Membrane Bioreactor Technology: The Effect of Membrane Filtration on Biogas Potential of the Excess Sludge
Abstract
1. Introduction
2. Materials and Methods
2.1. Characteristics of WWTP
2.2. Membrane Filtration
2.3. Analytical Methods
2.4. Calculation Methods
3. Results and Discussion
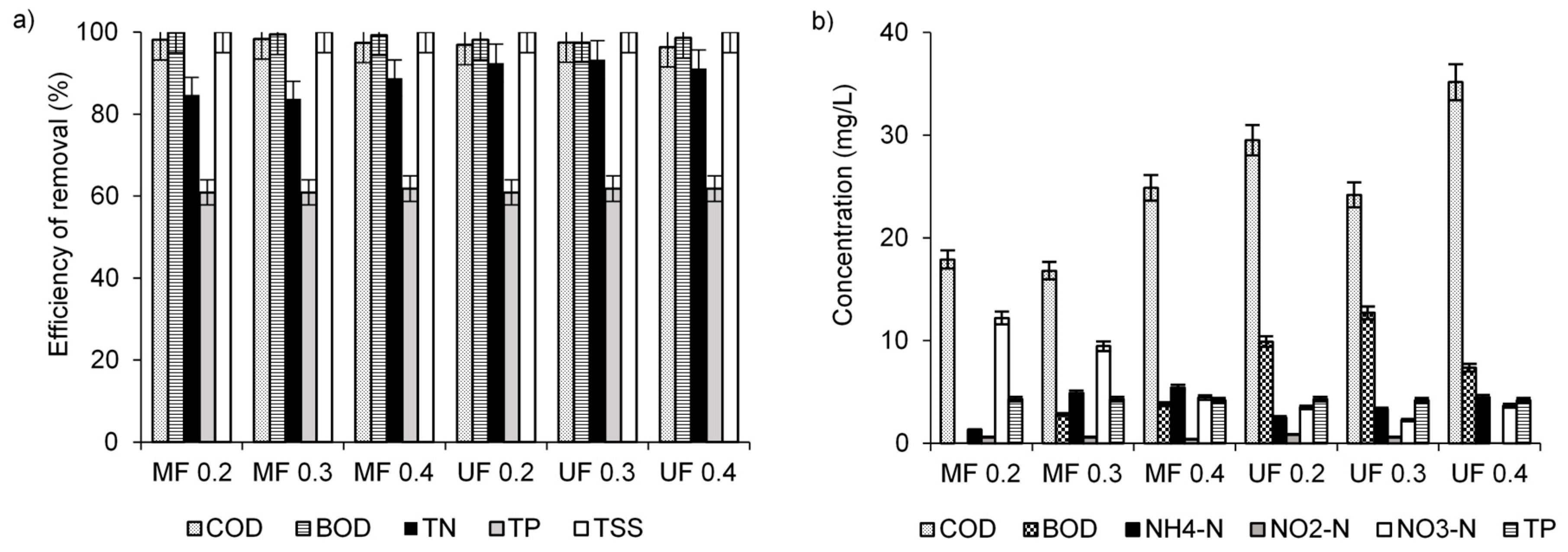
3.1. Efficiency of Wastewater Treatment with the Use of Membrane Separation
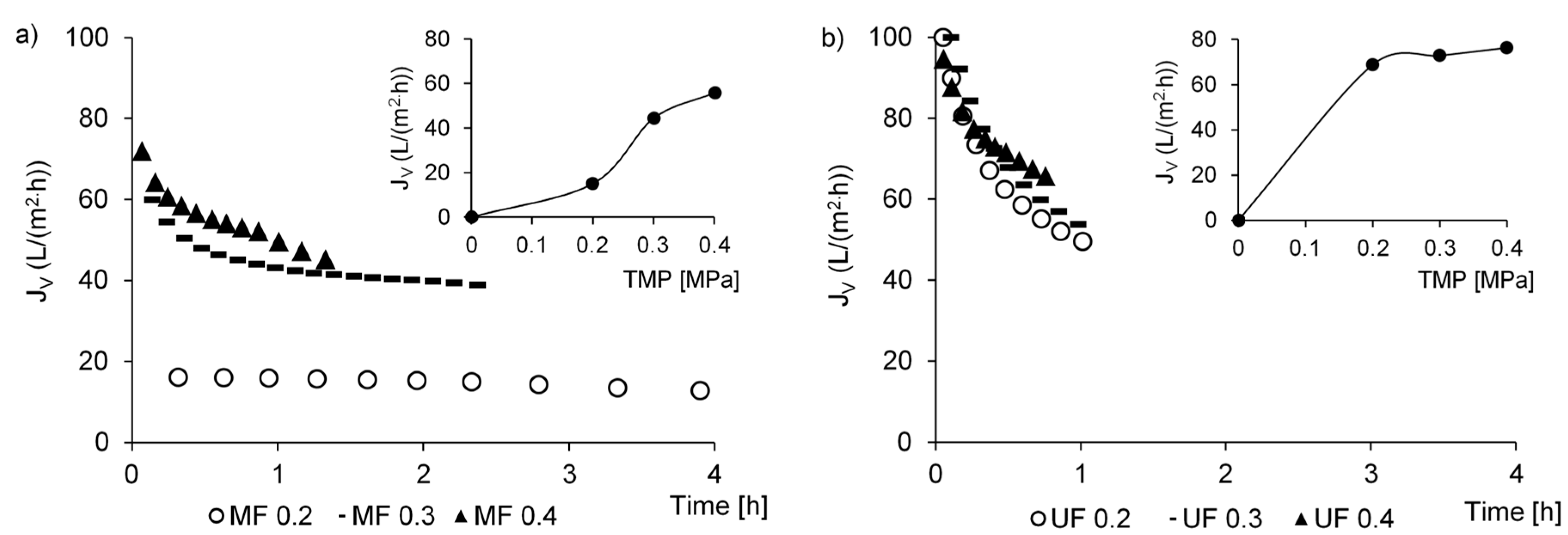
3.2. Hydraulic Capacity of the Membrane Installation
3.3. Biogas Production
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Judd, S.; Judd, C. The MBR Book, Principles and Applications of Membrane Bioreactors for Water and Wastewater Treatment, 2nd ed.; Butterworth-Heinemann, Cranfield University: Oxford, UK, 2011. [Google Scholar]
- Baresel, C.; Westling, K.; Samuelsson, O.; Andersson, S.; Royen, H.; Andersson, S.; Dahlén, N. Membrane bioreactor processes to meet todays and future municipal sewage treatment requirements? Int. J. Water Wastewater Treat. 2017, 3, 1–7. [Google Scholar]
- Judd, S. The status of membrane bioreactor technology. Trends Biotechnol. 2008, 26, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Melin, T.; Jefferson, B.; Bixio, D.; Thoeye, C.; De Wilde, W.; De Koning, J.; van der Graaf, J.; Wintgens, T. Membrane bioreactor technology for wastewater treatment and reuse. Desalination 2006, 187, 271–282. [Google Scholar] [CrossRef]
- Laurinonyte, J.; Meulepas, R.J.W.; van den Brink, P.; Temmink, H. Membrane bioreactor (MBR) as alternative to a conventional activated sludge system followed by ultrafiltration (CAS-UF) for the treatment of Fischer-Tropsch reaction water from gas-to-liquids industries. Water Air Soil Pollut. 2017, 228, 137. [Google Scholar] [CrossRef]
- Witzig, R.; Manz, W.; Rosenberger, S.; Krüger, U.; Kraume, M.; Szewzyk, U. Microbiological aspects of a bioreactor with submerged membranes for aerobic treatment of municipal wastewater. Water Res. 2002, 36, 394–402. [Google Scholar] [CrossRef]
- Wang, L.; Liang, W.; Chen, W.; Zhang, W.; Mo, J.; Liang, K.; Tang, B.; Zheng, Y.; Jiang, F. Integrated aerobic granular sludge and membrane process for enabling municipal wastewater treatment and reuse water production. Chem. Eng. J. 2018, 337, 300–311. [Google Scholar] [CrossRef]
- Yang, Y.; Xia, S.; Hermanowicz, S.W. Full-scale engineering application of MBR system in municipal wastewater treatment plants around China’s Tai Lake basin. Fresenius Environ. Bull. 2015, 24, 3616–3626. [Google Scholar]
- Sun, F.Y.; Wang, X.M.; Li, X.Y. An innovative membrane bioreactor (MBR) system for simultaneous nitrogen and phosphorus removal. Process Biochem. 2013, 48, 1749–1759. [Google Scholar] [CrossRef]
- Meng, F.; Chae, S.-R.; Drews, A.; Kraume, M.; Shin, H.-S.; Yang, F. Recent advances in membrane bioreactors (MBRs): Membrane fouling and membrane material. Water Res. 2009, 43, 1489–1512. [Google Scholar] [CrossRef]
- Lee, S.J.; Dilaver, M.; Park, P.K.; Kim, J.H. Comparative analysis of fouling characteristics of ceramic and polymeric microfiltration membranes using filtration models. J. Membr. Sci. 2013, 432, 97–105. [Google Scholar] [CrossRef]
- Rezakazemi, M.; Mirzaei, S.; Asghari, M.; Ivakpour, J. Aluminum oxide nanoparticles for highly efficient asphaltene separation from crude oil using ceramic membrane technology. Oil Gas Sci. Technol. 2017, 72, 1–9. [Google Scholar] [CrossRef]
- Mestre, S.; Gozalbo, A.; Lorente-Ayza, M.M.; Sanchez, E. Low-cost ceramic membranes: A research opportunity for industrial application. J. Eur. Ceram. Soc. 2019, 39, 3392–3407. [Google Scholar] [CrossRef]
- Xiao, K.; Liang, S.; Wang, X.; Chen, C.; Huang, X. Current state and challenges of full-scale membrane bioreactor applications: A critical review. Bioresour. Technol. 2019, 271, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Van Zyl, P.J.; Wentzel, M.C.; Ekama, G.A.; Riedel, K.J. Design and start-up of a high rate anaerobic membrane bioreactor for the treatment of a low pH, high-strength, dissolved organic wastewater. Water Sci. Technol. 2008, 57, 291–295. [Google Scholar] [CrossRef]
- Liao, B.Q.; Xie, K.; Lin, H.J.; Bertoldo, D. Treatment of kraft evaporator condensate using a thermophilic submerged anaerobic membrane bioreactor. Water Sci. Technol. 2010, 61, 2177–2183. [Google Scholar] [CrossRef]
- Krzeminski, P.; van der Graaf, J.H.J.M.; van Lier, J.B. Specific energy consumption of membrane bioreactor (MBR) for sewage treatment. Water Sci. Technol. 2012, 65, 380–392. [Google Scholar] [CrossRef]
- Lin, H.; Gao, W.; Meng, F.; Liao, B.-Q.; Leung, K.-T.; Zhao, L.; Chen, J.; Hong, H. Membrane bioreactors for industrial wastewater treatment: A critical review. Crit. Rev. Environ. Sci. Technol. 2012, 42, 677–740. [Google Scholar] [CrossRef]
- Bolzonella, D.; Silviadi, F.F.; Cecchi, F.F. Application of membrane bioreactor technology for wastewater treatment and reuse in the Mediterranean region: Focusing on removal efficiency of non-conventional pollutants. J. Environ. Manag. 2010, 91, 2424–2431. [Google Scholar] [CrossRef]
- Bolzonella, D.; Pavan, P.; Battistoni, P.; Cecchi, F. Mesophilic anaerobic digestion of waste activated sludge: Influence of the solid retention time in the wastewater treatment process. Process Biochem. 2005, 40, 1453. [Google Scholar] [CrossRef]
- Zielińska, M.; Rusanowska, P.; Jarząbek, J.; Nielsen, J.L. Community dynamics of denitrifying bacteria in full-scale wastewater treatment plants. Environ. Technol. 2016, 37, 2358–2367. [Google Scholar] [CrossRef]
- Rai, C.L.; Rao, P.G. Influence of sludge disintegration by high pressure homogenizer on microbial growth in sewage sludge: An approach for excess sludge reduction. Clean Technol. Environ. 2009, 11, 437–446. [Google Scholar] [CrossRef]
- Onyeche, T.I. Economic benefits of low pressure sludge homogenization for wastewater treatment plants. In Proceedings of the IWA Specialist Conferences Moving Forward Wastewater Biosolids Sustainability, Moncton, NB, Canada, 24–27 June 2007; pp. 417–422. [Google Scholar]
- Brockmann, M.; Seyfried, C.F. Sludge activity under the conditions of crossflow microfiltration. Water Sci. Technol. 1997, 35, 173–181. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, C.H.; Chang, I.S. Effect of pump shear on the performance of a crossflow membrane bioreactor. Water Res. 2001, 35, 2137–2144. [Google Scholar] [CrossRef]
- Hermanowicz, W.; Dożańska, W.; Dojlido, J.; Koziorowski, B. Fizyczno-Chemiczne Badanie Wody i Ścieków; Arkady: Warszawa, Poland, 1999. (In Polish) [Google Scholar]
- Heerenklage, J.; Stegmann, R. Analytical methods for the determination of the biological stability of waste samples. In Proceedings of the Tenth International Waste Management and Landfill Symposium, Padova, Italy, 3–7 October 2005. [Google Scholar]
- Sun, X.; Wang, C.; Li, Y.; Wang, W.; Wei, J. Treatment of phenolic wastewater by combined UF and NF/RO processes. Desalination 2015, 355, 68–74. [Google Scholar] [CrossRef]
- LaPara, T.M.; Klatt, C.G.; Chen, R. Adaptations in bacterial catabolic enzyme activity and community structure in membrane-coupled bioreactors fed simple synthetic wastewater. J. Biotechnol. 2006, 121, 368. [Google Scholar] [CrossRef]
- Elcik, H.; Cakmakci, M.; Ozkaya, B. The fouling effects of microalgal cells on crossflow membrane filtration. J. Membr. Sci. 2016, 499, 116–125. [Google Scholar] [CrossRef]
- Gienau, T.; Brüß, U.; Kraume, M.; Rosenberger, S. Nutrient recovery from biogas digestate by optimised membrane treatment. Waste Biomass Valoriz. 2018, 9, 2337–2347. [Google Scholar] [CrossRef]
- Aouni, A.; Fersi, C.; Cuartas-Uribe, B.; Bes-Piá, A.; Alcaina-Miranda, M.I.; Dhahbi, M. Study of membrane fouling using synthetic model solutions in UF and NF processes. Chem. Eng. J. 2011, 175, 192–200. [Google Scholar] [CrossRef]
- Kumar, R.V.; Goswami, L.; Pakshirajan, K.; Pugazhenthi, G. Dairy wastewater treatment using a novel low cost tubular ceramic membrane and membrane fouling mechanism using pore blocking models. J. Water Process Eng. 2016, 13, 168. [Google Scholar] [CrossRef]
- Environmental Protection Agency. Guidelines for Water Reuse; EPA: Washington, DC, USA, 2012.
- Cowan, J.A.C.; MacTavish, F.; Brouckaert, C.J.; Jacobs, E.P. Membrane treatment strategies for red meat abattoir effluents. Water Sci. Technol. 1992, 25, 137–148. [Google Scholar] [CrossRef]
- Bick, A.; Gillerman, L.; Manor, Y.; Oron, G. Economic assessment of an integrated membrane system for secondary effluent polishing for unrestricted reuse. Water 2012, 4, 219. [Google Scholar] [CrossRef]
- Bergamasco, R.; Konradt-Moraes, L.C.; Vieira, M.F.; Fagundes-Klen, M.R.; Vieira, A.M.S. Performance of a coagulation-ultrafiltration hybrid process for water supply treatment. Chem. Eng. J. 2011, 166, 483–489. [Google Scholar] [CrossRef]
- Hwang, K.J.; Liao, C.Y.; Tung, K.L. Effect of membrane pore size on the particle fouling in membrane filtration. Desalination 2008, 234, 16. [Google Scholar] [CrossRef]
- Lim, A.L.; Bai, R. Membrane fouling and cleaning in microfiltration of activated sludge wastewater. J. Membr. Sci. 2003, 216, 279–290. [Google Scholar] [CrossRef]
- Qu, F.; Liang, H.; Zhou, J.; Nan, J.; Shao, S.; Zhang, J.; Li, G. Ultrafiltration membrane fouling caused by extracellular organic matter (EOM) from Microcystis aeruginosa: Effects of membrane pore size and surface hydrophobicity. J. Membr. Sci. 2014, 449, 58–66. [Google Scholar] [CrossRef]
- Zawieja, I.; Włodarczyk, R.; Kowalczyk, M. Biogas generation from sonicated excess sludge. Water 2019, 11, 2127. [Google Scholar] [CrossRef]
- Ruffino, B.; Campo, G.; Genon, G.; Lorenzi, E.; Novarino, D.; Scibilia, G.; Zanetti, M. Improvement of anaerobic digestion of sewage sludge in a wastewater treatment plant by means of mechanical and thermal pre-treatments: Performance, energy and economical assessment. Bioresour. Technol. 2015, 175, 298–308. [Google Scholar] [CrossRef]
- Astals, S.; Esteban-Gutiérrez, M.; Fernández-Arévalo, T.; Aymerich, E.; García-Heras, J.L.; Mata-Alvarez, J. Anaerobic digestion of seven different sewage sludges: A biodegradability and modeling study. Water Res. 2013, 47, 6033–6043. [Google Scholar] [CrossRef]
- Angelidaki, I.; Sanders, W. Assessment of the anaerobic biodegradability of macropollutants. Rev. Environ. Sci. Biotechnol. 2004, 3, 117–129. [Google Scholar] [CrossRef]
- Triolo, J.M.; Sommer, S.G.; Moller, H.B.; Weisbjerg, M.R.; Jiang, X.Y. A new algorithm to characterize biodegradability of biomass during anaerobic digestion: Influence of lignin concentration on methane production potential. Bioresour. Technol. 2011, 102, 9395–9402. [Google Scholar] [CrossRef]
- Bernat, K.; Cydzik-Kwiatkowska, A.; Wojnowska-Baryła, I.; Karczewska, M. Physicochemical properties and biogas productivity of aerobic granular sludge and activated sludge. Biochem. Eng. J. 2017, 117, 43–51. [Google Scholar] [CrossRef]
- Gao, D.-W.; Zhang, T.; Tang, C.-Y.Y.; Wu, W.-M.; Wong, C.-Y.; Lee, Y.H.; Yeh, D.H.; Criddle, C.S. Membrane fouling in an anaerobic dynamic membrane bioreactor (AnDMBR) for municipal wastewater treatment: Characteristics of membrane foulants and bulk sludge. Process Biochem. 2011, 46, 1538–1544. [Google Scholar]
- Kim, D.H.; Cho, S.K.; Lee, M.K.; Kim, M.S. Increased solubilization of excess sludge does not always result in enhanced anaerobic digestion efficiency. Bioresour. Technol. 2013, 143, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Stasinakis, A.S. Review on the fate of emerging contaminants during sludge anaerobic digestion. Bioresour. Technol. 2012, 121, 432. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, R.; Masse, D.I.; Singh, G. A critical review on inhibition of anaerobic digestion process by excess ammonia. Bioresour. Technol. 2013, 143, 632–641. [Google Scholar] [CrossRef]
- Visvanathan, C.; Abeynayaka, A. Developments and future potentials of anaerobic membrane bioreactors. Water Treat. 2012, 3, 1–23. [Google Scholar] [CrossRef]
- Zhen, G.; Lu, X.; Kato, H.; Zhao, Y.; Li, Y.T. Overview of pretreatment strategies for enhancing sewage sludge disintegration and subsequent anaerobic digestion: Current advances, full-scale application and future perspectives. Renew. Sustain. Energ. Rev. 2017, 69, 559–577. [Google Scholar] [CrossRef]
- Manser, R.; Gujer, W.; Siegrist, H. Membrane bioreactor versus conventional activated sludge system: Population dynamics of nitrifiers. Water Sci. Technol. 2005, 52, 417–425. [Google Scholar] [CrossRef]
- Seyfried, C.F. Verfahrcnstechmk der Anaeroben Abwasserreinigung—Theorie und Praxis, Verfahrcnstechnik der Mechanischen, Thermischen, Chemischen und Biologischen Abwasserreinigung, Preprints Band 2: Biologische Verfahren; Tagung der VDI-Gesellschaft Verfahrenstechnik und Chemiemgenieurwesen: Baden-Baden, Germany, 1997. (In German) [Google Scholar]
- Padmasiri, S.I.; Zhang, J.; Fitch, M.; Norddahl, B.; Morgenroth, E.; Raskin, L. Methanogenic population dynamics and performance of an anaerobic membrane bioreactor (AnMBR) treating swine manure under high shear conditions. Water Res. 2007, 41, 134–144. [Google Scholar] [CrossRef]
- Westlund, A.D.; Hagland, E.; Rothman, M. Operational aspects on foaming in digesters caused by Microthrix parvicella. Water Sci. Technol. 1998, 38, 29–34. [Google Scholar] [CrossRef]


| Series | Type of Membrane | Pore Size (µm)/Membrane Cut-off (kDa) | TMP (MPa) |
|---|---|---|---|
| MF 0.2 | MF | 0.45 µm | 0.2 |
| MF 0.3 | MF | 0.45 µm | 0.3 |
| MF 0.4 | MF | 0.45 µm | 0.4 |
| UF 0.2 | UF | 150 kDa | 0.2 |
| UF 0.3 | UF | 150 kDa | 0.3 |
| UF 0.4 | UF | 150 kDa | 0.4 |
| Series | JV (L/(m2·h)) | α (–) | Rt (m−1) | Rp (m−1) | Rc (m−1) | Ads-TSS (%) | Ads-COD (%) |
|---|---|---|---|---|---|---|---|
| MF 0.2 | 15.0 | 0.005 | 4.8 × 1013 | 9.6 × 1011 | 4.7 × 1013 | 67.2 | 67.5 |
| MF 0.3 | 44.3 | 0.009 | 2.4 × 1013 | 9.8 × 1011 | 2.3 × 1013 | 35.7 | 44.6 |
| MF 0.4 | 55.7 | 0.009 | 2.6 × 1013 | 9.5 × 1011 | 2.5 × 1013 | 41.4 | 47.7 |
| UF 0.2 | 68.9 | 0.070 | 1.0 × 1013 | 8.5 × 1011 | 8.9 × 1012 | 36.8 | 57.0 |
| UF 0.3 | 72.9 | 0.050 | 1.5 × 1013 | 8.6 × 1011 | 1.3 × 1013 | 58.1 | 61.4 |
| UF 0.4 | 76.4 | 0.040 | 1.9 × 1013 | 8.5 × 1011 | 1.7 × 1013 | 46.1 | 53.6 |
| Parameters | SS | MF 0.2 | MF 0.3 | MF 0.4 | UF 0.2 | UF 0.3 | UF 0.4 | |
|---|---|---|---|---|---|---|---|---|
| Phase I | Cumulative biogas production after phase I (L/kg COD) | 205 ± 6 | 160 ± 5 | 168 ± 7 | 178 ± 4 | 130 ± 4 | 124 ± 5 | 128 ± 6 |
| kI (d−1) | 0.28 ± 0.01 | 0.16 ± 0.01 | 0.14 ± 0.01 | 0.13 ± 0.01 | 0.29 ± 0.01 | 0.46 ± 0.01 | 0.46 ± 0.01 | |
| Duration of phase I (tI) (d) | 21 | 21 | 21 | 21 | 9 | 8 | 7 | |
| * rI (L/(kg COD⋅d)) | 57.1 ± 2.1 | 25.9 ± 3.2 | 23.5 ± 1.1 | 23.1 ± 0.9 | 37.7 ± 1.0 | 57.0 ± 2.4 | 58.9 ± 1.8 | |
| Phase II | Cumulative biogas production after phase II (L/kg COD) | – | – | – | – | 208 ± 5 | 208 ± 6 | 214 ± 3 |
| kII (d−1) | – | – | – | – | 0.22 ± 0.01 | 0.20 ± 0.01 | 0.17 ± 0.01 | |
| Duration of phase II (tII) (d) | – | – | – | – | 12 | 13 | 14 | |
| * rII (L/(kg COD d)) | – | – | – | – | 17.2 ± 0.9 | 17.6 ± 1.1 | 14.6 ± 0.8 | |
| Energy yield (kWh/m3 feedstock) | 5.0 | 2.5 | 4.5 | 4.5 | 4.4 | 4.5 | 4.8 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zielińska, M.; Bernat, K.; Mikucka, W. Membrane Bioreactor Technology: The Effect of Membrane Filtration on Biogas Potential of the Excess Sludge. Membranes 2020, 10, 397. https://doi.org/10.3390/membranes10120397
Zielińska M, Bernat K, Mikucka W. Membrane Bioreactor Technology: The Effect of Membrane Filtration on Biogas Potential of the Excess Sludge. Membranes. 2020; 10(12):397. https://doi.org/10.3390/membranes10120397
Chicago/Turabian StyleZielińska, Magdalena, Katarzyna Bernat, and Wioleta Mikucka. 2020. "Membrane Bioreactor Technology: The Effect of Membrane Filtration on Biogas Potential of the Excess Sludge" Membranes 10, no. 12: 397. https://doi.org/10.3390/membranes10120397
APA StyleZielińska, M., Bernat, K., & Mikucka, W. (2020). Membrane Bioreactor Technology: The Effect of Membrane Filtration on Biogas Potential of the Excess Sludge. Membranes, 10(12), 397. https://doi.org/10.3390/membranes10120397





