desalination 2009, 245, 675–679. [Google Scholar] [CrossRef]
- Regel-Rosocka, M.; Cieszynska, A.; Wiśniewski, M. Methods of regeneration of spent pickling solutions from steel treatment plants. Pol. J. Chem. Technol. 2007, 9, 42–45. [Google Scholar] [CrossRef] [Green Version]
- Mansur, M.B. Solvent extraction for metal and water recovery from industrial wastes and effluents Extração por solventes aplicada à recuperação de metais e da água a partir de resíduos industriais e efluentes líquidos. REM Rev. Esc. Minas 2011, 64, 51–55. [Google Scholar] [CrossRef] [Green Version]
- Cook, S.J.; Perera, J.M.; Stevens, G.W.; Kentish, S.E. The screening of extractants for the separation of Zn(II) from Australian hot-dip galvanizing effluent. Sep. Sci. Technol. 2011, 46, 2066–2074. [Google Scholar] [CrossRef]
- Regel-Rosocka, M.; Wiśniewski, M. Selective removal of zinc(II) from spent pickling solutions in the presence of iron ions with phosphonium ionic liquid Cyphos IL 101. Hydrometallurgy 2011, 110, 85–90. [Google Scholar] [CrossRef]
- Wieszczycka, K. Recovery of Zn(II) from multielemental acidic chloride solution with hydrophobic 3-pyridineketoxime. Sep. Purif. Technol. 2013, 114, 17–23. [Google Scholar] [CrossRef]
- Román, M.S.; Gándara, I.O.; Ibáñez, R.; Ortiz, I. Hybrid membrane process for the recovery of major components (zinc, iron and HCl) from spent pickling effluents. J. Membr. Sci. 2012, 415, 616–623. [Google Scholar] [CrossRef]
- Sinha, M.K.; Sahu, S.K.; Meshram, P.; Pandey, B.D. Solvent extraction and separation of zinc and iron from spent pickle liquor. Hydrometallurgy 2014, 147–148, 103–111. [Google Scholar] [CrossRef]
- Abad, J.C.; Gabaldón, M.G.; Pérez-Herranz, V. Study of the zinc recovery from spent pickling baths by means of an electrochemical membrane reactor using a cation-exchange membrane under galvanostatic control. Sep. Purif. Technol. 2014, 132, 479–486. [Google Scholar] [CrossRef]
- Abad, J.C.; Gabaldón, M.G.; Pérez-Herranz, V. Treatment of spent pickling baths coming from hot dip galvanizing by means of an electrochemical membrane reactor. Desalination 2014, 343, 38–47. [Google Scholar] [CrossRef] [Green Version]
- Lum, K.H.; Cook, S.J.; Stevens, G.W.; Perera, J.M.; Kentish, S.E. Zinc chloride and hydrochloric acid coextraction from galvanizing pickling waste in the presence of Iron(II). results with hollow fiber membrane contactors. Ind. Eng. Chem. Res. 2014, 53, 4453–4461. [Google Scholar] [CrossRef]
- Laso, J.; García, V.; Bringas, E.; Urtiaga, A.; Ortiz, I. Selective recovery of zinc over iron from spent pickling wastes by different membrane-based solvent extraction process configurations. Ind. Eng. Chem. Res. 2015, 54, 3218–3224. [Google Scholar] [CrossRef]
- Diban, N.; Mediavilla, R.; Urtiaga, A.; Ortiz, I. Zinc recovery and waste sludge minimization from chromium passivation baths. J. Hazard. Mater. 2011, 192, 801–807. [Google Scholar] [CrossRef]
- Wieszczycka, K.; Regel-Rosocka, M.; Staszak, K.; Wojciechowska, A.; Carvalho, J.M.; Ismael, M.R.C.; Gameiro, M.L.F.; Carvalho, J.M. Recovery of zinc(II) from chloride solutions using pseudo-emulsion based hollow fiber strip dispersion (PEHFSD) with 1-(3-pyridyl)undecan-1-one oxime or tributylphosphate. Sep. Purif. Technol. 2015, 154, 204–210. [Google Scholar] [CrossRef]
- Van Genderen, E.; Wildnauer, M.; Santero, N.; Sidi, N. A global life cycle assessment for primary zinc production. Int. J. Life Cycle Assess. 2016, 21, 1580–1593. [Google Scholar] [CrossRef] [Green Version]
- London Metal Exchange LME Zinc. Available online: https://www.lme.com/en-GB/Metals/Non-ferrous/Zinc#tabIndex=0 (accessed on 19 November 2020).
- Ortiz-Gándara, I. Recovery of HCl from Galvanizing Effluents by Ion. Exchange Membranes. Ph.D. Thesis, University of Cantabria, Santander, Spain, 2016. [Google Scholar]
- Técnicas Reunidas Historia: División de Desarrollo de Tecnologías Propias. Available online: https://ddtp.tecnicasreunidas.es/quienes-somos/historia/ (accessed on 18 November 2020).
- Urtiaga, A.; Bringas, E.; Mediavilla, R.; Ortiz, I. The role of liquid membranes in the selective separation and recovery of zinc for the regeneration of Cr(III) passivation baths. J. Membr. Sci. 2010, 356, 88–95. [Google Scholar] [CrossRef]
- Regel, M.; Sastre, A.M.; Szymanowski, J. Recovery of zinc(II) from HCl spent pickling solutions by solvent extraction. Environ. Sci. Technol. 2001, 35, 630–635. [Google Scholar] [CrossRef]
- Cierpiszewski, R.; MiesiacI, I.; Regel-Rosocka, M.; Sastre, A.M.; Szymanowski, J. Removal of Zinc(II) from spent hydrochloric acid solutions from zinc hot galvanizing plants. Ind. Eng. Chem. Res. 2002, 41, 598–603. [Google Scholar] [CrossRef]
- Regel-Rosocka, M.; Szymanowski, J. Iron(II) transfer to the organic phase during Zinc(II) extraction from spent pickling solutions with tributyl phosphate. Solvent Extr. Ion. Exch. 2005, 23, 411–424. [Google Scholar] [CrossRef]
- Sayar, N.A.; Filiz, M.; Sayar, A. Extraction of Zn(II) from aqueous hydrochloric acid solutions into Alamine 336-m-xylene systems. Modeling considerations to predict optimum operational conditions. Hydrometallurgy 2007, 86, 27–36. [Google Scholar] [CrossRef]
- Mansur, M.B.; Rocha, S.D.; Magalhães, F.S.; Benedetto, J.D.S. Selective extraction of zinc(II) over iron(II) from spent hydrochloric acid pickling effluents by liquid–liquid extraction. J. Hazard. Mater. 2008, 150, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Lum, K.H.; Stevens, G.W.; Perera, J.M.; Kentish, S.E. The modelling of ZnCl2 extraction and HCl co-extraction by TBP diluted in ShellSol 2046. Hydrometallurgy 2013, 133, 64–74. [Google Scholar] [CrossRef]
- Lum, K.H.; Stevens, G.W.; Kentish, S.E. Development of a process for the recovery of zinc sulphate from hot-dip galvanizing spent pickling liquor via two solvent extraction steps. Hydrometallurgy 2014, 142, 108–115. [Google Scholar] [CrossRef]
- Lee, K.-R.; Kim, J.; Jang, J.-G. Recovery of zinc in spent pickling solution with oxalic acid. Korean Chem. Eng. Res. 2017, 55, 785–790. [Google Scholar] [CrossRef]
- Le, M.N.; Nguyen, T.H.; Lee, M.S. Extraction and stripping behavior of hydrochloric acid from aqueous solution by Cyanex 923/TEHA and its mixtures. Geosystem Eng. 2018, 22, 129–137. [Google Scholar] [CrossRef]
- Wang, Y.; He, Y.; Yin, S.; Long, H.; Li, S. Research on extraction of zinc from spent pickling solution using Aliquat 336. Hydrometallurgy 2020, 193, 105322. [Google Scholar] [CrossRef]
- Regel-Rosocka, M.; Nowak, Ł.; Wiśniewski, M. Removal of zinc(II) and iron ions from chloride solutions with phosphonium ionic liquids. Sep. Purif. Technol. 2012, 97, 158–163. [Google Scholar] [CrossRef]
- Román, M.F.S.; Ortiz-Gándara, I.; Bringas, E.; Ibáñez, R.; Ortiz, I. Membrane selective recovery of HCl, zinc and iron from simulated mining effluents. Desalination 2018, 440, 78–87. [Google Scholar] [CrossRef]
- Urtiaga, A.; Abellán, M.; Irabien, A.; Ortiz, I. Membrane contactors for the recovery of metallic compounds. J. Membr. Sci. 2005, 257, 161–170. [Google Scholar] [CrossRef]
- Pabby, A.K.; Swain, B.; Sastre, A.M. Recent advances in smart integrated membrane assisted liquid extraction technology. Chem. Eng. Process. Process. Intensif. 2017, 120, 27–56. [Google Scholar] [CrossRef]
- Alonso, A.; Urtiaga, A.; Zamacona, S.; Irabien, A.; Ortiz, I. Kinetic modelling of cadmium removal from phosphoric acid by non-dispersive solvent extraction. J. Membr. Sci. 1997, 130, 193–203. [Google Scholar] [CrossRef]
- Abad, J.C.; Gabaldón, M.G.; Ortiz-Gandara, I.; Bringas, E.; Urtiaga, A.; Ortiz, I.; Pérez-Herranz, V. Selective recovery of zinc from spent pickling baths by the combination of membrane-based solvent extraction and electrowinning technologies. Sep. Purif. Technol. 2015, 151, 232–242. [Google Scholar] [CrossRef]
- 3M TM Liqui-Cel TM Membrane Contactors-Data Sheets. Available online: https://www.3m.com.es/3M/es_ES/liquicel-technology-gas-control-es/resources/data-sheets/ (accessed on 7 September 2020).
- Puigdomenech, I. HYDRA (Hydrochemical Equilibrium-Constant Database) and MEDUSA (Make Equilibrium Diagrams Using Sophisticated Algorithms) Programs; Royal Institute of Technology: Stockholm, Sweden, 2006; Available online: http//www.kemi.kth.se/medusa2006 (accessed on 16 September 2020).
- Samaniego, H.; Román, M.F.S.; Ortiz, I. Modelling of the extraction and back-extraction equilibria of zinc from spent pickling solutions. Sep. Sci. Technol. 2006, 41, 757–769. [Google Scholar] [CrossRef]
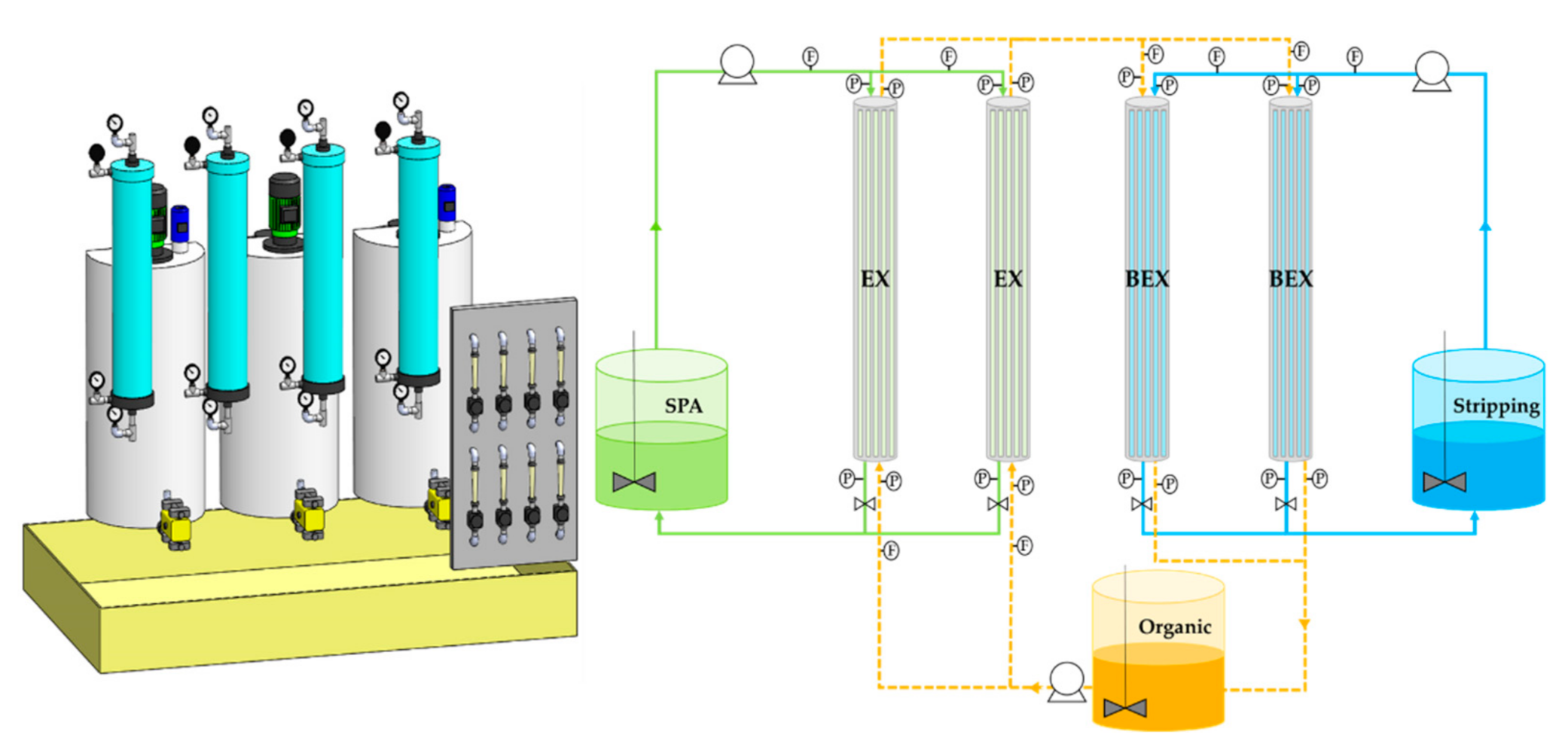
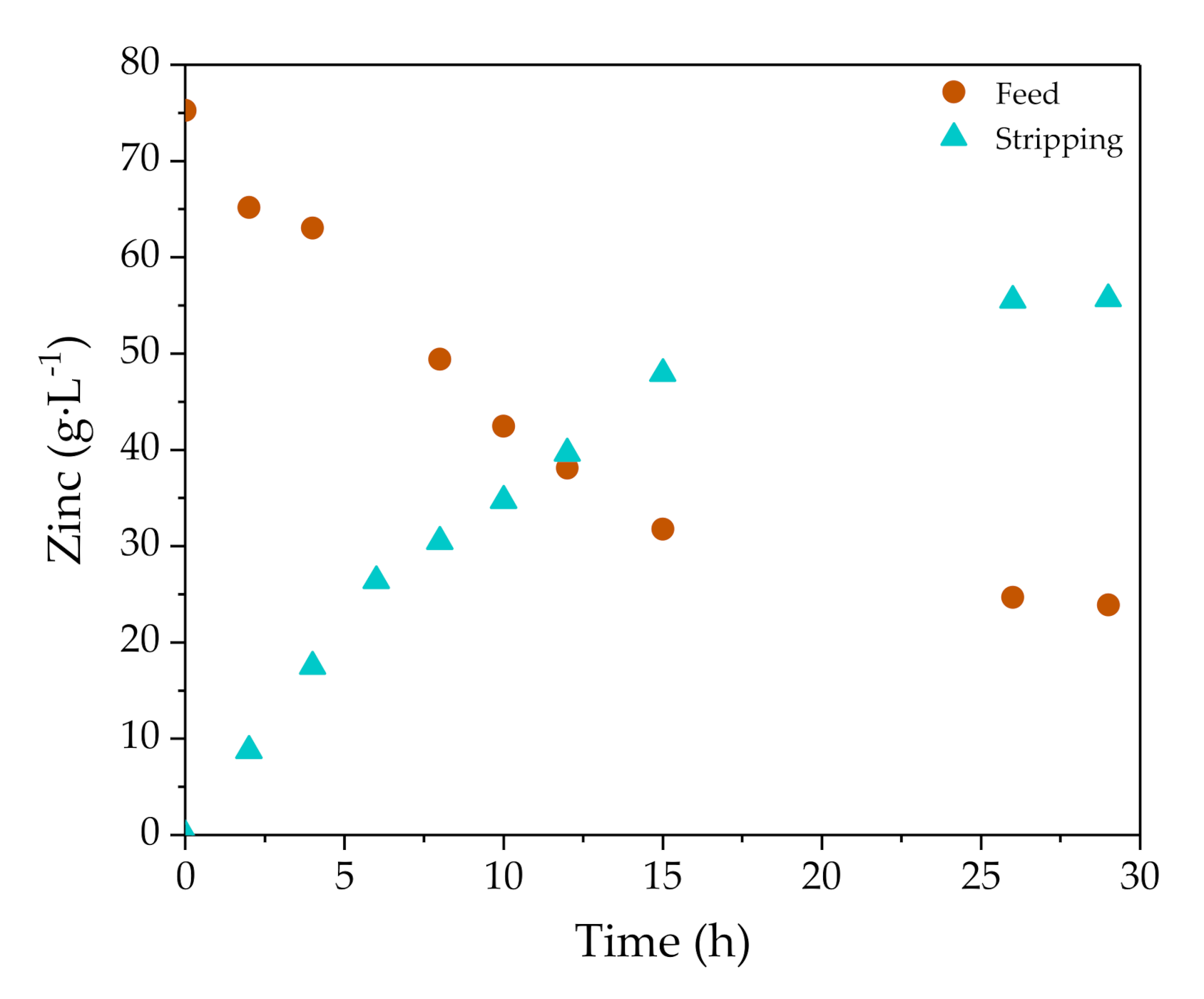
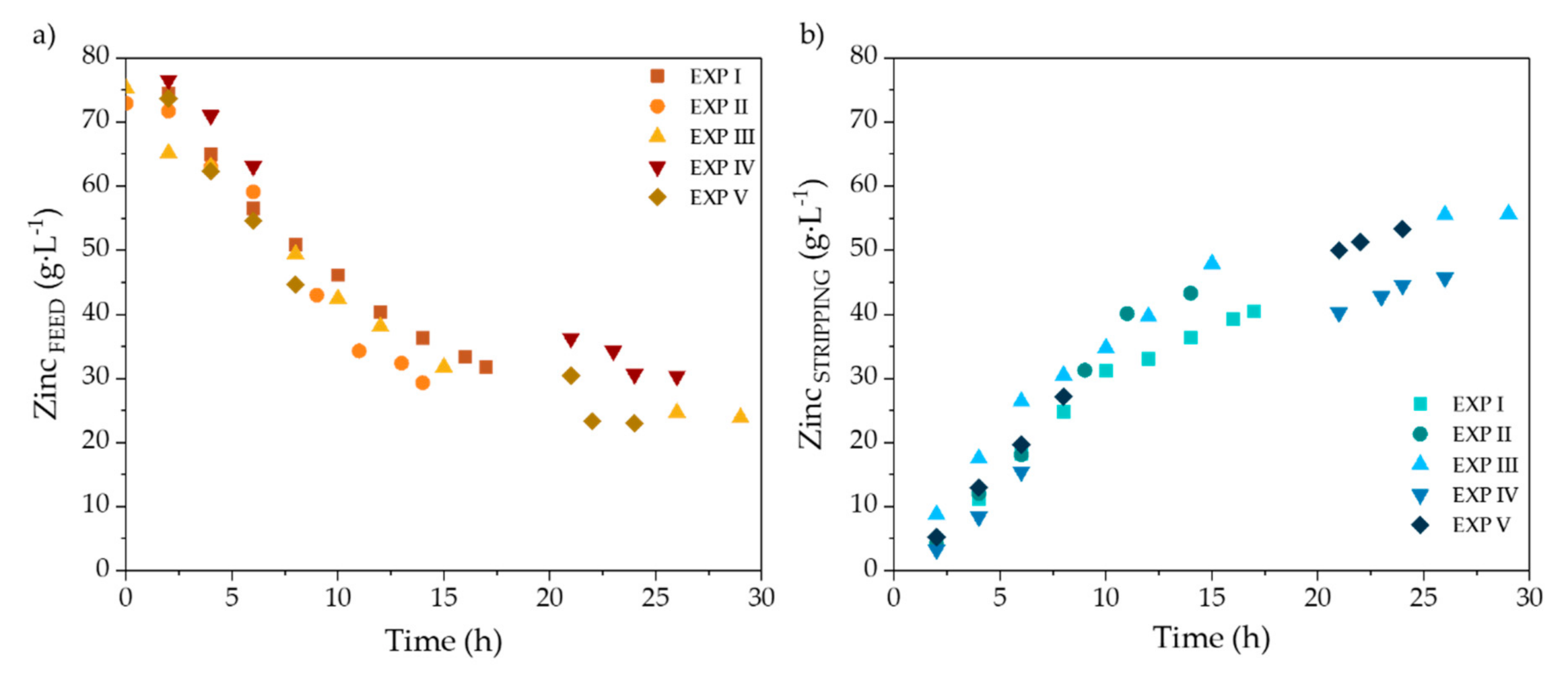
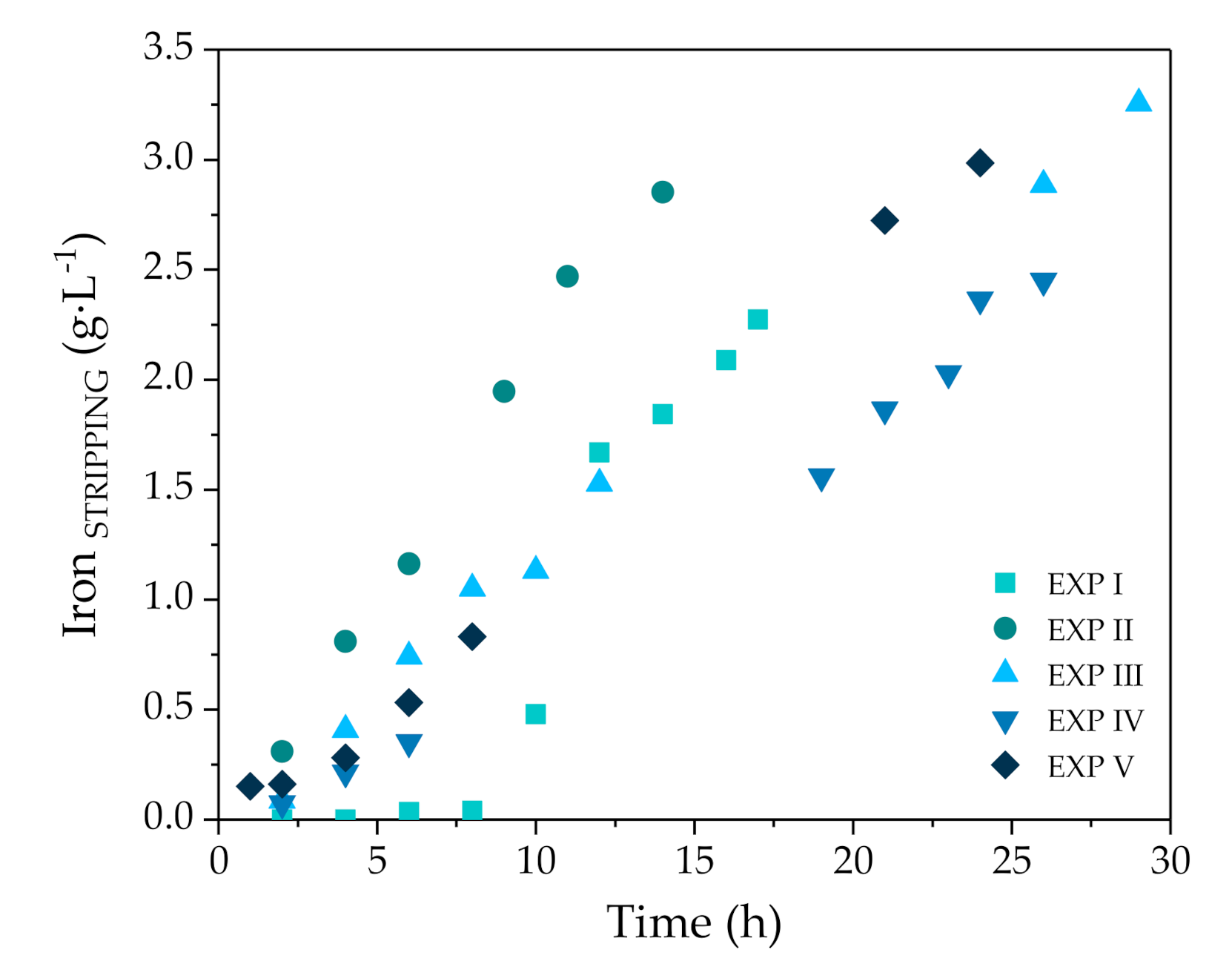
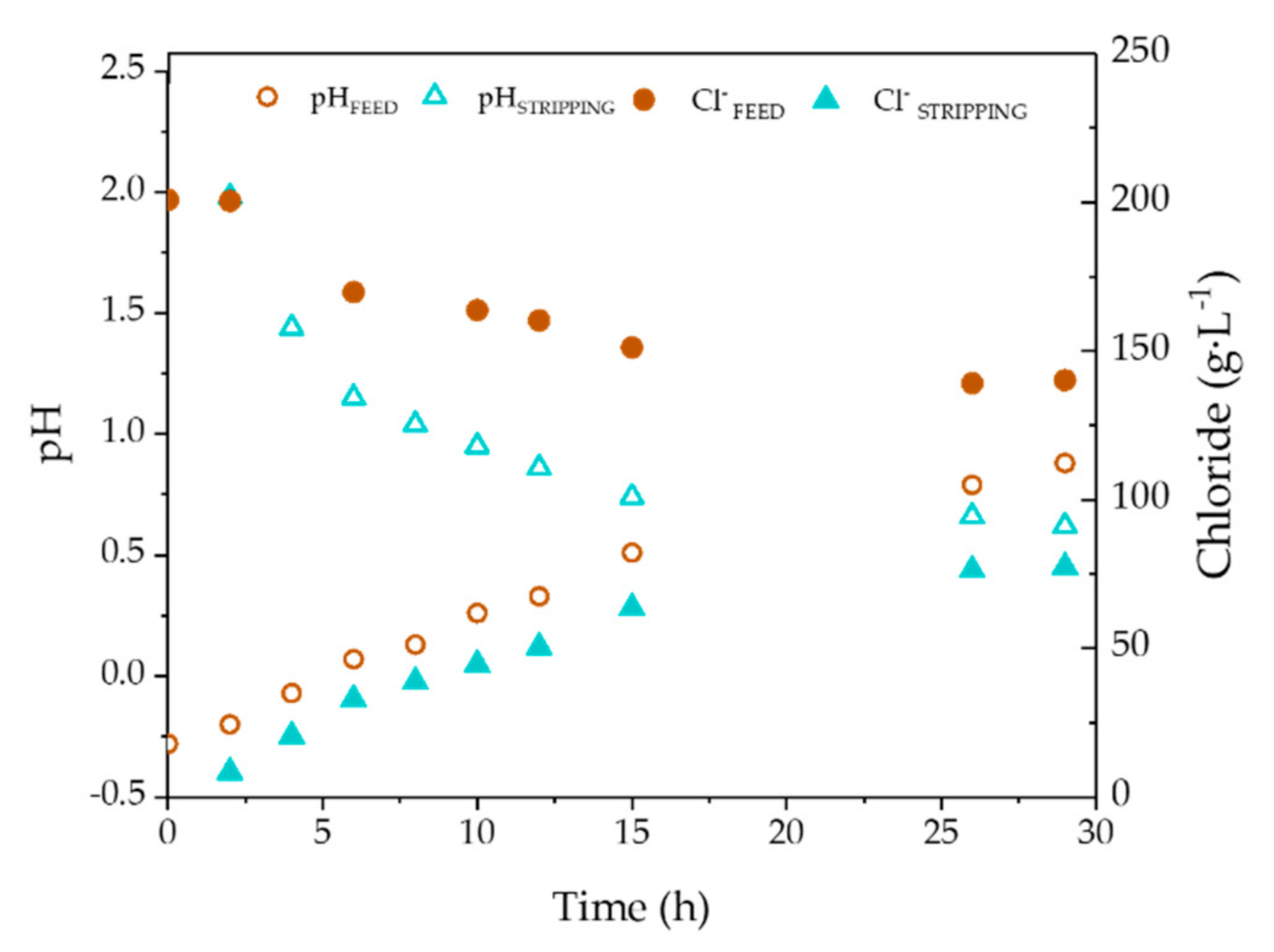
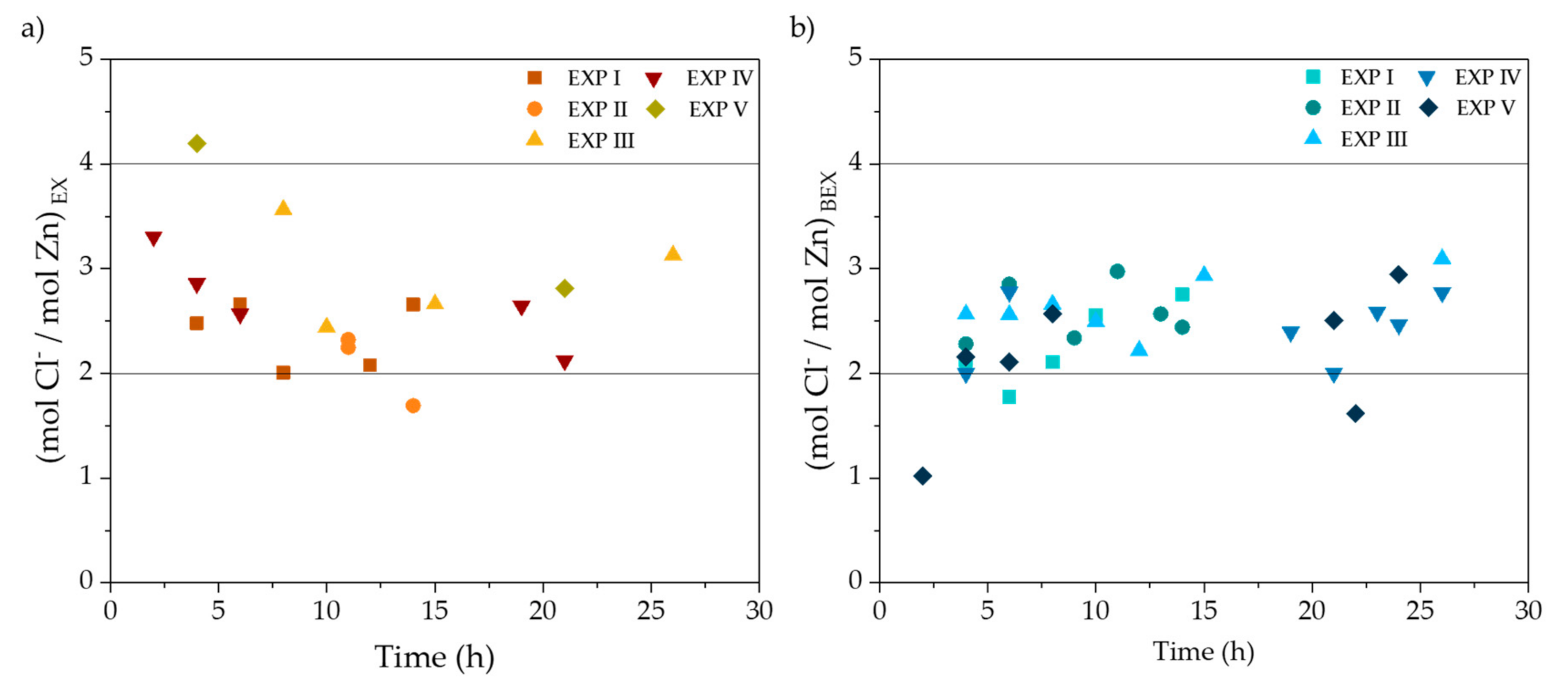
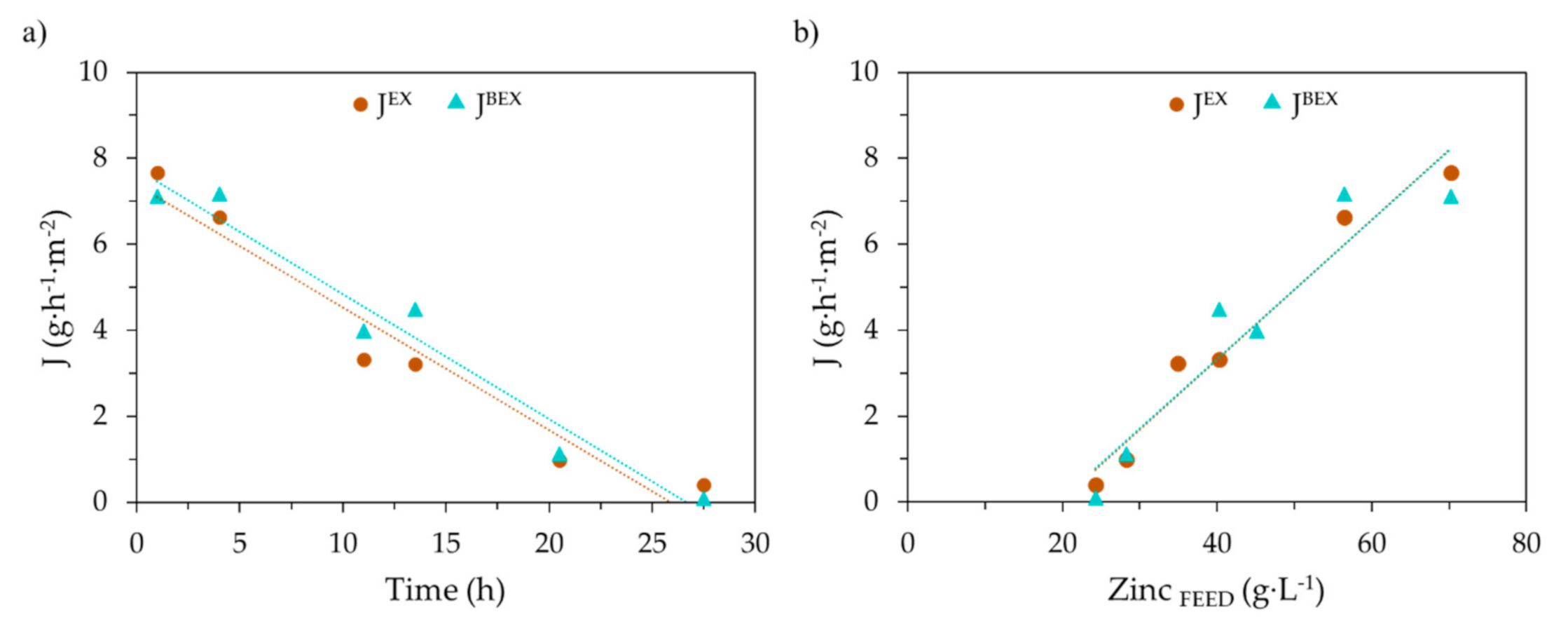
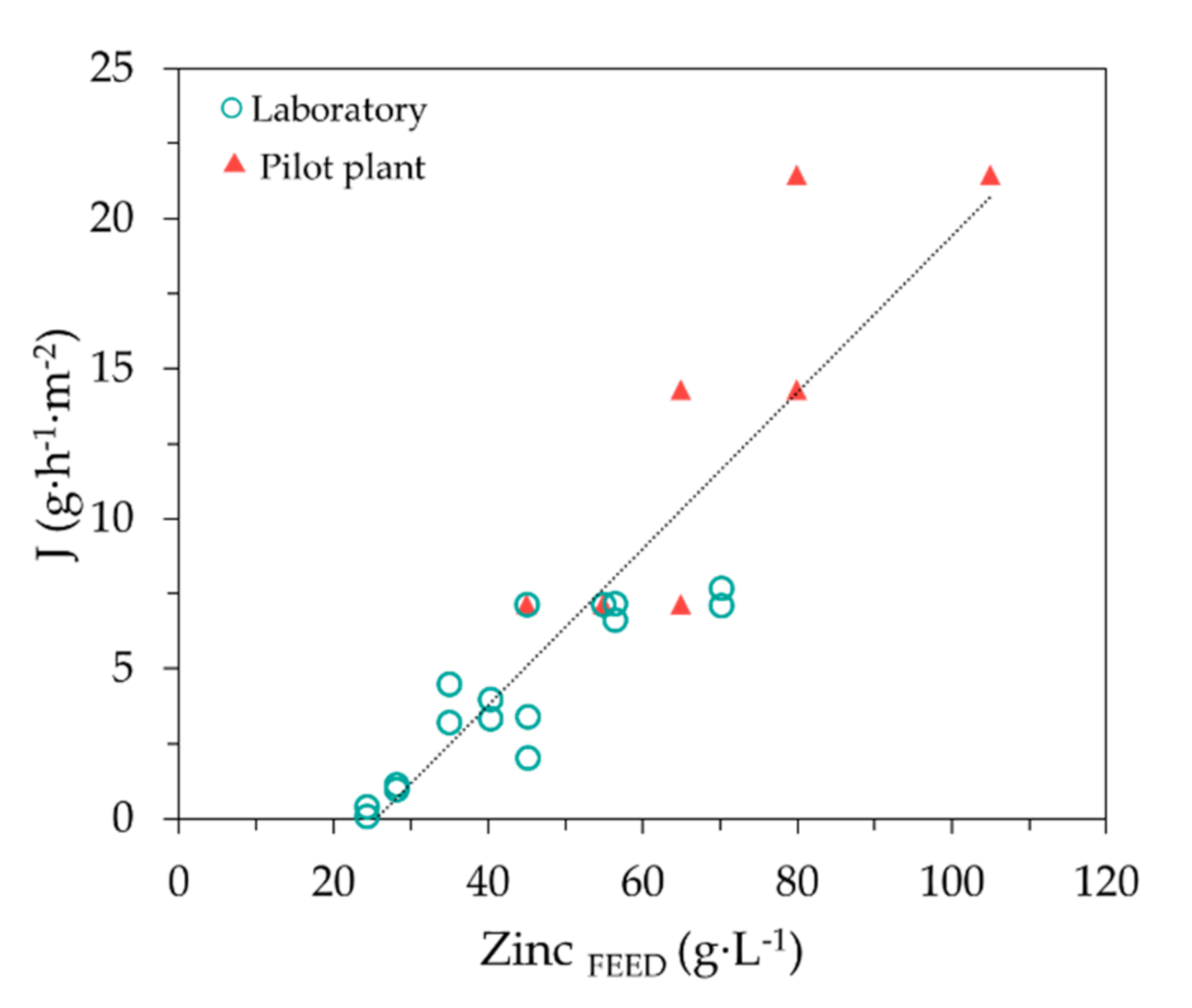
| Zn (g·L−1) | FeTOTAL (g·L−1) | Fe2+ (g·L−1) | Fe3+ (g·L−1) | HCl (g·L−1) | H+ (mol·L−1) | Cl− (g·L−1) | pH | Ref. |
|---|---|---|---|---|---|---|---|---|
| 26.1 | 137.5 | - | - | 17.9 | - | - | - | [11] |
| 82 | - | 96 | - | - | - | 227 | ≈0 | [10,12,13,14] |
| <130 | <100 | - | - | 10% | - | - | - | [15] |
| 20–120 | 100–130 | - | - | 36.5–219 | - | - | - | [2] |
| 70.2 | 92.2 | - | - | 9.1 | - | - | - | [16] |
| 5–150 | 8–150 | - | - | 10–80 | - | - | - | [1,9] |
| <150 | - | - | - | 3.6% | - | - | - | [17] |
| <120 | <204 | - | - | - | - | - | - | [18,19] |
| 78.5 | 89.4 | - | - | 237 | - | - | - | [20] |
| - | - | 35–45 | - | - | - | - | - | [7] |
| 100–120 | - | 30–32 | 1–2 | 90–100 | - | 230–250 | - | [21] |
| 129.3 | 58.8 | - | - | 78 | - | - | - | [22,23] |
| 134 | - | 74 | <1 | 36 | - | 300 | - | [24] |
| 122 ± 3 | 95.6 ± 3 | 92.6 ± 2 | 3 ± 2 | - | 1.1 ± 0.04 | 301 ± 3 | - | [25] |
| Method/Configuration | Extractant | Diluent | Stripping Agent | Ref. |
|---|---|---|---|---|
| SX | Cyanex 921/Cyanex 923/Cyanex 302/TBP/ALAMINE336 | Kerosene | Water | [33] |
| TBP/ DEHPA/HOE F 2562/ ALIQUAT 336/ALAMINE 304-308-310-336/CYANEX 301 | Kerosene Exxsol D220/230 | Water/HCl | [34] | |
| TBP | Exxsol D 220/230 | Water | [35] | |
| Alamine 336 | m-Xylene | Na2CO3 (aq.) | [36] | |
| TBP/Cyanex 272/Cyanex 301/Cyanex 302 | Kerosene | Water/HCl | [37] | |
| TBP/Hostarex A226, A324, and A327 | Kerosene | Water | [17] | |
| TBP/Cyanex 301/Cyanex 272 | Exxsol D-80 | H2SO4 | [16] | |
| Cyphos IL 101 | Toluene | H2SO4 | [18] | |
| 1-(3-pyridyl)undecan-1-one | Decan-1-ol | Na2SO4 | [19] | |
| TBP | ShellSol 2046 | Water | [38] | |
| Tri-iso-octyl amine (TiOA)/tris(2-ethylhexyl) amine (TEHA) | Di-(2-ethylhexyl) phosphoric acid (DEHPA) | Water/H2SO4 | [21] | |
| TBP, D2EHPA | Shellsol 2046 | Water | [39] | |
| TBP | - | Oxalic acid | [40] | |
| TBP/D2EHPA | Kerosene | Water/HCl | [3] | |
| Cyanex 923/TEHA | Kerosene | Diluted ammonia | [41] | |
| Aliquat 336 | Sulfonated kerosene | Water/H2SO4/NaOH/NH3·H2O | [42] | |
| NDSX | TBP | - | Water | [13] |
| TBP/Cyanex 272/DEHPA | Kerosene | Water/H2SO4 | [2] | |
| TBP | Shellsol 2046 | Water | [24] | |
| TBP | - | Water | [12] | |
| EPT | TBP | - | Water | [14] |
| 1-(3-pyridyl)undecan-1-one oxime/TBP | Toluene, ShellSol D70 and decan-1-ol | Na2SO4/Water | [27] | |
| NDSX/EPT | TBP | Shellsol D70 | Water | [25] |
| SX and Polymer inclusion membranes (PIM) | Cyphos IL 101/ Cyphos IL 104 | Toluene | H2SO4 | [43] |
| EPT and DD | TBP | - | Water/NaOH | [20] |
| NDSX and ED | TBP | - | Water | [44] |
| pH | - | ≈0 |
|---|---|---|
| Free acidity (HCl) | g·L−1 | 13.4 ± 3.4 |
| Chloride | 212.6 ± 41.6 | |
| Zinc | 71.7 ± 4.3 | |
| Iron | 82.9 ± 5.0 | |
| Manganese | 0.91 ± 0.01 | |
| Chromium | 0.07 ± 0.01 | |
| Aluminium | 0.04 ± 0.01 | |
| Molybdenum | 0.05 ± 0.01 | |
| Nickel | <0.0025 | |
| Tin | ||
| Bismuth | ||
| Lead | ||
| Wolfram | ||
| NH4+ | 0.050 | |
| Total organic carbon (TOC) | 2.93 |
| Characteristic | Unit | Pilot Plant (Liqui-Cel Extra-Flow 4 in. × 28 in.) |
|---|---|---|
| Cartridge dimensions (D × L) | cm | 11.6 × 88.9 |
| Num. fibers | - | 36,675 |
| Effective membrane surface area | m2 | 20 |
| Effective length | m | 0.789 |
| Fiber type | - | ×50 |
| Fiber material | - | Polypropylene |
| Inner diameter (fibers) | µm | 220 |
| Wall thickness (fibers) | µm | 80 |
| Porosity | % | 40 |
| EXP. | ||||||
|---|---|---|---|---|---|---|
| Exp. Conditions | Units | I | II | III | IV | V |
| Feed volume | L | 69 | 57 | 61 | 91 | 71 |
| Organic volume | 83 | 86 | 76 | 76 | 75 | |
| Stripping volume | 97 | 59 | 65 | 125 | 94 | |
| Feed phase (SPA) flowrate | L·h−1 | 200 | 50 | 50 | 50 | 100 |
| Organic extractant phase flowrate | 200 | 150 | 150 | 150 | 200 | |
| Stripping phase (water) flowrate | 200 | 50 | 100 | 100 | 150 | |
| Hollow fiber membrane contactors | - | 4 | ||||
| Extraction membrane area | m2 | 40 | ||||
| Backextraction membrane area | 40 | |||||
| EXP. | ||||||
|---|---|---|---|---|---|---|
| Units | I | II | III | IV | V | |
| ZincTOTAL | g·L−1 | 40.5 | 43.3 | 55.7 | 45.7 | 53.3 |
| IronTOTAL | 2.3 | 2.9 | 3.3 | 2.4 | 3.0 | |
| Chloride (Cl−) | 55.9 | 58.6 | 77.4 | 59.3 | 67.4 | |
| Free acidity (H+) | 0.173 | 0.223 | 0.272 | 0.416 | 0.128 | |
| Manganese | 0.026 | 0.010 | 0.047 | 0.040 | 0.018 | |
| Molybdenum | 0.003 | 0.005 | 0.008 | 0.006 | 0.001 | |
| Tin | 0.001 | 0.002 | 0.002 | 0.002 | 0.001 | |
| pH | 0.80 | 0.69 | 0.62 | 0.73 | 0.72 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arguillarena, A.; Margallo, M.; Arruti-Fernández, A.; Pinedo, J.; Gómez, P.; Urtiaga, A. Scale-Up of Membrane-Based Zinc Recovery from Spent Pickling Acids of Hot-Dip Galvanizing. Membranes 2020, 10, 444. https://doi.org/10.3390/membranes10120444
Arguillarena A, Margallo M, Arruti-Fernández A, Pinedo J, Gómez P, Urtiaga A. Scale-Up of Membrane-Based Zinc Recovery from Spent Pickling Acids of Hot-Dip Galvanizing. Membranes. 2020; 10(12):444. https://doi.org/10.3390/membranes10120444
Chicago/Turabian StyleArguillarena, Andrea, María Margallo, Axel Arruti-Fernández, Javier Pinedo, Pedro Gómez, and Ane Urtiaga. 2020. "Scale-Up of Membrane-Based Zinc Recovery from Spent Pickling Acids of Hot-Dip Galvanizing" Membranes 10, no. 12: 444. https://doi.org/10.3390/membranes10120444
APA StyleArguillarena, A., Margallo, M., Arruti-Fernández, A., Pinedo, J., Gómez, P., & Urtiaga, A. (2020). Scale-Up of Membrane-Based Zinc Recovery from Spent Pickling Acids of Hot-Dip Galvanizing. Membranes, 10(12), 444. https://doi.org/10.3390/membranes10120444






